Abstract
This article is an overview of a biosystem of food-industry wastewater (WW) treatment using microalgae towards circular bioeconomy through biosynthesis of compounds of added-value. Focusing on circular bioeconomy with concern to environmental pollution, the management of water-resource and energy-crisis could be combined; by upgrading conventional WW treatment and simultaneously producing a renewable and sustainable source of energy algal-lipids for biodiesel production. Phyco-remediation of food WW using microalgae has revealed many advantages that can fulfill new demands for the WW treatment. WWs can be valuable resources of micronutrients and organic content (carbon source) for algal cultivation. In this review, prospective routes for the production of value-added compounds (polysaccharides, amino acids, biofuels, and biopigments) along with the bioremediation of food industry WW have been discussed. Furthermore, limitations and issues of phyco-remediation of WW using microalgae have also been reviewed with perspectives for further research and development.
Keywords:
food; agriculture; environment; biofuel; biodiesel; Algae; wastewater; lipid; circular bioeconomy 1. Introduction
Currently urbanization, industrialization and modernization are three key factors, which are responsible for increasing the demand for energy for the development of the world’s economy. Fossil fuel and WW are two substantial concerns across the world due to increasing population, climate change and modern lifestyle. It cannot be denied that energy is one of the significant elementary and essential factors for the survival and development of human society [1,2,3]. Worldwide consumption of primary energy in 2018 was 13,864.9 MTOE (million tonnes oil equivalent), which was 1.4% higher than that in 2017 [4]. It has been predicted that world energy consumption will grow by 56% between 2010 and 2040 [5]. The limited reserve of fossil fuels has been a matter of global concern as these are under threat of extinction due to overexploitation. According to the prediction of the World Energy Forum fossil fuel, coal and gas reserves will be depleted in less than another 10 decades [6]. Currently, the combustion of fossil fuels is the dominant global source of CO2 emission and efforts are going on around the world to save the environment from further deterioration. Factors, such as the energy crisis associated with irreversible depletion of traditional sources of fossil fuels, coupled with the atmospheric accumulation of greenhouse gases have led to an innovative global search for renewable sources of energy [7]. It is estimated that total energy consumption by developing countries will exceed the consumption by developed countries by 2030 dramatically. In this review article, information is presented on the use of different food processing wastewater for microalgae biomass cultivation. Food industry wastewater is rich in both macro and micronutrients, which could support the growth of microalgae. This approach would tremendously reduce the cost and chemical expenditure consumed on the culture medium and make the process economically viable.
2. Prospects of Renewable Energy Sources
Renewable energy derived from regenerative natural resources does not diminish over time and emits fewer emissions as compared to fossil fuels. Fuel cell technology, biomass energy, ocean energy, solar energy and geothermal energy are some sources of renewable energy that can be utilized to overcome energy shortages with lesser emission of greenhouse gases.
Renewable energy represents an area of tremendous opportunity globally for circular bioeconomy. In addition, renewable energy has the potential to create many employment opportunities, accelerate economic development, decrease local air pollution, improve public health, and reduce carbon emissions.
2.1. Biofuel for Energy Sustainability
Biomass could satisfy future energy requirements and play a crucial role in reducing environmental pollution by lowering greenhouse gas emissions (GHG). It has been estimated that bioenergy produced using biomass-based sources would fulfill about 15–25% of the total world’s energy demand in 2050. Presently about 40% of the world’s population depends on biomass for its energy requirements. Instead of direct combustion of biomass, it can be converted into biofuels through different biochemical, chemical and thermal methodologies. Biofuels can be classified into different generations based on their biomass feedstock. First generation biofuels are being produced from animal fats, rapeseed oil, sunflower oil, soybean oil, sugarcane, corn, traditional food crops and other edible oils. The main drawbacks of the first generation of biofuels are food security, sustainability and lower yield [7]. To overcome these constraints second generation biofuels have been established, which are being produced from wastes (organic components of municipal solid wastes), non-edible oils (Karanj, Mahua, Calophyllum, Jatropha, etc.) and lignocellulosic biomass (cereal straw, forest residues and bagasse) [8,9,10,11].
However, the sustainability of biodiesel production from these crops is a major tailback for commercialization. Third generation biofuels have been derived from microalgae, bacteria and yeast biomass [8]. Biofuels can be produced in the form of bioethanol, biogas, biohydrogen and biodiesel (Figure 1). At an industrial scale, only two attractive forms of biofuels, biodiesel and bioethanol are being produced. Wheat, maize (corn), sorghum and sugarcane are the most typical feedstocks used for the production of bioethanol. Currently, biodiesel has been recognized as an alternative form of renewable and clean diesel fuel with its advantages, such as biodegradability, non-toxicity and lower emission of GHG. Moreover, without any engine modification, it can be used in existing diesel vehicles. Biodiesel is free from aromatics and other chemical substances, which are dangerous to the environment and mankind. Recent research on microalgal biofuel production showed new ways for sustainable feedstock for biodiesel production.
2.2. Potential of Microalgae for Bioeconomy
Biodiesel is an alternative to diesel fuel used in transportation [12]. Currently, jatropha is the most appropriate and emerging crop for biodiesel production. Microalgae are fast-growing microorganisms by doubling in biomass within 24 h. It has 2-to-5-fold higher mass productivity as compared to traditional crops. Microalgae may contain more than 80% oil on a dry biomass weight basis. Hossain et al. [13] have reported 7 to 31% higher oil yields of microalgae as compared to palm oil.
2.2.1. Phyco-Remediation of Food Industry WW
The food industry is one of the most resource-demanding industries due to the diverse range of functional food products, which establishes a significant problem of compositions of WW generated in the food industry. Large amounts of water are consumed for different stages starting from raw materials to finished products. Meat based products require large amounts of water as compared to vegetables, for example, around 112 L, 31 L, and 29 L of water are needed to make 1 g of animal protein from meat, milk, and egg, respectively [14]. Food industry WW contains a high amount of organic matter, nitrogen, phosphorus, and chlorides, which can be the potential source for microalgae cultivation (Figure 1). Biological WW treatment using microorganisms got the attention of the scientific community due to the easy operation in a basic bioreactor, environment-friendly, and efficient WW purification. Microorganisms use the nutrition present in WW as nitrogen and carbon sources during the metabolism process. Bacteria (aerobic and anaerobic) and microalgae are two biological treatment technologies that have been researched for WW purification and also for high value products, such as biodiesel production [15]. Table 1 shows biomass and lipid productivity in different food processing WW.

Table 1.
Biomass and lipid yield by algal strains cultivated in food industry wastewater.
