Distribution, Population Size, and Habitat Characteristics of the Endangered European Ground Squirrel (Spermophilus citellus, Rodentia, Mammalia) in Its Southernmost Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spatial Distribution and Population Size
2.2. Spatial Analysis of Site-Attributes
3. Results
3.1. Spatial Distribution and Population Size
3.2. Spatial Analysis of Site-Attributes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-Lara, N.; Koprowski, J.L.; Kryštufek, B.; Hoffmann, I.E. Spermophilus citellus (Rodentia: Sciuridae). Mamm. Species 2014, 46, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Lindtner, P.; Gömöryová, E.; Gömöry, D.; Stašiov, S.; Kubovčík, V. Development of physico-chemical and biological soil properties on the European ground squirrel mounds. Geoderma 2019, 339, 85–93. [Google Scholar] [CrossRef]
- Janák, M.; Marhoul, P.; Matějů, J. Action Plan for the Conservation of the European Ground Squirrel Spermophilus Citellus in the European Union; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Laundré, J.W.; Reynolds, T.D. Effects of soil structure on burrow characteristics of five small mammal species. Gt. Basin Nat. 1993, 53, 358–366. [Google Scholar]
- Davidson, A.D.; Lightfoot, D.C. Interactive effects of keystone rodents on the structure of desert grassland arthropod communities. Ecography 2007, 30, 515–525. [Google Scholar] [CrossRef]
- Hegyeli, Z. Spermophilus citellus. The IUCN Red List of Threatened Species 2020: E.T20472A91282380. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T20472A91282380.en (accessed on 22 June 2021).
- Hoffmann, I.E.; Millesi, E.; Pieta, K.; Dittami, J.P. Anthropogenic effects on the population ecology of European ground squirrels (Spermophilus citellus) at the periphery of their geographic range. Mamm. Biol. 2003, 68, 205–213. [Google Scholar] [CrossRef]
- Gedeon, C.I.; Hoffmann, I.E.; Váczi, O.; Knauer, F.; Ben Slimen, H.; Stefanović, M.; Lehoczky, É.; Laborczi, A.; Suchentrunk, F. The role of landscape history in determining allelic richness of European ground squirrels (Spermophilus citellus) in Central Europe. Hystrix Ital. J. Mammal. 2017, 28, 231–239. [Google Scholar] [CrossRef]
- Říčanová, Š.; Bryja, J.; Cosson, J.-F.; Gedeon, C.; Choleva, L.; Ambros, M.; Sedláček, F. Depleted genetic variation of the European ground squirrel in Central Europe in both microsatellites and the major histocompatibility complex gene: Implications for conservation. Conserv. Genet. 2011, 12, 1115–1129. [Google Scholar] [CrossRef]
- Matějů, J.; Nová, P.; Uhlíkovà, J.; Hulová, Š.; Cepáková, E. Distribution of the European ground squirrel (Spermophilus citellus) in the Czech Republic in 2002–2008. Lynx 2008, 39, 277–294. [Google Scholar]
- Hoffmann, I.E.; Millesi, E.; Huber, S.; Everts, L.G.; Dittami, J.P. Population dynamics of European ground squirrels (Spermophilus citellus) in a suburban area. J. Mammal. 2003, 84, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, I.E.; Turrini, T.; Brenner, M. Do European ground squirrels in Austria adjust their life history to anthropogenic influence? Lynx 2008, 39, 27–36. [Google Scholar]
- Turrini, T.A.; Brenner, M.; Millesi, E.; Hoffmann, I.E. Home ranges of European ground squirrels (Spermophilus citellus) in two habitats exposed to different degrees of human impact. Lynx 2008, 39, 323–332. [Google Scholar]
- Youlatos, D.; Boutsis, Y.; Pantis, J.D.; Hadjicharalambous, H. Activity patterns of European ground squirrels (Spermophilus citellus) in a cultivated field in northern Greece. Mammalia 2007, 71, 183–186. [Google Scholar] [CrossRef]
