Abstract
Urban gardening has become increasingly popular, creating green oases in cities; however, many of these activities are undertaken in areas of high traffic density or on ex-brown field sites. As a consequence, there are still some barriers to the adoption of these urban gardening practices for food production. One of the public concerns is the transfer of urban pollutants such as heavy metals into the consumer’s food chain, however, city-wide data is often difficult and expensive to collect. In the citizen science project described herein, we conducted simple citizen-led common collaborative experiments in urban community gardens. These data provided information on the potential risk of heavy metal contaminants and ways in which to mitigate those risks in an urban gardening context. Generally, values were below guideline thresholds, however, at a few garden sites, soil trace metal concentrations (Pb, Cd, Zn) exceeded Austrian recommended limits. Moreover, only at two sites were plant trace metal concentrations shown to be above European food standards limits. Given the citizen’s positive response to the project, we suggest expanding this study to the whole of Vienna, giving newly established gardens a chance to predetermine the risks posed by their local soils.
1. Introduction
The world food supply will need to be doubled by 2050 to cope with the increasing human population [1], creating pressure on limited soil resources [2]. Although soils are slowly being recognized as a valuable finite natural resource on par with air and water, past activities, particularly in cities, may have resulted in degraded soils [3]. All around the world, urban agriculture is booming, fulfilling diverse functions including food production, provision of ecosystem services, community building, reduction in socio-economic tensions and food millage [4,5,6,7,8,9,10,11]. However, with production of food in urban areas there will be a tension as a result of the historical legacy or current pollution of the city landscape, often originating from anthropogenic pollution sources such as traffic, industry, domestic combustion or the use of pesticides [12,13,14]. For example 67% of the crops sampled from the Berlin inner city area vegetable gardens, and situated in the vicinity of high traffic volume, exceeded EU standards for Pb concentration in food crops [15]. Furthermore, soil Pb concentrations above regulatory limits in Baltimore City were found to be related to the proximity to busy roads; however, legacy effects such as former use of paint containing Pb were also suspected as causes of high concentrations [16]. Enrichment of Zn, Pb and Cu in urban garden topsoils from the outskirts of Szeged (Hungary) were shown to be caused by anthropogenic sources derived from traffic and copper pesticides [13].
Even though these citywide studies present evidence that contamination of urban soils is an emerging human health concern, awareness among urban gardeners about this risk and awareness of management strategies to mitigate such risks might be lacking [17,18]. A survey conducted in St. Louis City revealed that 68.8% of community gardeners were not concerned about potential soil contamination and 62.4% were not worried about associated health risks. Further, it has been shown that gardeners generally had little knowledge about soil contamination [18]. However, if there was a perceived risk among gardeners, Pb in particular was found to be the pollutant of greatest health concern [17,18]. This perceived risk of urban food contamination may act as a sufficient mental barrier to individual uptake of community gardening, dissuading community members in participating in such multi-level-beneficial urban gardening activities [19,20].
In the last three decades there has been a shift towards both the development and acceptance of heterogeneous science practices that encourage public participation on a number of levels [21], this has led to the rise of the citizen science movement. Engagement of the public has shown to be successful in gathering large data sets about urban soil trace metal contamination [22]; however, it also allows data collection at property scales and can thus directly reflect the exposure risk of individual households [23]. Therefore, such studies are particularly useful in addressing citizens’ concerns about their personal exposure risk, whilst providing educational opportunities about environmental contaminants using a two-way dialogue [22,24,25]. Citizen science can provide reassurance to the public about the rigor and process of scientific enquiry [26]. In doing so it can inspire confidence and understanding of the nuances of political bias; putting contextual knowledge together in learning by doing. This citizen-knowledge does not necessarily reject technical information; however, such information may be incorporated into specific contexts in an ad-hoc manner, so the participation of “academic scientists” is particularly important to stress how objectivity is ensured and evidence based outcomes are achieved [26,27]. Within the realm of our citizen science project, we sought to identify potential problem areas in Vienna and develop simple strategies to overcome such issues. This citizen science study strived to publicize the importance of rigorous un-biased science but also to underpin and publicize the peripheral gains of participating in the urban gardening movement.
Across governments and in academia there has been a shift towards defining a broader framework of pollution exposure that can be integrated with health data and act as an integrated model for examining exogenous and endogenous source-exposure relationships at population levels [28,29,30]. It is generally agreed that there are five social determinants of health: (i) economic stability; (ii) education; (iii) health and healthcare; (iv) neighborhood and built environment; (v) the social and community context [31]. From an urban gardening perspective, the positive impact of “grow your own” can reduce economic burden, enhance gardening related exercise and nutrition, improve the environment and create a sense of community and cohesion [5,6,7]. Furthermore, many urban greening initiatives are rooted in Ecosystem-based Adaptation (EbA) and natural climate change adaptation [11,32] and a number are citizen driven such as community urban gardening or guerrilla gardening. Given the scope of the future urban challenges of climate change, population growth and diversification [33], with paradoxical increases in loneliness and anxiety [34], the potential social and environmental benefits [6] of these urban gardening initiatives is clear.
Although in some Austrian urban areas there are data on the soil contamination [35,36], there are few on the transfer of those heavy metals into growing crops. The transfer factor from soil to plant is expressed as the ratio of plant concentration divided by the total concentration in soil, which gives an indication of the plant accumulation behavior [37]. It is well known that organic amendments such as compost and farmyard manure may effectively reduce the bio-availability of heavy metals in soils due to their high content of organic matter and high concentrations of Fe [38], through the processes of immobilization, chelation or dilution. In Vienna, top soils are monitored every three years by the Vienna Magistrate—Department of the Environment—collecting soil samples at 286 locations and analyzing heavy metal contents [35], however, there are few studies looking at the bioavailability of these contaminants in real gardening contexts across the urban areas of Austria. Specifically, where vegetables are grown in potting mixtures, with composts or other soil additives and in structures such as raised beds. Therefore, our objectives in this study were to provide community gardeners with sound scientific data on which to base their decision making, giving them the tools and confidence to share their knowledge and practices in the community as follows: (1) to engage, inspire and involve the public in the process of scientific enquiry in order to emphasize the objectivity of the scientific process; (2) to gather data on potential heavy metal risk hot spots in urban areas and create an easily accessible, visible and frequented data base/map for urban gardeners to access in order to aid rational decision making; (3) to improve local and global models of heavy metal pollution, particularly addressing issues such as protective measures against pollution and feasible reduction of individual exposure. We chose a citizen science approach as it allowed us to gather a wealth of data and jointly generate useful information for the greater public good, which can contribute towards creating green sustainable cities.
2. Materials and Methods
We used a simple experimental approach based on a hub and spoke design. The citizen scientists established common replicated randomized experiments in buried pots and then carefully tendered and harvested the plants, and returned the soil and plant samples to the laboratory at the Institute of Soil Research at the University of Natural Resources and Life Sciences Vienna for heavy metal analysis.
In order to recruit citizen scientists, we used E-Mail addresses provided online by an association aimed at urban gardener collectives (http://gartenpolylog.org/; accessed on 8 January 2020) and invited around fifty randomly chosen Viennese community gardens listed on this platform to participate in our project. Finally, we were able to engage eleven community gardens located at different sites across Vienna (Figure 1) to establish one or more pot experiments in their garden initiatives, resulting in thirty-two citizen science pot experiments in total.