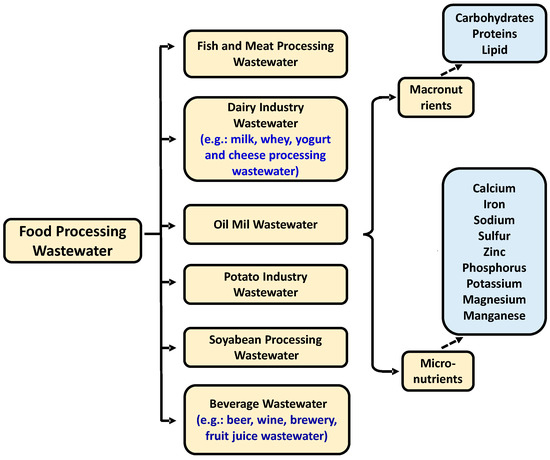
Figure 1.
Overview of types of wastewater generated from food processing [16].
Microalgae require the micronutrients (iron, magnesium, and calcium), and macronutrients (nitrogen and phosphorus) for their growth, which are present in food processing WW. Along with the remediation, several potential value-added products such as biofuel, protein, amino acid, polysaccharide, and functional pigment, can also be produced (a representative pictorial diagram has been provided in Figure 2. Previous research works demonstrated that microalgae could grow in different WWs leading to simultaneous treatment and biomass production. Thus, microalgae may be the most promising feedstock for producing biodiesel to replace conventional diesel fuel.
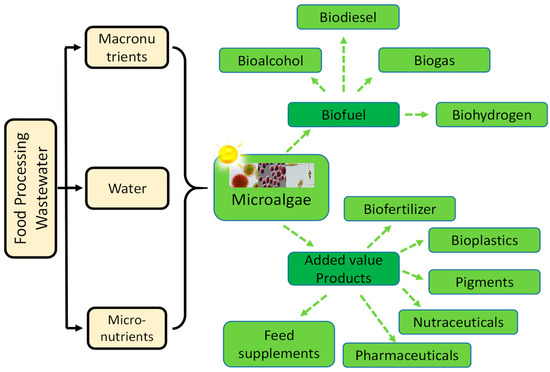
Figure 2.
Utilization of food industry WW for value-added products using microalgae.
2.2.2. Microalgae: An Emerging Feedstock for Biodiesel Production
Microalgae are photosynthetic microorganisms with chlorophyll-a as a primary pigment, have no sterile covering cells over reproductive cells [28]. Microalgae structures are primarily for energy conversion and their simple cellular development allows them to adapt to prevailing environmental conditions and prosper in the long term. While the photosynthesis mechanism in microalgae is similar to other plants, they are sunlight-driven cell factories that convert carbon dioxide to various types of renewable biofuels. Microalgae have the following advantages as a feedstock of biodiesel:
- Need less area as compared to other feedstock.
- Higher growth rate and lesser doubling time.
- Can be cultivated in saline water, freshwater, and WW.
- Can synthesize and accumulate lipids up to 10–70% on a dry cell weight basis.
- Have higher photosynthesis efficiency (3–8%) than terrestrial plants (0.5%).
- Can reduce GHG and sequestrate CO2 produced from power plants, combustion of fossil fuels and other sources as 1 kg of microalgal biomass needs more than 1.8 kg of CO2 for their respiration.
- Can cultivate in both closed (photobioreactor) and open systems (raceway pond) with higher biomass growth rate throughout the year.
- After extraction of biodiesel the remaining biomass can be further used for bioethanol or biogas production.
- Microalgal biomass can also be used to produce other value-added products such as protein, fertilizer, etc.
- Apart from biodiesel production, simultaneous WW remediation is possible as microalgae can grow in different WWs, especially nitrogen and phosphorus rich.
- Microalgae have the potential for rapid growth with less incubation period.
- Cultivation of microalgae does not require the application of pesticides and herbicides.
2.2.3. Techno-Economy Analysis of Biodiesel
Techno-economic analysis (TEA) is a powerful and useful tool, it should be integrated along with another tool termed life cycle assessment (LCA) since both the economic performance and the environmental impact of the process require to be optimized. In this way, the development of critical processes for biodiesel production might be developed within the framework of sustainability, which also takes into account social and ethical aspects. The main factors to consider are raw materials, energy use and environmental, social and ethical impacts. Consequently, the process should be not only economically viable but also energetic and ecological, with good social acceptance, to achieve sustainability.
The economic analysis of microalgae biodiesel production is depending on the earnings or sales, operating costs, and estimation of capital investment [29]. Additionally, for the sale of biodiesel, the two by-products of the process (glycerol and residual biomass) should also be considered. A study carried out by Branco-Vieira et al. [29] aimed at evaluating the technical and economic feasibility of biodiesel production from Phaeodactylum tricornutum. A total of 1811 tons of microalgae biomass was produced at 2.01 EUR kg−1 and 171,705 L of biodiesel per year at the production cost of 0.33 EUR L−1. In a different study, Heo et al. [30] studied three transesterification technologies, catalytic, enzymatic, and in situ transesterification and compared them for the production of microalgal-biodiesel through a comprehensive techno-economic analysis. Results showed that the lowest biodiesel production cost was achieved by catalytic transesterification ($4.77/kg), followed by the in situ ($9.92/kg), and enzymatic transesterification ($12.53/kg). However, numerous authors advocated that the cost of biofuels production remains higher compared to conventional fuel sources unless greater technological maturity is achieved to make the process more economical.
3. Biochemistry of Microalgae
Microalgae are primitive plants (thallophytes) having no roots, leaves and stems. It has been reported that around 30,000 out of more than 50,000 existing microalgal species have been studied and analyzed. Biosynthesis of microalgal lipid is a three-step process (1) fatty acid biosynthesis, (2) formation of glycerol-lipid into the cytoplasm of the cell, (3) packaging into the lipid droplets. For the synthesis of biomass, microalgae utilize sunlight, water and CO2 through photosynthesis. Both CO2 and solar energy are absorbed from the environment by chloroplasts present in algal-cells and are converted into oxygen and adenosine triphosphate (ATP). This generated energy is utilized in respiration and assists in cell development. Following Equation (1) shows the overall process of photosynthesis [31].
