Carbon Nanotube/Pt Cathode Nanocomposite Electrode in Microbial Fuel Cells for Wastewater Treatment and Bioenergy Production
Abstract
1. Introduction
2. Materials and Methods
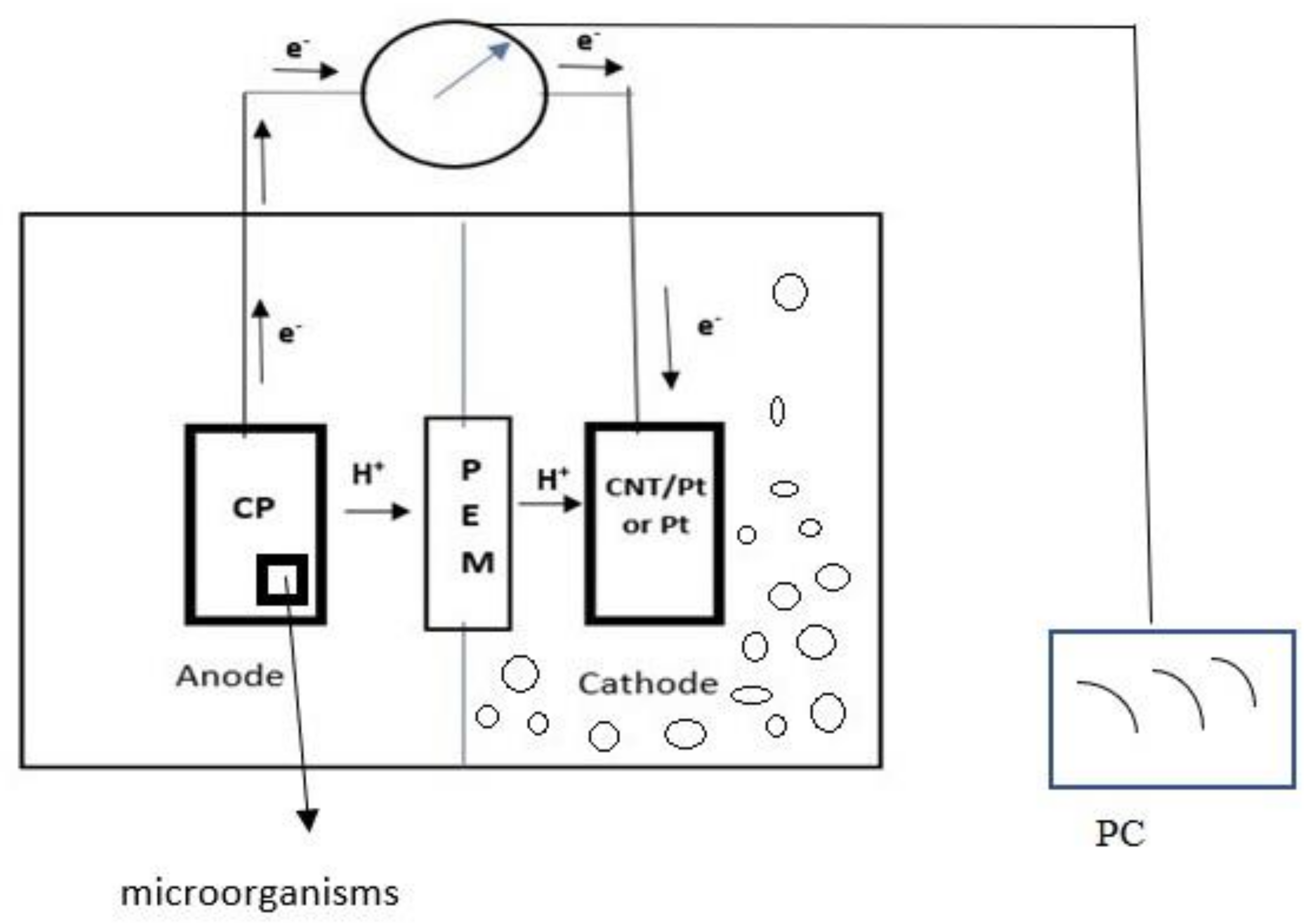
2.1. MFC Configuration
2.2. Electrode Preparation
2.2.1. Pt Electrode
2.2.2. CNT/Pt Nanocomposite Electrode
2.3. Analysis and Calculation
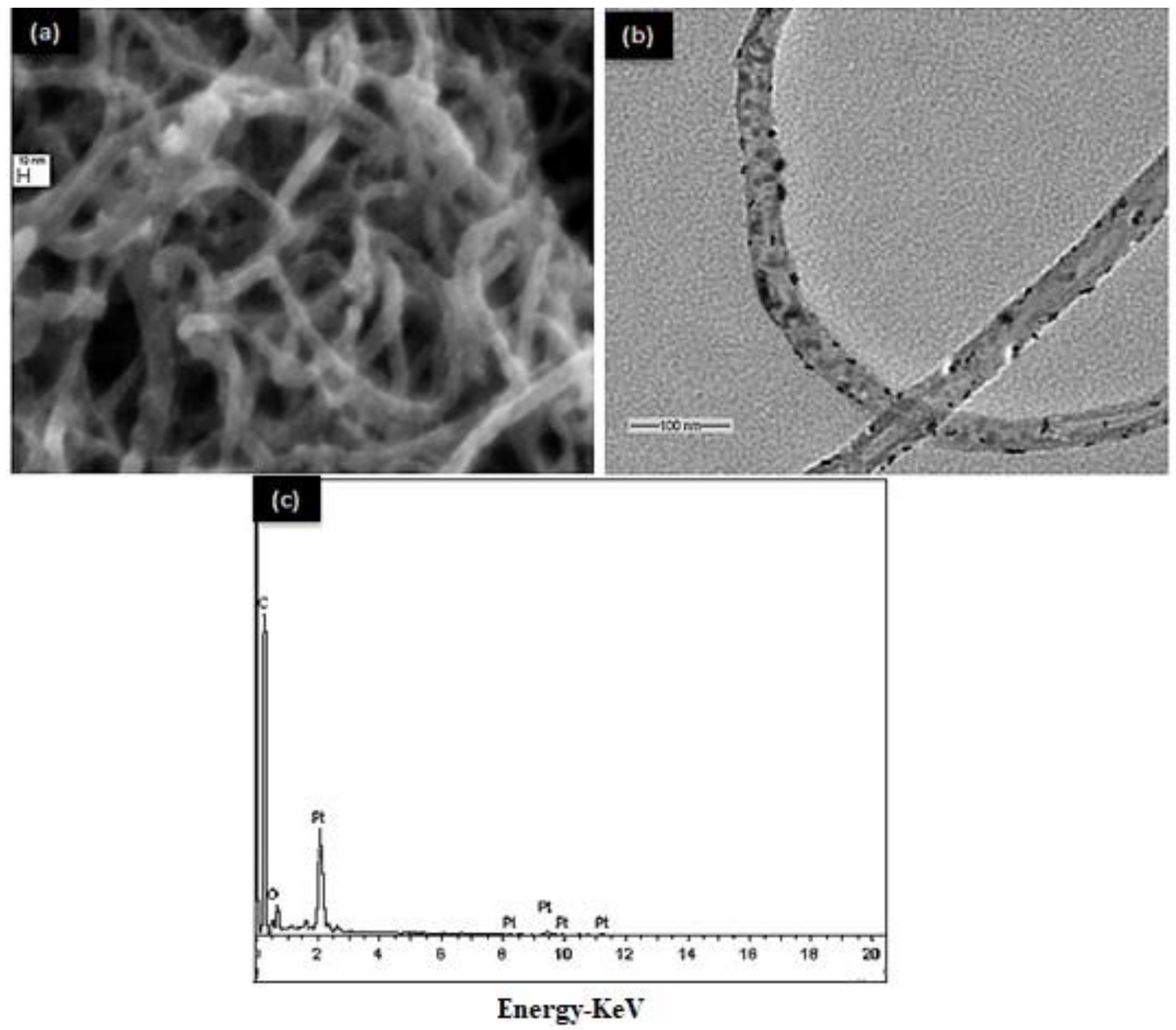
2.4. Electrodes Morphology and Catalytic Activity
3. Results and Discussion
3.1. Electrode Characterization
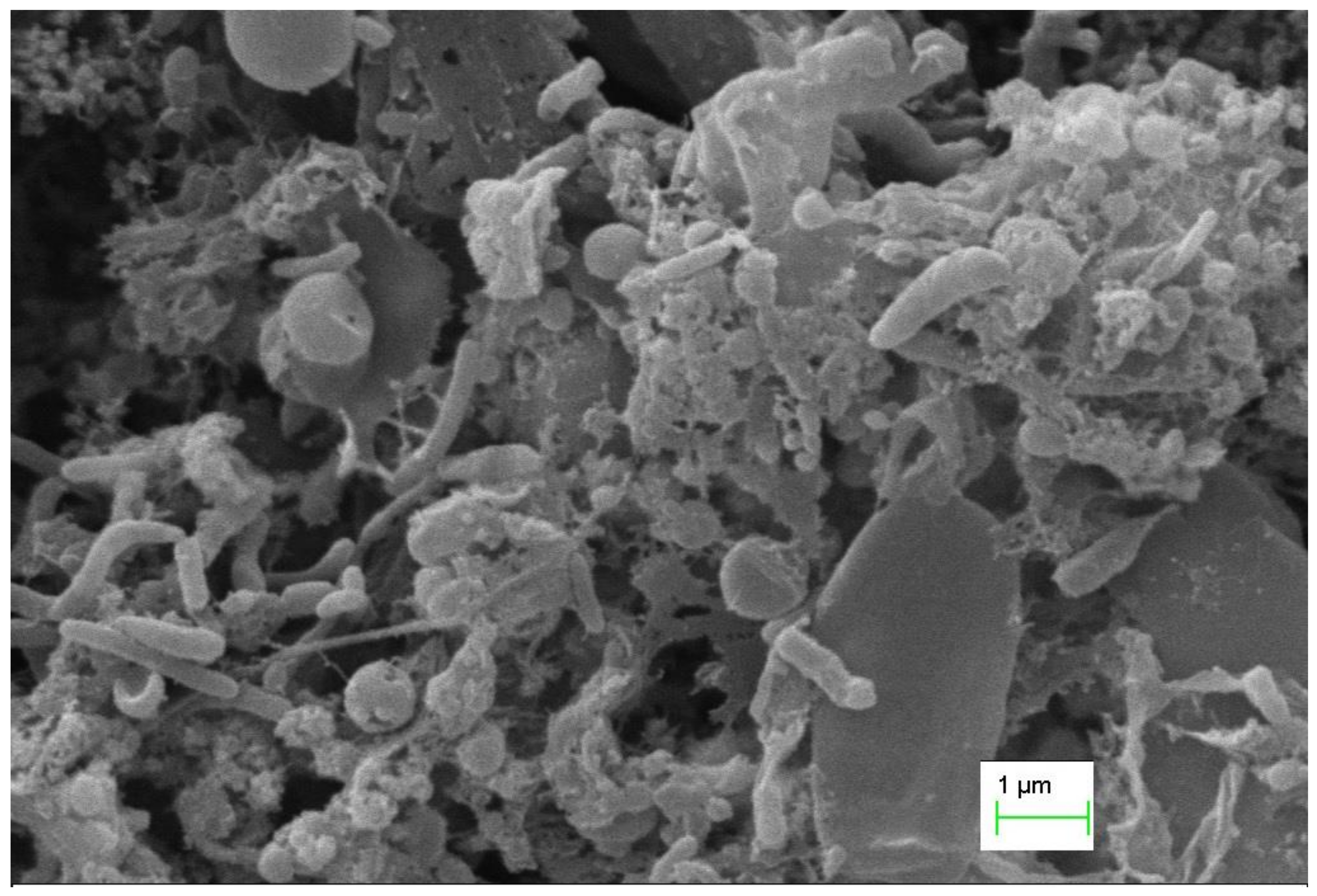
3.2. Attachment of Microorganisms on the Anode Electrode
3.3. Power Density
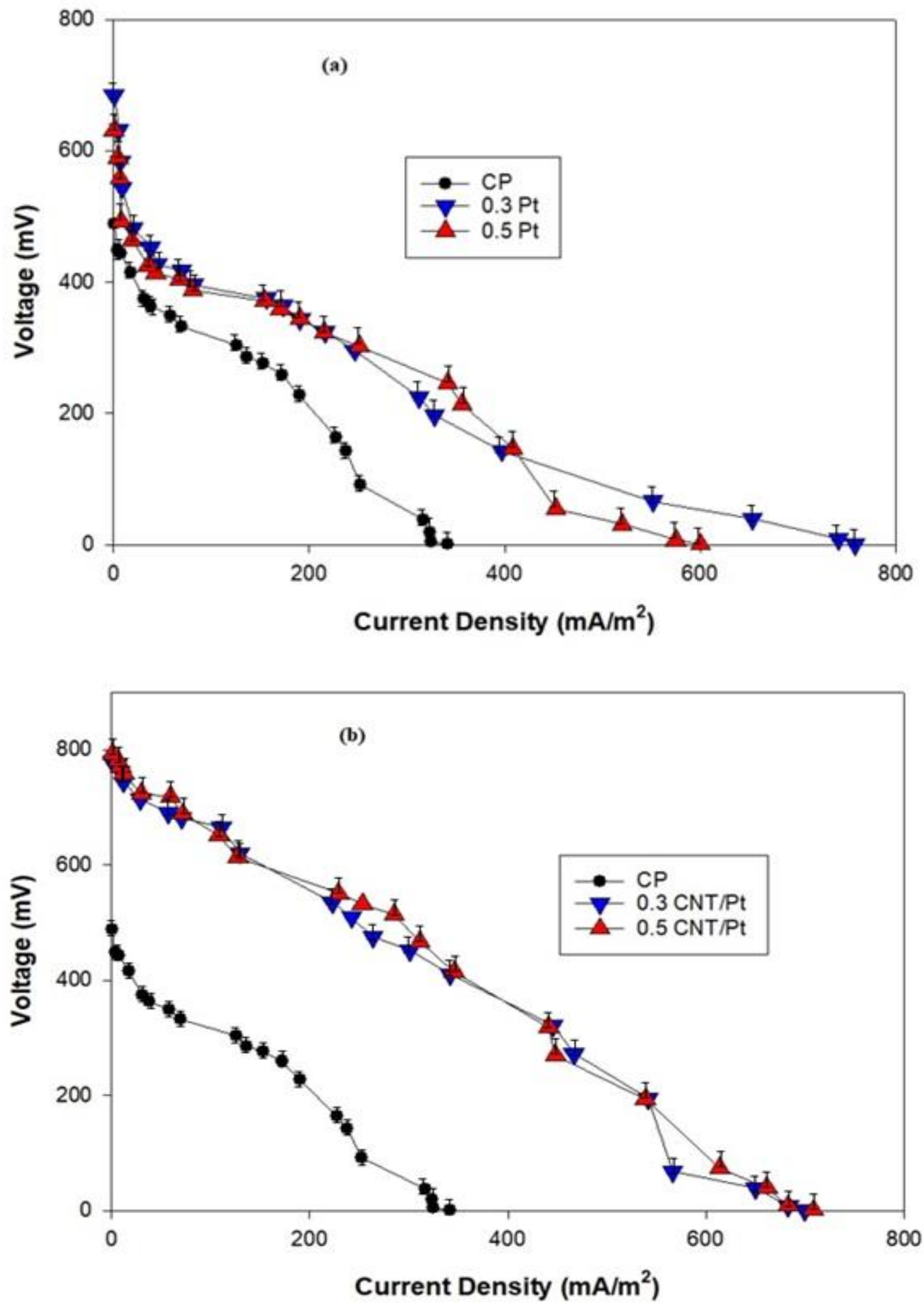
3.4. Polarization Curve
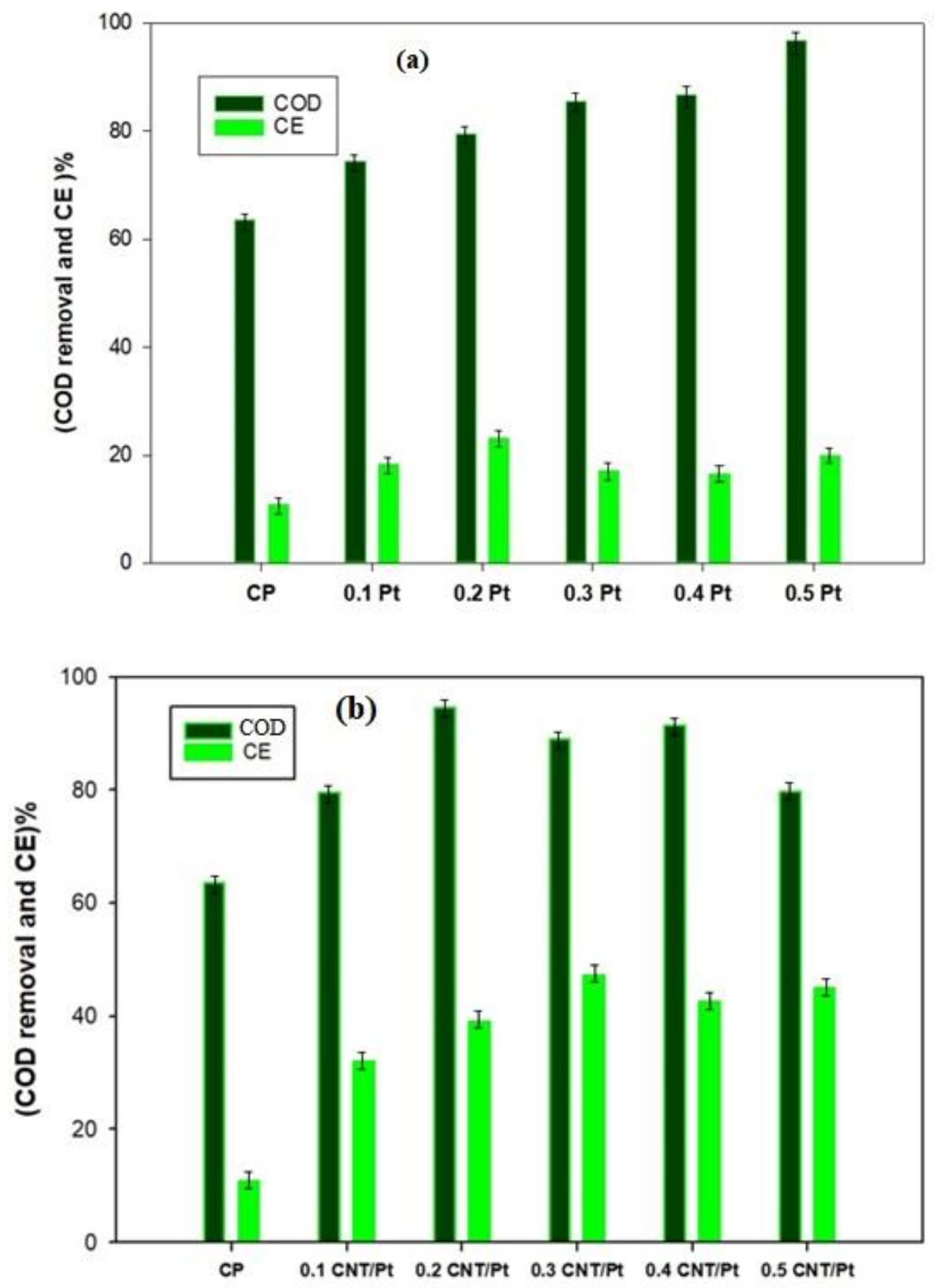
3.5. COD Removal and Coulombic Efficiency
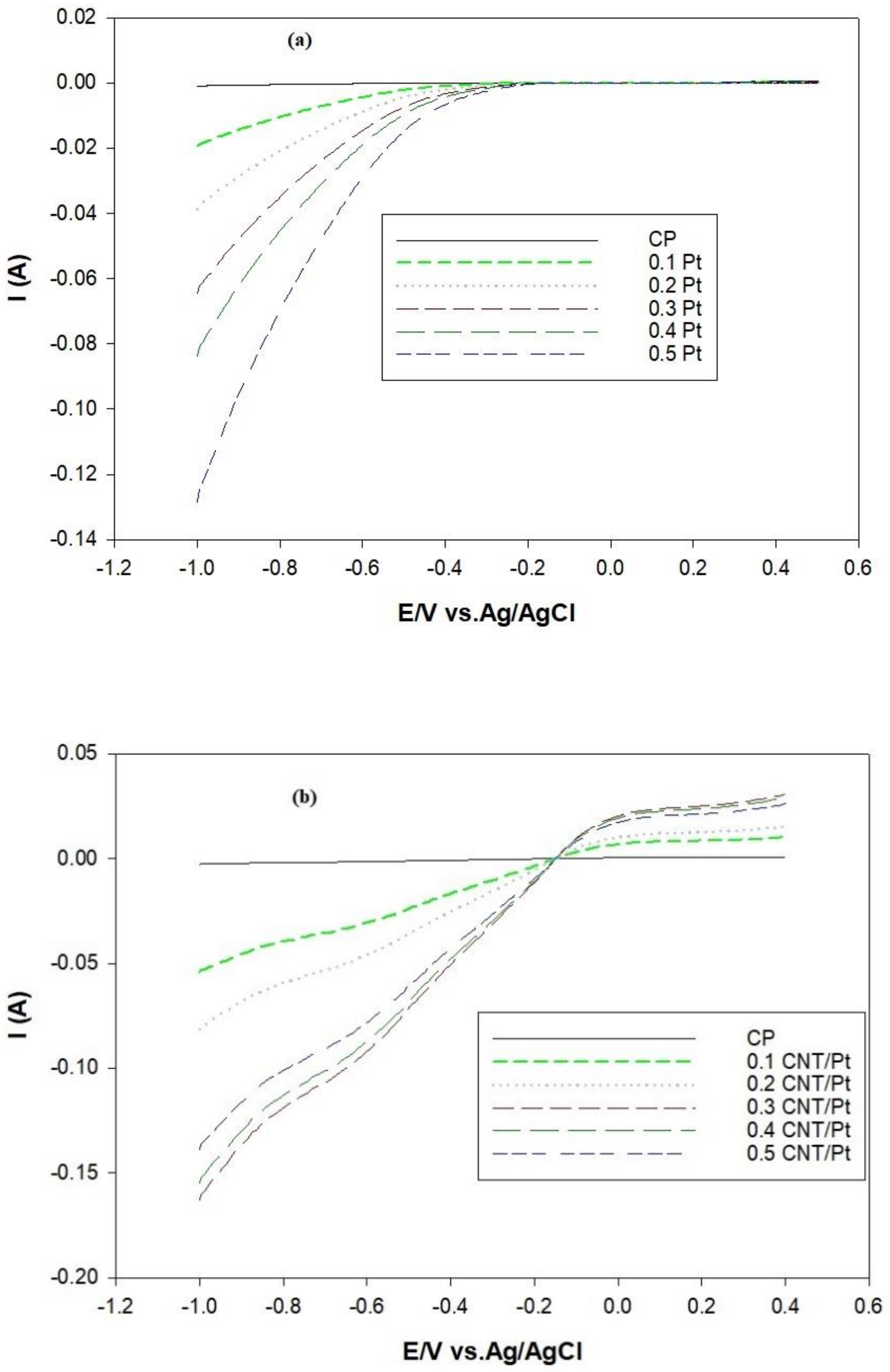
3.6. LSV Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Arulmani, S.R.B.; Gnanamuthu, H.L.; Kandasamy, S.; Govindarajan, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A.; Zhang, H. Sustainable bioelectricity production from Amaranthus viridis and Triticum aestivum mediated plant microbial fuel cells with efficient electrogenic bacteria selections. Process. Biochem. 2021, 107, 27–37. [Google Scholar] [CrossRef]
- Sedighi, M.; Mohammadi, M. CO2 hydrogenation to light olefins over Cu-CeO2/SAPO-34 catalysts: Product distribution and optimization. J. CO2 Util. 2020, 35, 236–244. [Google Scholar] [CrossRef]
- Sedighi, M.; Ghasemi, M.; Mohammadi, M.; Hassan, S.H.A. A novel application of a neuro–fuzzy computational technique in modeling of thermal cracking of heavy feedstock to light olefin. RSC Adv. 2014, 4, 28390–28399. [Google Scholar] [CrossRef]
- Sallam, E.; Khairy, H.; Elnouby, M.; Fetouh, H. Sustainable electricity production from seawater using Spirulina platensis microbial fuel cell catalyzed by silver nanoparticles-activated carbon composite prepared by a new modified photolysis method. Biomass Bioenergy 2021, 148, 106038. [Google Scholar] [CrossRef]