Figure 1.
City Map of Vienna. Maps Data: Google My Maps, ©2021. Orange symbols indicate locations of community gardens which participated in the project.
2.1. Citizen Science Pot Experiments
In March 2020 (prior to the Covid-19 restrictions), we ran hands-on training sessions and face to face consultations by visiting every single participating garden. We provided the citizen scientists with a Heavy Metal City-Zen starter pack, which explained the background and basics of the experiment and provided all materials to conduct the experiment. This was in order to ensure that every experiment was run in a comparable manner.
Between the end of April and the beginning of May 2020 (as Covid-19 restrictions eased), the citizen scientists collected soil from their neighborhood or soil adjacent to their vegetable/raised beds (to act as the control variant) in order to compare heavy metal concentrations with a compost mixture of their choice collected within their own vegetable or raised beds. There was no specification regarding the composition of the compost so that the citizen scientists were able to obtain results from their own mixtures used in the gardens. Control and amended soils were collected and sampled as composite samples consisting of three to five subsamples of the top 10 cm within a radius of 2 to 4 m. Before starting the experiments, one soil sample of each treatment was placed into a labelled plastic bag and stored at 4 °C until we picked them up and brought them to the laboratory. The citizen scientists filled the standard pots provided (11 × 11 × 12 cm) with the collected soils, four replicates per treatment, buried them randomly in the beds used in their community gardens and planted the seeds we provided. We gave the gardeners the opportunity to choose between two crops, either spinach (Spinaca oleracea L. cultivar Butterfly) or radish (Raphnus sativus L. cultivar Topsi), as they represent a leaf and a bulb crop and are commonly grown in urban gardens in Austria. Eight seeds were sown directly in each pot (representing one replicate) and thinned out after germination to two plants (radish) or four plants (spinach), respectively. The gardeners used our detailed instructions on how to water and treat the plants daily, ensuring we had a standard protocol. No additional fertilizer or pesticides were recommended as part of the protocol. Upon maturation of the plants (after a growth period of five to eight weeks—depending on the garden site) we came back to the gardens and relative chlorophyll content was measured by the citizen scientists with a chlorophyll meter, SPAD 502 (Minolta Camera Co., Osaka, Japan), from ten randomly chosen leaves per pot. Thereafter, plants were harvested, rinsed with water available in the gardens (tap- or well-water), blotted dry with tissue paper and cut into small pieces and fresh weight (f.w.) was determined and noted separately for each plant organ (spinach-leaves, radish-leaves and radish-bulbs) as a composite sample per pot (replicate).
2.2. Sample Preparation and Chemical Analyses
In the laboratory, soil pH was determined with a pH-meter in a soil:solution (0.01 M CaCl2) w/v ratio of 1:2.5. A 0.5 g finely grounded subsample of each air-dried composite soil sample was digested in 4.5 mL HCl 37% and 1.5 mL HNO3 65% (aqua regia) in a digestion block at 135 °C for 3 h. A certified reference soil (ISE 885 Braunerde pseudoclay; Wageningen Evaluating Programs for Analytical Laboratories), as well as two blanks, were included in each digestion run. Digestives were subsequently cooled, diluted to 50 mL with ultra pure water and filtered prior to analyses of Pb and Cd concentrations by using GF-AAS (HGA 900 coupled with AAnalyst 400, Perkin Elmer, Shelton, CT, USA) and Zn concentrations by using F-AAS (AAnalyst 400 Flame, Perkin Elmer, Shelton, CT, USA). Recovery rates ranged between 88–110%. The limit of detection was 0.4 ppb, 0.05 ppb and 2.2 ppm for Pb, Cd and Zn, respectively.
Dry weight (d.w.) of plant samples was determined and noted after drying at 50 °C until constant weight. Three replicated samples per treatment, if available, of each citizen science experiment were randomly chosen to measure Pb, Cd and Zn concentrations in the plant tissues, this was to overcome the fact that, in many experiments, one replicate failed to germinate or died due to diseases or pests. Prior to analysis, 0.2 g of a grounded and homogenized subsample from each replicate was digested in 5 mL HNO3 65% and 1 mL H2O2 30% in a digestion block at 155 °C for 2.5 h. Again, blanks and reference material (IPE 815 Sunflower, Wageningen Evaluating Programs for Analytical Laboratories) were included in each digestion run and digestates were diluted to 50 mL with ultra pure water and filtered. The element concentrations (Pb, Cd, Zn) in the filtrates were analyzed by using ICP-OES (Thermo Scientific iCAP 7000 Plus Series, Waltham, MA, USA).
2.3. Data Analysis
Soil trace metal concentrations (mg kg−1 d.w.) were compared with agricultural and horticultural Austrian guideline values [39].
Plant trace metal concentrations (mg kg−1 d.w.) were related to the European regulation, which sets maximum levels for certain contaminants in foodstuffs [40]. Because maximum levels of metals based on European food standards are reported on a fresh weight basis, we converted these maximum levels to a dry weight basis to facilitate comparison to the measured dry weight concentrations of our plant samples. Due to difficult conditions in the field (e.g., often windy conditions), sometimes inaccurate determination of plant fresh weight occurred. Therefore, to obtain corresponding maximum levels, we used a species-specific conversion, where the average f.w./d.w. ratio of all plant samples was multiplied by maximum levels defined in the Commission Regulation. Measured values with a large deviation were systematically eliminated before conversion.
A Welch’s t-test was conducted to compare trace metal concentrations in plants grown either in amended or control soils in the respective experiments. Experiments with less than three replicated samples were excluded from statistical analyses. One-way ANOVA or Kruskall–Wallis tests were performed to determine differences between trace metal concentrations in plants grown in control soils among all experiments.
Correlations between dry plant biomass and SPAD-values, as well as between trace metal concentrations of plants and soils, were evaluated using Spearman correlation coefficients.
Statistical analyses were carried out and data were plotted using SigmaPlot 14.0 (Systat Software, Inc., San Jose, CA, USA). Significance was assessed at the 5% level throughout.
3. Results
The pH of the soil samples, both control and amended soils, were similar and in the neutral to slightly alkaline range (ranging from 6.8 to 7.7; Table 1). This might be explained by the similar characteristics of the underlying parent materials of the Viennese soils used in the experiments, the majority being derived from Danube basin river sediments of loam, loess character [36]. Trace metal contents in the soils among the different garden sites showed a high variability, with a range from 9.0 to 133.0 mg kg−1 for Pb, from 0.12 to 0.74 mg kg−1 for Cd and from 55.2 to 269.0 mg kg−1 for Zn in control soils, respectively. In amended soils, trace metal concentrations varied for Pb from 4.04 to 164.0 mg kg−1, for Cd from 0.12 to 0.54 mg kg−1 and for Zn from 43.4 to 521 mg kg−1. Moreover, a high variability of soil trace metal concentrations was even observed within some garden sites when multiple trials were conducted (Figure 2, Figure 3 and Figure 4).

Table 1.
PH values of soil samples in citizen science experiments where radish or spinach was grown either in an amended variant (compost mixture) or in a control variant, i.e., in an untreated urban soil collected from in the vicinity of the vegetable bed.