6 CO2 + 12 H2O + Photons → C6H12O6 + 6 O2 + 6 H2O
Photosynthesis consists of light phase and dark phase reactions. Light phase reactions supply energy molecules ATP and reducing NADPH. In this phase, chlorophyll absorbs photons from sunlight and it is used to drive the process of electron stripping from water. Firstly, electrons pass to quinone and pheophytin molecules and finally, electron transport occurs through the electron transport chain (ETC) till reduced NADPH is produced. Meanwhile, protons generated during water photolysis produce chemiosmosis potential which is being utilized by ATPase for ATP generation. CO2 captured by RuBisCO (ribulose-1,5-bisphosphate carboxylase oxygenase) in the presence of ATP and NADPH are directly used in the dark reactions to produce three-carbon sugars (glycerate-3-phosphate) which subsequently convert to carbohydrates. Pyruvate is a vital compound in biochemistry and is produced in glycolysis, an important intermediary in the conversion of carbohydrates into fatty acids. Decarboxylation of pyruvate occurs in presence of the pyruvate dehydrogenase (PDH) enzyme to form acetyl coenzyme A, which is an important precursor molecule for the synthesis of fatty acids in the chloroplast of the cell [32]. In the presence of acetyl-CoA carboxylase (a multifunctional enzyme), malonyl-CoA is produced by the reaction of acetyl-CoA with bicarbonate, during the synthesis of fatty acid. The obtained malonyl-CoA further converted into malonyl-acetyl carrier protein (malonyl-ACP) by acyl carrier protein (malonyltransferase). Malonyl-ACP plays an important role during the fatty acid synthesis in desaturation and lengthening to obtain C16 and C18 carbon chains. These fatty acids are available in organelle membranes, synthetic and TGA [32,33,34]. TGA is a vital molecule for microalgal-based biodiesel production and attained by the enzyme catalyzed sequential acylation of glycerol-3-phosphate (G3P) backbone with three acyl-CoA molecules.
Biochemical Composition of Microalgae
Microalgae constitute proteins, carbohydrates and lipids at varying compositions depending upon the type of species used. They may have a balanced composition of lipids, proteins and carbohydrates, and can be rich in lipids or in proteins.
Protein: Another important component of microalgae cell walls is protein as high as 60% of algal biomass. Proteins containing essential amino acids are necessary for human beings, as such amino acids cannot be synthesized by our body. In the past few decades, more than 75% of the annual microalgae biomass has been used by the health and food market for the formulation of powders, tablets, and capsules. Spirulina, a filamentous blue-green alga is used worldwide as a food supplement in the form of tablets, flakes or powder. Derivation of biofuel from protei4ns is not a feasible process due to problems associated with the diminution of protein hydrolysate.
Carbohydrates: During nitrogen fixation by microalgae carbohydrates are produced. Cellulose is the main component of the microalgae cell wall, whereas starch is mainly present as a reserve material. It has been observed that different types of polysaccharides are produced by different species of microalgae. Starch containing amylose and amylopectin are produced as energy stock by green microalgae. Under nutrient deplete and replete conditions, green alga Tetraselmis suecica stores 47% and 11% starch on dry biomass weight, respectively. It has been reported that microalgae strains like Tetraselmis, chlorella, Chlamydomonas, Scenedesmus and Dunaliella store more than 40% carbohydrate on dry weight biomass. The algal carbohydrate can be easily converted into ethanol and sugars [35].
Lipids: Lipid content of microalgal species varies from species to species such as Neochloris oleoabundans, Botryococcus braunii, Porphyridium cruentum, Nannochloropsis sp., Dunaliella tertiolecta, Chlorella emersonii, Chlorella sp., accumulating lipid content up to 60% on a dry weight biomass basis [36]. Signaling and energy storage are the main biological functions of lipid molecules. Triacylglycerol (TAGs) is a storage lipid that is made up of three fatty acid molecules bound to a glycerol backbone and is used in the synthesis of the biological parts of plant species [37]. Biological synthesis of lipid is a three-step process i.e., (1) fatty acids synthesis in plastids, (2) accumulation of glycerolipid in the cytoplasm of the cells, and (3) finally wrapping into the oil bodies [32]. The algal lipids including TAG transform into biofuel [38].
The selection of microalgae is a crucial step in biodiesel production. Our ecosystem is composed of diverse range of microalgae such as Chlorella vulgaris, Chlorella Minutissima, Chlorella sp., Chlorella protothecoides, Crypthecodinium cohnii, Dunaliella salina, Isochrysis galbana, Nannochloropsis oculate, Nannochloris sp., Nitzschia sp., Phaeodactylum tricornutum, Scenedesmus dimorphus, Scenedesmus obliquus, Schizochytrium sp. and Skeletonema costatum, etc., with lipid content in the range of 40–60%. However, in some microalgal strains, low lipid content of less than 40% on dry weight biomass can also be seen. On a color basis, the order of lipid content is algae green > algae yellow-green > algae red > algae blood-red > algae blue-green [39].
4. Cultivation of Microalgae
A suitably designed setup for the cultivation of microalgae is very important to enhance the biomass yield of the selected microalgal strain. It is important to know recent methodologies employed for the cultivation and harvesting of microalgae so that further production can be maximized at a low cost. Optimization of experimental conditions is important for achieving enhanced biomass production [40].
Microalgae cultivation generally employs both semi-continuous and continuous culture systems and follows a similar growth pattern [41]. However, batch culture systems have a limited supply of nutrients as no supplements can be added or removed during microalgae cultivation, whereas feeding of fresh medium for increased microalgae cultivation and harvesting is done continuously.
Continuous culture can be operated in three modes: cyclostat, chemostat and turbidostat. Cyclostat shows steady cyclical illumination allowing microalgae cultivation under intermittent light illumination for 24 h. Chemostat signifies the process where culture removal is performed at a rate similar to the addition rate of the medium so that the volume remains constant. A turbidostat is an extended chemostat in which microalgal culture suspension is maintained at the same turbidity. Semi continuous culture, also known as fed-batch culture, where the medium is added continuously or intermittently, and the biomass is periodically removed. Thus, the volume of culture is not constant in semi-continuous mode.
4.1. Culture Conditions
There are four types of cultivation conditions: heterotrophic, photoautotrophic, photoheterotrophic and mixotrophic. In photoautotrophic cultivation, microalgae utilize sunlight as an energy source and inorganic carbon in the form of CO2 to produce chemical energy via photosynthesis. This cultivation condition is generally used for microalgal growth. It has been found that under phototrophic cultivation conditions, there is a huge difference in lipid content ranging between 5 to 68% depending on microalgal species. In literature, the maximum lipid productivity of 179 mg/L-d using 2% CO2 by Chlorella sp. was achieved under phototrophic cultivation conditions [42].
