Figure 1.
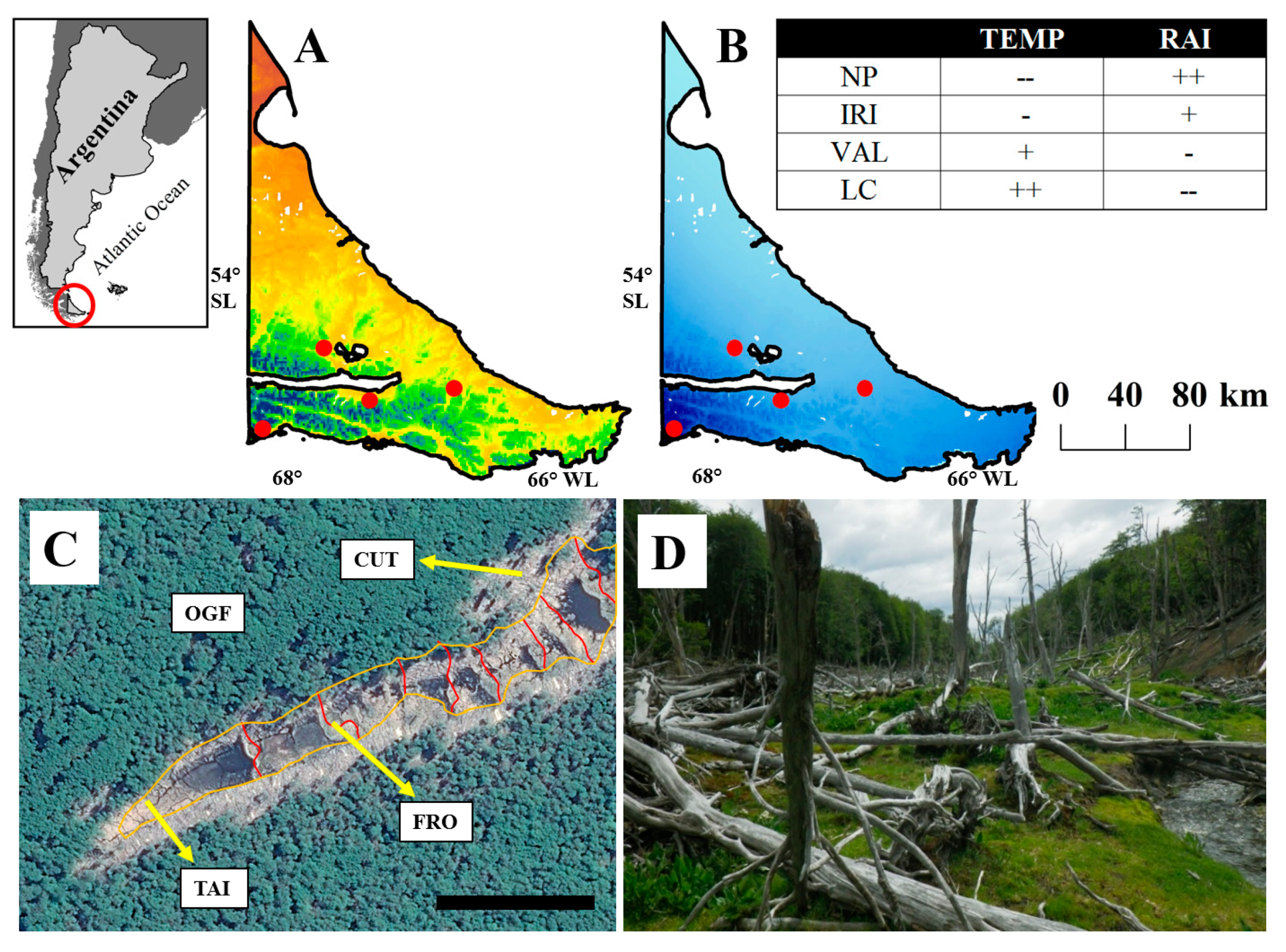
Location of the study area at the southern portion of Argentina (red circle) and locations (red dots) where NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros, showing (A) mean annual temperature (0.1 to 6.9 °C) (red is high and blue is low), (B) mean annual rainfall (253 to 721 mm yr−1) (dark colour means higher values), (C) forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest), where red lines identify old and new dams, orange line identifies boundary within harvested and flooded areas, and black line representing 100 m, and (D) general view of one abandoned meadow where the plantations were conducted.