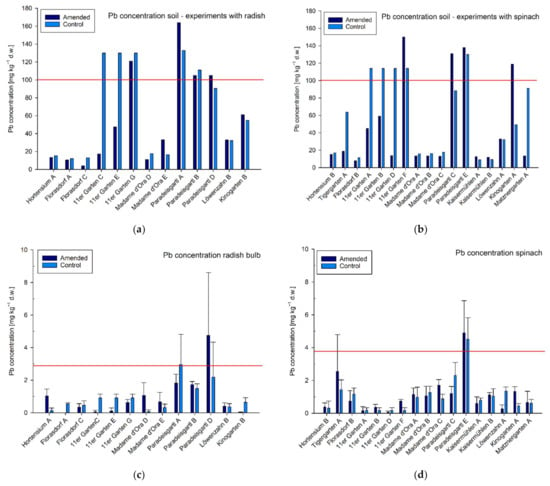
Figure 2.
Pb concentrations in mg kg−1 dry weight (d.w.) in soil samples from single garden experiments (a) conducted with radish (b) and spinach, n = 1. Agricultural and horticultural Austrian guideline values are indicated by red lines. Pb concentrations (mg kg−1 d.w.) of (c) radish bulbs and (d) spinach leaves of the individual experiments are shown below the Pb concentrations of the corresponding soil samples. Means ± standard deviation, n = 3 (target value). The red line in the graphs of the plant samples in (c,d) indicates maximum levels defined in the Commission Regulation converted in mg kg−1 plant biomass d.w. Note: experiments conducted in the 11er Garten were tested only against one control variant and Löwenzahngarten used the same soil samples for both experiments (A and B).
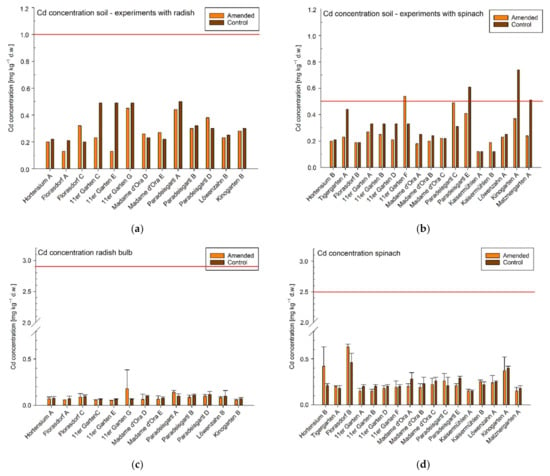
Figure 3.
Cd concentrations in mg kg−1 dry weight (d.w.) in soil samples from single garden experiments (a) conducted with radish and (b) spinach, n = 1. Agricultural and horticultural Austrian guideline values are indicated by red lines. Cd concentrations (mg kg−1 d.w.) of (c) radish bulbs and (d) spinach leaves of the individual experiments are shown below the Cd concentrations of the corresponding soil samples. Means ± standard deviation, n = 3 (target value). The red line in the graphs of the plant samples in (c,d) indicates maximum levels defined in the Commission Regulation converted in mg kg−1 plant biomass d.w. Note: experiments conducted in the 11er Garten were tested only against one control variant and Löwenzahngarten used the same soil samples for both experiments (A and B).
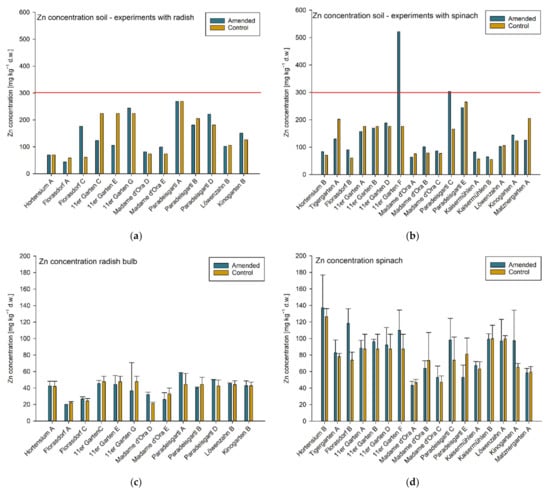
Figure 4.
Zn concentrations in mg kg−1 dry weight (d.w.) in soil samples from single garden experiments (a) conducted with radish and (b) spinach, n = 1. Agricultural and horticultural Austrian guideline values are indicated by red lines. Zn concentrations (mg kg−1 d.w.) of (c) radish bulbs (d) and spinach leaves of the individual experiments are shown below the Zn concentrations of the corresponding soil samples. Means ± standard deviation, n = 3 (target value). Note: experiments conducted in the 11er Garten were tested only against one control variant and Löwenzahngarten used same the soil samples for both experiments (A and B).
Eight experiments resulting from three garden sites (11er Garten, Paradeisgartl, Kinogarten) exceeded the use-specific guideline value in the amended variant for soil Pb concentration (soil pH ≥ 6; 100 mg kg−1 d.w.; Figure 2). In two of these three garden sites (11er Garten, Paradeisgartl), the use-specific guideline value for soil Zn concentration (soil pH ≥ 6; 300 mg kg−1 d.w) was also exceeded. Nevertheless, one of those soil samples (Paradeisgartl) exceeded the guideline value only by 1% and can therefore be omitted. The soil Zn concentration in the other sample (11er Garten) was 74% above this threshold and thus obviously stands out in comparison to the concentrations in the other gardens (Figure 4). As spinach is a cadmium accumulating vegetable, the use-specific guideline value for soil Cd concentrations for growing spinach is typically lower (0.5 mg kg−1 d.w.) than it is for growing radish (soil pH ≥ 6; 1.0 mg kg−1 d.w.; Figure 3). Experiments conducted with radish did not exceed the agricultural Austrian guideline value for soil Cd concentrations; however, in terms of spinach, three control soils but only one amended soil (and only slightly with 8%)—but again the experiment in the 11er Garten—exceeded the guideline value (Figure 3).
Plant dry biomass and SPAD values varied between garden sites (Figure 5), and both spinach and radish leaf dry matter correlated significantly but only moderately with measured SPAD-values (rs = 0.47, p < 0.01, N = 118; rs = 0,71, p < 0.01, N = 91); this suggests SPAD values were not a good predictor of plant production in these systems and that different parameters other than the relative chlorophyll content (e.g., intensity of light, temperature, water supply) were also influencing the plant growth stage when the measurements took place.
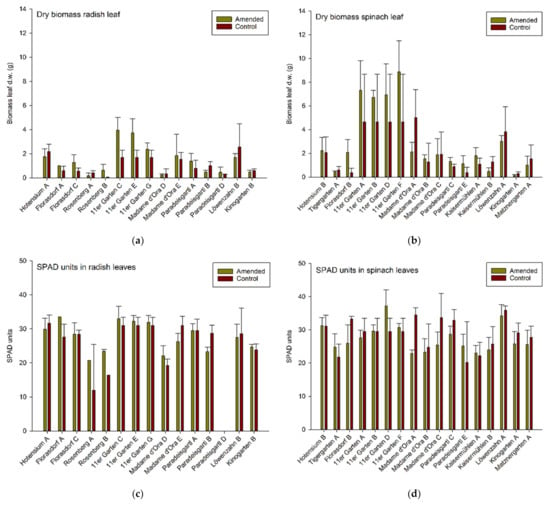
Figure 5.