Heterotrophic cultivation occurs when microalgae utilize organic carbon both as carbon and energy source in the dark cycle [43]. Such a cultivation condition is ideal for large scale microalgae production in a photobioreactor, as problems related to partial light exposure leading to obstruction of high cell density can be avoided [44]. It has been reported that some microalgae strains showed higher biomass production as well as higher lipid content under heterotrophic cultivation conditions. For example, Chlorella protothecoides revealed a 40% increment in lipid content when cultivation conditions were changed from phototrophic to heterotrophic [45].
In mixotrophic cultivation, the microalgal cell utilizes both the inorganic (CO2) and organic carbon source and undergoes photosynthesis for growth. This means that microalgae followed either heterotrophic or phototrophic conditions or both. CO2 released in this process is captured and reused under phototrophic cultivation conditions.
Photoheterotrophic cultivation conditions refer to the condition where microalgal cells need both light and organic carbon as light and carbon sources for their growth.
The major difference between the photoheterotrophic and mixotrophic cultivation conditions is energy source; while in mixotrophic cultivation organic compounds are used as an energy source, both light and organic compounds are needed in phototrophic cultivation. However, the production of several light-regulated metabolites can be enhanced under photoheterotrophic cultivation conditions [46]. Table 2 shows types of cultivation modes, which are suitable for different strains of algae, giving optimum productivity of lipid.

Table 2.
Types of algal-cultivation modes.
4.2. Cultivation Parameters
Specific environmental conditions are necessary to achieve efficient microalgae growth. Factors affecting the growth of microalgae include biotic, abiotic and operational. Biotic factors are fungi, viruses, bacteria and abiotic factors include nutrients (P, N, K), pH, salinity, light intensity, O2, CO2 and toxins. Operational factors refer to the experimental parameters of stirring or mixing rate, depth and width of cultivation vessel, dilution rate, harvesting frequency and addition of bicarbonate. Table 3 highlights the typical composition of the food processing industry.

Table 3.
Typical composition of wastewater generated in large-scale food industries **.
Light: Light is the main source of energy for the photosynthesis process of microalgal species. Intensity and accessibility of light are some of the crucial parameters affecting the growth of microalgae. There are two types of light sources used for microalgae cultivation such as natural and artificial light. The site location of the photobioreactor (PBR) in respect of the availability of light affects biomass productivity. To minimize the capital cost, freely available sunlight is the most suitable option for the cultivation of microalgae. For example, Chlorella pyrenoidosa shows autotrophic and heterotrophic growth in daylight and in the dark, respectively which is a cost-effective technique as compared to only autotrophic growth which demands a continuous supply of light [42].
Variation in light intensity on a daily or seasonal basis affects algal growth. In the autotrophic mode, sunlight is given in daytime only. However, to prevent the settling down of biomass generated during daylight hours, the biomass culture should be mixed unceasingly in the dark period. During the dark period to sustain cell growth, more than 25% of biomass is self-utilized [58].
Artificial light is provided in the indoor system for the cultivation of microalgae, and it is also used to compensate for the short supply of sunlight due to seasonal changes in some countries. Sunlight is harvested using light collectors and transferred through a bundle of fibers to decrease the use of artificial light [44]. It has been reported that the use of light intensity can be different for different microalgal species. For example, 120 and 30 μmol m−2 s−1 light intensity was used for Chlorella kessleri and Chlorella protothecoides, respectively when concentrated WW was used as a growth medium [59]. Under the exposure of 100 μmol m−2 s−1 light intensity, Nannochloropsis sp. achieved the highest growth rate with maximum cell concentration for 18 h/6 h light/dark period. The variation in illumination from 215 to 330 μmol m−2 s−1 increased the starch content of Chlorella vulgaris from 8.5 to 40% on a dry weight basis [60]. The presence of UVB in the sunlight radiation affects the composition and lipid content of algal cells.
Temperature: A crucial cultivation parameter regulating metabolic and photosynthesis processes of microalgae is temperature. It has been reported that the optimal growth of microalgae can be achieved at 20–35 °C [58]. The efficiency of photosynthesis and the specific growth rate of C. sorokiniana decreased at 20 °C drastically than at 38 °C its optimal growth temperature [66]. Temperature also influences the composition and lipid content of microalgae. Cultivation temperature can be easily maintained in a closed system for achieving optimum microalgae growth. However, in the open system, maintaining the optimal temperature for microalgae growth is not very easy due to changes in seasonal daylight periods. Studies also revealed that abrupt changes in temperature may cause declination in microalgae growth [67]. Temperature also governs the mass transfer of gas and solubility of micro or macro nutrients in water. Only a few microalgae, like Chlorella, are thermo tolerant species that can grow at 42 °C.
Mixing: Another important parameter controlling the growth of microalgae is mixing. It can be achieved effectively by sparging, mechanical stirring or pumping [68]. Proper mixing avoids sedimentation and aggregation of microalgae cells and facilitates uniform mass transfer for a higher uptake of CO2 and nutrients. Mixing also favors the uniform distribution of light to microalgal cells. Effective mixing plays a crucial role in removing excess oxygen from the cultivation medium.
4.3. Culture Cultivation Systems
Open or closed ponds and well-designed photobioreactors are commonly used for phyco-remediation of WW and biodiesel production using microalgae open pond cultivation includes raceway ponds, circular ponds, natural ponds, and inclined systems. It is the most common culture system for large scale production of microalgae biomass. These systems are generally made up of concrete or clay coated with porcelain tiles or polyvinyl chloride to avoid loss of nutrients and media. Depths of these systems are maintained in the range of 0.15 to 0.45 m to facilitate maximum penetration of sunlight [69]. Paddlewheel and air pumps are used for mixing and circulation of growth medium. However, an open pond system also has some drawbacks such as low mass transfer rate, insufficient mixing, low biomass productivity, water loss due to evaporation and difficulty in achieving microalgae monoculture due to occurrence of contamination of bacteria, and other microalgae culture in the medium. It is also not suitable in frequently changing environmental conditions.
Photobioreactors (PBRs) are used as closed pond systems permitting the exchange of light as well as energy but do not allow the exchange of any material with the surroundings. They are generally employed for bulk production of microalgae biomass. PBRs generally allow the production of monoculture of microalgae species for the elongated duration with a low possibility of any contamination. PBRs are designed for higher productivity of microalgae as flat panel, column and tubular. PBRs are usually more versatile than open ponds as they can utilize sunlight, artificial light or numerous combinations of light sources. Increased photoperiod and constant light intensity provided by the artificial light source improved yearly total oil yields up to 25–42% [70,71].
4.4. Nutrients for Microalgae Growth
The cultivation medium must contain macronutrients, micronutrients and other inorganic salts necessary for algal growth. The demand of the constituents required in the cultivation medium varies from species to species.
