Rhizobacteria Inoculation and Caffeic Acid Alleviated Drought Stress in Lentil Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Site
2.2. ACC-Deaminase Containing Rhizobacterial Strains and Inoculum Preparation
- Lysinibacillus fusiform [29]
2.3. Seed Dressing
2.4. Caffeic Acid (CA)
2.5. Drought Level
2.6. Treatments
- T1
- uninoculated + 100% field capacity
- T2
- uninoculated + drought
- T3
- Bacillus amyloliquefaciens + drought
- T4
- Lysinibacillus fusiform + drought
- T5
- CA (20 ppm) + drought
- T6
- CA (50 ppm) + drought
- T7
- CA (100 ppm) + drought
- T8
- CA (20 ppm) + Bacillus amyloliquefaciens plus drought
- T9
- CA (50 ppm) + Bacillus amyloliquefaciens plus drought
- T10
- CA (100 ppm) + Bacillus amyloliquefaciens plus drought
- T11
- CA (20 ppm) + Lysinibacillus fusiform plus drought
- T12
- CA (50 ppm) + Lysinibacillus fusiform plus drought
- T13
- CA (100 ppm) + Lysinibacillus fusiform plus drought
2.7. Sowing and Harvesting
2.8. Pre-Sowing Soil Analysis
2.9. Plant Analyses
2.10. Statistical Analysis
3. Results
3.1. Plant Height, Number of Pods Per Plant, 1000-Grain Weight and Chlorophyll Contents
3.2. Nutrients Concentration, Protein and Relative Water Contents
3.3. Electrolyte Leakage, Proline Contents, and Hydrogen Peroxide Contents Decreased, while Membrane Stability Increased
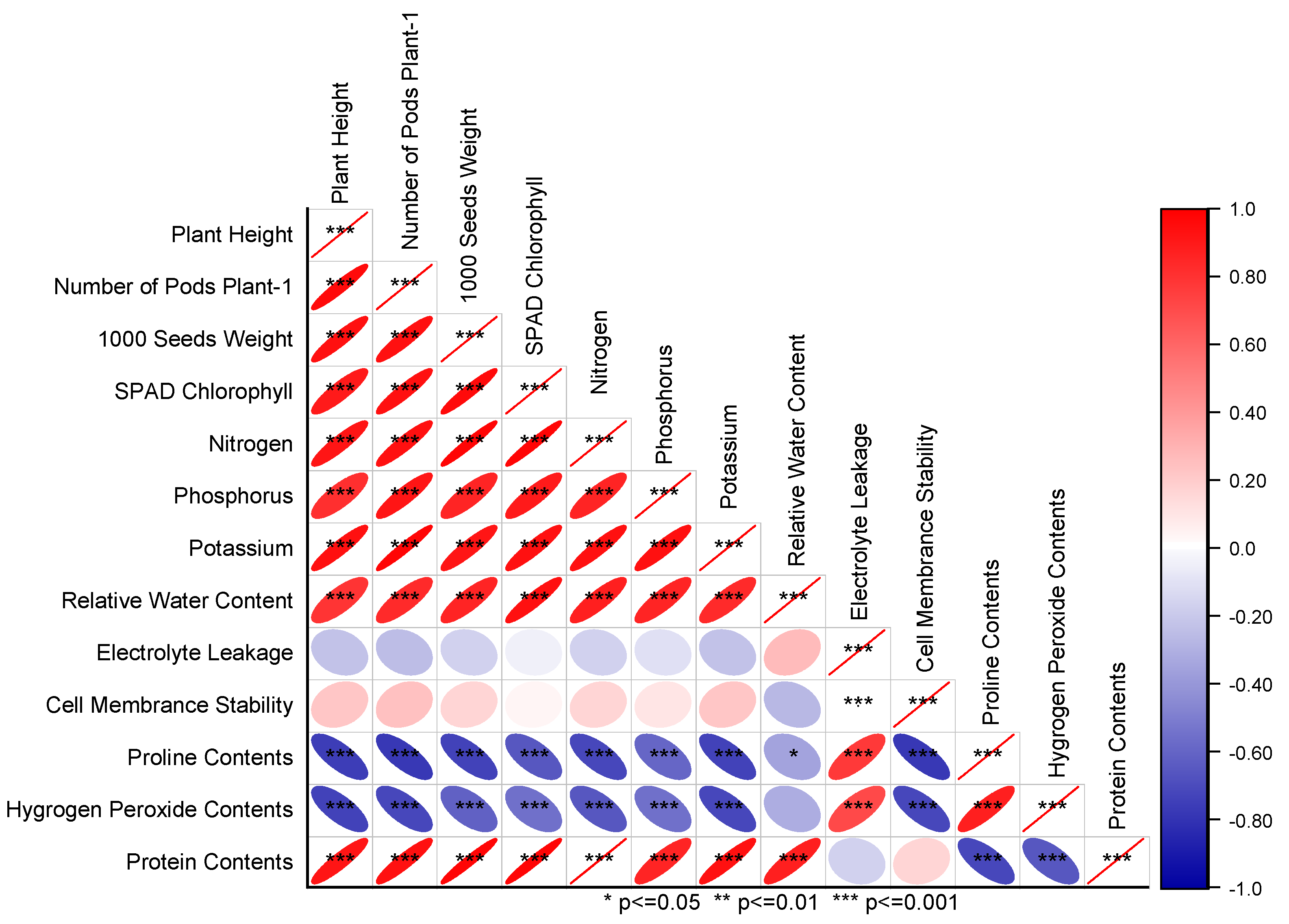
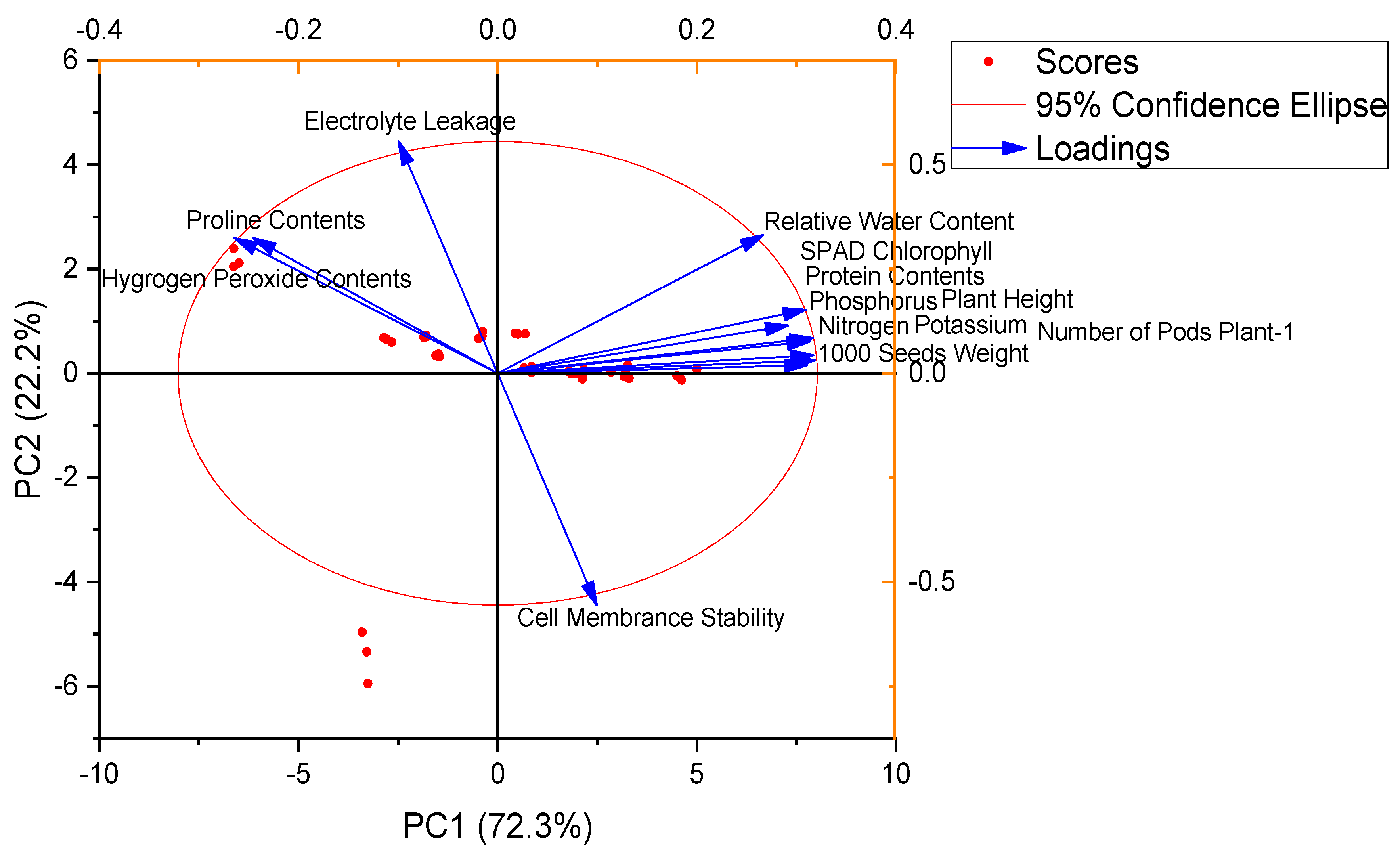
3.4. Pearson Correlation and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erskine, W.; Nesbitt, H. How can agriculture research make a difference in countries emerging from conflict? Exp. Agric. 2009, 45, 313–321. [Google Scholar] [CrossRef]
- Shah, S.; Ramanan, V.V.; Singh, A.; Singh, A.K. Potential and Prospect of Plant Growth Promoting Rhizobacteria in Lentil; Satish Serial Publishing House: Delhi, India, 2018. [Google Scholar]
- Voisin, A.-S.; Gastal, F. Nitrogen nutrition and specific agrophysiological functioning of legumes. In Legumes for Sustainable Agricultural and Food Systems; Editions Quae: Versailles, France, 2015; p. 01259103. [Google Scholar]
- Malhotra, R.S.; Sarker, A.; Saxena, M.C. Drought Tolerance in Chickpea and Lentil Present Status and Future Strategies. Chall. Strateg. Dryl. Agric. 2011, 257. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; uz Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pak. J. Agric. Res. 2019, 32, 8. [Google Scholar] [CrossRef]