- Brenner, M.; Turrini, T.; Hoffmann, I.E.; Millesi, E. Stress load in European ground squirrels living in habitats with high and low human impact. J. Wildl. Biodivers 2017, 1, 94–109. [Google Scholar]
- Lehrer, E.W.; Schooley, R.L.; Whittington, J.K. Survival and antipredator behavior of woodchucks (Marmota monax) along an urban-agricultural gradient. Can. J. Zool. 2012, 90, 12–21. [Google Scholar] [CrossRef]
- Sherman, P.W.; Runge, M.C. Demography of a population collapse: The Northern Idaho ground squirrel (Spermophilus brunneus brunneus). Ecology 2002, 83, 2816–2831. [Google Scholar] [CrossRef]
- Fraguedakis-Tsolis, S.E.; Ondrias, J.C. Geographic variation of the ground squirrel Citellus citellus (Mammalia: Rodentia) in Greece with description of a new subspecies. Säugetierkundliche Mitt. 1985, 32, 185–198. [Google Scholar]
- Ondrias, J.C. The taxonomy and geographical distribution of the rodents of Greece. Säugetierkundliche Mitteilungen 1966, 14, 1–134. [Google Scholar]
- Youlatos, D. Spermophilus citellus (Linnaeus, 1766). In The Red Book of Endangered Animals of Greece; Legakis, A., Maragou, P., Eds.; Hellenic Zoological Society: Athens, Greece, 2009; p. 528. [Google Scholar]
- Říčanová, Š.; Koshev, Y.; Říčan, O.; Ćosić, N.; Ćirović, D.; Sedláček, F.; Bryja, J. Multilocus phylogeography of the European ground squirrel: Cryptic interglacial refugia of continental climate in Europe. Mol. Ecol. 2013, 22, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Cepáková, E.; Hulová, Š. Current distribution of the European souslik (Spermophilus citellus) in the Czech Republic. Lynx 2002, 33, 89–103. [Google Scholar]
- Katona, K.; Váczi, O.; Altbäcker, V. Topographic distribution and daily activity of the European ground squirrel population in Bugacpuszta, Hungary. Acta Theriol. 2002, 47, 45–54. [Google Scholar] [CrossRef]
- Hubbs, A.H.; Karels, T.; Boonstra, R. Indices of Population Size for Burrowing Mammals. J. Wildl. Manag. 2000, 64, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Nydegger, N.C.; Smith, G.W. Prey Populations in Relation to Artemisia Vegetation. USDA Forest Service General Technical Report INT-Intermountain Forest and Range Experiment Station (USA). 1986. Available online: https://agris.fao.org/agris-search/search.do?recordID=US8640102 (accessed on 25 May 2021).
- Owings, D.H.; Borchert, M. Correlates of burrow location in beechey ground squirrels. Gt. Basin Nat. 1975, 35, 402–404. [Google Scholar]
- Van Horne, B.; Schooley, R.L.; Knick, S.T.; Olson, G.S.; Burnham, K.P. Use of Burrow Entrances to Indicate Densities of Townsend’s Ground Squirrels. J. Wildl. Manag. 1997, 61, 92–101. [Google Scholar] [CrossRef]
- Grulich, I. Sysel obecný Citellus citellus L. v ČSSR. Práce Brněnské Základny ČSAV 1960, 32, 473–563. [Google Scholar]
- Matějů, J.; Hulová, Š.; Nová, P.; Cepáková, E.; Marhoul, P.; Uhlíková, J. Action plan for the European Ground Squirrel (Spermophilus citellus) in the Czech Republic; Charles University and Agency for Nature and Landscape Protection of the Czech Republic: Prague, Czech Republic, 2010. [Google Scholar]
- Koshev, Y.S. Distribution and status of the European Ground Squirrel (Spermophilus citellus) in Bulgaria. Lynx 2008, 39, 251–261. [Google Scholar]
- Bischl, B.; Lang, M.; Bossek, J.; Horn, D.; Richter, J.; Surmann, D. BBmisc: Miscellaneous Helper Functions for B. Bischl. 2017. Available online: https://github.com/berndbischl/BBmisc (accessed on 15 April 2021).