Figure 1.
Location of the study area at the southern portion of Argentina (red circle) and locations (red dots) where NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros, showing (A) mean annual temperature (0.1 to 6.9 °C) (red is high and blue is low), (B) mean annual rainfall (253 to 721 mm yr−1) (dark colour means higher values), (C) forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest), where red lines identify old and new dams, orange line identifies boundary within harvested and flooded areas, and black line representing 100 m, and (D) general view of one abandoned meadow where the plantations were conducted.
Figure 2.
Examples of hemispherical photos to illustrate the overstory cover in the forest treatments: Left = adjacent unimpacted old-growth forest (OGF), Middle = area harvested, but not flooded, by beavers (CUT), and Right = beaver meadow (FRO = area just upstream of the old dam, and TAI = area where stream enters beaver meadow).
Figure 2.
Examples of hemispherical photos to illustrate the overstory cover in the forest treatments: Left = adjacent unimpacted old-growth forest (OGF), Middle = area harvested, but not flooded, by beavers (CUT), and Right = beaver meadow (FRO = area just upstream of the old dam, and TAI = area where stream enters beaver meadow).
Figure 3.
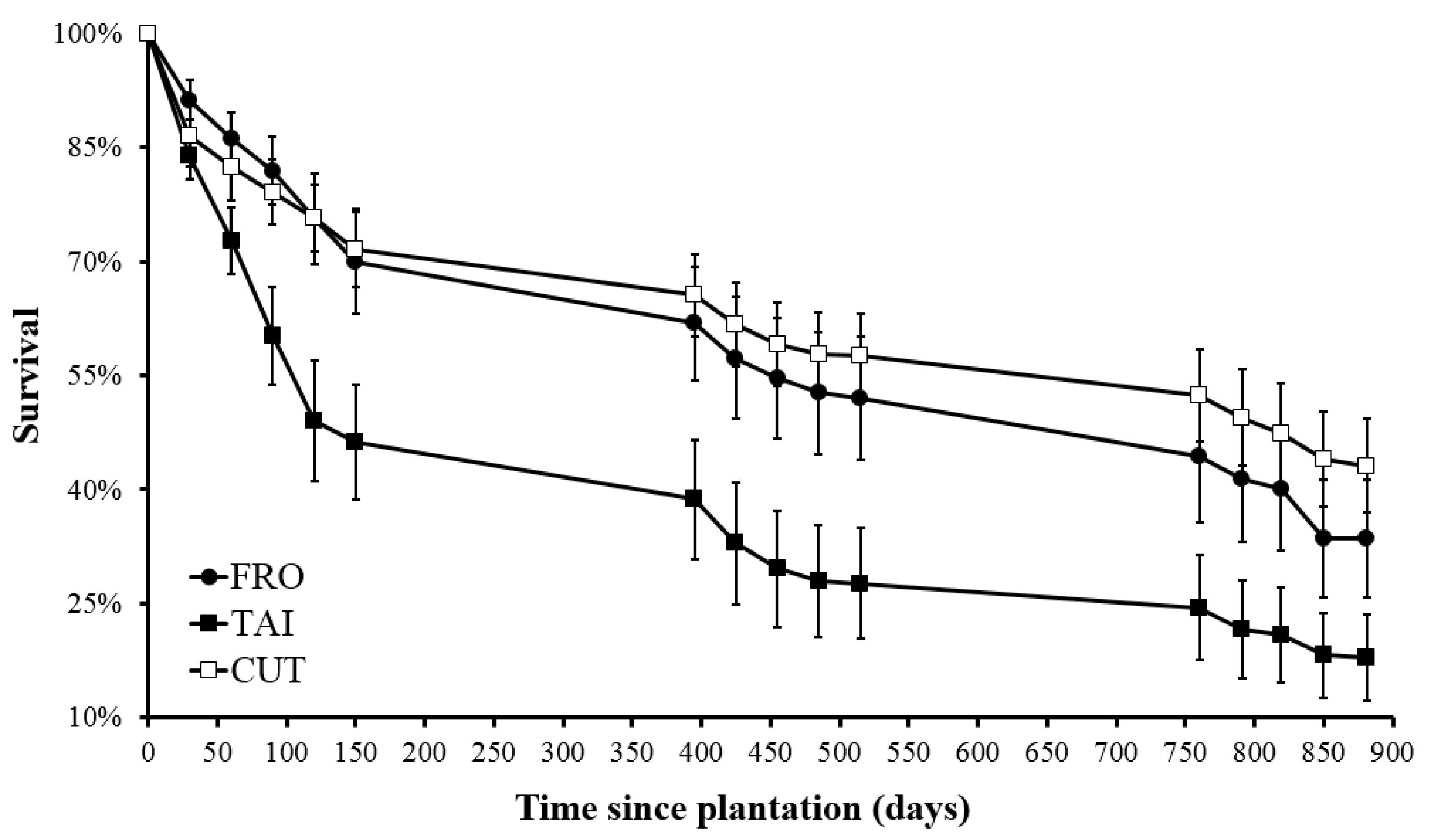
Survival of seedlings plantation of Nothofagus pumilio in Río Irigoyen among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers) and time since plantation.
Figure 3.