Algal cells may be classified into autotrophs or heterotrophs based on the carbon source they are consuming. Effects of CO2 concentration on the growth of various microalgal strains and accumulation of metabolites have been reported by Kim et al. [72]. A higher concentration of NaHCO3 leads to increased algal biomass productivity and specific growth rate. Both heterotrophic and mixotrophic modes utilize organic carbon in the form of acetic acid, fructose, glucose and sucrose [73]. Different pathways to capture and internalize CO2 by algae are (i) through the atmosphere (ii) feeding on industrial flue gases (iii) by solubilizing carbonates (NaHCO3 or Na2CO3) and (iv) uptake of organic carbon sources [74].
Microalgae consume nitrogen in the form of nitrate, urea, ammonia or their appropriate combination. It has been observed that high-lipid cells are grown under nitrogen stress conditions. Some of the microalgae strains use ammonium instead of nitrate which causes microalgae cells to multiply rapidly and grow fast. Moreover, cyanobacteria have the potential to convert atmospheric nitrogen into ammonia by the means of fixation.
Phosphorus is an essential macronutrient needed for microalgae growth as it helps in the synthesis of functional and structural components of microalgal cells. For balanced growth of microalgal cells, the N/P ratio in the medium is very crucial and plays an important role in WW treatment. Phosphorus is commonly provided in the form of orthophosphate (PO43−) in the culture medium. H2PO4− and HPO42− both are present in synthetic medium and act as a buffer to maintain the pH of the medium within the range. Microalgae store the excess amounts of phosphorus within its cell as polyphosphate granules. This reserve can be used by microalgae during phosphate starvation conditions for cell growth [75].
Micronutrients are important for the growth of microalgae and play a vital role in the activation of various metabolic pathways [76]. Different micronutrients such as Mg, S, Fe, Mn, Zn, Co, Mo and Cu are required in the synthetic medium for healthy microalgal growth. Magnesium promotes the activity of enzyme acetyl–CoA carboxylase which regulates the first performing step of microalgae lipid synthesis.
4.5. Harvesting of Microalgae
Microalgae biomass is separate from the medium, which is a key step for large scale biodiesel production contributing 20–30% of total biodiesel production cost [77,78,79,80]. Due to their microscopic size (1–10 μm diameter), the separation of microalgal biomass from the medium is the most challenging step [81]. Many harvesting techniques like coagulation, centrifugation, filtration, electro-flotation, electrophoresis, flocculation flotation and ultrasound have been employed [82]. In lab-scale experiments, centrifugation is the commonly used technique for harvesting. However, it is not practical to centrifuge large volumes of algal culture in pilot-scale or large-scale cultivations. Traditional filtration is not much effective for microalgae separation [76]. Fouling is the major issue during the filtration process which causes deposition of extracellular polymer substance (present in microalgae culture media) on the surface of filters. Due to fouling, replacement of membrane and backwashing is required which increases the overall process cost. Microfiltration and ultrafiltration are some improved techniques for microalgae harvesting [83].
Flocculation is an efficient harvesting method with lower capital costs. Microalgae cells are negatively charged in the growth medium and repel each other due to electrostatic force. This helps microalgae cells to make a stable system. When flocculants are added into the microalgae culture medium, the surface charge is blocked so that microalgae cells can adhere to form stable flocs. The flocculants utilized must be low-cost, nontoxic, and effective in low application doses [84]. Several chemicals are identified to be utilized as flocculants for this purpose. Inorganic salts containing metal ions (Al3+ and Fe3+) are generally used as flocculants. A high concentration of metal flocculants for biomass harvesting results in toxicity and affects the biodiesel production process. However, some organic polymers such as chitosan, cationic starch and grafted starch are being studied to replace inorganic flocculants [85].
In the flocculation process pH also plays a crucial role. Microalgae cells have a natural tendency to flocculate at pH 9 or above. Matos et al. [86] achieved 97% harvesting efficiency of marine Nannochloropsis sp. by using electro-coagulation. Bio-flocculation is also induced by utilizing one flocculating microalga to concentrate non-flocculating microalgae of interest, or by using microalgae-associated bacteria to enhance algal flocculation.
5. Extraction of Lipid and Biodiesel Production
The method used for oil extraction dried algal biomass should be effective, fast and scalable and at the same time, it should not cause any harm to the lipid cells. Physical, chemical and mechanical methods have been tested for the extraction of oil from microalgal biomass [87,88,89,90]. For large scale biodiesel production from microalgae, a mechanical pressing method may be used, however, the single cell nature of microalgae and stiff cell walls of some microalgae strains are the main drawbacks of mechanical pressing [91]. The choice of the best extraction technology greatly depends on the microalgae strain [92].
A chemical method is the most common method used for microalgal oil extraction. Both polar (ethanol, acetone, methanol, ethyl acetate) and non-polar (benzene, chloroform, toluene, hexane, diethyl ether) solvents can be used for the oil extraction from microalgae. Non-polar solvent helps in disrupting microalgal cell wall which has hydrophobic interaction between the natural and non-polar lipids. The mixture of chloroform and methanol in the ratio of 2:1; v/v is used for the extraction of lipid from animal tissues [93]. However, a 1:2 v/v volume ratio of chloroform and methanol may also be used for total lipid extraction and purification [94].
Lipid extraction from marine microalgae is highly influenced by the factors like biomass drying, moisture content and solvent systems like chloroform:methanol (2:1) and hexane:methanol (3:2). Lipids were categorized into three classes, e.g., neutral lipids, free fatty acids (FFA) and polar lipids using solid-phase extraction column [95].
Physical methods like ultrasonication, microwaves, osmotic shocks, autoclave, etc., are used to disrupt the cell wall of microalgae. Supercritical fluid extraction is a trending green technology that has the ability to extract nearly 100% of oil from biomass.
The microalgal crude oil is further processed for biodiesel production using a transesterification reaction. Due to its highly viscous nature, there is a need to convert extracted crude oil into lower molecular weight compounds in the form of fatty acid methyl esters. In this process, extracted lipids (triglyceride) are converted into renewable, biodegradable and nontoxic compounds. Lipid to alcohol ratio, type of catalyst, free fatty acids (FFA), temperature and time are some of the crucial factors which can influence the efficiency of transesterification [96,97]. Direct esterification only gives better results in laboratory scale experiments [98] and still has many challenges to scale up.
