Fermentative Production of Lasiodiplodan by Lasiodiplodia theobromae CCT3966 from Pretreated Sugarcane Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Microorganism
2.3. Pretreatment of Sugarcane Straw
2.4. Enzymatic Hydrolysis
2.5. Production of Lasiodiplodan
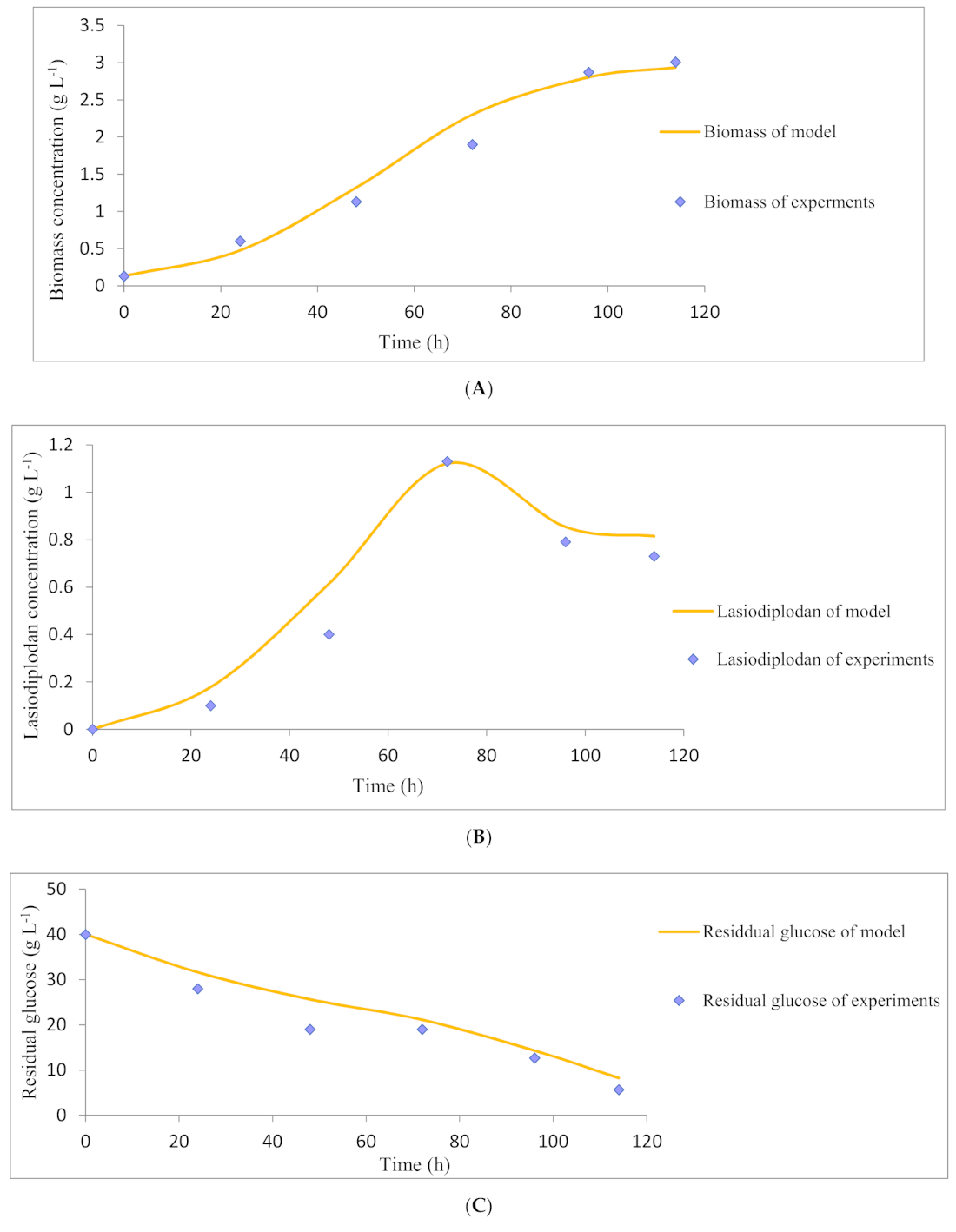
2.6. Kinetic Models of Lasiodiplodan Production Using Sugarcan Straw Hydrolysate
2.7. Measurement of Biomass and Lasiodiplodan
2.8. Chemical Characterization of Pretreated Sugarcane Straw
2.9. Scanning Electron Microscopy (SEM) of Pretreated Biomass
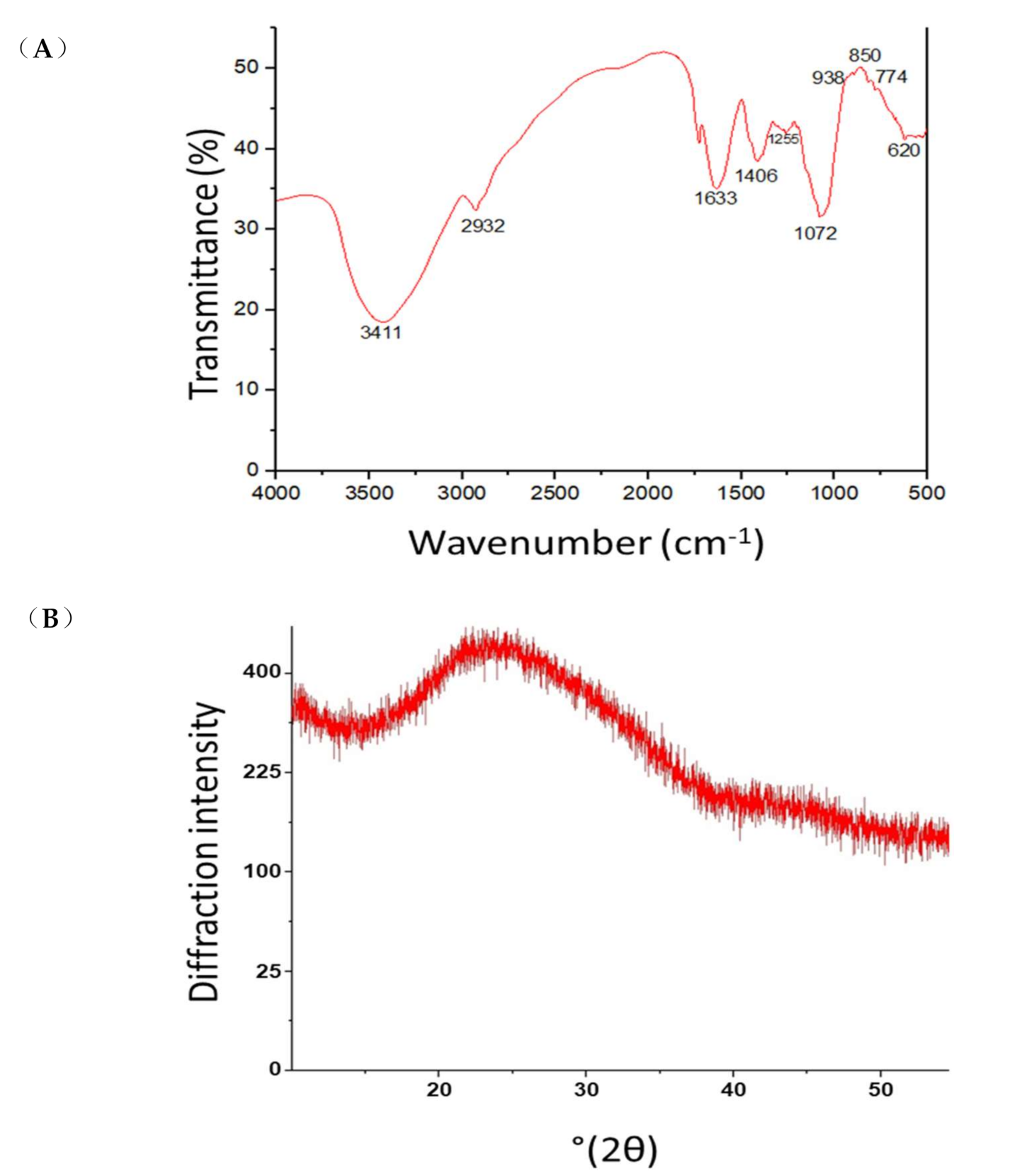
2.10. Fourier Transform Infrared (FTIR) Spectroscopy and X-ray Diffraction Analysis (XRD)
2.11. Measurement of Glucose in Hydrolysate
2.12. Statistical Analysis
3. Results
3.1. Chemical Composition of Pretreated Sugarcane Straw
3.2. Pretreatment Effect on Structural Modification of Sugarcane Straw
3.3. Enzymatic Hydrolysis of Raw and Pretreated Sugarcane Straw
3.4. Lasiodiplodan Production Using Sugarcane Straw Hydrolysate
3.5. Chemical Structure of the Lasiodiplodan Produced by L. theobromae CCT3966
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunha, M.A.A.; Turmina, J.A.; Ivanov, R.C.; Barroso, R.R.; Marques, P.T.; Fonseca, E.A.; Fortes, Z.B.; Dekker, R.F.; Khaper, N.; Barbosa, A.M. Lasiodiplodan, an exocellular (1-6)-b-D-glucan from Lasiodiplodia theobromae MMPI: Production on glucose, fermentation kinetics, rheology and anti-proliferative activity. J. Ind. Microbiol. Biotechnol. 2012, 39, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Valorization of winery spent yeast waste biomass as a new source for the production of β-glucan. Waste Biomass Valorization 2016, 7, 807–817. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; de Carvalho, A.S.; dos Santos, J.C.; da Silva, S.S. Valorization of lignocellulosic biomass and agri-food processing wastes for production of glucan polymer. Waste Biomass Valorization 2021, 12, 2915–2931. [Google Scholar] [CrossRef]
- Oliveira, K.S.; Di Bastiani, M.; Cordeiro, L.M.; Costa, M.F.; Toledo, K.A.; Iacomini, M.; Babosa, A.M.; Dekker, R.F.; Nascimento, V.M. (1→6)- and (1→3) (1→6)-β-glucans from Lasiodiplodia theobromae MMBJ: Structural characterization and pro-inflammatory activity. Carbohydr. Polym. 2015, 20, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.A.A.; Santos, V.A.Q.; Calegari, G.C.; Luna, W.N.S.; Marin, S.L.A.; Dekker, R.F.H.; Barbosa-Dekker, A.M. Structure and biological properties of lasiodiplodan: Anuncommon fungal exopolysaccharide of the (1→6)-β-D-glucan type. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 409–432. [Google Scholar]