- Ghasemi, M.; Mohammadi, M.; Sedighi, M. Sustainable production of light olefins from greenhouse gas CO2 over SAPO-34 supported modified cerium oxide. Microporous Mesoporous Mater. 2020, 297, 110029. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sedighi, M.; Natarajan, R.; Hassan, S.H.A.; Ghasemi, M. Microbial fuel cell for oilfield produced water treatment and reuse: Modelling and process optimization. Korean J. Chem. Eng. 2020, 38, 72–80. [Google Scholar] [CrossRef]
- Shamshiri, A.; Alimohammadi, V.; Sedighi, M.; Jabbari, E.; Mohammadi, M. Enhanced removal of phosphate and nitrate from aqueous solution using novel modified natural clinoptilolite nanoparticles: Process optimization and assessment. Int. J. Environ. Anal. Chem. 2020, 1–20. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sedighi, M.; Ghasemi, M. Systematic investigation of simultaneous removal of phosphate/nitrate from water using Ag/rGO nanocomposite: Development, characterization, performance and mechanism. Res. Chem. Intermed. 2021, 47, 1377–1395. [Google Scholar] [CrossRef]
- Sedighi, M.; Mohammadi, M. Application of green novel NiO/ZSM-5 for removal of lead and mercury ions from aqueous solution: Investigation of adsorption parameters. J. Water Environ. Nanotechnol. 2018, 3, 301–310. [Google Scholar] [CrossRef]
- Shahveh, S.; Sedighi, M.; Mohammadi, M. A Novel Application of Combined Biological and Physical Method for Nitrate and Nitrite Removal from Water. J. Environ. Sci. Technol. 2020, 22, 183–192. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lai, C.-Y.; Liu, S.-H.; Chen, Y.-R.; Alfanti, L.K. Enhancing bioelectricity generation and removal of copper in microbial fuel cells with a laccase-catalyzed biocathode. J. Clean. Prod. 2021, 298, 126726. [Google Scholar] [CrossRef]
- Ghasemi, M.; Ahmad, A.; Jafary, T.; Azad, A.K.; Kakooei, S.; Daud, W.R.W.; Sedighi, M. Assessment of immobilized cell reactor and microbial fuel cell for simultaneous cheese whey treatment and lactic acid/electricity production. Int. J. Hydrogen Energy 2017, 42, 9107–9115. [Google Scholar] [CrossRef]
