Hydroponic Phytoremediation of Ni, Co, and Pb by Iris Sibirica L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydroponic Culture
2.2. Sample Collection and Preparation
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
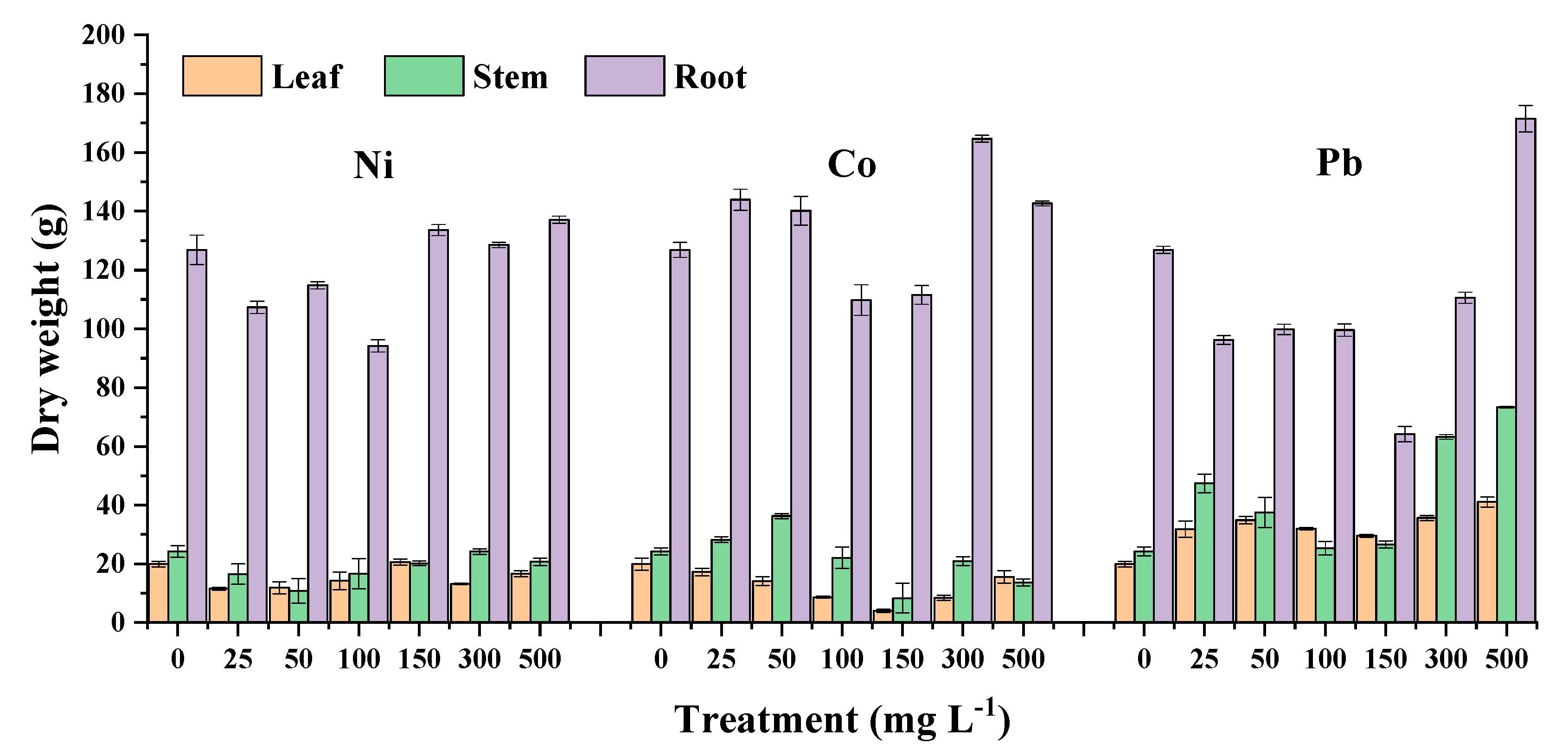
3.1. Physiological Responses of Emergent Wetland Plants
3.2. Ni, Co, and Pb Concentrations in Plant Tissues
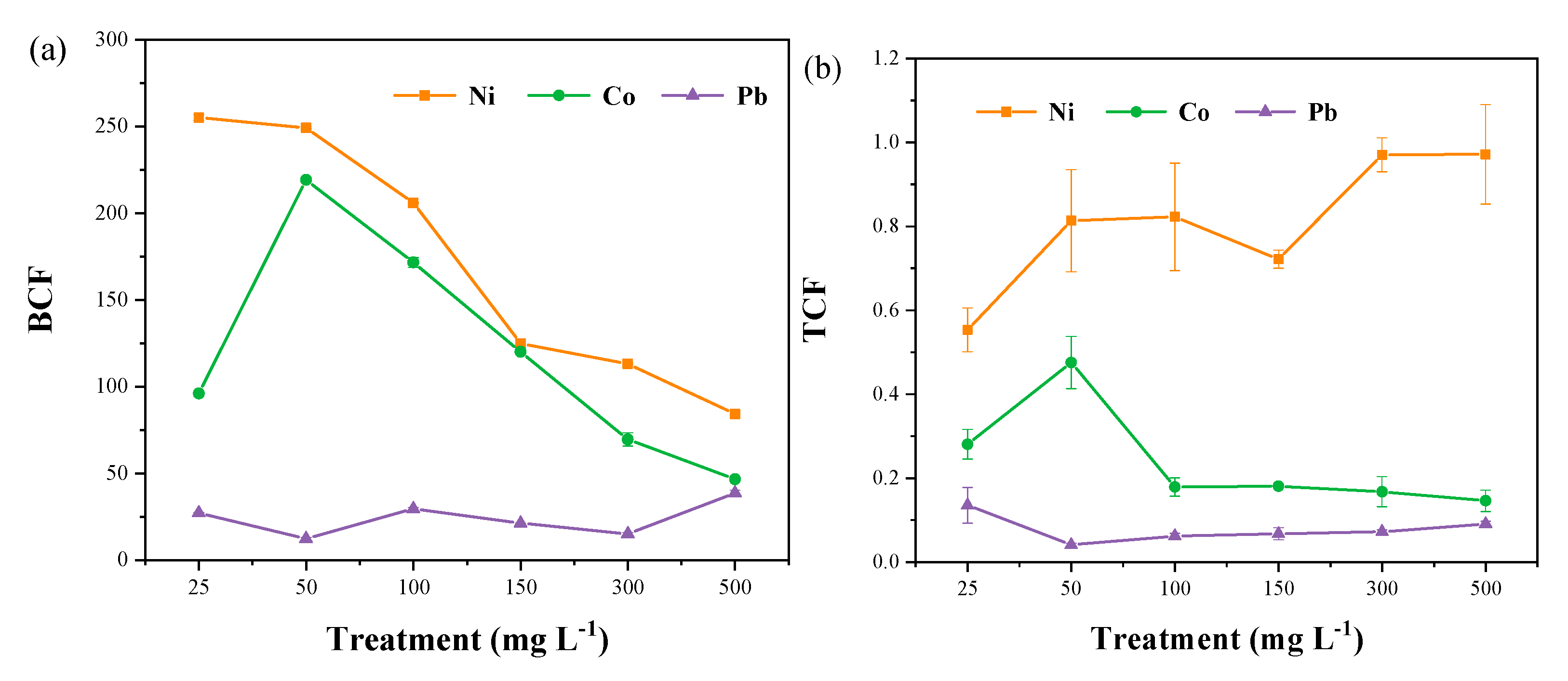
3.3. Bioconcentration Factor (BCF) and Translocation Factor (TCF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of Soil Heavy Metal Pollution on Food Safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Lin, C.; Cheng, H.; Duan, X.; Lei, K. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicol. Environ. Saf. 2015, 113, 391–399. [Google Scholar] [CrossRef]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Andrades-Moreno, L. Accumulation and tolerance characteristics of cadmium in a halophytic Cd-hyperaccumulator, Arthrocnemum macrostachyum. J. Hazard. Mater. 2010, 184, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Biosorption of nickel onto treated alga (Oedogonium hatei): Application of isotherm and kinetic models. J. Colloid Interface Sci. 2010, 342, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.L.; Cruz, C.C.; Luna, A.S.; Henriques, C.A. Sorption and desorption of Pb2+ ions by dead Sargassum sp. biomass. Biochem. Eng. J. 2006, 27, 310–314. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Lei, K.; Giubilato, E.; Critto, A.; Pan, H.; Lin, C. Contamination and human health risk of lead in soils around lead/zinc smelting areas in China. Environ. Sci. Pollut. Res. 2016, 23, 13128–13136. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Farooq Qayyum, M.; Abbas, F.; Hannan, F.; Rinklebe, J.; Sik Ok, Y. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-accumulation Metals in Plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2012, 362, 319–334. [Google Scholar] [CrossRef]