- Theis, T.V.; Calegari, G.C.; Santos, V.A.Q.; Junior, H.E.Z.; Barbosa, A.M.; Dekker, R.F.H.; Cunha, M.A.A. Exocellular (1→6)-β-D-glucan (Lasiodiplodan): Carboxymethylation, thermal behavior, antioxidant and antimicrobial activity. Am. J. Immunol. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Freitas, S. Review on the production cost of lignocellulose-degrading enzymes. Biofuel Bioprod. Biorefin. 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; Cunha, M.A.A.; Theis, T.V.; Barbosa, A.M.; Teixeira, S.D.; Dekker, R.F.H. Production of the exopolysaccharide lasiodiplodan in a stirred-tank bioreactor. Synerg. Scyentífica UTFPR 2015, 10, 1–8. [Google Scholar]
- Tabuchi, S.C.T.; Martiniano, S.E.; Cunha, M.A.A.; Barbosa-Dekke, A.M.; Dekker, R.F.H.; Prata, A.M.R. Kinetic Study of Lasiodiplodan Production by Lasiodiplodia theobromae MMPI in a Low-Shear Aerated and Agitated Bioreactor. J. Polym. Environ. 2021, 29, 89–102. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.M.; Dekker, R.F.H.; Barbosa, A.M.; Teixeira, S.D.; Salomé, K. Carboxymethylation of (1 → 6)- β-glucan (lasiodiplodan): Preparation, characterization and antioxidant evaluation. Carbohydr. Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflügl, S.; Marx, H.; Mattanovich, D.; Sauer, M. Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1,3-propanediol by Lactobacillus diolivorans. Bioresour. Technol. 2014, 152, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, S.; Ge, X. Co-production of 1,3-propanediol and 2,3-butanediol from waste lard by co-cultivation of Pseudomonas alcaligenes and Klebsiella pneumonia. Curr. Microbiol. 2019, 76, 415–424. [Google Scholar] [CrossRef]
- Philippini, R.R.; Martiniano, S.E.; Marcelino, P.R.F.; Chandel, A.K.; dos Santos, J.C.; da Silva, S.S. Production of β-glucan exopolysaccharide lasiodiplodan by Lasiodiplodia theobromae CCT3966 from corn bran acid hydrolysate. Appl. Microbiol. Biotechnol. 2021, 105, 2319–2332. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, Y.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Sarsaiya, S.; Jaina, A.; Awasthic, S.K.; Duanc, Y.; Awasthic, M.K.; Shia, J. Microbial dynamics for lignocellulosic waste bioconversion and its importance with modern circular economy, challenges and future perspectives. Bioresour. Technol. 