Leaf dry biomass in gram (g) of (a) radish plants and (b) spinach plants and corresponding chlorophyll meter readings (SPAD units) in (c) radish leaves and (d) spinach leaves. Means ± standard deviation, n = 4 (target value).
Total trace metal concentrations in the soil—across all gardens and treatments—showed no or only moderate linear correlations with the averaged concentrations in the edible plant parts. Spinach leaves showed no correlation at all, however, mean concentrations in radish bulbs correlated at least moderately with total soil Zn-concentration (rs = 0.69, p < 0.01, N = 26) and total soil Pb-concentration (rs = 0.57, p < 0.01, N = 26), respectively. This suggests a clear link of soil to plant concentrations but a low predictive capacity.
Even though eight experiments exceeded the use specific guideline value for soil Pb-concentrations, only four experiments, conducted at two garden sites (Paradeisgartl, Tigergarten), exceeded the maximum levels in plant tissues (converted in mg kg−1 d.w.) reported for European food standards (Table 2 and Figure 2).

Table 2.
Maximum levels of Pb and Cd concentrations on a fresh weight (f.w.) basis (mg kg−1) according to legal European values (EC) No 1881/2006 and corresponding maximum levels on a dry weight (d.w.) basis (mg kg−1), calculated with the average f.w./d.w. ratio of all plant samples per vegetable type obtained in this study.
In the Paradeisgartl, the highest Pb concentration in the soil was determined to be 164 mg kg−1. Even here, however, the concentration of the radish bulbs grown in the amended variant was below the maximum level of the Commission Regulation. However, in two other trials in this garden, the maximum concentrations in the plants were exceeded and should therefore not be offered for sale according to EU food law. It should be noted that the main risk from Pb in soils is direct ingestion of soil particles and not necessarily through the food due to its low mobility in soils [41].
Although the soil Pb concentration in the amended variant of the Tigergarten was relatively low, with 18.7 mg kg−1., one replicate plant grown in this variant exceeded the maximum level of the Commission Regulation and had a concentration of 5.12 mg kg−1 (Figure 2).
A variable transfer of trace metals from soil to plant across all garden sites was also observed in terms of Cd and Zn. In none of the experiments was the Cd concentration in the plant samples above the Commissions’ maximum level for foodstuff, even in those experiments with soil concentrations above the use-specific guideline value. The highest Cd concentration in the soil samples (0.74 mg kg−1) was measured in the Kinogarten with a trace metal concentration in plants grown therein of 0.40 ± 0.02 mg kg−1 d.w. (mean ± SD), whereas the highest concentration in the plant samples (0.63 ± 0.03 mg kg−1 d.w.; mean ± SD) was observed in the Florasdorf community garden, with a concentration of only 0.20 mg kg−1 in the corresponding soil sample (Figure 3).
The highest Zn concentration of 182.3 mg kg−1 dry weight (equivalent to 14.3 mg kg−1 fresh weight) was measured in spinach leaves from a trial in the Hortensium garden. However, the corresponding soil sample did not exceed the use-specific guideline value. For the radish samples, the highest Zn concentration was measured in a trial in the 11er Garten with 67.3 mg kg−1 dry weight and corresponds to a concentration of 2.3 mg kg−1 in fresh radish bulbs. The Commission Regulation does not specify maximum levels for Zn in food; however, the D-A-CH reference values for Zn intake (at a medium level of phytate intake) are 14 mg day−1 for male adults and 8 mg day−1 for female adults [42]. Zn is one of the essential trace elements and therefore is generally supplied as part of the normal diet. However, excess Zn intake and simultaneously low Cu intake may impair the absorption of Cu in humans according to The Scientific Committee on Food [43], and it has therefore set a tolerable upper intake level (UL) for Zn of 25 mg day−1 for adults. Depending on the body weight, tolerable upper intake levels for children range from 7 mg day−1 for 1- to 3-year-olds to 22 mg day−1 for 15- to 17-year-olds. According to an Austrian report on nutrition, the average Zn intake was 12.2 mg day−1 for men and 10 mg day−1 for women [44]. Eating 250 g of the spinach from the experiment with the highest Zn concentration would correspond to an additional Zn intake of 3.6 mg (14.3 mg kg−1 × 0.25 kg). Eating 300 g of fresh radish tubers from the experiment with the highest Zn concentration would result in an additional Zn intake of 0.7 mg (2.3 mg kg−1 × 0.3 kg). In both cases, the consumption of these plants would theoretically not exceed the tolerable upper intake level of 25 mg per day for adults (e.g., for men: 12.2 + 3.6 mg day−1 or 12.2 + 0.7 mg day−1).
As a proxy for untreated urban soil and to test for the effects of their heterogeneity on the metal uptake of the plants, we compared the metal concentrations in plants among all garden sites in the control variant. The results revealed the variability in the trace metal concentrations in soils across the city, and this was reflected in the values of both the spinach and radish samples, as values were significantly different between the garden sites (Table 3). The Cd contents in the radish bulbs were however, not significantly different across the city (H9 = 14.06, p = 0.12) as determined by Kruskall-Wallis test.

Table 3.
ANOVA table of F- and p-values of the effects of control soils among all experiments and garden sites on heavy metal concentrations (Zn, Pb, Cd) in mg kg−1 dry weight in radish bulbs and spinach, respectively.
Interestingly, the amended variant—where citizen scientists used the soil from their vegetable gardens—showed that these remedial efforts had hardly any positive effects on the heavy metal concentrations in the plants. In only three out of twenty-five statistically analyzed trials was a significantly lower Pb concentration found in the amended plant samples compared to plants which were grown in untreated soils collected from in the vicinity of the vegetable beds (control variant). Moreover, in three trials, the plants grown in the control soil exhibited significantly lower Pb concentrations than plants grown in the amended soils. For Cd and Zn, no positive effect of the amended variant could be observed in any of the trials. However, one trial showed a negative effect of the amended soil on the Zn uptake of the plants (Table 4 and Table 5).

Table 4.
Welch’s t-test results. Comparing heavy metal concentrations in mg kg−1 dry weight (d.w.) in radish bulbs grown in amended or control soils in single citizen science experiments at different garden sites (n = 3). Significant p-values (<0.05) are given in bold. SD = Standard Deviation.

Table 5.
Welch’s t-test results. Comparing heavy metal concentrations in mg kg−1 dry weight (d.w.) in spinach leaves grown in amended or control soils in single citizen science experiments at different garden sites (n = 3). Significant p-values (<0.05) are given in bold. SD = Standard Deviation.