Survival of seedlings plantation of Nothofagus pumilio in Río Irigoyen among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers) and time since plantation.
Figure 4.
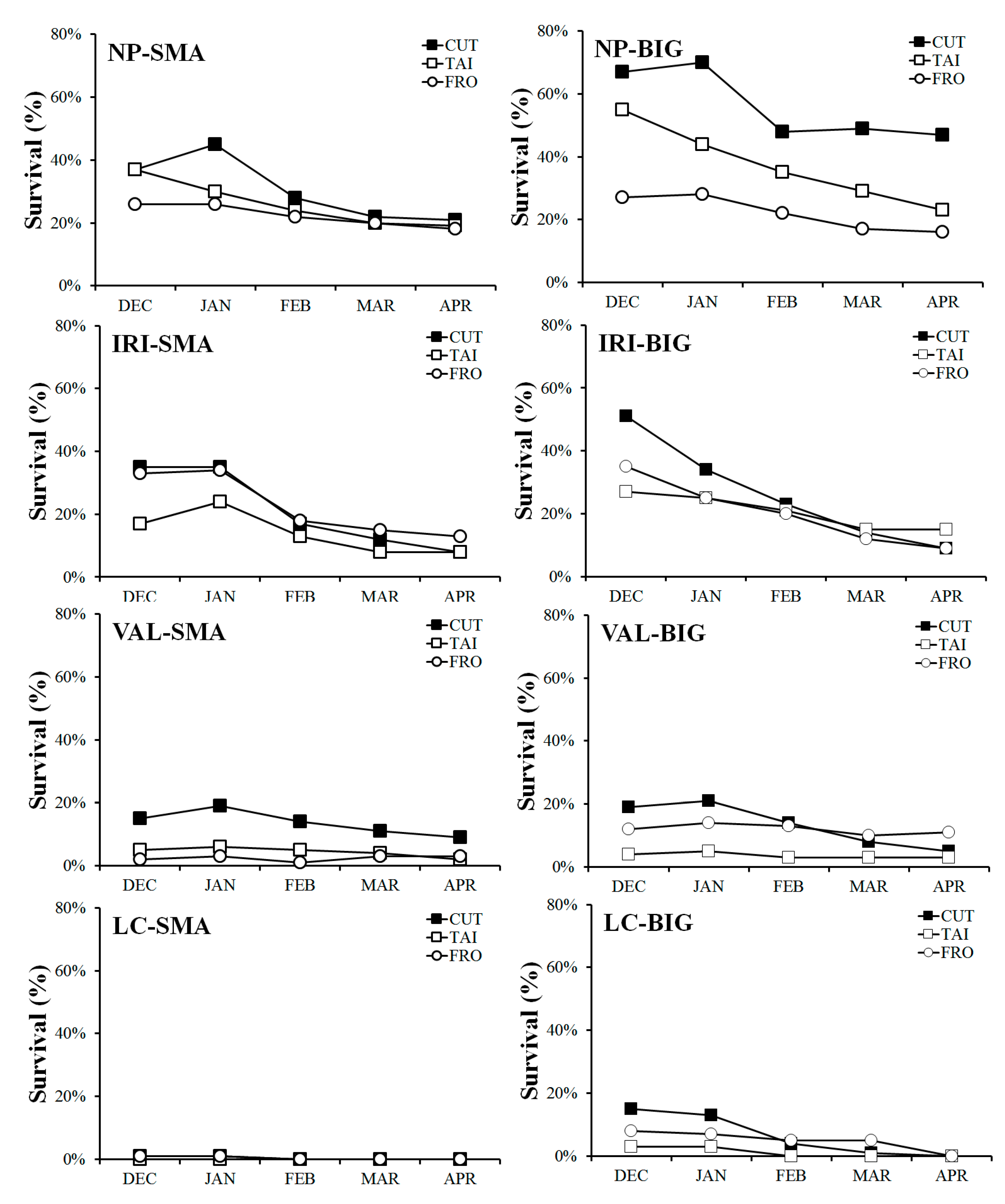
Survival of saplings plantation of Nothofagus antarctica among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers), locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros), plant type (BIG = big plants, SMA = small plants), and time (month) during the first growing season.
Figure 4.
Survival of saplings plantation of Nothofagus antarctica among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers), locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros), plant type (BIG = big plants, SMA = small plants), and time (month) during the first growing season.
Table 1.
Multiple ANOVAs of forest structure, environmental characteristics and natural regeneration among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros) analysing: DH = dominant overstory height (m), BA = basal area (m2 ha−1), CC = crown overstory cover (%), RLAI = relative leaf area index, TR = incidence of total radiation (%), SM = soil moisture (%), SBD = soil bulk density (g cm−3), C = carbon (%), NRD = natural regeneration density (n m−2), and BRO = damage plants by browsing (%).
Table 1.
Multiple ANOVAs of forest structure, environmental characteristics and natural regeneration among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros) analysing: DH = dominant overstory height (m), BA = basal area (m2 ha−1), CC = crown overstory cover (%), RLAI = relative leaf area index, TR = incidence of total radiation (%), SM = soil moisture (%), SBD = soil bulk density (g cm−3), C = carbon (%), NRD = natural regeneration density (n m−2), and BRO = damage plants by browsing (%).