2019, 291, 121905. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, J.J.; Philippini, R.R.; Gomes, F.M.; Pereira, F.M.; da Silva, S.S.; Kumar, V.; Chandel, A.K. Comparative highly efficient production of β-glucan by Lasiodiplodia theobromae CCT3966 and its multiscale characterization. Fermentation 2021, 7, 108. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.F.; de Arruda, P.V.; Felipe, M.G.A. Sugarcane straw as a feedstock for xylitol production by Candida guilliermondii FTI 20037. Braz. J. Microbiol. 2016, 47, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Aquino, G.S.; de Conti Medina, C.; da Costa, D.C.; Shahab, M.; Santiago, A.D. Sugarcane straw management and its impact on production and development of rations. Ind. Crops Prod. 2017, 102, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.M.; Sevastyanova, O.; Penna, L.S.; Silva, B.P.; Lindstromb, M.E.; Colodette, J.L. Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind. Crops Prod. 2015, 73, 118–126. [Google Scholar] [CrossRef]
- Production of Sugar Cane in Brazil from Crop Year 2010/11 to 2020/21. Statista. Available online: https://www.statista.com/statistics/742530/sugar-cane-production-volume-brazil/ (accessed on 15 June 2021).
- Menandro, L.M.S.; Cantarella, H.; Franco, H.C.J.; Kölln, O.T.; Pimenta, M.T.B.; Sanches, G.M.; Rabelo, S.C.; Carvalho, J.L.N. Comprehensive assessment of sugarcane straw: Implications for biomass and bioenergy production. Biofuel Bioprod. Biorefin. 2017, 11, 488–504. [Google Scholar] [CrossRef]
- Moutta, R.O.; Silva, M.C.; Corrales, R.C.N.R.; Cerullo, M.A.S.; Ferreira-Leitão, V.S.; Bon, E.P.S. Comparative response and structural characterization of sugarcane bagasse, straw and bagasse-straw 1:1 mixtures subjected to hydrothermal pretreatment and enzymatic conversion. J. Microb. Biochem. Technol. 2013, 12, 1–8. [Google Scholar]
- Gómez, E.O.; Souza, R.T.G.; Rocha, G.J.M.; Almeida, E.; Cortez, L.A.B. Sugarcane trash as feedstock for second generation processes. In Sugarcane Bioethanol—R&D for Productivity and Sustainability; Cortez, L.A.B., Ed.; Blucher: São Paulo, Brazil, 2014; pp. 637–660. [Google Scholar]