6. Biodiesel Quality
Suitability of microalgal biodiesel as an alternative to diesel fuel strongly depends on compliance with known standards such as EN 14214:2008, IS:15607 and ASTM D6751. Mostly the properties of biodiesel depend on fatty acid compositions [99]. Typical fatty acids present in biodiesel are palmitic, stearic, oleic, linoleic, and linolenic acid. Proportion of these fatty acids varies from feedstock to feedstock of microalgae. However, esters are rich in saturated fatty acids, which have high viscosity with high probability of clogging the nozzle of the engine and high cloud point. On the other hand, esters rich in polyunsaturated fatty acids lead to better cloud point and poor oxidation stability [100]. Miao and Wu [101] reported cold filter plugging point (CFPP), kinematic viscosity and density as, −13 °C, 4.43 cSt and 882 g/cm3, respectively. Amin, [102] reported 0.864 g/cm3 density, 5.2 cSt, kinematic viscosity, and −11 °C CFPP in microalgae oil biodiesel.
7. Algal Cultivation for Circular Bioeconomy
Discharge of wastewater from food industries can cause serious water and environmental pollution due to fouling. In the current scenario, this nutrient load can be utilized by developing biological systems of actively growing algae. Effluents from a variety of food-processing industries, summarized in Table 1, Table 2, Table 3 and Table 4 of this review, could be an alternative source of both organic and inorganic nutrients for the cultivation of microalgae [103]. Algae can reduce the pollution load providing a bioresource for the economy with improved CO2 balance [104,105]. Table 4 shows the biomass yield of microalgae on effluents from different food processing industries. The use of WWs from industries is a cheaper option as compared to the cost of the synthetic medium, to reduce the operating cost of microalgae cultivation [106] for biodiesel production.

Table 4.
Cultivation of microalgae on wastewaters generated globally in food industries.
8. Conclusions
In this review, the remediation of food processing WW has been analyzed for multiple aspects, such as 1. water resource management, 2. environmental protection, and 3. recovery of valuable material; an approach for contribution in circular bioeconomy. Microalgal based biofuels are being considered as an alternative sustainable renewable bioenergy to fossil fuels. Microalgae has the potential to accumulate the nutrients present in the WW for growth and their biomass can be used as a prospective source of biofuel production. Biofuel is a clean and sustainable alternative to fossil fuel and is becoming a true opponent of fossil fuel. Employment of microalgae for WW treatment and simultaneous biodiesel production can contribute to a circular bioeconomy.
Author Contributions
Conceptualization: A., D.D., A.K.J., P.S.N., U.K.G.; draft preparation: A.; writing, editing: A., D.D., A.K.J., P.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This review writing received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agency, I.E.; Birol, F. World Energy Outlook 2013; International Energy Agency: Paris, France, 2013.
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Ananthi, V.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. Impact of abiotic factors on biodiesel production by microalgae. Fuel 2021, 284, 118962. [Google Scholar] [CrossRef]
- BP. Statistical Review of World Energy 2019. 2019. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 10 July 2021).
- Kalaimurugan, K.; Karthikeyan, S.; Periyasamy, M.; Mahendran, G.; Dharmaprabhakaran, T. Experimental studies on the influence of copper oxide nanoparticle on biodiesel-diesel fuel blend in CI engine. Energy Sources Part A Recov. Util. Environ. Eff. 2019, 1–16. [Google Scholar] [CrossRef]
- Sharma, Y.; Singh, B. Development of biodiesel: Current scenario. Renew. Sustain. Energy Rev. 2009, 13, 1646–1651. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I.; Nigam, P.S. A viable technology to generate third-generation biofuel. J. Chem. Technol. Biotechnol. 2011, 86, 1349–1353. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Energy Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef]
- Satari, B.; Jaiswal, A.K. Green fractionation of 2G and 3G feedstocks for ethanol production: Advances, incentives and barriers. Curr. Opin. Food Sci. 2020, 37, 1–9. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and agricultural wastes as substrates for bioelectrochemical system (BES): The synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]
- Hossain, A.S.; Salleh, A.; Boyce, A.N.; Chowdhury, P.; Naqiuddin, M. Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. The green, blue and grey water footprint of farm animals and animal products. Appendices 2010, 2. Available online: https://www.waterfootprint.org/media/downloads/Report-48-WaterFootprint-AnimalProducts-Vol1.pdf (accessed on 21 July 2021).
- Nigam, P.S.; Singh, D.; Pandey, A. Biotechnological Treatment of Pollutants. Chem. Ind. Digest 2001, 14, 93–96. [Google Scholar]
- Li, S.; Zhao, S.; Yan, S.; Qiu, Y.; Song, C.; Li, Y.; Kitamura, Y. Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production—A review. Chin. J. Chem. Eng. 2019, 27, 2845–2856. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Choi, H.-J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res. 2016, 21, 393–400. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Z.; Wang, X.; Yuan, Z. Cultivation of Chlorella sp. using raw dairy wastewater for nutrient removal and biodiesel production: Characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Bioresour. Technol. 2015, 192, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Lee, I.; Jeon, S.H.; Han, J.-I. Efficient conversion from cheese whey to lipid using Cryptococcus curvatus. Biochem. Eng. J. 2014, 90, 149–153. [Google Scholar] [CrossRef]
- Sreekanth, D.; Pooja, K.; Seeta, Y.; Himabindu, V.; Reddy, P.M. Bioremediation of dairy wastewater using microalgae for the production of biodiesel. IJSEAT 2014, 2, 783–791. [Google Scholar]
- Hongyang, S.; Yalei, Z.; Chunmin, Z.; Xuefei, Z.; Jinpeng, L. Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour. Technol. 2011, 102, 9884–9890. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Cho, H.U.; Utomo, J.C.; Choi, Y.-N.; Xu, X.; Park, J.M. Biodiesel production from Scenedesmus bijuga grown in anaerobically digested food wastewater effluent. Bioresour. Technol. 2015, 184, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, G.; Scortica, A.; Scafati, V.; Ferella, F.; Gurrieri, L.; Giovannoni, M.; Bassi, R.; Sparla, F.; Mattei, B.; Benedetti, M. Exploring the potential of microalgae in the recycling of dairy wastes. Bioresour. Technol. Rep. 2020, 12, 100604. [Google Scholar] [CrossRef]