- Hisham, S.; Khan, F.A.; Aljlil, S.A.; Ghasemi, M. Investigating new techniques for the treatment of oil field produced water and energy production. SN Appl. Sci. 2019, 1, 646. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Alam, J.; Ilbeygi, H.; Sedighi, M.; Ismail, A.F.; Yazdi, M.H.; Aljlil, S.A. Treatment of two different water resources in desalination and microbial fuel cell processes by poly sulfone/Sulfonated poly ether ether ketone hybrid membrane. Energy 2016, 96, 303–313. [Google Scholar] [CrossRef]
- Yu, B.; Tian, J.; Feng, L. Remediation of PAH polluted soils using a soil microbial fuel cell: Influence of electrode interval and role of microbial community. J. Hazard. Mater. 2017, 336, 110–118. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Ismail, M.; Rahimnejad, M.; Ismail, A.F.; Leong, J.X.; Miskan, M.; Ben Liew, K. Effect of pre-treatment and biofouling of proton exchange membrane on microbial fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 5480–5484. [Google Scholar] [CrossRef]
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Kim, B.H.; Yaakob, Z.; Daud, W.R.W. Activated carbon nanofibers as an alternative cathode catalyst to platinum in a two-chamber microbial fuel cell. Int. J. Hydrogen Energy 2011, 36, 13746–13752. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.; Oh, S.-E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloys Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L. A microbial fuel cell system with manganese dioxide/titanium dioxide/graphitic carbon nitride coated granular activated carbon cathode successfully treated organic acids industrial wastewater with residual nitric acid. Bioresour. Technol. 2020, 304, 122992. [Google Scholar] [CrossRef]
- Ali, J.; Wang, L.; Waseem, H.; Djellabi, R.; Oladoja, N.; Pan, G. FeS@rGO nanocomposites as electrocatalysts for enhanced chromium removal and clean energy generation by microbial fuel cell. Chem. Eng. J. 2020, 384, 123335. [Google Scholar] [CrossRef]