| Treatment | Level | DH | BA | CC | RLAI | TR | SM | SBD | C | NRD | BRO |
|---|
| A: Forest | FRO | 19.30 | 0.81a | 36.38a | 0.13a | 82.0c | 72.4c | 0.27a | 23.7bc | 1.25a | 0.00a |
| TAI | 17.93 | 2.87a | 34.41a | 0.12a | 83.6c | 73.5c | 0.23a | 25.2c | 0.62a | 0.00a |
| CUT | 19.16 | 13.56b | 48.56b | 0.54b | 65.5b | 34.7a | 0.57c | 9.9a | 94.37b | 16.25b |
| OGF | 17.70 | 58.25c | 81.84c | 1.99c | 22.8a | 43.6b | 0.44b | 17.3b | 55.00b | 3.12a |
| | F | 2.61 | 318.38 | 380.68 | 166.96 | 420.84 | 86.06 | 24.42 | 16.47 | 14.96 | 21.18 |
| | (p) | (0.061) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) |
| B: Location | NP | 12.39a | 13.25a | 49.70b | 0.50a | 68.0b | 71.7c | 0.24a | 22.4bc | 6.87a | 0.00a |
| VAL | 17.84b | 20.75bc | 44.58a | 0.66a | 65.5b | 57.1b | 0.33a | 23.8c | 20.62a | 3.75a |
| IRI | 21.14c | 24.56c | 63.83c | 0.96b | 52.1a | 49.8ab | 0.45b | 16.5ab | 96.87b | 10.62b |
| LC | 22.71c | 16.93ab | 43.09a | 0.66a | 68.5b | 45.7a | 0.49b | 13.4a | 26.87a | 5.00ab |
| | F | 80.51 | 10.50 | 70.71 | 7.84 | 31.39 | 28.63 | 16.33 | 8.15 | 11.7 | 6.84 |
| | (p) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) |
| AxB | F | 1.45 | 2.51 | 5.08 | 2.09 | 2.46 | 4.77 | 2.83 | 3.65 | 6.09 | 3.47 |
| | (p) | (0.194) | (0.019) | (<0.001) | (0.049) | (0.022) | (<0.001) | (0.009) | (0.015) | (<0.001) | (0.002) |
Table 2.