- Talapatra, N.; Gautam, R.; Mittal, V.; Ghosh, U. A comparative study of the growth of microalgae-bacteria symbiotic consortium with the axenic culture of microalgae in dairy wastewater through extraction and quantification of chlorophyll. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Zkeri, E.; Iliopoulou, A.; Katsara, A.; Korda, A.; Aloupi, M.; Gatidou, G.; Fountoulakis, M.S.; Stasinakis, A.S. Comparing the use of a two-stage MBBR system with a methanogenic MBBR coupled with a microalgae reactor for medium-strength dairy wastewater treatment. Bioresour. Technol. 2021, 323, 124629. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, M.; Palabhanvi, B.; Misra, S.; Kumar, V.; Sivalingavasu, K.; Das, D. Flux balance analysis of Chlorella sp. FC2 IITG under photoautotrophic and heterotrophic growth conditions. Photosynth. Res. 2013, 118, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Branco-Vieira, M.; Mata, T.; Martins, A.; Freitas, M.; Caetano, N. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Heo, H.Y.; Heo, S.; Lee, J.H. Comparative Techno-Economic Analysis of Transesterification Technologies for Microalgal Biodiesel Production. Ind. Eng. Chem. Res. 2019, 58, 18772–18779. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Biology of microalgae. In Microalgae in Health and Disease Prevention; Elsevier: Cham, Switzerland, 2018; pp. 23–72. [Google Scholar]
- Fan, J.; Andre, C.; Xu, C. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett. 2011, 585, 1985–1991. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, D. Biodiesel Production from Microalgal Biomass: Challenges and Perspectives. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 51–62. [Google Scholar]
- Cagliari, A.; Margis, R.; dos Santos Maraschin, F.; Turchetto-Zolet, A.C.; Loss, G.; Margis-Pinheiro, M. Biosynthesis of triacylglycerols (TAGs) in plants and algae. Int. J. Plant Biol. 2011, 2, e10. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Maity, J.P.; Hou, C.-P.; Majumder, D.; Bundschuh, J.; Kulp, T.R.; Chen, C.-Y.; Chuang, L.-T.; Chen, C.-N.N.; Jean, J.-S.; Yang, T.-C. The production of biofuel and bioelectricity associated with wastewater treatment by green algae. Energy 2014, 78, 94–103. [Google Scholar] [CrossRef]
- Liang, M.-H.; Jiang, J.-G. Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog. in Lipid Res 2013, 52, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, S.; Duan, X.; Wang, W.; Yang, F.; Xiong, J.; Wang, T.; Wang, C. A novel approach for enhancing lipid recovery for biodiesel production from wet energy biomass using surfactants-assisted extraction. Renew. Energy 2021, 170, 462–470. [Google Scholar] [CrossRef]
- Baunillo, K.E.; Tan, R.S.; Barros, H.R.; Luque, R. Investigations on microalgal oil production from Arthrospira platensis: Towards more sustainable biodiesel production. RSC Adv. 2012, 2, 11267–11272. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.; Murphy, J.D. Mechanism & Challenges in Commercialisation of Algal Biofuels. Bioresour. Technol. 2011, 102, 26–34. [Google Scholar]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Marquez-Rocha, F.-J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 2004, 3, 21–34. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Ogbonna, D.; Ekweozor, I.; Igwe, F. Waste management: A tool for environmental protection in Nigeria. Ambio 2002, 31, 55–57. [Google Scholar] [CrossRef]
- Devi, M.P.; Mohan, S.V. CO2 supplementation to domestic wastewater enhances microalgae lipid accumulation under mixotrophic microenvironment: Effect of sparging period and interval. Bioresour. Technol. 2012, 112, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Devi, M.P. Salinity stress induced lipid synthesis to harness biodiesel during dual mode cultivation of mixotrophic microalgae. Bioresour. Technol. 2014, 165, 288–294. [Google Scholar] [CrossRef]
- Mitra, D.; van Leeuwen, J.H.; Lamsal, B. Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res. 2012, 1, 40–48. [Google Scholar] [CrossRef]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sukumaran, R.K. Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresour. Technol. 2014, 165, 295–301. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Ma, C.; Zhao, L.; Ren, N.-Q. A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: Effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Hu, B.; Min, M.; Zhou, W.; Li, Y.; Mohr, M.; Cheng, Y.; Lei, H.; Liu, Y.; Lin, X.; Chen, P. Influence of exogenous CO2 on biomass and lipid accumulation of microalgae Auxenochlorella protothecoides cultivated in concentrated municipal wastewater. App. Biochem. Biotechnol. 2012, 166, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Voltolina, D.; Cordero, B.; Nieves, M.; Soto, L.P. Growth of Scenedesmus sp. in artificial wastewater. Bioresour. Technol. 1999, 68, 265–268. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, H.; Hui, Z.; Zeng, X. Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour. Technol. 2012, 104, 215–220. [Google Scholar] [CrossRef]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 282, 245–253. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Effect of light intensity on algal biomass accumulation and biodiesel production for mixotrophic strains Chlorella kessleri and Chlorella protothecoide cultivated in highly concentrated municipal wastewater. Biotechnol. Bioeng. 2012, 109, 2222–2229. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Sanz, S.; Ortiz, J.M.; Esteve-Núñez, A. Merging microbial electrochemical systems with electrocoagulation pretreatment for achieving a complete treatment of brewery wastewater. Chem. Eng. J. 2017, 330, 1068–1074. [Google Scholar] [CrossRef]
- Ioannou, L.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, H.; Zhong, Y.; Zhang, C.; Shen, Z.; Sang, W.; Yan, G.; Zhou, X. The effect of bacterial contamination on the heterotrophic cultivation of Chlorella pyrenoidosa in wastewater from the production of soybean products. Water Res. 2012, 46, 5509–5516. [Google Scholar] [CrossRef] [PubMed]
- Alver, A.; Baştürk, E.; Kılıç, A.; Karataş, M. Use of advance oxidation process to improve the biodegradability of olive oil mill effluents. Process. Saf. Environ. Protec. 2015, 98, 319–324. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Franco, M.C.; Buffing, M.F.; Janssen, M.; Lobato, C.V.; Wijffels, R.H. Performance of Chlorella sorokiniana under simulated extreme winter conditions. J. App. Phycol. 2012, 24, 693–699. [Google Scholar] [CrossRef]
- Park, J.; Craggs, R.; Shilton, A. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef]
