Mix Design Effects on the Durability of Alkali-Activated Slag Concrete in a Hydrochloric Acid Environment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
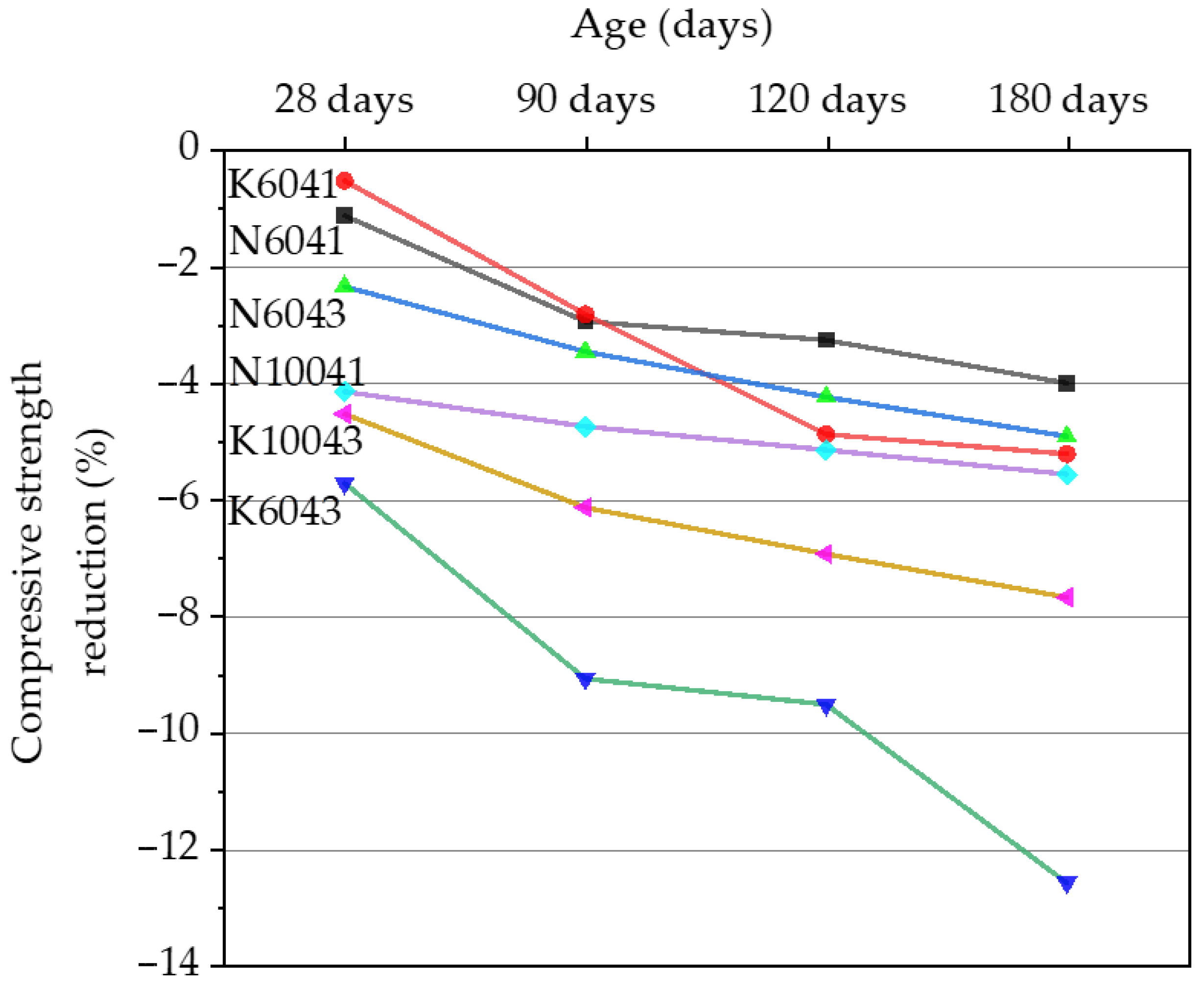
3.1. Compressive Strength of AASC Samples before Acid Exposure
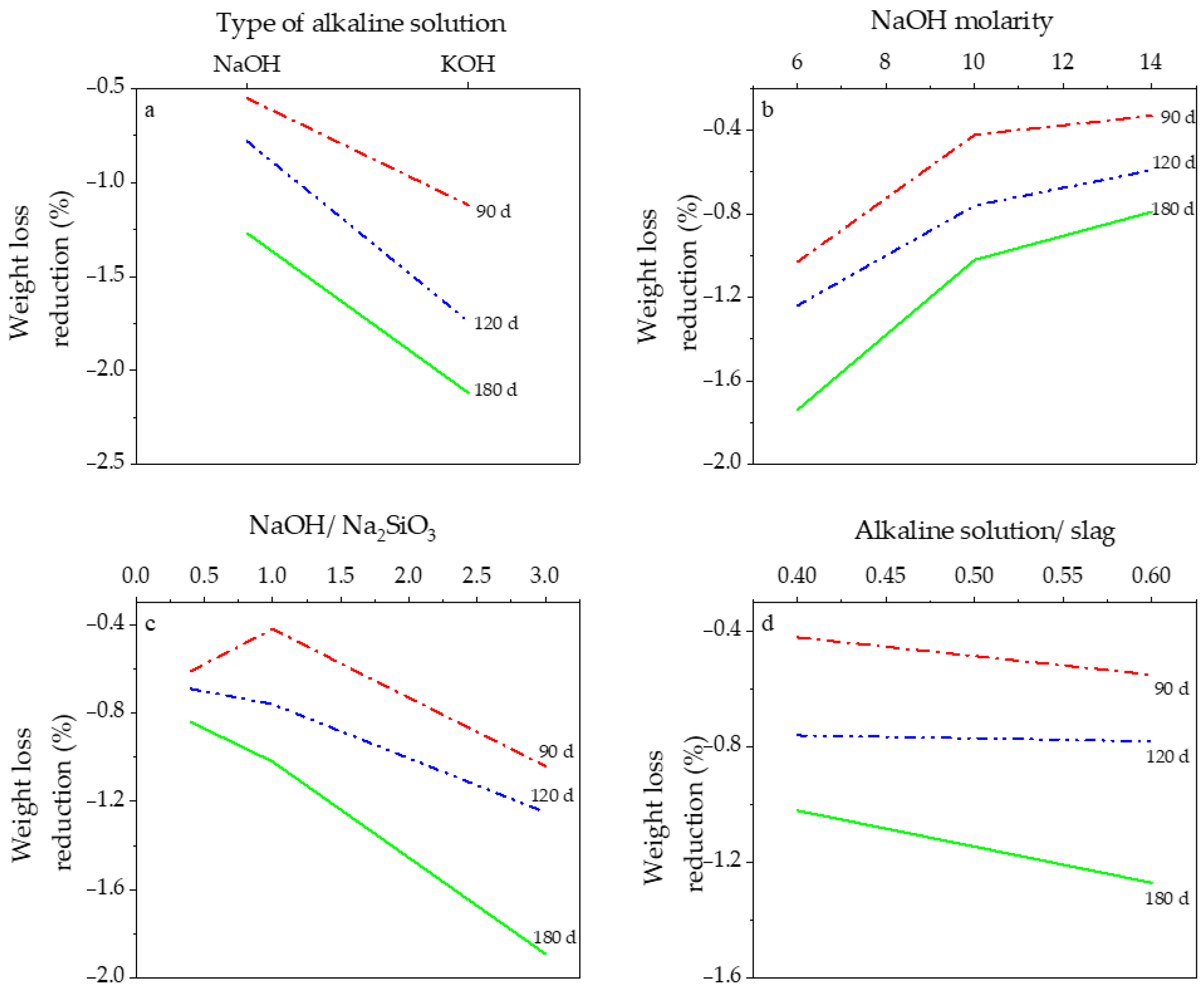
3.2. Effect of Mix Design Parameters on Performance in Acid Attack
3.2.1. Type of Alkaline Activator
3.2.2. Sodium Hydroxide Molarity
3.2.3. Weight Ratio of Sodium Hydroxide to Sodium Silicate
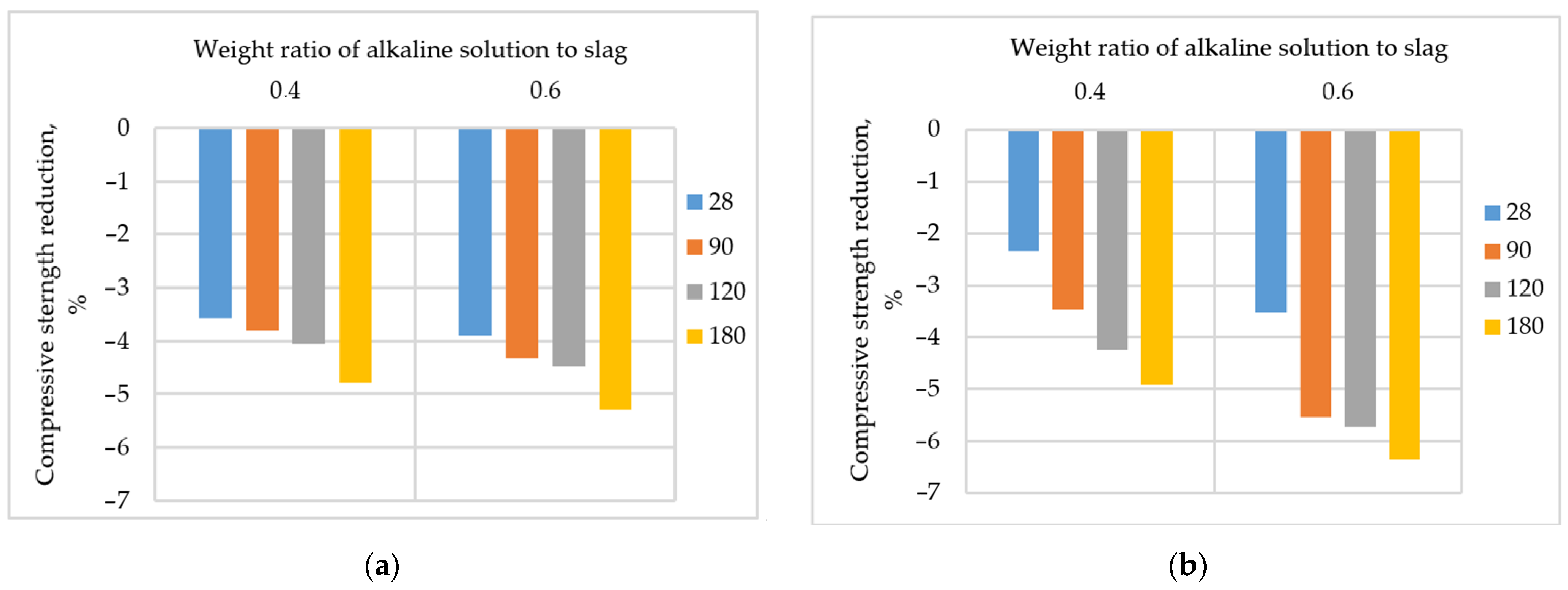
3.2.4. Weight Ratio of Alkaline Solution to Slag
4. Comparison of OPC Concrete and AASC in HCl Acid Solution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonebi, M.; Ammar, Y.; Diederich, P. 15-Sustainability of cement, concrete and cement replacement materials in construction. In Sustainability of Construction Materials, 2nd ed.; Khatib, J.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 371–396. [Google Scholar] [CrossRef]
- Raju, P.S.N.; Dayaratnam, P. Durability of concrete exposed to dilute sulphuric acid. Build. Environ. 1984, 19, 75–79. [Google Scholar] [CrossRef]
- Zivica, V.; Bajza, A. Acidic attack of cement based materials—A review: Part 1. Principle of acidic attack. Constr. Build. Mater. 2001, 15, 331–340. [Google Scholar] [CrossRef]
- Mohamed, O.A. A review of durability and strength characteristics of alkali-activated slag concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Ling, T.-C.; Mo, K.H. Functions and impacts of plastic/rubber wastes as eco-friendly aggregate in concrete—A review. Constr. Build. Mater. 2020, 240, 117869. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Emam, M.A. Factors affecting the mechanical properties of alkali activated ground granulated blast furnace slag concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M.J. Investigation of the properties of alkali-activated slag mixes involving the use of nanoclay and nucleation seeds for 3D printing. Compos. Part B Eng. 2020, 186, 107826. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.-H.; Song, J.-K. Workability Loss and Compressive Strength Development of Cementless Mortars Activated by Combination of Sodium Silicate and Sodium Hydroxide. J. Mater. Civ. Eng. 2009, 21, 119–127. [Google Scholar] [CrossRef]