- Pattanayak, P.; Papiya, F.; Kumar, V.; Singh, A.; Kundu, P.P.; Pattanayak, P.; Papiya, F.; Kumar, V.; Singh, A.; Kundu, P.P. Performance evaluation of poly(aniline-co-pyrrole) wrapped titanium dioxide nanocomposite as an air-cathode catalyst material for microbial fuel cell. Mater. Sci. Eng. C 2021, 118, 111492. [Google Scholar] [CrossRef] [PubMed]
- Türk, K.; Kruusenberg, I.; Kibena-Põldsepp, E.; Bhowmick, G.D.; Kook, M.; Tammeveski, K.; Matisen, L.; Merisalu, M.; Sammelselg, V.; Ghangrekar, M.; et al. Novel multi walled carbon nanotube based nitrogen impregnated Co and Fe cathode catalysts for improved microbial fuel cell performance. Int. J. Hydrogen Energy 2018, 43, 23027–23035. [Google Scholar] [CrossRef]
- Ghasemi, M.; Nassef, A.M.; Al-Dhaifallah, M.; Rezk, H. Performance improvement of microbial fuel cell through artificial intelligence. Int. J. Energy Res. 2020, 45, 342–354. [Google Scholar] [CrossRef]
- Du, Y.; Ma, F.-X.; Xu, C.-Y.; Yu, J.; Li, D.; Feng, Y.; Zhen, L. Nitrogen-doped carbon nanotubes/reduced graphene oxide nanosheet hybrids towards enhanced cathodic oxygen reduction and power generation of microbial fuel cells. Nano Energy 2019, 61, 533–539. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, K.; Li, H.; Dai, Y.; Zhang, H.; Zuo, J.; Yan, J.; Xiao, T.; Liu, X.; Lu, Y.; et al. Bimetallic hybrids modified with carbon nanotubes as cathode catalysts for microbial fuel cell: Effective oxygen reduction catalysis and inhibition of biofilm formation. J. Power Sources 2021, 485, 229273. [Google Scholar] [CrossRef]
- Majidi, M.R.; Farahani, F.S.; Hosseini, M.G.; Ahadzadeh, I. Low-cost nanowired α-MnO2/C as an ORR catalyst in air-cathode microbial fuel cell. Bioelectrochemistry 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Babanova, S.; Santoro, C.; Jones, J.; Phan, T.; Serov, A.; Atanassov, P.; Bretschger, O.; Babanova, S.; Santoro, C.; Jones, J.; et al. Practical demonstration of applicability and efficiency of platinum group metal-free based catalysts in microbial fuel cells for wastewater treatment. J. Power Sources 2021, 491, 229582. [Google Scholar] [CrossRef]