- Kumar, A.; Ergas, S.; Yuan, X.; Sahu, A.; Zhang, Q.; Dewulf, J.; Malcata, F.X.; Van Langenhove, H. Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Murthy, G.S. Overview and assessment of algal biofuels production technologies. In Biofuels; Elsevier: Cham, Switzerland, 2011; pp. 415–437. [Google Scholar]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Advances and perspectives in using microalgae to produce biodiesel. App. Energy 2011, 88, 3402–3410. [Google Scholar] [CrossRef]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.; Abdullah, M.O.; San Chan, Y.; Khalid, M. Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, C.-S.; Park, Y.-H.; Lee, S.-T.; Oh, H.-M. Effect of CO2 concentration on growth and photosynthesis of Spirulina platensis. In Studies in Surface Science and Catalysis; Elsevier: Cham, Switzerland, 2004; Volume 153, pp. 295–298. [Google Scholar]
- Yeh, K.-L.; Chen, C.-Y.; Chang, J.-S. pH-stat photoheterotrophic cultivation of indigenous Chlorella vulgaris ESP-31 for biomass and lipid production using acetic acid as the carbon source. Biochem. Eng. J. 2012, 64, 1–7. [Google Scholar] [CrossRef]
- Patil, V.; Tran, K.-Q.; Giselrød, H.R. Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 2008, 9, 1188–1195. [Google Scholar] [CrossRef]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Ananthi, V.; Balaji, P.; Sindhu, R.; Kim, S.-H.; Pugazhendhi, A.; Arun, A. A critical review on different harvesting techniques for algal based biodiesel production. Sci. Total Environ. 2021, 780, 146467. [Google Scholar] [CrossRef]
- Khatib, W.A.; Ayari, A.; Yasir, A.T.; Talhami, M.; Das, P.; Quadir, M.; Hawari, A.H. Enhancing the electrocoagulation process for harvesting marine microalgae (Tetraselmis sp.) using interdigitated electrodes. J. Environ. Manag. 2021, 292, 112761. [Google Scholar] [CrossRef]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sustain. Energy Rev. 2020, 137, 110498. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L. Harvesting freshwater microalgae with natural polymer flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Gao, T.; Teng, J. Impacts of applied voltage on forward osmosis process harvesting microalgae: Filtration behaviors and lipid extraction efficiency. Sci. Total Environ. 2021, 773, 145678. [Google Scholar] [CrossRef]
- Zhu, L.; Takala, J.; Hiltunen, E.; Wang, Z. Recycling harvest water to cultivate Chlorella zofingiensis under nutrient limitation for biodiesel production. Bioresour. Technol. 2013, 144, 14–20. [Google Scholar] [CrossRef]
- Ogbonna, C.N.; Nwoba, E.G. Bio-based flocculants for sustainable harvesting of microalgae for biofuel production. A review. Renew. Sustain. Energy Rev. 2021, 139, 110690. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y.; Cui, Y.-L. Chitosan and its derivatives applied in harvesting microalgae for biodiesel production: An outlook. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Matos, C.T.; Santos, M.; Nobre, B.P.; Gouveia, L. Nannochloropsis sp. biomass recovery by Electro-Coagulation for biodiesel and pigment production. Bioresour. Technol. 2013, 134, 219–226. [Google Scholar] [CrossRef][Green Version]
- Karpagam, R.; Jawaharraj, K.; Gnanam, R. Review on integrated biofuel production from microalgal biomass through the outset of transesterification route: A cascade approach for sustainable bioenergy. Sci. Total Environ. 2021, 766, 144236. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Ferreira, G.F.; Moreira, L.S.; Maciel Filho, R. Biodiesel production from microalgae by direct transesterification using green solvents. Renew. Energy 2020, 160, 1283–1294. [Google Scholar] [CrossRef]
- Kim, B.; Heo, H.Y.; Son, J.; Yang, J.; Chang, Y.-K.; Lee, J.H.; Lee, J.W. Simplifying biodiesel production from microalgae via wet in situ transesterification: A review in current research and future prospects. Algal Res. 2019, 41, 101557. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Xu, G.; Fu, X. Improving the lipid extraction yield from Chlorella based on the controllable electroporation of cell membrane by pulsed electric field. Bioresour. Technol. 2021, 330, 124933. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.F.; Chen, P.C.; Lee, C.M. The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. Int. Biodeterior. Biodegrad. 2013, 85, 506–510. [Google Scholar] [CrossRef]
- Kumar, V.; Muthuraj, M.; Palabhanvi, B.; Ghoshal, A.K.; Das, D. Evaluation and optimization of two stage sequential in situ transesterification process for fatty acid methyl ester quantification from microalgae. Renew. Energy 2014, 68, 560–569. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.K.; Doan, T.T.Y.; Obbard, J.P. Factors affecting cellular lipid extraction from marine microalgae. Chem. Eng. J. 2013, 215, 929–936. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Watheyb, B.; Westmana, J. Microwave assisted organic synthesisÐa review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G.; Mondala, A.; Holmes, W.; Hernandez, R. Microwave and ultrasound enhanced extractive-transesterification of algal lipids. App. Energy 2014, 129, 354–363. [Google Scholar] [CrossRef]
- Ehimen, E.; Sun, Z.; Carrington, C. Variables affecting the in situ transesterification of microalgae lipids. Fuel 2010, 89, 677–684. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Rehan, M.; Saeed, M.; Lam, S.S.; Nizami, A.; Waseem, A.; Sultana, S.; Zafar, M. Production of high quality biodiesel from novel non-edible Raphnus raphanistrum L. seed oil using copper modified montmorillonite clay catalyst. Environ. Res. 2021, 193, 110398. [Google Scholar] [CrossRef]
- Srivastava, N.; Nandan, N.K. Microbial Growth Control in Diesel by Optimization of Sulphur. Int. J. Environ. Pollut. Remed. 2012, 1, 119–125. [Google Scholar] [CrossRef][Green Version]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Amin, S. Review on biofuel oil and gas production processes from microalgae. Energy Convers. Manag. 2009, 50, 1834–1840. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Srivastava, N.; Parhi, S.; Jha, M.; Sreekrishnan, T. Optimization of effect of pre-treatment on chromium removal by algal biomass using response surface methodology. Int. J. Eng. Res. 2014, 3, 167–171. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Ciurli, A.; Chiellini, C.; Di Caprio, F.; Concas, A.; Dunford, N.T. Latest developments in wastewater treatment and biopolymer production by microalgae. J. Environ. Chem. Eng. 2020, 9, 104926. [Google Scholar] [CrossRef]
- Manirethan, V.; Raval, K.; Rajan, R.; Thaira, H.; Balakrishnan, R.M. Kinetic and thermodynamic studies on the adsorption of heavy metals from aqueous solution by melanin nanopigment obtained from marine source: Pseudomonas stutzeri. J. Environ. Manag. 2018, 214, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meneses, Y.E.; Stratton, J.; Lau, S.K.; Subbiah, J. Integration of ozone with co-immobilized microalgae-activated sludge bacterial symbiosis for efficient on-site treatment of meat processing wastewater. J. Environ. Manag. 2021, 285, 112152. [Google Scholar] [CrossRef]
- Kalra, R.; Gaur, S.; Goel, M. Microalgae bioremediation: A perspective towards wastewater treatment along with industrial carotenoids production. J. Water Process Eng. 2021, 40, 101794. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).