Mineralization of Farm Manures and Slurries under Aerobic and Anaerobic Conditions for Subsequent Release of Phosphorus and Sulphur in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection and Preparation
2.2. Analysis of Initial Soil Samples
2.3. Collection of Different Manures and Determination of Their Chemical Composition
2.4. Experimental Setup
2.5. Organic Fertilizer Incubation in Soil
2.6. Analysis of Soil Samples after Manure Application
2.7. Statistical Analysis
3. Results
3.1. Chemical Compostion of the Manures
3.2. Phosphorus Release Pattern from Mineralization of OM in Soils
3.2.1. Total P Release
3.2.2. Net P Release
3.2.3. Cumulative P Release
3.2.4. Cumulative Mineralization Kinetic Model and Rates for P
3.2.5. Phosphorus Release from Manures
3.2.6. Relation between Net Available P and Manure C/P Ratio
3.3. Sulphur Release Pattern from Mineralization of OM in Soils
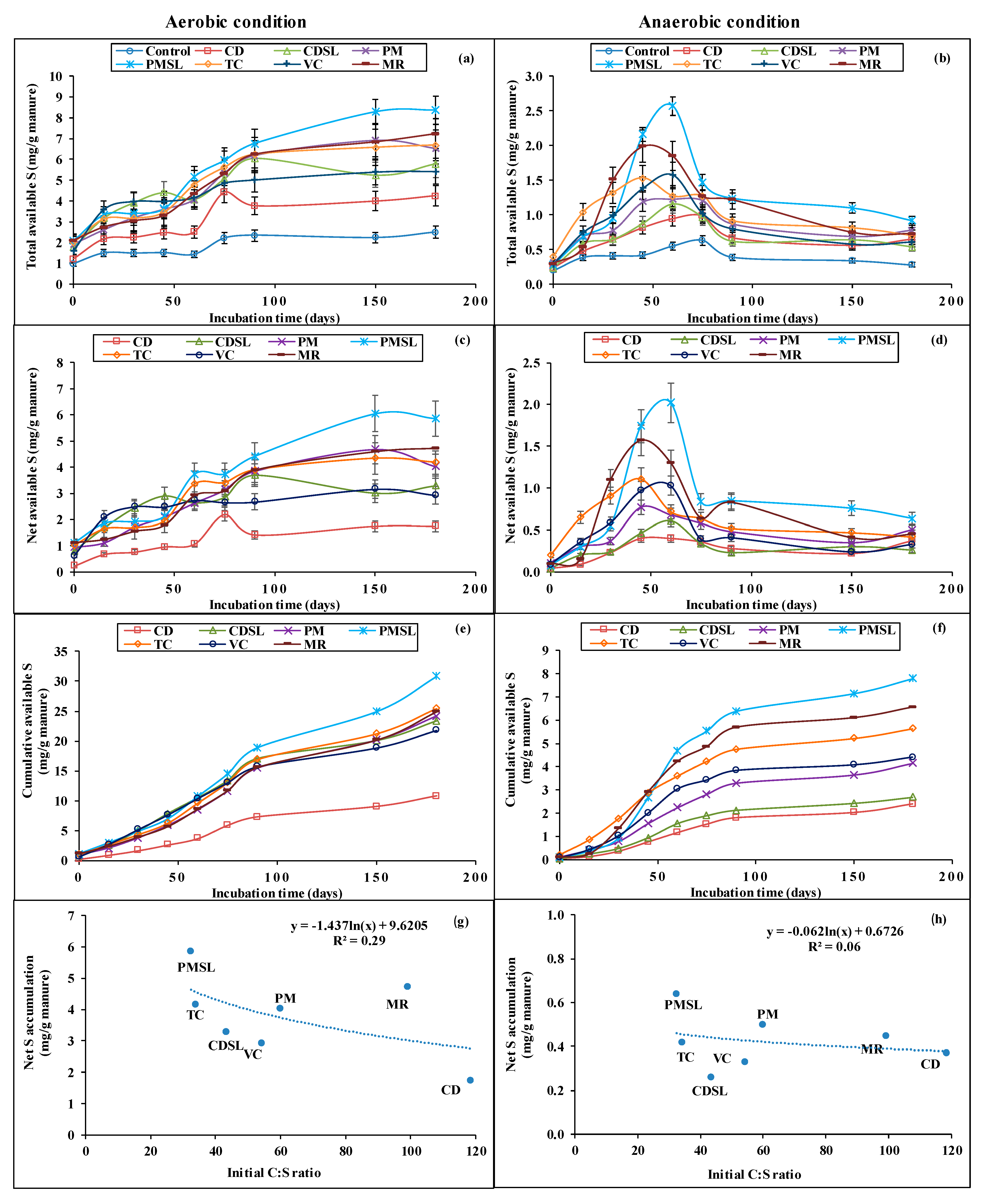
3.3.1. Total S Release
3.3.2. Net S Release
3.3.3. Cumulative S Release
3.3.4. Cumulative Mineralization Kinetic Model and Rates for S
3.3.5. Sulphur release from Manures
3.3.6. Relation between Net S Accumulation and Manure C/S Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bangladesh Economic Review. Bangladesh Economic Review, Dhaka: Finance Division; Ministry of Finance, Government of Peoples’ Republic of Bangladesh: Dhaka, Bangladesh, 2018.
- Statistical Yearbook Bangladesh. Statistical Yearbook Bangladesh, Bangladesh Bureau of Statistics, 38th ed.; Statistics and Informatics Division, Ministry of Planning, Government of People’s Republic of Bangladesh: Dhaka, Bangladesh, 2018.
- Agriculture Sector Review. Agriculture Sector Review, Actionable Policy Brief and Resource Implications; Ministry of Agriculture, Government of Republic of Bangladesh: Dhaka, Bangladesh, 2006; pp. 14–51.
- Wani, S.P.; Rupela, O.P.; Lee, K.K. Sustainable agriculture in the semi-arid tropics through biological nitrogen fixation in grain legumes. Plant Soil 1995, 174, 29–49. [Google Scholar] [CrossRef]
- Escobar, M.E.O.; Hue, N.V. Temporal changes of selected chemical properties in three manure—Amended soils of Hawaii. Bioresour. Technol. 2008, 99, 8649–8654. [Google Scholar] [CrossRef]
- Rahman, M.H.; Islam, M.R.; Jahiruddin, M.; Puteh, A.B.; Mondal, M.M.A. Influence of organic matter on nitrogen mineralization pattern in soils under different moisture regimes. Int. J. Agric. Biol. 2013, 15, 55–61. [Google Scholar]
- Weeraratna, C.S. Pattern of nitrogen release during decomposition of some green manures in a tropical alluvial soil. Plant Soil 1979, 53, 287–294. [Google Scholar] [CrossRef]