- EEA (European Environment Agency); R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; EEA: København, Denmark, 2020. [Google Scholar]
- Pfaffel, O. R: A Language and Environment for Statistical Computing. 2021. Available online: https://CRAN.R-project.org/package=FeatureImpCluster (accessed on 16 April 2021).
- Leisch, F. Neighborhood graphs, stripes and shadow plots for cluster visualization. Stat. Comput. 2010, 20, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, G.C.; Braga, R.P. Evaluation of NASA POWER Reanalysis Products to Estimate Daily Weather Variables in a Hot Summer Mediterranean Climate. Agronomy 2021, 11, 1207. [Google Scholar] [CrossRef]
- Coroiu, C.; Kryštufek, B.; Vohralík, V.; Zagorodnyuk, I. Spermophilus citellus. IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. Available online: http://www.iucnredlist.org/details/20472/0 (accessed on 20 June 2018).
- Baltag, E.Ş.; Zaharia, G.; Fasolă, L.; Ion, C. European Ground Squirrel (Mammalia: Rodentia) population from Eastern Romania: Density, distribution and threats. Eur. Sci. J. 2014, 10, 94–101. [Google Scholar]
- Kryštufek, B.; Glasnović, P.; Petkovski, S. The status of a rare phylogeographic lineage of the Vulnerable European souslik Spermophilus citellus, endemic to central Macedonia. Oryx 2012, 46, 442–445. [Google Scholar] [CrossRef] [Green Version]
- McEachern, M.B.; Van Vuren, D.H.; Floyd, C.H.; May, B.; Eadie, J.M. Bottlenecks and rescue effects in a fluctuating population of golden-mantled ground squirrels (Spermophilus lateralis). Conserv. Genet. 2011, 12, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Hostetler, J.A.; Kneip, E.; Van Vuren, D.H.; Oli, M.K. Stochastic Population Dynamics of a Montane Ground-Dwelling Squirrel. PLoS ONE 2012, 7, e34379. [Google Scholar] [CrossRef] [Green Version]
- Ricankova, V.; Fric, Z.; Chlachula, J.; Stastna, P.; Faltynkova, A.; Zemek, F. Habitat requirements of the long-tailed ground squirrel (Spermophilus undulatus) in the southern Altai. J. Zool. 2006, 270, 1–8. [Google Scholar] [CrossRef]
- Kis, J.; Váczi, O.; Katona, K.; Altbäcker, V. A növényzet magasságának hatása a cinegési ürgék élőhelyválasztására. The effect of vegetation height to habitat selection of ground squirrels in Cinegés. Természetvédelmi Közlemények 1998, 7, 117–123. [Google Scholar]