- Adesina, A. Properties of Alkali Activated Slag Concrete Incorporating Waste Materials as Aggregate: A Review. Mater. Sci. Forum 2019, 967, 214–220. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Shojaei, M.; Behfarnia, K.; Mohebi, R. Application of alkali-activated slag concrete in railway sleepers. Mater. Des. 2015, 69, 89–95. [Google Scholar] [CrossRef]
- Mohebi, R.; Behfarnia, K.; Shojaei, M. Abrasion resistance of alkali-activated slag concrete designed by Taguchi method. Constr. Build. Mater. 2015, 98, 792–798. [Google Scholar] [CrossRef]
- Surul, O.; Bilir, T.; Gholampour, A.; Sutcu, M.; Ozbakkaloglu, T.; Gencel, O. Recycle of ground granulated blast furnace slag and fly ash on eco-friendly brick production. Eur. J. Environ. Civ. Eng. 2020, 1–19. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. Durability Properties of Alkali Activated Slag Composites: Short Overview. Silicon 2020, 12, 987–996. [Google Scholar] [CrossRef]
- Bondar, D.; Ma, Q.; Soutsos, M.; Basheer, M.; Provis, J.L.; Nanukuttan, S. Alkali activated slag concretes designed for a desired slump, strength and chloride diffusivity. Constr. Build. Mater. 2018, 190, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F.; González-Fonteboa, B.; González-Taboada, I.; Alonso, M.M.; Torres-Carrasco, M.; Rojo, G.; Martínez-Abella, F. Alkali-activated slag concrete: Fresh and hardened behaviour. Cem. Concr. Compos. 2018, 85, 22–31. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Tang, D.; Yang, K.; Zhang, Z.; Li, Q.; Pan, Q.; Yang, C. Effect of Ca(OH)2 on shrinkage characteristics and microstructures of alkali-activated slag concrete. Constr. Build. Mater. 2018, 175, 467–482. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- He, J.; Gao, Q.; Wu, Y.; He, J.; Pu, X. Study on improvement of carbonation resistance of alkali-activated slag concrete. Constr. Build. Mater. 2018, 176, 60–67. [Google Scholar] [CrossRef]
- Mohabbi Yadollahi, M.; Dener, M. Investigation of elevated temperature on compressive strength and microstructure of alkali activated slag based cements. Eur. J. Environ. Civ. Eng. 2019, 1–15. [Google Scholar] [CrossRef]
- Shabani, A.; Kioumarsi, M.; Plevris, V.; Stamatopoulos, H. Structural Vulnerability Assessment of Heritage Timber Buildings: A Methodological Proposal. Forests 2020, 11, 881. [Google Scholar] [CrossRef]