- Eghball, B.; Wienhold, B.J.; Gilley, J.E.; Eigenberg, R.A. Mineralization of manurenutrients. J. Soil Water Conserv. 2002, 57, 470–473. [Google Scholar]
- Moharana, P.C.; Biswas, D.R.; Datta, S.C. Mineralization of nitrogen, phosphorus and sulphur in soil as influenced by rock phosphate enriched compost and chemical fertilizers. J. Indian Soc. Soil Sci. 2015, 63, 283–293. [Google Scholar] [CrossRef]
- Hadas, A.; Portnoy, R. Nitrogen and carbon mineralization rates of composted manures incubated in soil. J. Environ. Qual. 1994, 23, 1184–1189. [Google Scholar] [CrossRef]
- Jalali, M.; Mahdvi, S.; Ranjbar, F. Nitrogen, phosphorus and sulphur mineralization as affected by soil depth in rangeland ecosystems. Environ. Earth Sci. 2014, 72, 1775–1788. [Google Scholar] [CrossRef]
- Xiao, R.; Bai, J.; Gao, H.; Huang, L.; Deng, W. Spatial distribution of phosphorus in marsh soils of a typical land/inland water ecotone along a hydrological gradient. Catena 2012, 98, 96–103. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Bennett, E.M. Reconsideration of the planetary boundary for phosphorus. Environ. Res. Lett. 2011, 6, 104013. [Google Scholar] [CrossRef]
- Reddy, K.S.; Muneshwar, S.; Tripathi, A.K.; Swarup, A.; Dwivedi, A.K. Changes in organic and inorganic sulphur fractions and S mineralisation in a Typic Haplustert after long-term cropping with different fertilizer and organic manure inputs. Aust. J. Soil Res. 2001, 39, 737–748. [Google Scholar] [CrossRef]
- Ghani, A.; Mclaren, R.G.; Swifts, R.S. Sulfur mineralization in some New Zealand soils. Biol. Fert. Soils 1991, 11, 68–74. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Goh, K.M. Sulphur mineralization and release of soluble organic S from camp and non-camp soils of grazed pastures receiving long term super phosphate applications. Biol. Fertil Soils 1992, 14, 272–279. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Chae, Y.M. Mineralization of sulphur in soil amended with organic wastes. J. Environ. Qual. 1991, 20, 684–690. [Google Scholar] [CrossRef]
- Islam, M.M.; Dick, R.P. Effect of organic residue amendment on mineralization of sulphur in flooded rice soils under laboratory conditions. Commun. Soil Sci. Plant Anal. 1998, 29, 955–969. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Land Resources Appraisal of Bangladesh for Agricultural Development. Report 2. Agro- Ecological Regions of Bangladesh; Food and Agriculture Organization: Rome, Italy, 1988; pp. 212–221. [Google Scholar]
- Marfo, T.D.; Datta, R.; Pathan, S.I.; Vranová, V. Ecotone dynamics and stability from soil scientific point of view. Diversity 2019, 11, 53. [Google Scholar] [CrossRef]