Multiple ANOVAs of soil properties, floor cover and mean distance to forest edge in Río Irigoyen among treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and meadows (1 to 3), analysing: N = nitrogen (%), C/N = ratio carbon/nitrogen, P = phosphorous (ppm), CEC = cation exchange capacity (meq 100 gr), MONO = monocot plants (%), DICO = dicot plants (%), NV = non-vascular plants (%), TREE = overstory trees (%), BS = bare soil (%), and DIST = mean distance to the forest edge (m).
Table 2.
Multiple ANOVAs of soil properties, floor cover and mean distance to forest edge in Río Irigoyen among treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and meadows (1 to 3), analysing: N = nitrogen (%), C/N = ratio carbon/nitrogen, P = phosphorous (ppm), CEC = cation exchange capacity (meq 100 gr), MONO = monocot plants (%), DICO = dicot plants (%), NV = non-vascular plants (%), TREE = overstory trees (%), BS = bare soil (%), and DIST = mean distance to the forest edge (m).
| | Level | N | C/N | P | CEC | MONO | DICO | NV | TREE | BS | DIST |
|---|
| A: Forest | FRO | 1.46b | 12.05 | 10.31a | 87.52c | 31.9b | 42.9 | 21.7b | 0.0a | 3.5a | 19.4b |
| TAI | 1.28ab | 11.88 | 10.56a | 68.83ab | 39.8b | 55.4 | 3.5a | 1.2ab | 0.0a | 20.6b |
| CUT | 1.44ab | 11.90 | 15.75ab | 71.46bc | 2.0a | 54.1 | 4.6a | 7.2b | 30.1b | 4.7a |
| OGF | 1.09a | 12.23 | 25.04b | 52.62a | -- | -- | -- | -- | -- | -- |
| | F | 3.18 | 0.50 | 3.85 | 9.63 | 43.50 | 1.39 | 11.42 | 4.06 | 11.83 | 98.56 |
| | (p) | (0.035) | (0.682) | (0.017) | (<0.001) | (<0.001) | (0.266) | (<0.001) | (0.029) | (<0.001) | (<0.001) |
| B: Meadow | 1 | 1.26b | 11.80 | 14.28 | 73.82b | 19.9 | 40.0 | 17.2b | 3.4 | 19.41 | 16.15 |
| 2 | 0.93a | 11.88 | 13.84 | 43.70a | 24.7 | 60.3 | 0.4a | 1.7 | 11.25 | 15.44 |
| 3 | 1.76c | 12.36 | 18.13 | 92.80c | 29.1 | 52.1 | 12.1b | 3.3 | 2.92 | 13.12 |
| | F | 25.02 | 2.36 | 0.60 | 38.51 | 2.34 | 3.09 | 8.18 | 0.27 | 2.97 | 3.15 |
| | (p) | (<0.001) | (0.108) | (0.552) | (<0.001) | (0.115) | (0.062) | (0.002) | (0.765) | (0.068) | (0.059) |
| AxB | F | 5.18 | 0.46 | 3.10 | 7.97 | 2.32 | 5.13 | 4.39 | 0.43 | 1.93 | 11.61 |
| | (p) | (0.001) | (0.832) | (0.015) | (<0.001) | (0.083) | (0.003) | (0.007) | (0.786) | (0.134) | (<0.001) |
Table 3.
Multiple ANOVAs of natural seed production and litter among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros) analysing: S = total seeds (thousand ha−1 yr−1), SI = seeds foraged by insects (thousand ha−1 yr−1), SB = seeds foraged by birds (thousand ha−1 yr−1), SW = seed weight (kg ha−1 yr−1), LW = leaves weight of the litter (kg ha−1 yr−1), and BW = small branches (<1 cm) of the litter (kg ha−1 yr−1).