4. Discussion
Since the Austrian ban of leaded gasoline in 1993, decreasing Pb soil concentrations have been observed, even along roads with heavy traffic in the main city, Vienna [35]. However, in Viennese urban soils, anthropogenic input sources are still evident. Especially, it seems, residential and industrial areas exhibit higher soil Pb and Cd contents compared to soils in parks [36]. Even though the soil trace metal concentrations in some community gardens surpassed the agricultural and horticultural Austrian guideline values for maximum Pb and Cd levels for European food standards in plant samples, these thresholds were only exceeded at two of the garden sites (Paradeisgartl, Tigergarten) and only in terms of Pb. The fact that, in the Tigergarten soil, Pb concentrations were below the guideline value and only one single plant replicate showed a relatively high Pb content, suggests that not only root uptake, but probably also soil splash or deposition from air may have contributed to elevated Pb concentrations in this plant sample. High levels of Pb in horticultural crop samples from the inner city in Berlin were substantially attributed to a high traffic volume in the surrounding areas [15]. However, the observed variable transfer of trace metals from soil to plants in the other experiments might not be explained by atmospheric deposition alone. The weak correlation between vegetable and soil metal concentrations found in urban gardens in New York City has been partly explained by soil characteristics that effect trace metal availability to plants [45]. The lack of predictability to calculate trace metal concentrations in plants from total concentrations in soils in our study was probably caused by the use of different compost mixtures in the amended variant and different history and characteristics of urban soils used for the control variant in each experiment. It is well known that sorption and desorption processes of trace metals in soils are controlled by parameters such as pH, cation exchange capacity, clay and organic matter content, as well as oxides, hydroxides and microorganisms [37]. However, a study from Copenhagen suggested that soil characteristics and high total soil metal contents do not automatically lead to a high phyto-availability [46]. The authors compared trace metal concentrations of soil and plant samples derived from urban garden sites and did not find Cd and Pb concentrations above the European food standards, even at sites with soil concentrations above the soil quality criteria. In contrast, Murray et al. [47] found elevated concentrations in plants grown in soils rated uncontaminated. However, the lack of strong correlations in our results might also be due to the high variability of plant biomass. The weak correlation with corresponding SPAD values (relative chlorophyll content) indicates differences in growth factors between experiments. In addition to soil properties, it is very likely that microclimatic conditions between experiments such as fertilization, solar radiation, water regime or insect damage may at least partly explain the differences in observed growth rates and subsequently might also alter trace metal uptake by plants [48].
The high variability among control soils could indicate a general heterogeneity of Viennese urban soils and our results suggest that plant trace metal concentrations could be affected by this heterogeneity. Even though we analyzed only one soil sample per experiment and treatment, it might be assumed that, generally, trace metal concentrations (Pb, Cd, Zn) in Viennese urban soils tend to be below critical guideline values; however, for some reason, probably industrial legacy effects, there exists a clear patchiness of these metals in the soil.
At a few garden sites we certainly measured critical metal concentrations in the soil, and we observed that these concentrations even varied within some garden sites where multiple trials were conducted. An inhomogeneous concentration of Pb and Ba in urban soils was also found in New York City [45], and the authors pointed out that this patchiness makes it difficult to determine trace metal uptake by plants and to correlate it with trace metal concentrations in soil. A high variability and heterogeneity of metal concentrations was also found in urban garden soils in a former coal mining area of northern France [49] and the inclusion of a greater number of soil samples in such studies was therefore suggested.
It proved difficult to detect any differences between trace metal concentrations in plants grown in the amended variants compared to the controls, in the single experiments. Probably, the inherent concentrations were generally too low to identify the effects. In addition, the differences in soil concentrations between the control and the amended variant seemed to be rather small in most cases. This might also indicate that the physicochemical parameters of Viennese urban soils have the capacity to adsorb trace metals in an efficient manner. However, this remains to be investigated.
Even though the evidence of elevated trace metal concentrations in plant samples at most garden sites was low, the pollution source for soils with concentrations above the guideline values has to be investigated in order to minimize possible health risks. In the experiment with the elevated zinc levels (11er Garten F), for example, it could be investigated whether the soil or the irrigation water was stored in galvanized containers for a longer period of time, so that zinc from these materials could possibly be transferred to the soil. Similarly, it is possible that elevated concentrations of heavy metals in the soil indicate a source of pollution associated with the historical use of the site. Indeed, this suspicion was reinforced when we discussed possible sources of the soil contamination with the gardeners in question. The gardeners of the Paradeisgartl obtained information from neighbors about the previous use of their garden and also made inquiries based on contamination maps. They found a number of potential causes that could have been responsible for the elevated Pb in the soil, such as the dismantling of a former glass house construction, which had Pb seals that were used to stabilize the glass. Although the Pb source could also have originated as a result of the proximity to former industrial sites. However, the actual source could not be determined, which shows how difficult the interpretation of such research can be, especially for non-specialists. On the other hand, the 11er Garten commissioned soil analysis at a certified laboratory for the most common trace metals before they started garden activities at their property. They were already aware of a potential legacy pollution in their garden, which might have been caused by a nearby former gasometer. Test results showed elevated soil Pb concentrations and, hence, the Viennese municipality recommended building raised beds with pollutant-free soil in their garden to prevent contamination of their self-produced vegetables. The experiments (11er Garten F and G) in which the concentrations exceeded the guidelines were carried out in the beds that were mainly composed of original soil on site and not the recommended substitute soil, which was used in the other vegetable beds in their garden (11er Garten A, B, C, D and E). Additionally, a residential building was demolished on this plot and the land had been abandoned for at least fifteen years before it was used for gardening. Many bricks and building materials, cutlery, etc., were found, as well as many metal pieces. This might be the explanation for the high Zn concentration found in this soil sample (11er Garten F).
The positive effect of the raised beds in the 11er Garten in terms of reducing contamination risks was additionally apparent by the high metal content measured in the control variant (untreated original urban soil) in contrast to the treated variant, where the substitute soil from the raised beds was used. The plants grown in the raised beds also showed a significantly lower Pb concentration compared to the plants grown in the control variant, at least in the experiments conducted with radish.
The take home message from our project is that even if the data suggest there is little risk of plant contamination in the urban area of Vienna, simple measures can be taken to further reduce or even eliminate those risks. The problem is that the gardeners often do not have low-threshold access to this information and the knowledge of the mitigation measures that can be implemented, as can be seen from the example of the Paradeisgartl, where the gardeners did not build raised beds and started growing their vegetables despite elevated soil Pb levels; simply because they did not know about it. Our findings with the Viennese urban gardeners resonate with the findings of a survey conducted with Baltimore community gardeners where the authors found that gardeners did not know about the soil measures available to reduce contamination, as well issues around gathering information on the history of garden plots or having access to soil tests [17]. An important outcome of the cooperation with the citizen science gardeners in Vienna was the design and production of a brochure which outlined clear measures to minimize the risk of contamination during gardening activities in the city, as well as information about institutions for soil testing or for inquiring about former site use. This brochure (in the German language) is online, accessible at our project homepage (www.heavymetalcityzen.com; accessed on 20 July 2021) or on the website of the community garden association “Gartenpolylog” (http://gartenpolylog.org/schwermetalle-stadtgemuese; accessed on 20 July 2021).
The results of our project clearly suggest that safe gardening in Vienna is easily attainable. The important thing, however, as in any gardening practice, is to know about the soil and its characteristics. Based on our findings, prospective and existing gardeners in Vienna are encouraged to first obtain information about the historical and current use of their garden sites. Soil tests can then be carried out based on this background of information. The constant exchange with our citizen scientists and their feedback showed the need for a bundled access to information and, also, support to deal with this information. The topic of having one’s own garden in the city of Vienna and the associated risk of contamination may be of varying concern; however, we experienced a large number of incoming requests and enquiries. Many Viennese gardeners were eager to participate in the project to learn more about their garden soils, and it was easy to expand our network, involving associations and institutions working in this field.