- Black, C.A. Methods of Soil Analysis: Part-1; American Society of Agronomy, Inc.: Madison, WI, USA, 1965. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis—Part 1; Klute, A., Ed.; Agronomy 9; ASA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt Ltd.: New Delhi, India, 1973; pp. 69–182. [Google Scholar]
- Chapman, H.D. Cation exchange capacity. In Methods of Soil Analysis—Part 2; Black, C.A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar]
- Walkey, A.J.; Black, A.I. Estimation of organic carbon by chromic acid titration method. J. Soil Sci. 1934, 25, 259–260. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen- total. In Methods of Soil Analysis—Part; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Cole, C.U.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soil by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954; p. 929.
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium and potassium. In Methods of Soil Analysis-Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 225–245. [Google Scholar]
- Williams, C.H.; Steinbergs, A. Soil sulphur fractions as chemical indices of available sulphur in some Australian soils. Aust. J. Agric. Res. 1959, 10, 340–352. [Google Scholar] [CrossRef]
- Soil Testing Procedure Manual. Soil Testing Procedure Manual; Marathwada Agricultural University: Parbhani, India, 2008; p. 22. [Google Scholar]
- Yamakawa, T. Laboratory Methods for Soil Science and Plant Nutrition. In Methods of Plant Analysis-Part-2, JICA-IPSA Project; American Society of Agronomy, Inc.: Madison, WI, USA, 1992; pp. 6–14. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis: A Laboratory Manual of Methods for the Examination of Soils and the Determination of the Inorganic Constituents of Plants; Hans Publishers: Bombay, India, 1966. [Google Scholar]
- Fox, R.L.; Olson, R.A.; Rhoades, H.F. Evaluating the sulfur status of soils by plant and soil tests. Soil Sci. Soc. Am. J. 1964, 28, 243–246. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management; Prentice Hall: London, UK, 1999; p. 499. [Google Scholar]
- Sleutel, S.; Vandenbruwane, J.; De Schrijver, A.; Wuyts, K.; Moeskops, B.; Verheyen, K.; De Neve, S. Patterns of dissolved organic carbon and nitrogen fluxes in deciduous and coniferous forests under historic high nitrogen deposition. Biogeosciences 2009, 6, 2743–2758. [Google Scholar] [CrossRef]
- Naher, U.A.; Hashem, M.A.; Mitra, B.K.; Uddin, M.K.; Saleque, M.A. Effect of rice straw and lime on phosphorus and potassium mineralization from cowdung and poultry manure under covered and uncovered conditions in the tropical environment. Pak. J. Biol. Sci. 2004, 7, 45–48. [Google Scholar] [CrossRef][Green Version]