- Johansen, C.; Baldev, B.; Brouwer, J.B.; Erskine, W.; Jermyn, W.A.; Li-Juan, L.; Malik, B.A.; Miah, A.A.; Silim, S.N. Biotic and abiotic stresses constraining productivity of cool season food legumes in Asia, Africa and Oceania. In Expanding the Production and Use of Cool Season Food Legumes; Springer: Berlin/Heidelberg, Germany, 1994; pp. 175–194. [Google Scholar]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Caffeic acid inhibits in vitro rooting in mung bean [Vigna radiata (L.) Wilczek] hypocotyls by inducing oxidative stress. Plant Growth Regul. 2009, 57, 21–30. [Google Scholar] [CrossRef]
- Delfine, S.; Alvino, A.; Zacchini, M.; Loreto, F. Consequences of salt stress on conductance to CO2 diffusion, Rubisco characteristics and anatomy of spinach leaves. Funct. Plant Biol. 1998, 25, 395–402. [Google Scholar] [CrossRef]
- Mishra, B.K.; Srivastava, J.P.; Lal, J.P.; Sheshshayee, M.S. Physiological and biochemical adaptations in lentil genotypes under drought stress. Russ. J. Plant Physiol. 2016, 63, 695–708. [Google Scholar] [CrossRef]
- Hussain, M.; Bashir, W.; Farooq, S.; Rehim, A. Root Development, Allometry and Productivity of Maize Hybrids under Terminal Drought Sown by Varying Method. Int. J. Agric. Biol. 2013, 15, 1244–1250. [Google Scholar]
- Shakir, M.A.; Bano, A.; Arshad, M. Rhizosphere bacteria containing ACC-deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ. 2012, 31, 108–112. [Google Scholar]
- Wenke, K.; Kai, M.; Piechulla, B. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 2010, 231, 499–506. [Google Scholar] [CrossRef]
- Van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.L. Plant growth promoting rhizobacteria: Fundamentals and applications. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–43. [Google Scholar]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Farooq, H.M.; Zahir, Z.A.; Hussain, M.; Hussain, A. Application of ACC-deaminase containing rhizobacteria with fertilizer improves maize production under drought and salinity stress. Int. J. Agric. Biol. 2014, 16, 591–596. [Google Scholar]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil 2020, 451, 57–73. [Google Scholar] [CrossRef]