- Shabani, A.; Kioumarsi, M.; Zucconi, M. State of the art of simplified analytical methods for seismic vulnerability assessment of unreinforced masonry buildings. Eng. Struct. 2021, 239, 112280. [Google Scholar] [CrossRef]
- Khitab, A.; Arshad, M.; Ffaisal, A.; Khan, I. Development of an Acid Resistant Concrete: A Review. Int. J. Sustain. Constr. Eng. Technol. 2013, 4, 33–38. [Google Scholar]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. External sulfate attack on alkali-activated slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Madhuri, G.; Srinivasa Rao, K. Performance of alkali-activated slag concrete against sulphuric acid attack. Asian J. Civ. Eng. 2018, 19, 451–461. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L.; San Nicolas, R. Degradation process of alkali-activated slag/fly ash and Portland cement-based pastes exposed to phosphoric acid. Constr. Build. Mater. 2020, 232, 117209. [Google Scholar] [CrossRef]
- Miyamoto, S.; Minagawa, H.; Hisada, M. Deterioration rate of hardened cement caused by high concentrated mixed acid attack. Constr. Build. Mater. 2014, 67, 47–54. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Acidic corrosion of hydrated cement based materials. Part 1. Mechanism of the phenomenon. Ceram. Silik. 2000, 44, 114–120. [Google Scholar]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Yun, T.; Kwak, S.-Y. Recovery of hydrochloric acid using positively-charged nanofiltration membrane with selective acid permeability and acid resistance. J. Environ. Manag. 2020, 260, 110001. [Google Scholar] [CrossRef]
- Türkel, S.; Felekoǧlu, B.; Dulluc, S. Influence of various acids on the physico-mechanical properties of pozzolanic cement mortars. Sadhana 2007, 32, 683–691. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Acidic corrosion of hydrated cement based materials—Part 2. Kinetics of the phenomenon and mathematical models. Ceram.Silik. 2000, 44, 152–160. [Google Scholar]
- Pavlík, V. Corrosion of hardened cement paste by acetic and nitric acids part I: Calculation of corrosion depth. Cem. Concr. Res. 1994, 24, 551–562. [Google Scholar] [CrossRef]
- Özcan, A.; Karakoç, M.B. The Resistance of Blast Furnace Slag- and Ferrochrome Slag-Based Geopolymer Concrete Against Acid Attack. Int. J. Civ. Eng. 2019, 17, 1571–1583. [Google Scholar] [CrossRef]