- Kenyeres, Z.; Bauer, N.; Nagy, L.; Szabó, S. Enhancement of a declining European ground squirrel (Spermophilus citellus) population with habitat restoration. J. Nat. Conserv. 2018, 45, 98–106. [Google Scholar] [CrossRef]
- Hannon, M.J.; Jenkins, S.H.; Crabtree, R.L.; Swanson, A.K. Visibility and Vigilance: Behavior and Population Ecology of Uinta Ground Squirrels (Spermophilus armatus) in Different Habitats. J. Mammal. 2006, 87, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J. Response of small rodents to manipulations of vegetation height in agro-ecosystems. Integr. Zool. 2008, 3, 3–10. [Google Scholar] [CrossRef]
- Van Andel, T.H.; Tzedakis, P.C. Palaeolithic landscapes of Europe and environs, 150,000-25,000 years ago: An overview. Quat. Sci. Rev. 1996, 15, 481–500. [Google Scholar] [CrossRef]
- Pärtel, M.; Bruun, H.H.; Sammul, M. Biodiversity in temperate European grasslands: Origin and conservation. Grassl. Sci. Eur. 2005, 10, 14. [Google Scholar]
- Popova, L.V.; Maul, L.C.; Zagorodniuk, I.V.; Veklych, Y.M.; Shydlovskiy, P.S.; Pogodina, N.V.; Bondar, K.M.; Strukova, T.V.; Parfitt, S.A. ‘Good fences make good neighbours’: Concepts and records of range dynamics in ground squirrels and geographical barriers in the Pleistocene of the Circum-Black Sea area. Quat. Int. 2019, 509, 103–120. [Google Scholar] [CrossRef]
- Surkova, E.; Popov, S.; Tchabovsky, A. Rodent burrow network dynamics under human-induced landscape transformation from desert to steppe in Kalmykian rangelands. Integr. Zool. 2019, 14, 410–420. [Google Scholar] [CrossRef]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Beneš, J. Origin and history of grasslands in Central Europe—A review. Grass Forage Sci. 2013, 68, 345–363. [Google Scholar] [CrossRef]
- Koshev, Y.S.; Kocheva, M. Environmental factors and distribution of European ground squirrel (Spermophilus citellus) in Bulgaria. Ecol. Saf. Int. Sci. Publ. 2007, 1, 277–287. [Google Scholar]
- Zaharia, G.; Petrencu, L.; Baltag, E.Ș. Site selection of european ground squirrels (Spermophilus citellus) in eastern Romania and how they are influenced by climate, relief, and vegetation. Turk. J. Zool. 2016, 40, 917–924. [Google Scholar] [CrossRef]