- Mayer, M.J.; Narbad, A.; Parr, A.J.; Parker, M.L.; Walton, N.J.; Mellon, F.A.; Michael, A.J. Rerouting the plant phenylpropanoid pathway by expression of a novel bacterial enoyl-CoA hydratase/lyase enzyme function. Plant Cell 2001, 13, 1669–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.A. Natural Fungicides Obtained from Plants. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Ed.; IntechOpen: London, UK, 2012; pp. 953–978. [Google Scholar]
- Klein, A.; Keyster, M.; Ludidi, N. Caffeic acid decreases salinity-induced root nodule superoxide radical accumulation and limits salinity-induced biomass reduction in soybean. Acta Physiol. Plant. 2013, 35, 3059–3066. [Google Scholar] [CrossRef]
- Gowri, G.; Bugos, R.C.; Campbell, W.H.; Maxwell, C.A.; Dixon, R.A. Stress responses in alfalfa (Medicago sativa L.) X. Molecular cloning and expression of S-adenosyl-L-methionine: Caffeic acid 3-O-methyltransferase, a key enzyme of lignin biosynthesis. Plant Physiol. 1991, 97, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippert, P.; Puyaubert, J.; Grisollet, D.; Derrier, L.; Matringe, M. Tyrosine and phenylalanine are synthesized within the plastids in Arabidopsis. Plant Physiol. 2009, 149, 1251–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achnine, L.; Blancaflor, E.B.; Rasmussen, S.; Dixon, R.A. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 2004, 16, 3098–3109. [Google Scholar] [CrossRef] [PubMed]
- Koshiro, Y.; Jackson, M.C.; Katahira, R.; Wang, M.-L.; Nagai, C.; Ashihara, H. Biosynthesis of chlorogenic acids in growing and ripening fruits of Coffea arabica and Coffea canephora plants. Z. Naturforsch. C 2007, 62, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Grzesiak, S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. J. Plant Physiol. 2009, 166, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, J.-P.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 2007, 17, 291–297. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Yaseen, R.; Akbar, M.N.; Asghar, H. Mitigating the effect of salinity stress through integrated application of ACC-deaminase containing rihizobacteria and biogas slurry to improve the productivity of wheat crop. Merit Res. J. Agric. Sci. Soil Sci. 2020, 8, 72–81. [Google Scholar]