- Thunuguntla, C.S.; Gunneswara Rao, T.D. Effect of mix design parameters on mechanical and durability properties of alkali activated slag concrete. Constr. Build. Mater. 2018, 193, 173–188. [Google Scholar] [CrossRef]
- Matalkah, F.; Salem, T.; Soroushian, P. Acid resistance and corrosion protection potential of concrete prepared with alkali aluminosilicate cement. J. Build. Eng. 2018, 20, 705–711. [Google Scholar] [CrossRef]
- Nagaraj, V.K.; Babu, D.L.V. Assessing the performance of molarity and alkaline activator ratio on engineering properties of self-compacting alkaline activated concrete at ambient temperature. J. Build. Eng. 2018, 20, 137–155. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated slag concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Collins, F.G.; Sanjayan, J.G. Workability and mechanical properties of alkali activated slag concrete. Cem. Concr. Res. 1999, 29, 455–458. [Google Scholar] [CrossRef]
- Rajamane, N.P.; Nataraja, M.C.; Lakshmanan, N.; Dattatreya, J.K.; Sabitha, D. Sulphuric acid resistant ecofriendly concrete from geopolymerisation of blast furnace slag. Indian J. Eng. Mater. Sci. 2012, 19, 357–367. [Google Scholar]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. A review on alkali-activated slag concrete. Ain Shams Eng. J. 2021. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Reddy, A.; Uppalapati, V. Performance of Alkali Activated Slag and Alkali Activated Slag + Fly Ash with various Alkali Activators. Int. J. Eng. Technol. Res. 2014, 2, 73–78. [Google Scholar]
- Behfarnia, K.; Taghvayi Yazeli, H.; Khalili Khasraghi, M.B. The Effect of Alkaline Activator on Workability and Compressive Strength of Alkali-Activated Slag Concrete. AUT J. Civ. Eng. 2017, 1, 55–60. [Google Scholar] [CrossRef]
- Manjunath, G.S.; Giridhar, C.; Jadhav, M. Compressive Strength Development in Ambient Cured Geo-Polymer Mortar. Int. J. Earth Sci. Eng. 2011, 4, 830–834. [Google Scholar]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Beltrame, N.A.M.; da Luz, C.A.; Perardt, M.; Hooton, R.D. Alkali activated cement made from blast furnace slag generated by charcoal: Resistance to attack by sodium and magnesium sulfates. Constr. Build. Mater. 2020, 238, 117710. [Google Scholar] [CrossRef]