- Zhao, F.; Xi, S.; Yang, X.; Yang, W.; Li, J.; Gu, B.; He, Z. Purifying eutrophic river waters with integrated floating island systems. Ecol. Eng. 2012, 40, 53–60. [Google Scholar] [CrossRef]
- Ma, N.; Wang, W.; Gao, J.; Chen, J. Removal of cadmium in subsurface vertical flow constructed wetlands planted with Iris sibirica in the low-temperature season. Ecol. Eng. 2017, 109, 48–56. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; Ma, N.; Wang, W.; Ma, C.; Zhang, R. Cadmium removal capability and growth characteristics of Iris sibirica in subsurface vertical flow constructed wetlands. Ecol. Eng. 2015, 84, 443–450. [Google Scholar] [CrossRef]
- Navarro, M.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Mânzatu, C.; Nagy, B.; Ceccarini, A.; Iannelli, R.; Giannarelli, S.; Majdik, C. Laboratory tests for the phytoextraction of heavy metals from polluted harbor sediments using aquatic plants. Mar. Pollut. Bull. 2015, 101, 605–611. [Google Scholar] [CrossRef]
- Hajar, E.W.I.; Bin Sulaiman, A.Z.; Sakinah, A.M. Assessment of Heavy Metals Tolerance in Leaves, Stems and Flowers of Stevia Rebaudiana Plant. Procedia Environ. Sci. 2014, 20, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, J.; Zhao, H.; Zhao, H. Stress induced plant resistance and enzyme activity varying in cucumber. Colloids Surfaces B Biointerfaces 2006, 48, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.; Liu, W.; An, J.; Xu, Z.; Wang, L. Joint effects of arsenic and cadmium on plant growth and metal bioaccumulation: A potential Cd-hyperaccumulator and As-excluder Bidens pilosa L. J. Hazard. Mater. 