- Zafar-ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Zafar-Ul-hye, M.; Farooq, U.; Danish, S.; Hussain, S.; Shaaban, M.; Qayyum, M.F.; Rehim, A. Bacillus amyloliquefaciens and alcaligenes faecalis with biogas slurry improved maize growth and yield in saline-sodic field. Pak. J. Bot. 2020, 52, 1839–1847. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J.W. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Naveed, M.; Mitter, B.; Sessitsch, A. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 2014, 21, 11054–11065. [Google Scholar] [CrossRef] [PubMed]
- Helali, A.K.; Islam, M.T.; Islam, M.T. Evaluation of some lentil genotypes under different soil moisture regimes. Bangladesh J. Nucl. Agric. 2002, 11, 58–66. [Google Scholar]
- Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.A.; Alhejeli, A.M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis, Part 3, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 417–435. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Soil pH and lime requirement. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1982; pp. 199–208. [Google Scholar]
- Bouyouces, G.J. Hydrometer method improved for making particle size analysis of soil. Agron. J. 1962, 53, 464–465. [Google Scholar] [CrossRef]
- Walkley, A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils. J. Agric. Sci. 1935, 25, 598. [Google Scholar] [CrossRef]
- Jackson, M.C. Soil Chemical Analysis; Prentice Hall. Inc.: Englewood, CO, USA, 1962. [Google Scholar]
- Pratt, P.F. Potassium. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2; Norman, A.G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1965; pp. 1022–1030. [Google Scholar]
- Hussain, F.; Bronson, K.F.; Yadvinder, S.; Singh, B.; Peng, S. Use of chlorophyll meter sufficiency indices for nitrogen management of irrigated rice in Asia. Agron. J. 2000, 92, 875–879. [Google Scholar]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Kirnak, H.; Kaya, C.; Higgs, D.; Gercek, S. A long-term experiment to study the role of mulches in the physiology and macro-nutrition of strawberry grown under water stress. Aust. J. Agric. Res. 2001, 52, 937–943. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance, Methods in Molecular Biology; Sunkar, R., Ed.; Humana Press: New York, NY, USA, 2010; pp. 291–297. [Google Scholar]
- Mishra, B.K.; Srivastava, J.P.; Lal, J.P. Drought resistance in lentil (Lens culinaris Medik.) in relation to morphological, physiological parameters and phenological developments. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2288–2304. [Google Scholar] [CrossRef]