- Shariati, M.; Shariati, A.; Trung, N.T.; Shoaei, P.; Ameri, F.; Bahrami, N.; Zamanabadi, S.N. Alkali-activated slag (AAS) paste: Correlation between durability and microstructural characteristics. Constr. Build. Mater. 2021, 267, 120886. [Google Scholar] [CrossRef]
- Wanger, V. Manual of Chemical Technology; Forgotten Books: London, UK, 2012. [Google Scholar]
- Idir, R.; Cyr, M.; Pavoine, A. Investigations on the durability of alkali-activated recycled glass. Constr. Build. Mater. 2020, 236, 117477. [Google Scholar] [CrossRef]
- Abdul Rahim, R.H.; Rahmiati, T.; Azizli, K.A.; Man, Z.; Nuruddin, M.F.; Ismail, L. Comparison of Using NaOH and KOH Activated Fly Ash-Based Geopolymer on the Mechanical Properties. Mater. Sci. Forum 2015, 803, 179–184. [Google Scholar] [CrossRef]
- Sabitha, D.; Dattatreya, J.K.; Sakthivel, N.; Bhuvaneshwari, M.; Sathik, S.A.J. Reactivity, workability and strength of potassium versus sodium-activated high volume fly ash-based geopolymers. Curr. Sci. 2012, 103, 1320–1327. [Google Scholar]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Provis, J.L. Performance of alkali-activated slag mortars exposed to acids. J. Sustain. Cem. Based Mater. 2012, 1, 138–151. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Aguirre-Guerrero, A.M.; Mejía de Gutiérrez, R. Carbonation-induced corrosion of alkali-activated binary concrete based on natural volcanic pozzolan. Constr. Build. Mater. 2020, 232, 117189. [Google Scholar] [CrossRef]
- Behfarnia, K.; Rostami, M. The Effect of Alkaline Solution-to-Slag Ratio on Permeability of Alkali Activated Slag Concrete. Int. J. Civ. Eng. 2017, 16. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Li, Y.; Shi, L.; Sun, D. The Durability of Alkali-Activated Materials in Comparison with Ordinary Portland Cements and Concretes: A Review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Fernando, P.-T.; Castro-Gomes, J.; Jalali, S. Durability and Environmental Performance of Alkali-Activated Tungsten Mine Waste Mud Mortars. J. Mater. Civ. Eng. 2010, 22, 897–904. [Google Scholar] [CrossRef]
- Robert Munn, X.S.; Marosszeky, M. Experimental Study of Geopolymer Concrete Resistance to Sulphuric Acid Attack. In Proceedings of the 11DBMC International Conference on Durability of Building Materials and Components ISTANBUL, Istanbul, Turkey, 11 May 2008. [Google Scholar]