- Meena, A.L.; Jha, P.; Dotaniya, M.L.; Kumar, B.; Meena, B.P.; Jat, R.L. Carbon, nitrogen and phosphorus mineralization as influenced by type of organic residues and soil contact variation in vertisol of Central India. Agric. Res. 2020, 9, 232–240. [Google Scholar] [CrossRef]
- Haque, A.M.; Jahiruddin, M.; Rahman, M.M.; Saleque, M.A. Phosphorus mineralization of bioslurry and other manures in soil. J. Environ. Waste Manage. 2015, 2, 79–83. [Google Scholar]
- Hegeman, G. The mineralization of organic materials under aerobic conditions. In Bacteria in Nature; Leadbetter, E.R., Poindexter, J.S., Eds.; Plenum Press: New York, NY, USA; London, UK, 1985; Volume 1, pp. 39–110. [Google Scholar]
- Pal, Y.; Ram, S.; Pradhan, S.; Singh, P.; Seema; Ghosh, A.K. Phosphorus mineralization in an alluvial soil as influenced by organic manure addition and time of incubation. Int. J. Chem. Stud. 2018, 6, 1727–1730. [Google Scholar]
- McDowell, R.W.; Sharpley, A.N. Phosphorus solubility and release kinetics as a function of soil test P concentration. Geoderma 2003, 112, 143–154. [Google Scholar] [CrossRef]
- Islas-Espinoza, M.; Solís-Mejía, L.; Estelle, M.V. Phosphorus release kinetics in a soil amended with biosolids and vermicompost. Environ. Earth Sci. 2013, 71, 1441–1451. [Google Scholar] [CrossRef]
- Gagnon, B.; Simard, R.R. Nitrogen and phosphorus release from on-farm and industrial composts. Can. J. Soil Sci. 1999, 79, 481–489. [Google Scholar] [CrossRef]
- Garg, S.; Bahl, G.G. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.D.; Biswas, D.R. Phosphorus and potassium transformations in soil amended with enriched compost and chemical fertilizers in a wheat–soybean cropping system. Commun. Soil Sci. Plant Anal. 2014, 45, 624–652. [Google Scholar] [CrossRef]
- Eriksen, J. Gross sulphurmineralisation-immobilisation turnover in soil amended with plant residues. Soil Biol. Biochem. 2005, 37, 2216–2224. [Google Scholar] [CrossRef]
- Singh, B.P.; Rengel, Z.; Bowden, J.W. Carbon, nitrogen and sulphur cycling following incorporation of canola residue of different sizes into a nutrient-poor sandy soil. Soil Biol. Biochem. 2006, 38, 32–42. [Google Scholar] [CrossRef]
- Niknahad-Gharmakher, H.; Piutti, S.; Machet, J.M.; Benizri, E.; Recous, S. Mineralization-immobilization of sulphur in a soil during decomposition of plant residues of varied chemical composition and S content. Plant Soil 2012, 360, 391–404. [Google Scholar] [CrossRef]