Table 3.
Multiple ANOVAs of natural seed production and litter among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros) analysing: S = total seeds (thousand ha−1 yr−1), SI = seeds foraged by insects (thousand ha−1 yr−1), SB = seeds foraged by birds (thousand ha−1 yr−1), SW = seed weight (kg ha−1 yr−1), LW = leaves weight of the litter (kg ha−1 yr−1), and BW = small branches (<1 cm) of the litter (kg ha−1 yr−1).
| Treatment | Level | S | SI | SB | SW | LW | BW |
|---|
| A: Forest | FRO | 31.3a | 0.0 | 0.0 | 0.15a | 84.7a | 19.0ab |
| TAI | 18.8a | 0.0 | 0.0 | 0.04a | 92.9a | 4.6a |
| CUT | 1241.3b | 34.7 | 29.9 | 14.21a | 862.0b | 39.8bc |
| OGF | 3181.4c | 21.7 | 32.8 | 33.57b | 3222.0c | 306.5c |
| | F | 26.37 | 1.26 | 2.25 | 14.92 | 114.39 | 11.15 |
| | (p) | (<0.001) | (0.293) | (0.086) | (<0.001) | (<0.001) | (<0.001) |
| B: Location | NP | 824.6a | 0.0 | 0.0a | 1.47a | 1056.5ab | 102.7ab |
| VAL | 1077.4a | 0.0 | 112.8b | 13.22a | 689.2a | 117.8ab |
| IRI | 2201.9b | 34.7 | 30.4ab | 28.19b | 1492.4b | 220.4b |
| LC | 368.9a | 21.7 | 0.0a | 5.08a | 1023.7a | 55.7a |
| | F | 8.53 | 1.30 | 3.39 | 8.94 | 7.43 | 3.14 |
| | (p) | (<0.001) | (0.277) | (0.021) | (<0.001) | (<0.001) | (0.028) |
| AxB | F | 3.01 | 1.28 | 2.17 | 3.32 | 5.86 | 1.81 |
| | (p) | (0.003) | (0.257) | (0.029) | (0.001) | (<0.001) | (0.073) |
Table 4.
Multiple ANOVAs of seedlings plantation of Nothofagus pumilio in Río Irigoyen among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest), meadows (1 to 3) and time measured by days (i) or years (ii), analysing: SUR = survival (%), H = seedling height (cm), and LEA = number of leaves.
Table 4.
Multiple ANOVAs of seedlings plantation of Nothofagus pumilio in Río Irigoyen among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest), meadows (1 to 3) and time measured by days (i) or years (ii), analysing: SUR = survival (%), H = seedling height (cm), and LEA = number of leaves.
| (i) | Level | SUR | (ii) | Level | H | LEA |
|---|
| A: Forest | FRO | 61.0b | A: Forest | FRO | 12.1a | 6.2 |
| | TAI | 41.9a | | TAI | 11.0a | 6.7 |
| | CUT | 64.5b | | CUT | 22.4b | 6.9 |
| | F | 84.57 | | F | 19.06 | 0.50 |
| | (p) | (<0.001) | | (p) | (<0.001) | (0.611) |
| B: Meadow | 1 | 66.7c | B: Meadow | 1 | 16.6 | 7.9 |
| | 2 | 40.7a | | 2 | 14.2 | 5.4 |
| | 3 | 60.1b | | 3 | 14.7 | 6.5 |
| | F | 104.62 | | F | 0.76 | 5.47 |
| | (p) | (<0.001) | | (p) | (0.469) | (0.005) |
| C: Time | 0 | 100.0i | C: Time | 2012 | 5.6a | 3.0a |
| | 30 | 87.2hi | | 2013 | 7.9a | 3.4a |
| | 60 | 80.4gh | | 2014 | 20.0b | 5.6b |
| | 90 | 73.7fgh | | 2015 | 27.1c | 14.4c |
| | 120 | 66.8efg | | F | 31.29 | 61.81 |
| | 150 | 62.6def | | (p) | (<0.001) | (<0.001) |
| | 395 | 55.4cde | AxBxC | F | 0.76 | 0.64 |
| | 425 | 50.6bcd | | (p) | (0.603) | (0.697) |
| | 455 | 47.8bcd | |
| | 485 | 46.1abc |
| | 515 | 45.7abc |
| | 760 | 40.4ab |
| | 791 | 37.5ab |
| | 819 | 36.2ab |
| | 850 | 31.9a |
| | 881 | 31.5a |
| | F | 45.99 |
| | (p) | (<0.001) |
| AxBxC | F | 0.29 |
| | (p) | (0.999) |
Table 5.