2009, 165, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Amari, T.; Ghnaya, T.; Debez, A.; Taamali, M.; Ben Youssef, N.; Lucchini, G.; Sacchi, G.A.; Abdelly, C. Comparative Ni tolerance and accumulation potentials between Mesembryanthemum crystallinum (halophyte) and Brassica juncea: Metal accumulation, nutrient status and photosynthetic activity. J. Plant Physiol. 2014, 171, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, E.; Przybyłowicz, W.J.; Orlowski, D.; Mongwaketsi, N.P.; Turnau, K.; Mesjasz-Przybyłowicz, J. Mycorrhizal colonization affects the elemental distribution in roots of Ni-hyperaccumulator Berkheya coddii Roessler. Environ. Pollut. 2013, 175, 100–109. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Zhang, Y.; Liu, Y.; Liu, S.; Guo, J.; Li, R.; Wu, S.; Chen, B. Growth and metal uptake of energy sugarcane (Saccharum spp.) in different metal mine tailings with soil amendments. J. Environ. Sci. 2014, 26, 1080–1089. [Google Scholar] [CrossRef]
- Mogopodi, D.; Mosetlha, K.; Torto, N.; Wibetoe, G. Accumulation patterns of Cu and Ni for Indigofera melanadenia and Tephrosia longipes plant species growing in Cu–Ni mining area in Botswana. J. Geochem. Explor. 2008, 97, 21–28. [Google Scholar] [CrossRef]
- Adamidis, G.; Aloupi, M.; Kazakou, E.; Dimitrakopoulos, P. Intra-specific variation in Ni tolerance, accumulation and translocation patterns in the Ni-hyperaccumulator Alyssum lesbiacum. Chemosphere 2014, 95, 496–502. [Google Scholar] [CrossRef]
- Hassan, Z.; Aarts, M.G. Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ. Exp. Bot. 2011, 72, 53–63. [Google Scholar] [CrossRef]