- Assefa, M.K.; von Tucher, S.; Schmidhalter, U. Soil sulfur availability due to mineralization of soil amended with biogas residues. J. Soil Sci. Environ. Manage. 2014, 5, 13–19. [Google Scholar]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Khan, R.I.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Izhar Shafi, M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hye, M.; Brtnicky, M.; Datta, R. Application of Single Superphosphate with Humic Acid Improves the Growth, Yield and Phosphorus Uptake of Wheat (Triticum aestivum L.) in Calcareous Soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Ullah, A.; Ali, M.; Shahzad, K.; Ahmad, F.; Iqbal, S.; Habib, M.; Rahman, M.; Ahmad, S.; Iqbal, M.; Danish, S.; et al. Impact of Seed Dressing and Soil Application of Potassium Humate on Cotton Plants Productivity and Fiber Quality. Plants 2020, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, G.S.; El-Esawi, M.A. Effect of Cadmium-Tolerant Rhizobacteria on Growth Attributes and Chlorophyll Contents of Bitter Gourd under Cadmium Toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Abid, M.; Fahad, S.; Brtnicky, M.; Dokulilova, T.; Datta, R.; Danish, S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Fahad, S.; Datta, R.; Abbas, M.; Rahi, A.A.; Brtnicky, M.; Holátko, J.; Tarar, Z.H.; et al. Alleviation of Cadmium Adverse Effects by Improving Nutrients Uptake in Bitter Gourd through Cadmium Tolerant Rhizobacteria. Environments 2020, 7, 54. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Fahad, S.; Saud, S.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Drought Stress Alleviation by ACC Deaminase Producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without Timber Waste Biochar in Maize. Sustainability 2020, 12, 6286. [Google Scholar] [CrossRef]
- Pathan, S.I.; Větrovský, T.; Giagnoni, L.; Datta, R.; Baldrian, P.; Nannipieri, P.; Renella, G. Microbial expression profiles in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil 2018, 433, 401–413. [Google Scholar] [CrossRef]

| Soil Characteristics | Values |
|---|---|
| Particle size distribution (USDA system) | |
| % Sand (0.2–0.05 mm) | 1.4 |
| % Silt (0.05–0.002 mm) | 80 |
| % Clay (<0.002 mm) | 18.6 |
| Textural class | Silt loam |
| Water holding capacity (%) | 51.5 |
| Bulk density (g cm−3) | 1.33 |
| Organic C (%) | 2.15 |
| Cation exchange capacity (cmol kg−1) | 12.1 |
| pH | 6.5 |
| Total N (%) | 0.119 |
| Available P (mg kg−1) | 9.1 |
| Exchangeable K (cmol kg−1) | 0.12 |
| Available S (mg kg−1) | 25.3 |
| Manure | C (%) | N (%) | P (%) | S (%) | C:N | C:P | C:S |
|---|---|---|---|---|---|---|---|
| CD | 33.14 ± 1.91 | 1.27 ± 0.07 | 0.50 ± 0.03 | 0.28 ± 0.02 | 26.2 ± 1.51 | 66.8 ± 3.86 | 118.4 ± 6.84 |