- Lachos-Perez, D.; Tompsett, G.A.; Guerra, P.; Timko, M.T.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Sugars and char formation on subcritical water hydrolysis of sugarcane straw. Bioresour. Technol. 2017, 243, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.G.; Mori, N.R.; Gonçalves, A.R. Sugarcane straw as feedstock for 2G ethanol: Evaluation of pretreatments and enzymatic hydrolysis. Ind. Crops Prod. 2019, 142, 111845. [Google Scholar] [CrossRef]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. Lignocellulolytic enzymes in biotechnological and industrial processes: A review. Sustainability 2020, 12, 7282. [Google Scholar] [CrossRef]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.S.; Paraschiv, G. Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Chaudhary, N.; Qazi, J.I.; Irfan, M. Isolation and identification of cellulolytic and ethanologenic bacteria from soil. Iran. J. Sci. Technol. Trans. Sci. 2017, 41, 551–555. [Google Scholar] [CrossRef]
- Mulyaningtyas, A.; Sediawan, W.B. Effect of combined pretreatment of lignocellulose and the kinetics of its subsequent bioconversion by Aspergillus niger. Biocatal. Agric. Biotechnol. 2019, 21, 101292. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. Sci. World J. 2014, 1–13. [Google Scholar] [CrossRef]
- Zaafouri, K.; Ziadi, M.; Trabelsi, A.B.H.; Mekni, S.; Aïssi, B.; Alaya, M.; Bergaoui, L.; Hamdi, M. Optimization of hydrothermal and diluted acid pretreatments of Tunisian Luffa cylindrica (L.) fibers for 2G bioethanol production through the cubic central composite experimental design CCD: Response surface methodology. BioMed. Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miléo, P.C.; Rodrigues, L.C.; Gandara, M.; Rocha, G.J.M.; Goncalves, A.R. Cellulose. In biorefinary: Fibres obtainment form sugarcane bagasse and pretreated by diluted acid and delignified by alkali. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 18–22 June 2012. [Google Scholar]
- De Jong, E.; Gosselink, R.J.A. lignocellulose-based chemical products. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 277–313. [Google Scholar]
- Jia, Z.; Zheng, Y.; Zhou, J. Effects of different pretreatment methods on the enzymatic hydrolysis of cassava residue. BioResources 2019, 14, 6060–6078. [Google Scholar] [CrossRef]
- Dzieko´nska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef] [Green Version]
- Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; dos Santos, J.C.; da Silva, S.S. Utilization of sugarcane straw for production of β-glucan biopolymer by Lasiodiplodia theobromae CCT3966 in batch fermentation process. Bioresour. Technol. 2020, 314, 123716. [Google Scholar] [CrossRef] [PubMed]