- Németh, I.; Nyitrai, V.; Altbäcker, V. Ambient temperature and annual timing affect torpor bouts and euthermic phases of hibernating European ground squirrels (Spermophilus citellus). Can. J. Zool. 2009, 87, 204–210. [Google Scholar] [CrossRef]
- Váczi, O.; Koósz, B.; Altbäcker, V. Modified Ambient Temperature Perception Affects Daily Activity Patterns in the European Ground Squirrel (Spermophilus citellus). J. Mammal. 2006, 87, 54–59. [Google Scholar] [CrossRef]
- Bennie, J.; Hill, M.O.; Baxter, R.; Huntley, B. Influence of slope and aspect on long-term vegetation change in British chalk grasslands. J. Ecol. 2006, 94, 355–368. [Google Scholar] [CrossRef]
- Hadjigeorgiou, I. Past, present and future of pastoralism in Greece. Pastor. Res. Policy Pract. 2011, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Sidiropoulou, A.; Karatassiou, M.; Galidaki, G.; Sklavou, P. Landscape Pattern Changes in Response to Transhumance Abandonment on Mountain Vermio (North Greece). Sustainability 2015, 7, 15652–15673. [Google Scholar] [CrossRef] [Green Version]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- Roerink, G.J.; Menenti, M.; Soepboer, W.; Su, Z. Assessment of climate impact on vegetation dynamics by using remote sensing. Phys. Chem. Earth Parts A/B/C 2003, 28, 103–109. [Google Scholar] [CrossRef]
- Thomas, W. A three-dimensional model for calculating reflection functions of inhomogeneous and orographically structured natural landscapes. Remote Sens. Environ. 1997, 59, 44–63. [Google Scholar] [CrossRef]
- Weber, D.; Schaepman-Strub, G.; Ecker, K. Predicting habitat quality of protected dry grasslands using Landsat NDVI phenology. Ecol. Indic. 2018, 91, 447–460. [Google Scholar] [CrossRef]
- Fernández, N.; Paruelo, J.M.; Delibes, M. Ecosystem functioning of protected and altered Mediterranean environments: A remote sensing classification in Doñana, Spain. Remote Sens. Environ. 2010, 114, 211–220. [Google Scholar] [CrossRef]
- Wheeler, H.C.; Chipperfield, J.D.; Roland, C.; Svenning, J.-C. How will the greening of the Arctic affect an important prey species and disturbance agent? Vegetation effects on arctic ground squirrels. Oecologia 2015, 178, 915–929. [Google Scholar] [CrossRef]