- Ashfaq, M.; Ali, S.; Hanif, M.A. Bioaccumulation of cobalt in silkworm (Bombyx mori L.) in relation to mulberry, soil and wastewater metal concentrations. Process Biochem. 2009, 44, 1179–1184. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- January, M.C.; Cutright, T.J.; Van Keulen, H.; Wei, R. Hydroponic phytoremediation of Cd, Cr, Ni, As, and Fe: Can Helianthus annuus hyperaccumulate multiple heavy metals? Chemosphere 2008, 70, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Mirzahossini, Z.; Shabani, L.; Sabzalian, M.R.; Sharifi-Tehrani, M. ABC transporter and metallothionein expression affected by NI and Epichloe endophyte infection in tall fescue. Ecotoxicol. Environ. Saf. 2015, 120, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kuzovkina, Y.A.; Volk, T.A. The characterization of willow (Salix L.) varieties for use in ecological engineering applications: Co-ordination of structure, function and autecology. Ecol. Eng. 2009, 35, 1178–1189. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Prasad, M. Accumulation of arsenic by aquatic plants in large-scale field conditions: Opportunities for phytoremediation and bioindication. Sci. Total Environ. 2012, 433, 390–397. [Google Scholar] [CrossRef]
- Pusz, A.; Wiśniewska, M.; Rogalski, D. Assessment of the Accumulation Ability of Festuca rubra L. and Alyssum saxatile L. Tested on Soils Contaminated with Zn, Cd, Ni, Pb, Cr, and Cu. Resources 2021, 10, 46. [Google Scholar] [CrossRef]
- Álvarez-López, V.; Prieto-Fernandez, A.; Cabello-Conejo, M.; Kidd, P. Organic amendments for improving biomass production and metal yield of Ni-hyperaccumulating plants. Sci. Total Environ. 2016, 548–549, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Cai, L.; Qi, S.; Wu, J.; Gu, X.S. A multi-technique phytoremediation approach to purify metals contaminated soil from e-waste recycling site. J. Environ. Manag. 2017, 204, 17. [Google Scholar] [CrossRef]