- Kusmiyati, K.; Anarki, S.T.; Nugroho, S.W.; Widiastutik, R.; Hadiyanto, H. Effects of dilute acid and alkaline pretreatments on enzymatic saccharification of palm tree trunk waste for bioethanol produc-tion. Bull. Chem. React. Eng. Catal. 2019, 14, 705–714. [Google Scholar] [CrossRef]
- Tri, C.L.; Khuong, L.D.; Kamei, I. The improvement of sodium hydroxide pretreatment in bioethanol production from Japanese bamboo Phyllostachys edulis using the white rot fungus Phlebia sp. MG-60. Int. Biodeterior. Biodegradation 2018, 133, 86–92. [Google Scholar] [CrossRef]
- Santos-Rocha, M.S.R.; Pratto, B.; Corrêa, L.J.; Badino, A.C.; Almeida, R.M.R.G.; Cruz, A.J.G. Assessment of different biomass feeding strategies for improving the enzymatic hydrolysis of sugarcane straw. Ind. Crops Prod. 2018, 125, 293–302. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, J.; Wang, Z.; Hang, H.; Zhao, W.; Zhuang, Y.; Chu, J. Kinetic analysis of curdlan production by Alcaligenes faecalis with maltose, sucrose, glucose and fructose as carbon sources. Bioresour. Technol. 2018, 259, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Luedeking, R.; Piret, E.L. A kinetic study of the lactic acid fermentation. Batch process at controlled pH. Biotechnol. Bioeng. 2000, 67, 636–644. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Moutta, R.O.; Ferreira-Leitao, V.S.; Bon, E.P.S. Enzymatic hydrolysis of sugarcane bagasse and straw mixtures pretreated with diluted acid. Biocatal. Biotransformation 2014, 32, 93–100. [Google Scholar] [CrossRef]
- Cortez, D.V.; Roberto, I.C.; Barbosa, M.H.P.; Milagres, A.M.F. Evaluation of cellulosic and hemicellulosic hydrolysate fermentability from sugarcane bagasse hyborids with different compositions. Biomass Convers. Biorefin. 2014, 4, 351–356. [Google Scholar] [CrossRef]
- Guilherme, A.A.; Dantas, P.V.F.; Soares, J.C.J.; Santos, E.S.; Fernandes, F.A.N.; Macedo, G.R. Pretreatments and enzymatic hydrolysis of sugarcane bagasse aiming at the enhancement of the yield of glucose and xylose. Braz. J. Chem. Eng. 2017, 34, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.S.R.S.; Pratto, B.; Júnior, R.S.; Almeida, R.M.R.G.; Cruz, A.J.G. A kinetic model for hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2017, 228, 176–185. [Google Scholar] [CrossRef]