- Nikolić, T.; Arok, M.; Radišić, D.; Mirč, M.; Velaja, L.; Milić, D.; Ćirović, D. Endangered species’ trait responses to environmental variability in agricultural settings. Arch. Biol. Sci. 2020, 72, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Arenz, C.L.; Leger, D.W. Antipredator vigilance of juvenile and adult thirteen-lined ground squirrels and the role of nutritional need. Anim. Behav. 2000, 59, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Hefty, K.L.; Stewart, K.M. Novel location data reveal spatiotemporal strategies used by a central-place forager. J. Mammal. 2018, 99, 333–340. [Google Scholar] [CrossRef]
- van der Merwe, M.; Brown, J.S. Mapping the Landscape of Fear of the Cape Ground Squirrel (Xerus inauris). J. Mammal. 2008, 89, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Hut, R.A.; Scharff, A. Endoscopic observations on tunnel blocking behaviour in the European ground squirrel. Mamm. Biol. 1998, 63, 377–380. [Google Scholar]
- Flower, C.E.; Dalton, J.E.; Whelan, C.J.; Brown, J.S.; Gonzalez-Meler, M.A. Patch use in the arctic ground squirrel: Effects of micro-topography and shrub encroachment in the Arctic Circle. Oecologia 2019, 190, 243–254. [Google Scholar] [CrossRef]
- Pe’er, G.; Dicks, L.V.; Visconti, P.; Arlettaz, R.; Báldi, A.; Benton, T.G.; Collins, S.; Dieterich, M.; Gregory, R.D.; Hartig, F.; et al. EU agricultural reform fails on biodiversity. Science 2014, 344, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T.; Radišić, D.; Ćosić, N.; Díaz-Delgado, R.; Milić, D.; Vujić, A.; Ćirović, D. Landscape heterogeneity effects on keystone rodent species: Agro-ecological zoning for conservation of open grasslands. Biodivers. Conserv. 2019, 28, 3139–3158. [Google Scholar] [CrossRef]
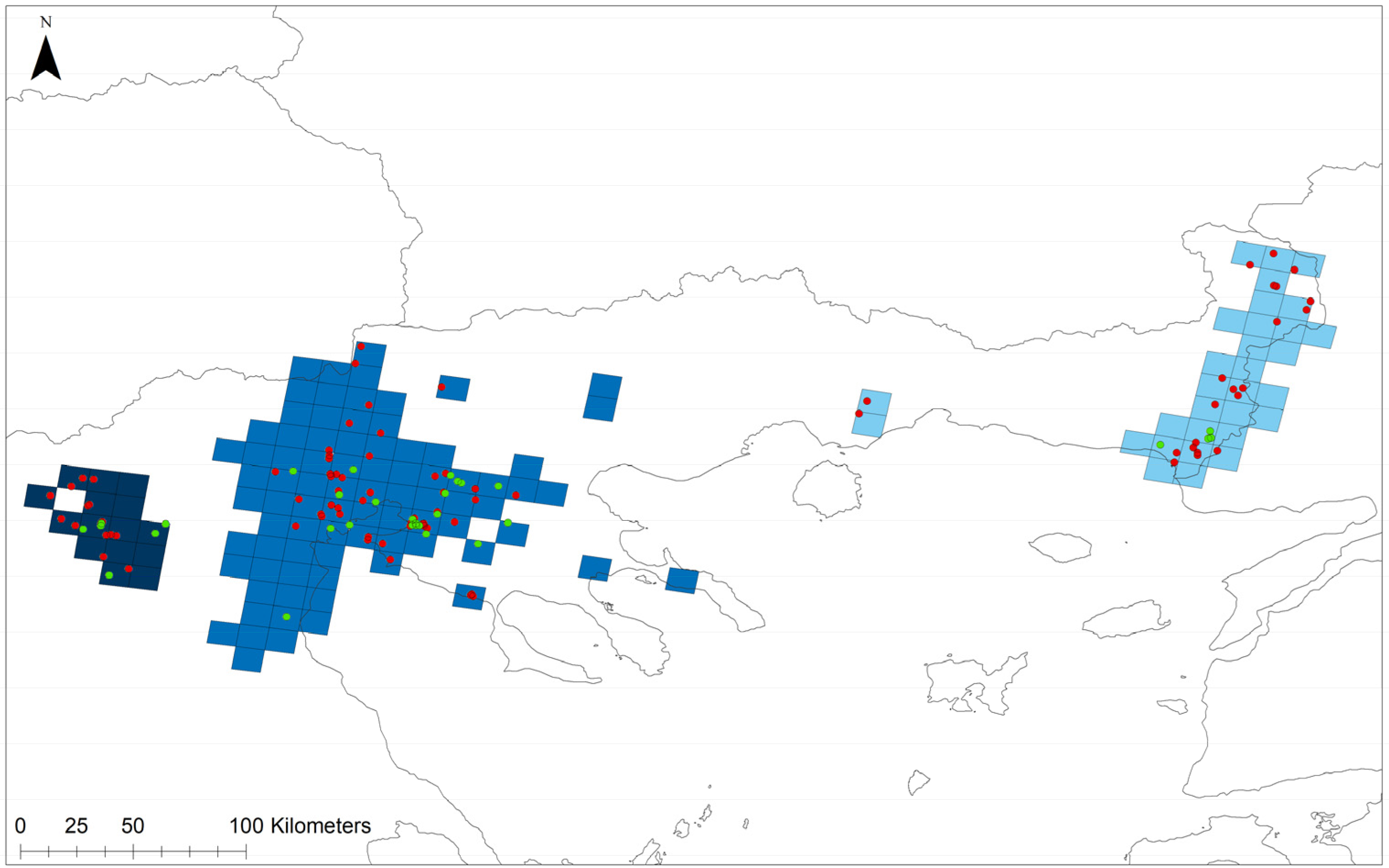
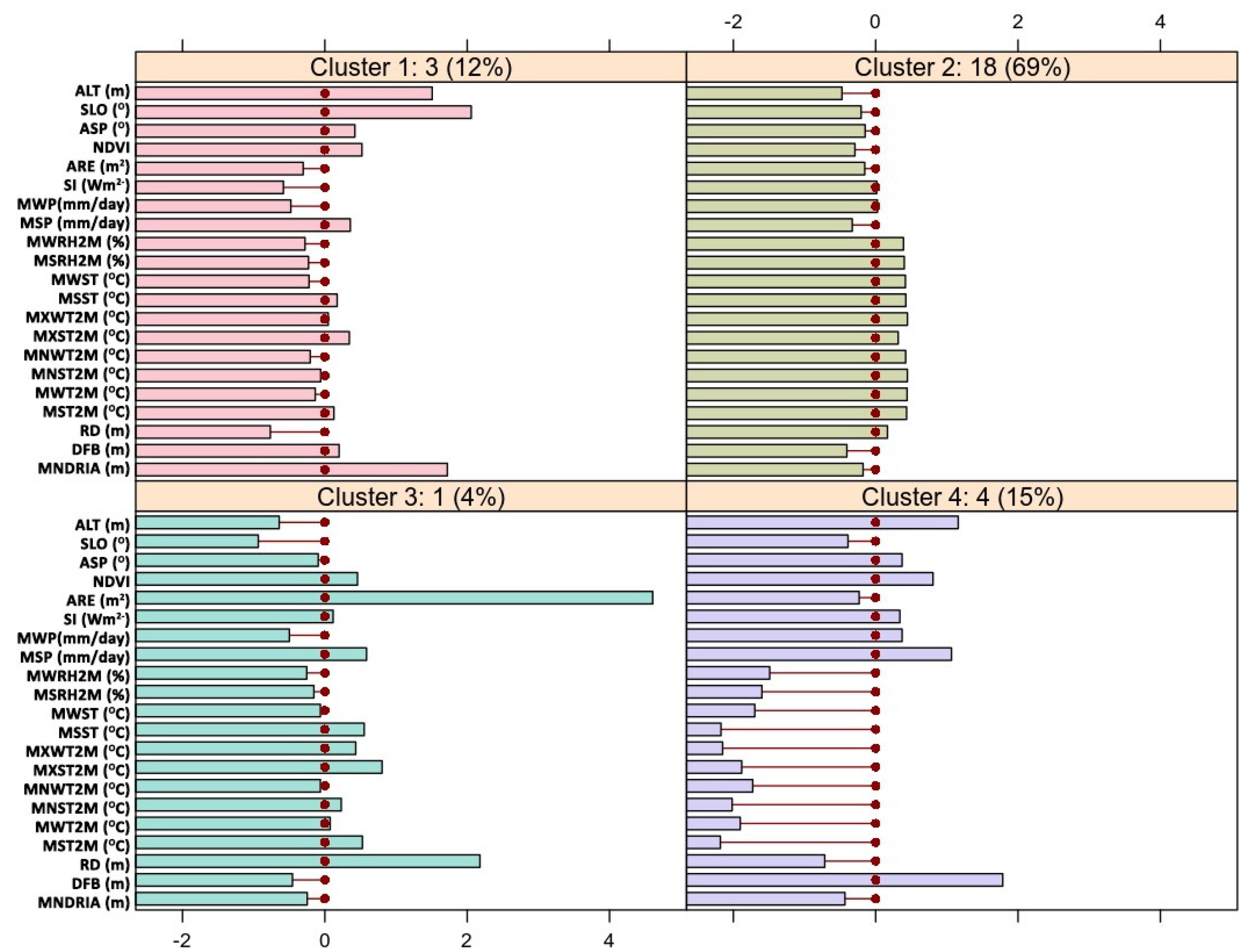
| Locality | Counted Burrows | Observed Individuals | Individual-Per-Burrow Entrance Ratio |
|---|---|---|---|
| 2019 | |||
| Alexandroupoli | 60 | 8 | 0.13 |
| Analipsi | 219 | 15 | 0.07 |
| AUTH School of Agriculture | 108 | 14 | 0.13 |
| Chortiatis | 15 | 1 | 0.07 |
| Dion | 107 | 24 | 0.22 |
| Drakontio | 18 | 3 | 0.17 |