- Yeoh, H.-H.; Wee, Y.-C. Leaf protein contents and nitrogen-to-protein conversion factors for 90 plant species. Food Chem. 1994, 49, 245–250. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mondal, R.; Choudhuri, M.A. Role of hydrogen peroxide in senescence of excised leaves of rice and maize. Biochem. Physiol. Pflanz. 1981, 176, 700–709. [Google Scholar] [CrossRef]
- Danish, S.; Younis, U.; Akhtar, N.; Ameer, A.; Ijaz, M.; Nasreen, S.; Huma, F.; Sharif, S.; Ehsanullah, M. Phosphorus solubilizing bacteria and rice straw biochar consequence on maize pigments synthesis. Int. J. Biosci. 2015, 5, 31–39. [Google Scholar]
- Han, H.S.; Lee, K.D. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar] [CrossRef]
- Silva, R.; Filgueiras, L.; Santos, B.; Coelho, M.; Silva, M.; Estrada-Bonilla, G.; Vidal, M.; Baldani, J.I.; Meneses, C. Gluconacetobacter diazotrophicus changes the molecular mechanisms of root development in Oryza sativa L. growing under water stress. Int. J. Mol. Sci. 2020, 21, 33. [Google Scholar] [CrossRef] [Green Version]
- Danish, S.; Zafar-ul-Hye, M.; Hussain, M.; Shaaban, M.; Núñez-delgado, A. Rhizobacteria with ACC-Deaminase Activity Improve Nutrient Uptake, Chlorophyll Contents and Early Seedling Growth of Wheat under PEG- Induced Osmotic Stress. Int. J. Agric. Biol. 2019, 21, 1212–1220. [Google Scholar]
- Danish, S.; Zafar-Ul-Hye, M.; Hussain, S.; Riaz, M.; Qayyum, M.F. Mitigation of drought stress in maize through inoculation with drought tolerant ACC deaminase containing PGPR under axenic conditions. Pak. J. Bot. 2020, 52, 49–60. [Google Scholar] [CrossRef]
- Amarowicz, R.; Weidner, S. Biological activity of grapevine phenolic compounds. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 389–405. [Google Scholar]
- Arshad, M.; Shaharoona, B.; Mahmood, T. Inoculation with Pseudomonas spp. Containing ACC-Deaminase Partially Eliminates the Effects of Drought Stress on Growth, Yield, and Ripening of Pea (Pisum sativum L.). Pedosphere 2008, 18, 611–620. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Zahra, M.B.; Danish, S.; Abbas, M.; Rehim, A.; Akbar, M.N.; Iftikhar, A.; Gul, M.; Nazir, I.; Abid, M.; et al. Multi-strain inoculation with pgpr producing acc deaminase is more effective than single-strain inoculation to improve wheat (Triticum aestivum) growth and yield. Phyton 2020, 89, 405–413. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought stress alleviation by ACC deaminase producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without timber waste biochar in maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Erdogan, U.; Çakmakçi, R.; Varmazyari, A.; Turan, M.; Erdogan, Y.; Kitir, N. Role of inoculation with multi-trait rhizobacteria on strawberries under water deficit stress. Zemdirb.-Agric. 2016, 103, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Biju, S.; Fuentes, S.; Gupta, D. Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol. Biochem. 2017, 119, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Srivastava, R.; Sharma, A.K. Influence of IAA and ACC Deaminase Producing Fluorescent Pseudomonads in Alleviating Drought Stress in Wheat (Triticum aestivum). Agric. Res. 2018, 7, 290–299. [Google Scholar] [CrossRef]
| Characteristics | Units | Soil |
|---|---|---|
| Textural class | ‒ | Silt loam |
| Sand | % | 21 |
| Silt | % | 60 |
| Clay | % | 19 |
| pHs | ‒ | 8.01 |
| ECe | dS m−1 | 1.03 |
| Organic matter | % | 0.63 |
| Total nitrogen | % | 0.02 |
| Available phosphorus | mg kg−1 | 4.5 |
| Extractable potassium | mg kg−1 | 107 |
| Treatment | Plant Height | Number of Pods | 1000 Grains Weight | Chlorophyll |
|---|---|---|---|---|
| (cm) | Plant−1 | (g) | (SPAD Value) | |
| Control | 14.32 ± 0.11 i | 22.27 ± 0.12 l | 27.15 ± 0.07 l | 17.42 ± 0.15 j |
| Drought | 12.14 ± 0.11 j | 20.37 ± 0.07 m | 25.21 ± 0.11 m | 18.32 ± 0.10 i |
| Strain1 + Drought | 16.03 ± 0.07 g | 24.19 ± 0.09 i | 29.31 ± 0.08 j | 21.13 ± 0.06 h |
| Strain2 + Drought | 15.25 ± 0.14 h | 23.56 ± 0.10 k | 28.22 ± 0.13 k | 20.61 ± 0.07 h |
| CA (20 ppm) + Drought | 17.08 ± 0.07 c | 25.86 ± 0.05 g | 33.52 ± 0.05 e | 24.75 ± 0.07 e |
| CA (50 ppm) + Drought | 16.81 ± 0.07 d | 24.89 ± 0.08 h | 32.57 ± 0.05 g | 23.68 ± 0.06 f |
| CA (100 ppm) + Drought | 15.43 ± 0.10 h | 23.82 ± 0.08 j | 31.44 ± 0.06 i | 22.92 ± 0.07 g |
| Strain1 + CA (20 ppm) + Drought | 18.84 ± 0.07 a | 30.86 ± 0.07 a | 36.73 ± 0.06 a | 28.24 ± 0.08 a |
| Strain1 + CA (50 ppm) + Drought | 17.48 ± 0.05 b | 28.69 ± 0.06 c | 34.83 ± 0.06 b | 27.79 ± 0.09 ab |
| Strain1 + CA (100 ppm) + Drought | 16.56 ± 0.05 e | 26.41 ± 0.08 e | 33.81 ± 0.08 d | 26.80 ± 0.16 c |