- Agnello, A.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef]
- Roccotiello, E.; Serrano, H.C.; Mariotti, M.G.; Branquinho, C. Nickel phytoremediation potential of the Mediterranean Alyssoides utriculata (L.) Medik. Chemosphere 2015, 119, 1372–1378. [Google Scholar] [CrossRef]
- Luo, J.; Wu, J.; Huo, S.; Qi, S.; Gu, X.S. A real scale phytoremediation of multi-metal contaminated e-waste recycling site with Eucalyptus globulus assisted by electrical fields. Chemosphere 2018, 201, 262–268. [Google Scholar] [CrossRef]

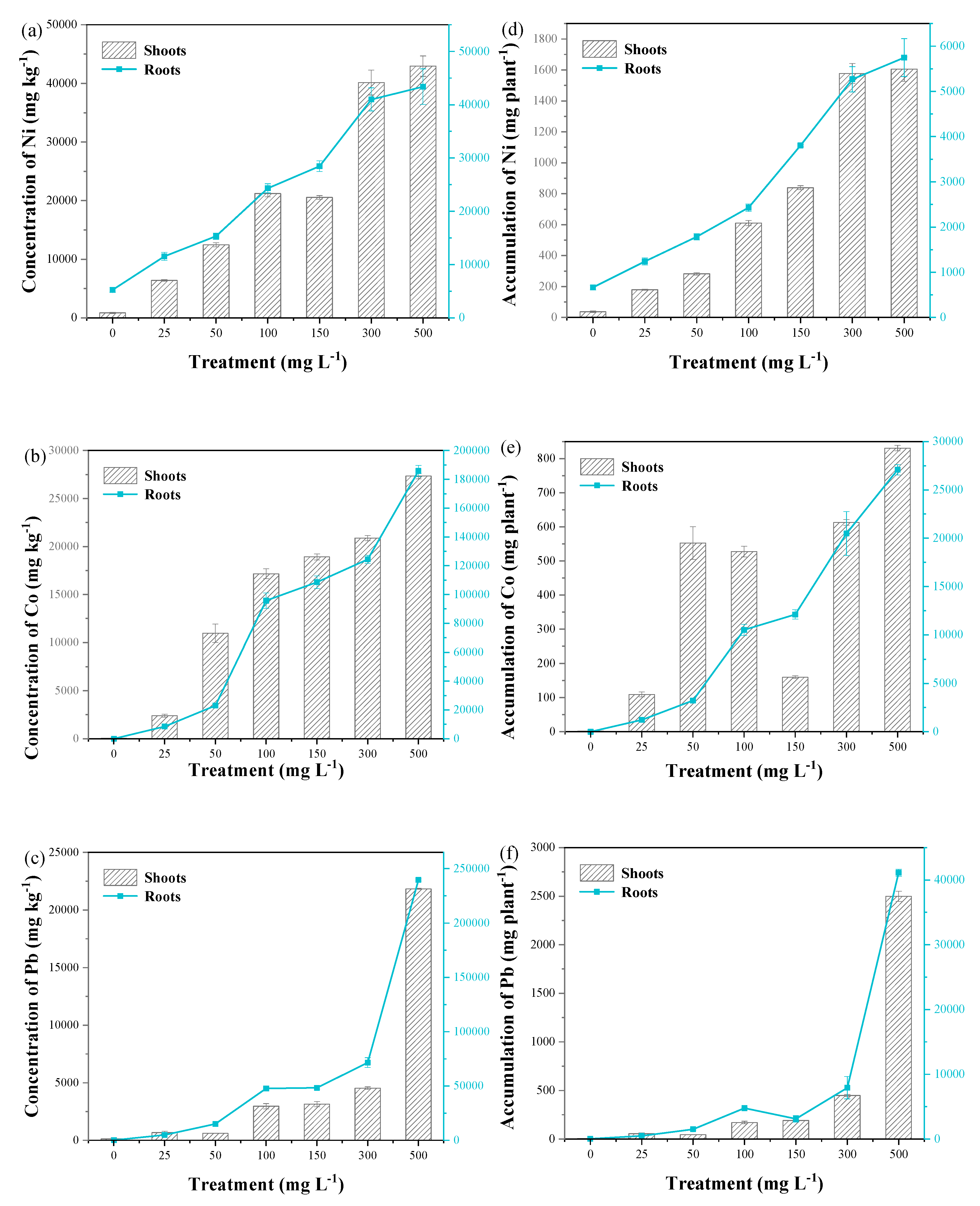
| Treatment mg/L | Leaf | Stem | Root | ||||
|---|---|---|---|---|---|---|---|
| Ni | ck | 471.39 ± 8.47 | a | 1136.51 ± 16.12 | a | 5224.77 ± 41.54 | a |
| 25 | 3740.77 ± 4.48 | b | 8221.67 ± 22.21 | b | 11,526.72 ± 74.49 | b | |
| 50 | 11,420.60 ± 33.34 | c | 13,602.18 ± 56.93 | c | 15,317.35 ± 60.67 | c | |
| 100 | 19,194.75 ± 1.64 | d | 22,199.13 ± 104.93 | d | 25,299.34 ± 85.46 | d | |
| 150 | 20,080.49 ± 12.75 | e | 20,998.29 ± 52.74 | e | 28,449.05 ± 100.63 | e | |
| 300 | 40,800.75 ± 147.96 | f | 39,193.93 ± 203.33 | f | 40,976.35 ± 216.99 | f | |
| 500 | 43,987.28 ± 241.52 | g | 40,638.43 ± 204.56 | g | 43,372.37 ± 333.63 | g | |
| Co | ck | 60.55 ± 3.87 | a | 28.16 ± 1.97 | a | 15.68 ± 4.39 | a |
| 25 | 367.04 ± 3.58 | b | 3644.68±26.68 | b | 8566.31 ± 51.09 | b | |
| 50 | 1660.75 ± 7.23 | c | 14 599.58 ± 130.66 | c | 23,059.15 ± 40.74 | c | |
| 100 | 3363.70 ± 11.34 | d | 22,558.34 ± 74.16 | d | 95,754.99 ± 533.49 | d | |