| Feres West | 52 | 6 | 0.12 |
| Galatista | 27 | 12 | 0.44 |
| International Hellenic University | 138 | 11 | 0.08 |
| Ptolemaida | 32 | 3 | 0.09 |
| Seli | 27 | 4 | 0.15 |
| 2020 | |||
| Agios Vasileios | 95 | 10 | 0.11 |
| Analipsi | 115 | 12 | 0.10 |
| Galatista | 84 | 9 | 0.11 |
| Kavisos | 79 | 16 | 0.20 |
| Nymfopetra | 13 | 1 | 0.08 |
| Pella | 347 | 26 | 0.07 |
| Mean | 0.14 |
| A/A | Locality * | Burrow Entrances | N | Occupied Area (ha) | Density (ind/ha) |
|---|---|---|---|---|---|
| Central Macedonia | |||||
| 1 | Agios Vasileios | 95 | 13 | 0.57 | 23.33 |
| 2 | Analipsi | 219 | 31 | 6.61 | 4.64 |
| 3 | Anatoliko (Thessaloniki) | 397 | 56 | 29.7 | 1.87 |
| 4 | Chortiatis | 15 | 2 | 0.65 | 3.23 |
| 5 | Dion | 107 | 15 | 2.5 | 5.99 |
| 6 | Drakontio | 18 | 3 | 0.52 | 4.85 |
| 7 | Galatista | 84 | 12 | 1.09 | 10.79 |
| 8 | IHU | 138 | 19 | 2.56 | 7.55 |
| 9 | Kalamoto | 2 | 1 | - | - |
| 10 | Kalochori | 429 | 60 | 40.76 | 1.47 |
| 11 | Loudias | 17 | 2 | 5.1 | 0.47 |
| 12 | Nea Mesimvria | 57 | 8 | 1.09 | 7.32 |
| 13 | Nea Raidestos | 2 | 1 | - | - |
| 14 | Noesis | 10 | 1 | 1.34 | 1.04 |
| 15 | Nymfopetra | 8 | 1 | 0.69 | 1.62 |
| 16 | Evangelismos | 51 | 7 | 1.13 | 6.32 |
| 17 | Agriculture, AUTh | 108 | 15 | 2.5 | 6.05 |
| 18 | Pella | 347 | 49 | 6.42 | 7.57 |
| 19 | Thermi | 198 | 28 | 0.74 | 37.46 |
| 20 | Axios | - | 1 | - | - |
| 21 | SEDES air base | - | - | - | - |
| West Macedonia | |||||
| 22 | Anatoliko South (Ptolemaida) | 9 | 1 | 0.11 | 11.45 |
| 23 | Anatoliko North (Ptolemaida) | 37 | 5 | 9.45 | 0.55 |
| 24 | Koila (Kozani) | 34 | 5 | 0.83 | 5.73 |
| 25 | Ptolemaida | 32 | 4 | 0.18 | 24.89 |
| 26 | Seli | 27 | 4 | 2.48 | 1.52 |
| 27 | Oros Vermio | - | - | - | - |
| Thrace | |||||
| 28 | Alexandroupoli | 60 | 8 | 3.17 | 2.65 |
| 29 | Feres West | 52 | 7 | 4.33 | 1.68 |
| 30 | Feres East | 50 | 7 | 1.21 | 5.79 |
| 31 | Kavisos | 79 | 11 | 1.5 | 7.37 |
| TOTAL | 2682 | 378 | |||
| Habitat Parameters | Median (Min, Max) |
|---|---|
| Altitude–ALT (m) | 0.04 (0, 0.13) |
| Slope-SLO (°) | 0.07 (0, 0.12) |
| Aspect–ASP (°) | 0.29 (0.25, 0.34) |
| NDVI | 0.126 (0.75, 0.16) |
| Area–ARE (m2) | 0.07 (0.07, 0.07) |
| Solar incidence–SI (Wm−2) | 0 (0, 0) |