In the future we hope to establish a cooperation with relevant institutions that will provide easy and affordable access to soil tests for community gardeners as well as appropriate and tailored advice on the results. Our experience with the citizen scientists showed that it was very important to interpret soil test results and explain the actual risk involved in a cautious way and to give them clear and consistent advice if necessary. To meet the concerns of gardeners with high Pb levels in plant and soil samples, and in order to encourage them to continue with their garden activities, it was important to put the Pb soil concentrations in a more global perspective so that the relative level of the risk could be assessed by the gardeners. Even though Pb concentrations in some experiments were above Austrian guideline values, these Pb concentrations were not comparable to contaminated areas of real concern. In a community science program conducted in Sydney, where soil Pb concentrations in over 5200 samples of residential gardens were analyzed [24], the legacy effect was more evident than it seems to be the case in the urban area of Vienna [35]. Soil Pb concentrations in these residential gardens exceeded the Australian guideline value of lead (300 mg kg−1) by 40%, whereas 15% showed even concentrations above 1000 mg kg−1 [24]. Moreover, after the closing of a Pb/Zn smelter in 1992 in Arnoldstein, Austria [50], Pb levels of agricultural soil in the surrounding area were investigated, and revealed Pb concentrations above 5000 mg kg−1 [51] and, today, concentrations above 1000 mg kg−1 can still be found in this area [52].
In the course of the study, it became apparent that the gardeners needed repeated reassurance from a diverse range of experts, who all came to a similar conclusion, that gardening activities would still be possible if some specific measures were adopted in their garden. In order to support reassurance with evidence-based findings, further testing and research was suggested by the experts. This included research about the historical use of the site, the self-initiated voluntary analysis of blood Pb concentrations of a small group of gardeners (all of which were under threshold levels) and further, more comprehensive investigations of soil, plant, compost and well water samples. Based on the outcomes of this testing, they initiated measures that will continuously improve their soil and their self-made compost by dilution with an adequate amount of uncontaminated compost and the cultivation of suitable plant species. In addition, they are planning to monitor these measures by periodically testing.
In potentially contaminated areas—as was the case in the 11er Garten and Paradeisgartl—regular plant screening and soil testing is recommended. This will provide certainty and reassurance among urban gardeners and will provide important indications as to whether measures have been effective or whether further remedial measures are needed. The direct benefit of scientific results can thus be demonstrated to the public by involving them in the process and allowing them to immediately experience and evaluate the effectiveness. However, such frequent tests might often be costly and not be affordable for everyone. This issue could be solved simply by establishing something such as a contact service for gardeners in Vienna, which provides information and access to affordable soil and plant tests and insightful support for the interpretation of soil tests [17]. Especially when it comes to complex issues such as risk assessment, we want to highlight the importance of a respectful cooperation between science and the broader public to promote mutual understanding and trust and to create an added value for the whole society. The risk concept of the public is usually more complex and goes beyond what is explainable by simple risk statistics. Communicating risk assessment can be challenging and is suggested to be sensitive to the risk conception of the public and should therefore be organized in a two-way process [53]. Otherwise, it is very likely that the risk perception among gardeners leads to inflated insecurity, resulting in avoidance of garden activities. Leake et al. [4] already highlighted the need for a more comprehensive risk management that also accounts for the health benefits of garden activities and of providing self-produced vegetables and fruits in urban areas.
We have seen that our project participants have a deep personal connection to their garden projects and that it is important for them to feel confident and secure about possible soil contaminations or the correct application of measures to reduce contaminations. We have also observed a great willingness among the gardeners to take on the effort of such measures, because these urban garden communities offer additional benefits to the people that go beyond simply growing vegetables, as these communities also provide potential social, health and environmental benefits e.g., [4,5,6,7,8,9,54]. The feedback we received from the citizen scientists on what they themselves consider to be the benefits of community gardens included not only growing and eating your own vegetables or better understanding of food production, but also the importance of community experience, neighborhood, social interaction, enjoying leisure time, greening areas or the possibility of activity and learning. These results are based on an online survey conducted at our final event, however less than ten participants responded, therefore not scientifically rigorous, although the results were in-line with the study conducted in south-east Toronto [7]. Here, the authors explored community gardeners’ personal experiences directly through observations, focus groups and in-depth interviews and came to the conclusion that community gardens have the potential to enhance access to food and improve gardeners’ diet but might also positively affect mental and social health. Furthermore, these urban green spaces have the capacity to improve our wellbeing through aesthetic aspects [9] while providing habitats for different species of insects, birds, reptiles and animals and thus play an important role in urban nature conservation and enhancing urban biodiversity [6,55]. Urban community gardens are considered as dynamic social-ecological systems which are able to offer important ecosystem services [56].
Even though our results were limited to only eleven sites in Vienna, and soil trace metal concentrations were generally within acceptable limits, there were still some indications of elevated levels resulting from previous use of the sites. Soils are slowly being acknowledged as a finite natural resource and their role in combating climate change is receiving more attention, e.g., the 4permil initiative. The potential benefits that community gardens might contribute to climate change mitigation in urban areas are diverse and ranging from carbon sequestration to provision of cooling effects, but also offer educational opportunities about global climate change [57]. Considering all of the benefits that these community gardens may provide to the overall urban environmental health or the “urban one health”, it becomes evident that urban soil protection is of crucial importance for the resilience of these garden initiatives, ensuring a sustainable safe garden environment to their citizens.
5. Conclusions
The results from the experiments of eleven community gardens suggest that the potential risk of heavy metal contamination of crops in the investigated Viennese urban gardens is generally rather low. However, it is recommended that before starting a garden project, the historical and current use of the site should be investigated and if there are any concerns, soil tests should be carried out. In potentially contaminated areas, measures such as building raised beds filled with pollutant-free soil, or using soil amendments such as compost as well as the choice of type of plant to be cultivated, washing or peeling crops, covering bare soil with mulch to avoid dust, etc., [41,58,59,60,61] are the basis for an effective way to further reduce or eliminate the health risk associated with urban gardening. If these measures are taken into consideration, the positive effects of gardening ventures in Vienna far outweigh the risks.
However, our project could not cover all community gardens and sites by far, although it provides a good starting point for further projects. For example, we identified the need for supportive public institutions to collaborate with the urban gardeners in terms of risk management associated with pollutants. Such collaborations may contribute to a sustainable existence of these valuable green urban spaces and thereby to stimulating sustainability in the urban area of Vienna in general. On the one hand, urban gardens create multifunctional green spaces with positive ecological effects and, on the other hand, urban gardening activities promote a healthy lifestyle and strengthen social interactions and the sense of community.
Author Contributions
Conceptualization, R.H.-N., E.Z., A.W., W.F.-H. and C.N.; methodology, E.Z., R.H.-N. and C.N.; formal analysis, E.Z., R.H.-N., S.G. and C.N.; data curation, E.Z. and S.G.; writing—original draft preparation, E.Z. and R.H.-N.; writing—review and editing, E.Z., R.H.-N., A.W., W.F.-H., C.N. and M.P.; supervision, R.H.-N., A.W., W.F.-H. and M.P.; project administration, E.Z., R.H.-N. and A.W.; funding acquisition, R.H.-N., E.Z., A.W. and W.F.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Austrian Science Fund (FWF), grant number TCS 74.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in [Zenodo] at [http://doi.org/10.5281/zenodo.4785321] accessed on 25 May 2021.