| 150 | 11,016.48 ± 64.99 | e | 23,833.24 ± 21.78 | e | 108, 553.93 ± 435.95 | e | |
| 300 | 14,176.08 ± 68.20 | f | 23,564.26 ± 57.06 | f | 124,405.28 ± 282.92 | f | |
| 500 | 26,140.43 ± 9.04 | g | 30,148.81 ± 51.24 | g | 191,748.30 ± 383.09 | g | |
| Pb | ck | 198.43 ± 10.98 | a | 61.15 ± 6.27 | a | 151.65 ± 4.90 | a |
| 25 | 211.02 ± 1.64 | b | 996.97 ± 11.36 | b | 5036.57 ± 5.37 | b | |
| 50 | 660.33 ± 2.37 | c | 579.22 ± 2.73 | c | 15,045.06 ± 78.42 | c | |
| 100 | 663.52 ± 3.40 | d | 5849.08 ± 47.78 | d | 47,747.68 ± 144.02 | d | |
| 150 | 760.70 ± 33.77 | e | 6061.20 ± 53.38 | e | 48,355.87 ± 125.95 | e | |
| 300 | 1892.23 ± 5.40 | f | 7022.88 ± 14.61 | f | 71,562.62 ± 449.44 | f | |
| 500 | 2721.30 ± 11.30 | g | 32,552.21 ± 67.95 | g | 239,736.98 ± 1010.22 | g | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, S.; Pang, J.; Li, Y.; Li, Y.; Zhu, J.; Wang, J.; Chang, M.; Wang, L. Hydroponic Phytoremediation of Ni, Co, and Pb by Iris Sibirica L. Sustainability 2021, 13, 9400. https://doi.org/10.3390/su13169400
Wan S, Pang J, Li Y, Li Y, Zhu J, Wang J, Chang M, Wang L. Hydroponic Phytoremediation of Ni, Co, and Pb by Iris Sibirica L. Sustainability. 2021; 13(16):9400. https://doi.org/10.3390/su13169400
Chicago/Turabian StyleWan, Shuming, Jun Pang, Yiwei Li, Yanping Li, Jia Zhu, Jinsheng Wang, Ming Chang, and Lei Wang. 2021. "Hydroponic Phytoremediation of Ni, Co, and Pb by Iris Sibirica L." Sustainability 13, no. 16: 9400. https://doi.org/10.3390/su13169400
APA StyleWan, S., Pang, J., Li, Y., Li, Y., Zhu, J., Wang, J., Chang, M., & Wang, L. (2021). Hydroponic Phytoremediation of Ni, Co, and Pb by Iris Sibirica L. Sustainability, 13(16), 9400. https://doi.org/10.3390/su13169400