| Mean winter precipitation–MWP (mm/day) | 0.01 (0, 0.01) |
| Mean summer precipitation–MSP (mm/day) | 0.01 (0, 0.01) |
| Mean winter relative humidity at 2 m height–MWRH2M (%) | 0 (0, 0) |
| Mean summer relative humidity at 2 m height–MSRH2M (%) | 0 (0, 0) |
| Mean winter surface temperature–MWST (°C) | 0 (0, 0) |
| Mean summer surface temperature–MSST (°C) | 0 (0, 0) |
| Maximum winter temperature at 2 m height–MXWT2M (°C) | 0 (0, 0) |
| Maximum summer temperature at 2 m height–MXST2M (°C) | 0 (0, 0) |
| Minimum winter temperature at 2 m height–MNWT2M (°C) | 0 (0, 0) |
| Minimum summer temperature at 2 m height–MNST2M (°C) | 0 (0, 0) |
| Mean winter temperature at 2 m height–MWT2M (°C) | 0, (0,0) |
| Mean summer temperature at 2 m height–MST2M (°C) | 0, (0,0) |
| Road distance–RD (m) | 0.04 (0, 0.07) |
| Distance from freshwater bodies–DFB (m) | 0.06 (0.04, 0.07) |
| Minimum distance from residential/industrial area–MNDRIA (m) | 0.07 (0.04, 0.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rammou, D.-L.; Kavroudakis, D.; Youlatos, D. Distribution, Population Size, and Habitat Characteristics of the Endangered European Ground Squirrel (Spermophilus citellus, Rodentia, Mammalia) in Its Southernmost Range. Sustainability 2021, 13, 8411. https://doi.org/10.3390/su13158411
Rammou D-L, Kavroudakis D, Youlatos D. Distribution, Population Size, and Habitat Characteristics of the Endangered European Ground Squirrel (Spermophilus citellus, Rodentia, Mammalia) in Its Southernmost Range. Sustainability. 2021; 13(15):8411. https://doi.org/10.3390/su13158411
Chicago/Turabian StyleRammou, Dimitra-Lida, Dimitris Kavroudakis, and Dionisios Youlatos. 2021. "Distribution, Population Size, and Habitat Characteristics of the Endangered European Ground Squirrel (Spermophilus citellus, Rodentia, Mammalia) in Its Southernmost Range" Sustainability 13, no. 15: 8411. https://doi.org/10.3390/su13158411
APA StyleRammou, D.-L., Kavroudakis, D., & Youlatos, D. (2021). Distribution, Population Size, and Habitat Characteristics of the Endangered European Ground Squirrel (Spermophilus citellus, Rodentia, Mammalia) in Its Southernmost Range. Sustainability, 13(15), 8411. https://doi.org/10.3390/su13158411