- Alrumman, S.A. Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, S.C.; Maehara, L.; Machado, C.M.M.; Farinas, C.S. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, L.R.M.; Nascimento, V.M.; Goncalves, A.R.; Rocha, G.J.M. Combined process system for the production of bioethanol fromsugarcane straw. Ind. Crops Prod. 2014, 58, 1–7. [Google Scholar] [CrossRef]
- Crognale, S.; Federici, F.; Petruccioli, M. β-Glucan production by Botryosphaeria rhodina on undiluted olive-mill wastewaters. Biotechnol. Lett. 2003, 25, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Prochazka, V.; Synytsya, A.; Blahovec, J.; Hejlova, A.; Čopikova, J. Yeast β (1-3), (1-6)-D-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]



| Component | Untreated Biomass (%) c | After Acid Pretreatment (%) | After Alkaline Pretreatment (%) |
|---|---|---|---|
| Cellulose | 41.25 ± 1.10 | 62.28 ± 1.11 | 82.22 ± 1.04 |
| Hemicelluloses | 35.17 ± 0.30 | 3.53 ± 0.40 | ND b |
| Lignin | 17.25 ± 3.39 | 32.15 ± 0.44 | 16.28 ± 1.35 |
| Ash | 2.81 ± 1.43 | 2.265 ± 0.007 | 1.73 ± 0.29 |
| Kinetic Parameter | Value |
|---|---|
| 0.13 gL−1 | |
| 3.01 gL−1 | |
| 0.06 h−1 | |
| 4.54 gg−1 | |
| 0.054 gg−1 | |
| 0.016 gL−1h−1 | |
| 0.09 gg−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; Ingle, A.P.; Abdeshahian, M.; Santos, J.C.d.; Silva, S.S.d. Fermentative Production of Lasiodiplodan by Lasiodiplodia theobromae CCT3966 from Pretreated Sugarcane Straw. Sustainability 2021, 13, 9697. https://doi.org/10.3390/su13179697
Abdeshahian P, Ascencio JJ, Philippini RR, Antunes FAF, Ingle AP, Abdeshahian M, Santos JCd, Silva SSd. Fermentative Production of Lasiodiplodan by Lasiodiplodia theobromae CCT3966 from Pretreated Sugarcane Straw. Sustainability. 2021; 13(17):9697. https://doi.org/10.3390/su13179697
Chicago/Turabian StyleAbdeshahian, Peyman, Jesús Jiménez Ascencio, Rafael R. Philippini, Felipe Antonio Fernandes Antunes, Avinash P. Ingle, Mojgan Abdeshahian, Júlio César dos Santos, and Silvio Silvério da Silva. 2021. "Fermentative Production of Lasiodiplodan by Lasiodiplodia theobromae CCT3966 from Pretreated Sugarcane Straw" Sustainability 13, no. 17: 9697. https://doi.org/10.3390/su13179697
APA StyleAbdeshahian, P., Ascencio, J. J., Philippini, R. R., Antunes, F. A. F., Ingle, A. P., Abdeshahian, M., Santos, J. C. d., & Silva, S. S. d. (2021). Fermentative Production of Lasiodiplodan by Lasiodiplodia theobromae CCT3966 from Pretreated Sugarcane Straw. Sustainability, 13(17), 9697. https://doi.org/10.3390/su13179697