- Ghasemi, M.; Halakoo, E.; Sedighi, M.; Alam, J.; Sadeqzadeh, M.; Ghasemi, M.; Halakoo, E.; Sedighi, M.; Alam, J.; Sadeqzadeh, M. Performance Comparison of Three Common Proton Exchange Membranes for Sustainable Bioenergy Production in Microbial Fuel Cell. Procedia CIRP 2015, 26, 162–166. [Google Scholar] [CrossRef]
- Khan, F.A.; Hisham, S.; Ghasemi, M. Oil field produced water recovery and boosting the quality for using in membrane less fuel cell. SN Appl. Sci. 2019, 1, 510. [Google Scholar] [CrossRef]
- Jafary, T.; Aljlil, S.A.; Alam, J.; Ghasemi, M. Effect of the Membrane Type and Resistance Load on the Performance of the Microbial Fuel Cell: A Step ahead of Microbial Desalination Cell Establishment. J. Jpn. Inst. Energy 2017, 96, 346–351. [Google Scholar] [CrossRef][Green Version]
- Shamshirgaran, S.; Al-Kayiem, H.; Sharma, K.; Ghasemi, M. State of the Art of Techno-Economics of Nanofluid-Laden Flat-Plate Solar Collectors for Sustainable Accomplishment. Sustainability 2020, 12, 9119. [Google Scholar] [CrossRef]
- Sedighi, M.; Aljlil, S.A.; Alsubei, M.D.; Ghasemi, M.; Mohammadi, M. Performance optimisation of microbial fuel cell for wastewater treatment and sustainable clean energy generation using response surface methodology. Alex. Eng. J. 2018, 57, 4243–4253. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Rahimnejad, M.; Rezayi, M.; Fatemi, A.; Jafari, Y.; Somalu, M.R.; Manzour, A. Copper-phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. Int. J. Hydrogen Energy 2013, 38, 9533–9540. [Google Scholar] [CrossRef]
- Bharti, A.; Cheruvally, G.; Muliankeezhu, S. Microwave assisted, facile synthesis of Pt/CNT catalyst for proton exchange membrane fuel cell application. Int. J. Hydrogen Energy 2017, 42, 11622–11631. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, Z.; Song, H.; Xie, Y.; Zhang, M. Enhancement of bioelectricity generation by synergistic modification of vertical carbon nanotubes/polypyrrole for the carbon fibers anode in microbial fuel cell. Electrochim. Acta 2019, 296, 69–74. [Google Scholar] [CrossRef]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.; Shao, C.; Zhuang, S.; Ye, J.; Li, B. Enhancing oxygen reduction reaction by using metal-free nitrogen-doped carbon black as cathode catalysts in microbial fuel cells treating wastewater. Environ. Res. 2020, 182, 109011. [Google Scholar] [CrossRef]
- Negassa, L.W.; Mohiuddin, M.; Tiruye, G.A. Treatment of brewery industrial wastewater and generation of sustainable bioelectricity by microbial fuel cell inoculated with locally isolated microorganisms. J. Water Process. Eng. 2021, 41, 102018. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X.; Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent insights into microalgae-assisted microbial fuel cells for generating sustainable bioelectricity. Int. J. Hydrogen Energy 2021, 46, 3135–3159. [Google Scholar] [CrossRef]
- Rossi, R.; Logan, B.E. Unraveling the contributions of internal resistance components in two-chamber microbial fuel cells using the electrode potential slope analysis. Electrochim. Acta 2020, 348, 136291. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Ghasemi, M.; Najafpour, G.; Ismail, M.; Mohammad, A.W.; Ghoreyshi, A.; Hassan, S.H. Synthesis, characterization and application studies of self-made Fe3O4/PES nanocomposite membranes in microbial fuel cell. Electrochim. Acta 2012, 85, 700–706. [Google Scholar] [CrossRef]
- Parkhey, P.; Sahu, R. Microfluidic microbial fuel cells: Recent advancements and future prospects. Int. J. Hydrogen Energy 2021, 46, 3105–3123. [Google Scholar] [CrossRef]
- Xin, S.; Shen, J.; Liu, G.; Chen, Q.; Xiao, Z.; Zhang, G.; Xin, Y. Electricity generation and microbial community of single-chamber microbial fuel cells in response to Cu2O nanoparticles/reduced graphene oxide as cathode catalyst. Chem. Eng. J. 2020, 380, 122446. [Google Scholar] [CrossRef]
- Ullah, Z.; Zeshan, S. Effect of substrate type and concentration on the performance of a double chamber microbial fuel cell. Water Sci. Technol. 2020, 81, 1336–1344. [Google Scholar] [CrossRef]