| CaO | SiO2 | Al2O3 | MgO | TiO2 | MnO | S | K2O | Fe2O3 | NaO2 | SO3 | L.O.I * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GGBFS | 36.52 | 38.35 | 10.88 | 8.77 | 1.48 | 1.25 | 1.21 | 0.93 | 0.52 | 0.49 | - | 0.26 |
| Cement | 63.50 | 21.50 | 5.10 | 2.30 | - | - | - | 0.93 | 3.80 | - | 2.00 | 0.70 |
| Type of Aggregate | Fineness Module | Sand Equality | SSD Specific Gravity (gr/cm3) | Water Absorption (%) |
|---|---|---|---|---|
| Fine | 2.99 | 77 | 2.47 | 2.06 |
| Course | - | - | 2.59 | 0.76 |
| Mix Code | Alkaline Solution (Kg/m3) | Slag (Kg/m3) | The Weight Ratio of NaOH (KOH) to Na2SiO3 | The Weight Ratio of Alkaline Solution to Slag | Molarity | Type of Alkaline Solution |
|---|---|---|---|---|---|---|
| N6041 | 158 | 394 | 1 | 0.4 | 6 | NaOH |
| N6043 | 158 | 394 | 3 | 0.4 | 6 | NaOH |
| N10041 | 158 | 394 | 1 | 0.4 | 10 | NaOH |
| N10043 | 158 | 394 | 3 | 0.4 | 10 | NaOH |
| N14041 | 158 | 394 | 1 | 0.4 | 14 | NaOH |
| N14043 | 158 | 394 | 3 | 0.4 | 14 | NaOH |
| N60404 | 158 | 394 | 0.4 | 0.4 | 6 | NaOH |
| N100404 | 158 | 394 | 0.4 | 0.4 | 10 | NaOH |
| N6061 | 207 | 345 | 1 | 0.6 | 6 | NaOH |
| N10063 | 207 | 345 | 3 | 0.6 | 10 | NaOH |
| K6041 | 158 | 394 | 1 | 0.4 | 6 | KOH |
| K6043 | 158 | 394 | 3 | 0.4 | 6 | KOH |
| K10043 | 158 | 394 | 3 | 0.4 | 10 | KOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teymouri, M.; Behfarnia, K.; Shabani, A. Mix Design Effects on the Durability of Alkali-Activated Slag Concrete in a Hydrochloric Acid Environment. Sustainability 2021, 13, 8096. https://doi.org/10.3390/su13148096
Teymouri M, Behfarnia K, Shabani A. Mix Design Effects on the Durability of Alkali-Activated Slag Concrete in a Hydrochloric Acid Environment. Sustainability. 2021; 13(14):8096. https://doi.org/10.3390/su13148096
Chicago/Turabian StyleTeymouri, Mohammad, Kiachehr Behfarnia, and Amirhosein Shabani. 2021. "Mix Design Effects on the Durability of Alkali-Activated Slag Concrete in a Hydrochloric Acid Environment" Sustainability 13, no. 14: 8096. https://doi.org/10.3390/su13148096
APA StyleTeymouri, M., Behfarnia, K., & Shabani, A. (2021). Mix Design Effects on the Durability of Alkali-Activated Slag Concrete in a Hydrochloric Acid Environment. Sustainability, 13(14), 8096. https://doi.org/10.3390/su13148096