| Strain2 + CA (20 ppm) + Drought | 18.62 ± 0.05 a | 29.65 ± 0.08 b | 34.21 ± 0.09 c | 27.48 ± 0.15 b |
| Strain2 + CA (50 ppm) + Drought | 17.30 ± 0.05 bc | 27.70 ± 0.11 d | 32.96 ± 0.10 f | 26.56 ± 0.22 c |
| Strain2 + CA (100 ppm) + Drought | 16.33 ± 0.08 f | 26.16 ± 0.08 f | 31.87 ± 0.09 h | 25.58 ± 0.20 d |
| Treatment | Nitrogen | Phosphorus | Potassium | Protein | RWC |
|---|---|---|---|---|---|
| (%) | (mg/g) | (%) | |||
| Control | 2.73 ± 0.06 h | 0.02136 ± 0.0050 j | 0.72 ± 0.024 fg | 12.11 ± 0.041 h | 22.39 ± 0.14 i |
| Drought | 2.50 ± 0.05 i | 0.02207 ± 0.0043 i | 0.69 ± 0.024 g | 11.11 ± 0.047 i | 34.51 ± 0.54 h |
| Strain1 + Drought | 3.15 ± 0.05 fg | 0.02329 ± 0.0027 fg | 0.76 ± 0.024 de | 13.99 ± 0.058 fg | 42.08 ± 0.04 f |
| Strain2 + Drought | 3.01 ± 0.06 g | 0.02259 ± 0.0035 h | 0.74 ± 0.024 ef | 13.36 ± 0.041 g | 39.93 ± 0.14 g |
| CA (20 ppm) + Drought | 3.43 ± 0.05 de | 0.02370 ± 0.0035 e | 0.78 ± 0.029 cd | 15.24 ± 0.025 de | 47.49 ± 0.12 d |
| CA (50 ppm) + Drought | 3.39 ± 0.07 e | 0.02299 ± 0.0038 gh | 0.76 ± 0.024 de | 15.03 ± 0.033 e | 44.26 ± 0.17 e |
| CA (100 ppm) + Drought | 3.29 ± 0.08 ef | 0.02162 ± 0.0043 j | 0.72 ± 0.033 f | 14.61 ± 0.025 ef | 42.16 ± 0.09 f |
| Strain1 + CA (20 ppm) + Drought | 3.90 ± 0.10 a | 0.02626 ± 0.0035 a | 0.84 ± 0.047 a | 17.32 ± 0.033 a | 54.95 ± 0.50 a |
| Strain1 + CA (50 ppm) + Drought | 3.80 ± 0.08 ab | 0.02514 ± 0.0043 b | 0.82 ± 0.033 ab | 16.86 ± 0.025 ab | 51.60 ± 0.10 b |
| Strain1 + CA (100 ppm) + Drought | 3.74 ± 0.08 b | 0.02504 ± 0.0035 bc | 0.80 ± 0.033 bc | 16.61 ± 0.041 b | 50.50 ± 0.30 bc |
| Strain2 + CA (20 ppm) + Drought | 3.66 ± 0.08 bc | 0.02470 ± 0.0035 cd | 0.82 ± 0.041 ab | 16.24 ± 0.033 bc | 51.84 ± 0.11 b |
| Strain2 + CA (50 ppm) + Drought | 3.57 ± 0.08 cd | 0.02433 ± 0.0043 d | 0.80 ± 0.024 bc | 15.86 ± 0.025 cd | 48.84 ± 0.13 cd |
| Strain2 + CA (100 ppm) + Drought | 3.54 ± 0.08 cd | 0.02348 ± 0.0035 ef | 0.78 ± 0.024 cd | 15.74 ± 0.041 cd | 47.27 ± 0.13 d |
| Treatment | Electrolyte Leakage | Cell Membrane Stability | Proline Contents | Hydrogen Peroxide Contents |
|---|---|---|---|---|
| (%) | µg g−1 | nmol g−1 | ||
| Control | 21.77 ± 0.57 i | 78.22 ± 0.57 a | 0.53 ± 0.04 ij | 1.66 ± 0.04 gh |
| Drought | 52.53 ± 0.20 a | 47.47 ± 0.20 i | 0.91 ± 0.05 a | 2.41 ± 0.06 a |
| Strain + Drought | 44.42 ± 0.17 bc | 55.58 ± 0.17 gh | 0.73 ± 0.06 c | 1.35 ± 0.07 e |