| CDSL | 20.88 ± 1.21 | 1.90 ± 0.11 | 1.23 ± 0.07 | 0.48 ± 0.03 | 11.0 ± 0.64 | 17.0 ± 0.98 | 43.5 ± 2.51 |
| PM | 33.54 ± 1.94 | 3.08 ± 0.18 | 2.33 ± 0.13 | 0.56 ± 0.03 | 10.9 ± 0.63 | 14.4 ± 0.83 | 59.9 ± 3.46 |
| PMSL | 22.34 ± 1.29 | 2.69 ± 0.16 | 2.49 ± 0.14 | 0.69 ± 0.04 | 8.3 ± 0.48 | 9.0 ± 0.52 | 32.4 ± 1.87 |
| TC | 19.41 ± 1.12 | 1.32 ± 0.08 | 1.76 ± 0.10 | 0.57 ± 0.03 | 14.7 ± 0.85 | 11.0 ± 0.64 | 34.1 ± 1.97 |
| VC | 22.81 ± 1.32 | 1.15 ± 0.07 | 0.52 ± 0.03 | 0.42 ± 0.02 | 19.9 ± 1.15 | 43.9 ± 2.53 | 54.3 ± 3.14 |
| MR | 45.60 ± 2.63 | 1.29 ± 0.07 | 0.45 ± 0.03 | 0.46 ± 0.03 | 35.4 ± 2.04 | 101.3 ± 5.85 | 99.1 ± 5.72 |
| Manures | Aerobic Incubation | Anaerobic Incubation | ||||||
|---|---|---|---|---|---|---|---|---|
| P0 (mg/g Manure) | k (mg/g Manure/Day) | R2 adj * | F | P0 (mg/g Manure) | k (mg/g Manure/Day) | R2 adj * | F | |
| CD | 1.38 | 0.003 | 0.505 | 9.166 (P < 0.019) | 0.63 | 0.009 | 0.479 | 8.346 (P < 0.020) |
| CDSL | 2.73 | 0.004 | 0.268 | 3.932(P < 0.088) | 1.18 | 0.011 | 0.281 | 4.126 (P < 0.080) |
| PM | 4.54 | 0.004 | 0.155 | 2.468 (P < 0.160) | 3.09 | 0.004 | 0.768 | 27.36 (P < 0.001) |
| PMSL | 5.31 | 0.004 | 0.214 | 3.174 (P < 0.118) | 3.28 | 0.006 | 0.642 | 15.37 (P < 0.006) |
| TC | 4.09 | 0.005 | 0.320 | 4.763 (P < 0.065) | 1.96 | 0.007 | 0.523 | 9.767 (P < 0.016) |
| VC | 2.72 | 0.002 | 0.601 | 13.06 (P < 0.009) | 0.94 | 0.011 | 0.820 | 37.39 (P < 0.001) |
| MR | 2.25 | 0.001 | 0.894 | 68.24 (P < 0.160) | 0.23 | 0.006 | 0.506 | 9.191 (P < 0.019) |
| Organic Manure | P Added (mg 100 g−1 Soil) | P Release in 180 Days (mg g−1 Manure) | % P Release | ||
|---|---|---|---|---|---|
| Aerobic | Anaerobic | Aerobic | Anaerobic | ||
| CD | 5.0 | 1.6 ± 0.2d | 0.7 ± 0.1e | 32.5 ± 3.8b | 14.3 ± 1.7ef |
| CDSL | 12.3 | 3.0 ± 0.4c | 1.6 ± 0.2d | 24.4 ± 2.8c | 12.9 ± 1.5f |
| PM | 23.3 | 5.0 ± 0.6a | 4.3 ± 0.5b | 21.4 ± 2.5cde | 18.5 ± 2.1cdef |
| PMSL | 24.9 | 5.4 ± 0.6a | 4.4 ± 0.5ab | 21.7 ± 2.5cde | 17.8 ± 2.1cdef |
| TC | 17.6 | 4.2 ± 0.5b | 2.8 ± 0.3c | 24.1 ± 2.8cd | 16.0 ± 1.9def |
| VC | 5.2 | 3.1 ± 0.4c | 1.1 ± 0.1de | 60.1 ± 6.9a | 21.5 ± 2.5cde |
| MR | 4.5 | 1.6 ± 0.2d | 0.7 ± 0.1e | 35.6 ± 4.1b | 14.4 ± 1.7ef |
| Manures | Aerobic Incubation | Anaerobic Incubation | ||||||
|---|---|---|---|---|---|---|---|---|
| S0 (mg/g Manure) | k (mg/g Manure/Day) | R2 adj * | F | S0 (mg/g Manure) | k (mg/g Manure/Day) | R2 adj * | F | |
| CD | 1.82 | 0.0020 | 0.809 | 34.57 (P < 0.001) | 0.32 | 0.0037 | 0.753 | 25.39 (P < 0.002) |
| CDSL | 3.2 | 0.0049 | 0.731 | 22.79 (P < 0.002) | 0.32 | 0.0074 | 0.739 | 23.67 (P < 0.002) |
| PM | 4.76 | 0.0015 | 0.787 | 30.51 (P < 0.001) | 0.51 | 0.0066 | 0.616 | 13.85 (P < 0.007) |