- Li, S.; Ho, S.-H.; Hua, T.; Zhou, Q.; Li, F.; Tang, J. Sustainable biochar as electrocatalysts for the oxygen reduction reaction in microbial fuel cells. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Gadkari, S.; Gu, S.; Sadhukhan, J. Two-dimensional mathematical model of an air-cathode microbial fuel cell with graphite fiber brush anode. J. Power Sources 2019, 441, 227145. [Google Scholar] [CrossRef]
- Khajeh, R.T.; Aber, S.; Zarei, M. Comparison of NiCo2O4, CoNiAl-LDH, and CoNiAl-LDH@NiCo2O4 performances as ORR catalysts in MFC cathode. Renew. Energy 2020, 154, 1263–1271. [Google Scholar] [CrossRef]
- Taskan, E.; Hasar, H.; Taskan, E.; Hasar, H. Comprehensive Comparison of a New Tin-Coated Copper Mesh and a Graphite Plate Electrode as an Anode Material in Microbial Fuel Cell. Appl. Biochem. Biotechnol. 2015, 175, 2300–2308. [Google Scholar] [CrossRef]
- Papiya, F.; Pattanayak, P.; Kumar, V.; Das, S.; Kundu, P.P.; Papiya, F.; Pattanayak, P.; Kumar, V.; Das, S.; Kundu, P.P. Sulfonated graphene oxide and titanium dioxide coated with nanostructured polyaniline nanocomposites as an efficient cathode catalyst in microbial fuel cells. Mater. Sci. Eng. C 2020, 108, 110498. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Ghangrekar, M. Application of bimetallic low-cost CuZn as oxygen reduction cathode catalyst in lab-scale and field-scale microbial fuel cell. Chem. Phys. Lett. 2020, 751, 137536. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Nandy, A.; Kundu, P. Fabrication of laminated and coated Nafion 117 membranes for reduced mass transfer in microbial fuel cells. RSC Adv. 2016, 6, 21526–21534. [Google Scholar] [CrossRef]

| Cathode Electrode | Internal Resistance (Ω) | Pmax (mW/m2) | Imax (mA/m2) at Pmax | OCV at SS Condition (mV) |
|---|---|---|---|---|
| CP | 1084 | 44.7 | 172.7 | 493 |
| 0.1 Pt | 894 | 53.4 | 159.5 | 587 |
| 0.2 Pt | 873 | 77 | 327.1 | 687 |
| 0.3 Pt | 812 | 73 | 246.7 | 693 |
| 0.4 Pt | 811 | 75.9 | 251.6 | 673 |