Acknowledgments
Especially we want to thank all garden initiatives which participated in our project: Gartenverein Hortensium, Tigergarten, Nachbarschaftsgarten Florasdorf, Gemeinschaftsgarten Rosenberg, 11er Garten, Gemeinschaftsgarten Madame d’Ora, Paradeisgartl, Nachbarschaftsgarten DonauCity/Kaisermühlen, Gemeinschaftsgarten Löwenzahn, Kinogarten and Matznergarten. We also want to thank Mathäus Steurer, Teresa Stockreiter, Dean Jordanski and Isabella Gehrken for their help in the lab.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [Green Version]
- Montanarella, L.; Vargas, R. Global governance of soil resources as a necessary condition for sustainable development. Curr. Opin. Environ. Sustain. 2012, 4, 559–564. [Google Scholar] [CrossRef]
- Blum, W.E.H. Soil and Land Resources for Agricultural Production: General Trends and Future Scenarios—A Worldwide Perspective. Int. Soil Water Conserv. Res. 2013, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Leake, J.R.; Adam-Bradford, A.; Rigby, J.E. Health benefits of ‘grow your own’ food in urban areas: Implications for contaminated land risk assessment and risk management? Environ. Health 2009, 8, S6. [Google Scholar] [CrossRef] [Green Version]
- McCormack, L.A.; Laska, M.; Larson, N.I.; Story, M. Review of the Nutritional Implications of Farmers’ Markets and Community Gardens: A Call for Evaluation and Research Efforts. J. Am. Diet. Assoc. 2010, 110, 399–408. [Google Scholar] [CrossRef]
- Brown, K.H.; Jameton, A.L. Public Health Implications of Urban Agriculture. J. Public Health Policy 2000, 21, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, S.; Yeudall, F.; Taron, C.; Reynolds, J.; Skinner, A. Growing urban health: Community gardening in South-East Toronto. Health Promot. Int. 2007, 22, 92–101. [Google Scholar] [CrossRef]
- Waliczek, T.; Zajicek, J.; Lineberger, R. The Influence of Gardening Activities on Consumer Perceptions of Life Satisfaction. HortScience 2005, 40, 1360–1365. [Google Scholar] [CrossRef] [Green Version]
- Litt, J.; Schmiege, S.; Hale, J.; Buchenau, M.; Sancar, F. Exploring ecological, emotional and social levers of self-rated health for urban gardeners and non-gardeners: A path analysis. Soc. Sci. Med. 2015, 144, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zasada, I. Multifunctional peri-urban agriculture—A review of societal demands and the provision of goods and services by farming. Land Use Policy 2011, 28, 639–648. [Google Scholar] [CrossRef]
- Russo, A.; Escobedo, F.J.; Cirella, G.T.; Zerbe, S. Edible green infrastructure: An approach and review of provisioning ecosystem services and disservices in urban environments. Agric. Ecosyst. Environ. 2017, 242, 53–66. [Google Scholar] [CrossRef]
- McIlwaine, R.; Doherty, R.; Cox, S.; Cave, M. The relationship between historical development and potentially toxic element concentrations in urban soils. Environ. Pollut. 2017, 220, 1036–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szolnoki, Z.; Farsang, A.; Puskás, I. Cumulative impacts of human activities on urban garden soils: Origin and accumulation of metals. Environ. Pollut. 2013, 177, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Antisari, L.V.; Orsini, F.; Marchetti, L.; Vianello, G.; Gianquinto, G.P. Heavy metal accumulation in vegetables grown in urban gardens. Agron. Sustain. Dev. 2015, 35, 1139–1147. [Google Scholar] [CrossRef]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Schwarz, K.; Pouyat, R.V.; Yesilonis, I. Legacies of Lead in Charm City’s Soil: Lessons from the Baltimore Ecosystem Study. Int. J. Environ. Res. Public Health 2016, 13, 209. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.F.; Poulsen, M.N.; Margulies, J.D.; Dix, K.L.; Palmer, A.M.; Nachman, K.E. Urban Community Gardeners’ Knowledge and Perceptions of Soil Contaminant Risks. PLoS ONE 2014, 9, e87913. [Google Scholar] [CrossRef]
- Wong, R.; Gable, L.; Nunez, Z.R. Perceived Benefits of Participation and Risks of Soil Contamination in St. Louis Urban Community Gardens. J. Community Health 2018, 43, 604–610. [Google Scholar] [CrossRef]
- Poulsen, M.N.; Hulland, K.R.S.; Gulas, C.A.; Pham, H.; Dalglish, S.L.; Wilkinson, R.K.; Winch, P.J. Growing an Urban Oasis: A Qualitative Study of the Perceived Benefits of Community Gardening in Baltimore, Maryland. Cult. Agric. Food Environ. 2014, 36, 69–82. [Google Scholar] [CrossRef]
- Paltseva, A.A.; Cheng, Z.; Egendorf, S.P.; Groffman, P.M. Remediation of an urban garden with elevated levels of soil contamination. Sci. Total Environ. 2020, 722, 137965. [Google Scholar] [CrossRef]
- Allen, A. Environmental planning and management of the peri-urban interface: Perspectives on an emerging field. Environ. Urban. 2003, 15, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.P.; Isley, C.F.; Fry, K.L.; Liu, X.; Gillings, M.M.; Rouillon, M.; Soltani, N.S.; Gore, D.B.; Filippelli, G.M. A citizen science approach to identifying trace metal contamination risks in urban gardens. Environ. Int. 2021, 155, 106582. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, G.M.; Adamic, J.; Nichols, D.; Shukle, J.; Frix, E. Mapping the Urban Lead Exposome: A Detailed Analysis of Soil Metal Concentrations at the Household Scale Using Citizen Science. Int. J. Environ. Res. Public Health 2018, 15, 1531. [Google Scholar] [CrossRef] [Green Version]
- Rouillon, M.; Harvey, P.; Kristensen, L.J.; George, S.G.; Taylor, M.P. VegeSafe: A community science program measuring soil-metal contamination, evaluating risk and providing advice for safe gardening. Environ. Pollut. 2017, 222, 557–566. [Google Scholar] [CrossRef]
- Sandhaus, S.; Kaufmann, D.; Ramirez-Andreotta, M. Public participation, trust and data sharing: Gardens as hubs for citizen science and environmental health literacy efforts. Int. J. Sci. Educ. 2019, 9, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Broeder, L.D.; Devilee, J.; Van Oers, H.; Schuit, J.; Wagemakers, A. Citizen Science for public health. Health Promot. Int. 2018, 33, 505–514. [Google Scholar] [CrossRef]
- Rüfenacht, S.; Woods, T.; Agnello, G.; Gold, M.; Hummer, P.; Land-Zandstra, A.; Sieber, A. Communication and Dissemination in Citizen Science. In The Science of Citizen Science; Vohland, K., Land-Zandstra, A., Ceccaroni, L., Lemmens, R., Perelló, J., Ponti, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 475–494. [Google Scholar] [CrossRef]