| PMSL | 7.02 | 0.0011 | 0.755 | 25.65 (P < 0.002) | 0.89 | 0.0046 | 0.592 | 12.60 (P < 0.009) |
| TC | 4.54 | 0.0055 | 0.069 | 1.596 (P < 0.247) | 0.65 | 0.0081 | 0.265 | 3.89 (P < 0.063) |
| VC | 2.77 | 0.0086 | 0.819 | 37.21 (P < 0.001) | 0.44 | 0.0150 | 0.327 | 4.89 (P < 0.063) |
| MR | 4.21 | 0.0083 | 0.389 | 6.102 (P < 0.043) | 0.65 | 0.0048 | 0.363 | 5.56 (P < 0.050) |
| Organic Manure | S Added (mg 100 g−1 Soil) | Maximum S Release (mg g−1 Manure) | % S Release | ||
|---|---|---|---|---|---|
| Aerobic | Anaerobic | Aerobic | Anaerobic | ||
| CD | 2.8 | 1.8 ± 0.2c | 0.4 ± 0.04cd | 62.5 ± 7.2b | 13.2 ± 1.5c |
| CDSL | 4.8 | 3.3 ± 0.4bc | 0.3 ± 0.03d | 68.5 ± 7.9ab | 5.4 ± 0.6c |
| PM | 5.6 | 4.0 ± 0.5b | 0.5 ± 0.06cd | 71.9 ± 8.3ab | 8.9 ± 1.0c |
| PMSL | 6.9 | 5.9 ± 0.7a | 0.6 ± 0.07cd | 85.1 ± 9.8ab | 9.2 ± 1.1c |
| TC | 4.7 | 4.2 ± 0.5b | 0.4 ± 0.05cd | 88.9 ± 9.5a | 9.0 ± 1.0c |
| VC | 3.4 | 2.9 ± 0.3bc | 0.3 ± 0.04cd | 86.2 ± 9.9a | 9.8 ± 1.1c |
| MR | 5.6 | 4.7 ± 0.4b | 0.5 ± 0.05cd | 84.5 ± 7.7ab | 8.1 ± 0.9c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.R.; Bilkis, S.; Hoque, T.S.; Uddin, S.; Jahiruddin, M.; Rahman, M.M.; Siddique, A.B.; Hossain, M.A.; Danso Marfo, T.; Danish, S.; et al. Mineralization of Farm Manures and Slurries under Aerobic and Anaerobic Conditions for Subsequent Release of Phosphorus and Sulphur in Soil. Sustainability 2021, 13, 8605. https://doi.org/10.3390/su13158605
Islam MR, Bilkis S, Hoque TS, Uddin S, Jahiruddin M, Rahman MM, Siddique AB, Hossain MA, Danso Marfo T, Danish S, et al. Mineralization of Farm Manures and Slurries under Aerobic and Anaerobic Conditions for Subsequent Release of Phosphorus and Sulphur in Soil. Sustainability. 2021; 13(15):8605. https://doi.org/10.3390/su13158605
Chicago/Turabian StyleIslam, Mohammad Rafiqul, Sultana Bilkis, Tahsina Sharmin Hoque, Shihab Uddin, Mohammad Jahiruddin, Mohammad Mazibur Rahman, Abu Bakkar Siddique, Mohammad Anwar Hossain, Theodore Danso Marfo, Subhan Danish, and et al. 2021. "Mineralization of Farm Manures and Slurries under Aerobic and Anaerobic Conditions for Subsequent Release of Phosphorus and Sulphur in Soil" Sustainability 13, no. 15: 8605. https://doi.org/10.3390/su13158605
APA StyleIslam, M. R., Bilkis, S., Hoque, T. S., Uddin, S., Jahiruddin, M., Rahman, M. M., Siddique, A. B., Hossain, M. A., Danso Marfo, T., Danish, S., & Datta, R. (2021). Mineralization of Farm Manures and Slurries under Aerobic and Anaerobic Conditions for Subsequent Release of Phosphorus and Sulphur in Soil. Sustainability, 13(15), 8605. https://doi.org/10.3390/su13158605