| 0.5 Pt | 810 | 84.1 | 341.7 | 672 |
| 0.1 CNT/Pt | 587 | 73.5 | 221.3 | 746 |
| 0.2 CNT/Pt | 593 | 125 | 288.7 | 787 |
| 0.3 CNT/Pt | 551 | 143.1 | 445.8 | 794 |
| 0.4 CNT/Pt | 564 | 146 | 312 | 811 |
| 0.5 CNT/Pt | 573 | 146.8 | 285.6 | 815 |
| Cathode Catalyst | PEM | Power Density (mW/m2) | COD Removal (%) | References |
|---|---|---|---|---|
| CoNiAl-LDH | Nafion N966 | 87.91 | 87.38 | [48] |
| CoNiAl-LDH@NiCo2O4 | 85.28 | 85.81 | ||
| Pt-coated titanium | Ultrex CMI-7000) | 271 | - | [49] |
| SGO-TiO2-PANi | Nafion 117 | 904.18 | - | [50] |
| TiO2-PANi | 561.5 | - | ||
| Pt | 483.5 | - | ||
| Pt | Clayware based ceramic cylinder | 110 | 90 | [51] |
| CuZn | 75.1 | 87 | ||
| C | 19.2 | 77 | ||
| Pt | Nafion 117 | 481.55 | 93.97 | [52] |
| Nafion 117-SPVDF | 446 | 90.27 | ||
| Laminated 117-SPVDF | 413 | 84.15 | ||
| Pt | Nafion 117 | 481 | 90.48 | [22] |
| (PANi-Co-PPy)@TiO2 | 987 | 81.2 | ||
| Pt | Nafion 117 | 84.1 | 96.8 | This study |
| CNT/Pt | 143.1 | 88.9 | ||
| C | 44.7 | 63.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, M.; Sedighi, M.; Tan, Y.H. Carbon Nanotube/Pt Cathode Nanocomposite Electrode in Microbial Fuel Cells for Wastewater Treatment and Bioenergy Production. Sustainability 2021, 13, 8057. https://doi.org/10.3390/su13148057
Ghasemi M, Sedighi M, Tan YH. Carbon Nanotube/Pt Cathode Nanocomposite Electrode in Microbial Fuel Cells for Wastewater Treatment and Bioenergy Production. Sustainability. 2021; 13(14):8057. https://doi.org/10.3390/su13148057
Chicago/Turabian StyleGhasemi, Mostafa, Mehdi Sedighi, and Yie Hua Tan. 2021. "Carbon Nanotube/Pt Cathode Nanocomposite Electrode in Microbial Fuel Cells for Wastewater Treatment and Bioenergy Production" Sustainability 13, no. 14: 8057. https://doi.org/10.3390/su13148057
APA StyleGhasemi, M., Sedighi, M., & Tan, Y. H. (2021). Carbon Nanotube/Pt Cathode Nanocomposite Electrode in Microbial Fuel Cells for Wastewater Treatment and Bioenergy Production. Sustainability, 13(14), 8057. https://doi.org/10.3390/su13148057