- Debord, D.G.; Carreón, T.; Lentz, T.J.; Middendorf, P.J.; Hoover, M.D.; Schulte, P.A. Use of the “Exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2016, 184, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Escher, B.I.; Hackermüller, J.; Polte, T.; Scholz, S.; Aigner, A.; Altenburger, R.; Böhme, A.; Bopp, S.K.; Brack, W.; Busch, W.; et al. From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ. Int. 2017, 99, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Juarez, P.D.; Matthews-Juarez, P.; Hood, D.B.; Im, W.; Levine, R.S.; Kilbourne, B.J.; Langston, M.A.; Al-Hamdan, M.Z.; Crosson, W.L.; Estes, M.G.; et al. The Public Health Exposome: A Population-Based, Exposure Science Approach to Health Disparities Research. Int. J. Environ. Res. Public Health 2014, 11, 12866–12895. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.; Gottlieb, L. The Social Determinants of Health: It’s Time to Consider the Causes of the Causes. Public Health Rep. 2014, 129, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Brink, E.; Aalders, J.T.; Ádám, D.; Feller, R.; Henselek, Y.; Hoffmann, A.; Ibe, K.; Matthey-Doret, A.; Meyer, M.; Negrut, N.L.; et al. Cascades of green: A review of ecosystem-based adaptation in urban areas. Glob. Environ. Chang. 2016, 36, 111–123. [Google Scholar] [CrossRef]
- Thierfelder, H.; Kabisch, N. Viewpoint Berlin: Strategic urban development in Berlin—Challenges for future urban green space development. Environ. Sci. Policy 2016, 62, 120–122. [Google Scholar] [CrossRef]
- Scharf, T.; Gierveld, J.D.J. Loneliness in urban neighbourhoods: An Anglo-Dutch comparison. Eur. J. Ageing 2008, 5, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Kreiner, P. Wiener Bodenbericht 2003. Untersuchung des Wiener Bodens Auf Schwermetalle und Polyaromatische Kohlenwasserstoffe. Beiträge zum Umweltschutz; Magistratsabteilung 22 Umweltschutz: Vienna, Austria, 2004; Volume 70. [Google Scholar]
- Pfleiderer, S.; Englisch, M.; Reiter, R. Current state of heavy metal contents in Vienna soils. Environ. Geochem. Health 2012, 34, 665–675. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Brown, S.; Chaney, R.L.; Hallfrisch, J.G.; Xue, Q. Effect of Biosolids Processing on Lead Bioavailability in an Urban Soil. J. Environ. Qual. 2003, 32, 100–108. [Google Scholar] [CrossRef]
- Austrian Standard L 1075: 2017 11 01. In Grundlagen Für Die Bewertung der Gehalte Ausgewählter Chemischer Elemente in Böden; Österreichisches Normungsinstitut: Wien, Austria, 2017.
- European Commission. Commission Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Brown, S.L.; Chaney, R.L.; Hettiarachchi, G.M. Lead in Urban Soils: A Real or Perceived Concern for Urban Agriculture? J. Environ. Qual. 2016, 45, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Ellinger, S.; Linseisen, J.; Neuhäuser-Berthold, M.; Richter, M. Revised D-A-CH-reference values for the intake of zinc. J. Trace Elem. Med. Biol. 2020, 61, 126536. [Google Scholar] [CrossRef]
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Zinc; Scientific Committee on Food: Brussels, Belgium, 2003. [Google Scholar]
- Elmadafa, I.; Freisling, H.; Nowak, V.; Hofstädter, D.; Hasenegger, V.; Ferge, M.; Fröhler, M.; Fritz, K.; Meyer, A.L.; Putz, P.; et al. Österreichischer Ernährungsbericht 2008; Institut für Ernährungswissenschaften, Universität Wien: Wien, Austria, 2009. [Google Scholar]
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Warming, M.; Hansen, M.G.; Holm, P.; Magid, J.; Hansen, T.; Trapp, S. Does intake of trace elements through urban gardening in Copenhagen pose a risk to human health? Environ. Pollut. 2015, 202, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.; Thompson, K.; MacFie, S.M. Site- and species-specific patterns of metal bioavailability in edible plants. Botany 2009, 87, 702–711. [Google Scholar] [CrossRef]
- Yi, Z.; Lehto, N.J.; Robinson, B.H.; Cavanagh, J.-A.E. Environmental and edaphic factors affecting soil cadmium uptake by spinach, potatoes, onion and wheat. Sci. Total Environ. 2020, 713, 136694. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Sahmer, K.; Waterlot, C.; Douay, F. From environmental data acquisition to assessment of gardeners’ exposure: Feedback in an urban context highly contaminated with metals. Environ. Sci. Pollut. Res. 2019, 26, 20107–20120. [Google Scholar] [CrossRef] [PubMed]
- Friesl, W.; Friedl, J.; Platzer, K.; Horak, O.; Gerzabek, M. Remediation of contaminated agricultural soils near a former Pb/Zn smelter in Austria: Batch, pot and field experiments. Environ. Pollut. 2006, 144, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Kasperowski, E. Schwermetalle in Böden Im Raum Arnoldstein. Umweltbundesamt, Monographien Bd. 33; Bundesministerium für Umwelt, Jugend und Familie: Wien, Austria, 1993. [Google Scholar]
- Friesl-Hanl, W.; Platzer, K.; Riesing, J.; Horak, O.; Waldner, G.; Watzinger, A.; Gerzabek, M.H. Non-destructive soil amendment application techniques on heavy metal-contaminated grassland: Success and long-term immobilising efficiency. J. Environ. Manag. 2017, 186, 167–174. [Google Scholar] [CrossRef]
- Slovic, P. Perception of risk. Science 1987, 236, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Litt, J.S.; Soobader, M.-J.; Turbin, M.S.; Hale, J.; Buchenau, M.; Marshall, J.A. The Influence of Social Involvement, Neighborhood Aesthetics, and Community Garden Participation on Fruit and Vegetable Consumption. Am. J. Public Health 2011, 101, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.; Dougill, A.; Benton, T. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.B.; Egerer, M.H. Global social and environmental change drives the management and delivery of ecosystem services from urban gardens: A case study from Central Coast, California. Glob. Environ. Chang. 2020, 60, 102006. [Google Scholar] [CrossRef]
- Okvat, H.A.; Zautra, A.J. Community Gardening: A Parsimonious Path to Individual, Community, and Environmental Resilience. Am. J. Community Psychol. 2011, 47, 374–387. [Google Scholar] [CrossRef]
- Alexander, P.; Alloway, B.; Dourado, A. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006, 144, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Nabulo, G.; Black, C.; Craigon, J.; Young, S. Does consumption of leafy vegetables grown in peri-urban agriculture pose a risk to human health? Environ. Pollut. 2012, 162, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Glass, K.; Morris, S.; Zhang, H.; McRae, I.; Anderson, N.; Alfieri, A.; Egendorf, S.P.; Holberton, S.; Owrang, S.; et al. Sediment exchange to mitigate pollutant exposure in urban soil. J. Environ. Manag. 2018, 214, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Defoe, P.P.; Hettiarachchi, G.M.; Benedict, C.; Martin, S. Safety of Gardening on Lead- and Arsenic-Contaminated Urban Brownfields. J. Environ. Qual. 2014, 43, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).