| Strain2 + Drought | 46.18 ± 0.04 b | 53.81 ± 0.04 h | 0.79 ± 0.04 b | 1.50 ± 0.04 d |
| CA (20 ppm) + Drought | 43.39 ± 0.05 cd | 56.60 ± 0.05 fg | 0.63 ± 0.03 ef | 1.56 ± 0.04 c |
| CA (50 ppm) + Drought | 44.00 ± 0.10 bc | 55.99 ± 0.10 gh | 0.67 ± 0.03 de | 1.64 ± 0.04 b |
| CA (100 ppm) + Drought | 45.15 ± 0.07 bc | 54.85 ± 0.07 gh | 0.71 ± 0.03 cd | 1.69 ± 0.03 b |
| Strain1 + CA (20 ppm) + Drought | 36.74 ± 0.06 h | 63.26 ± 0.06 b | 0.51 ± 0.03 j | 1.11 ± 0.04 h |
| Strain1 + CA (50 ppm) + Drought | 37.54 ± 0.05 gh | 62.45 ± 0.05 bc | 0.55 ± 0.03 hi | 1.19 ± 0.05 g |
| Strain1 + CA (100 ppm) + Drought | 38.21 ± 0.08 fgh | 61.79 ± 0.08 bcd | 0.58 ± 0.04 gh | 1.28 ± 0.04 f |
| Strain2 + CA (20 ppm) + Drought | 39.39 ± 0.05 efg | 60.60 ± 0.05 cde | 0.54 ± 0.03 ij | 1.18 ± 0.05 g |
| Strain2 + CA (50 ppm) + Drought | 40.16 ± 0.05 ef | 59.83 ± 0.05 de | 0.57 ± 0.03 ghi | 1.22 ± 0.04 g |
| Strain2 + CA (100 ppm) + Drought | 41.04 ± 0.09 de | 58.95 ± 0.09 ef | 0.60 ± 0.04 fg | 1.31 ± 0.04 ef |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar-ul-Hye, M.; Akbar, M.N.; Iftikhar, Y.; Abbas, M.; Zahid, A.; Fahad, S.; Datta, R.; Ali, M.; Elgorban, A.M.; Ansari, M.J.; et al. Rhizobacteria Inoculation and Caffeic Acid Alleviated Drought Stress in Lentil Plants. Sustainability 2021, 13, 9603. https://doi.org/10.3390/su13179603
Zafar-ul-Hye M, Akbar MN, Iftikhar Y, Abbas M, Zahid A, Fahad S, Datta R, Ali M, Elgorban AM, Ansari MJ, et al. Rhizobacteria Inoculation and Caffeic Acid Alleviated Drought Stress in Lentil Plants. Sustainability. 2021; 13(17):9603. https://doi.org/10.3390/su13179603
Chicago/Turabian StyleZafar-ul-Hye, Muhammad, Muhammad Naeem Akbar, Yasir Iftikhar, Mazhar Abbas, Atiqa Zahid, Shah Fahad, Rahul Datta, Muqarrab Ali, Abdallah M. Elgorban, Mohammad Javed Ansari, and et al. 2021. "Rhizobacteria Inoculation and Caffeic Acid Alleviated Drought Stress in Lentil Plants" Sustainability 13, no. 17: 9603. https://doi.org/10.3390/su13179603
APA StyleZafar-ul-Hye, M., Akbar, M. N., Iftikhar, Y., Abbas, M., Zahid, A., Fahad, S., Datta, R., Ali, M., Elgorban, A. M., Ansari, M. J., & Danish, S. (2021). Rhizobacteria Inoculation and Caffeic Acid Alleviated Drought Stress in Lentil Plants. Sustainability, 13(17), 9603. https://doi.org/10.3390/su13179603