Multiple ANOVAs of seedlings plantation of Nothofagus pumilio in Río Irigoyen after one-year among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and meadows (1 to 3), analysing: H = seedling height (cm), R = root length (cm), FA = foliar area (cm2), LW = leaves weight (gr), SW = steam weight (gr), RW = roots weight (gr), and S/R = steam/root weight ratio.
Table 5.
Multiple ANOVAs of seedlings plantation of Nothofagus pumilio in Río Irigoyen after one-year among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers, OGF = adjacent unimpacted old-growth forest) and meadows (1 to 3), analysing: H = seedling height (cm), R = root length (cm), FA = foliar area (cm2), LW = leaves weight (gr), SW = steam weight (gr), RW = roots weight (gr), and S/R = steam/root weight ratio.
| | Level | H | R | FA | LW | SW | RW | S/R |
|---|
| A: Forest | FRO | 4.7 | 6.2 | 2.3 | 0.04 | 0.04 | 0.06 | 78.1 |
| | TAI | 4.3 | 5.1 | 2.4 | 0.04 | 0.04 | 0.05 | 82.5 |
| | CUT | 4.9 | 5.4 | 2.3 | 0.03 | 0.05 | 0.06 | 97.1 |
| | F | 1.06 | 1.17 | 0.03 | 0.53 | 0.97 | 0.60 | 0.68 |
| | (p) | (0.359) | (0.326) | (0.968) | (0.597) | (0.390) | (0.556) | (0.517) |
| B: Meadow | 1 | 4.5 | 5.4 | 2.2 | 0.04 | 0.04 | 0.05 | 77.9 |
| | 2 | 5.0 | 5.6 | 2.4 | 0.04 | 0.04 | 0.06 | 86.1 |
| | 3 | 4.4 | 5.7 | 2.5 | 0.04 | 0.05 | 0.06 | 93.9 |
| | F | 1.69 | 0.09 | 0.47 | 0.08 | 0.65 | 0.11 | 0.43 |
| | (p) | (0.204) | (0.916) | (0.633) | (0.927) | (0.528) | (0.898) | (0.652) |
| AxB | F | 2.12 | 0.81 | 3.88 | 5.28 | 2.14 | 1.08 | 0.55 |
| | (p) | (0.105) | (0.528) | (0.013) | (0.003) | (0.103) | (0.385) | (0.703) |
Table 6.
Multiple ANOVAs of saplings plantation performance of Nothofagus antarctica after one-year among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers), locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros), plant type (BIG = big plants, SMA = small plants), an time (month), analysing: SUR = survival (%), H = sapling height (cm), and GRO = growth of the extended dominant branch across the studied growing season (cm month−1).
Table 6.
Multiple ANOVAs of saplings plantation performance of Nothofagus antarctica after one-year among forest treatments (FRO = area just upstream of the old dam, TAI = area where stream enters beaver meadow, CUT = area harvested, but not flooded, by beavers), locations (NP = Tierra del Fuego National Park, VAL = Río Valdez, IRI = Río Irigoyen, LC = Los Cerros), plant type (BIG = big plants, SMA = small plants), an time (month), analysing: SUR = survival (%), H = sapling height (cm), and GRO = growth of the extended dominant branch across the studied growing season (cm month−1).
| | Level | SUR | H | GRO |
|---|
| A: Forest | FRO | 13.3a | 17.5 | 0.70b |
| | TAI | 13.1a | 18.0 | 0.61ab |
| | CUT | 21.1b | 16.3 | 0.38a |
| | F | 32.40 | 1.94 | 4.36 |
| | (p) | (<0.001) | (0.144) | (0.014) |
| B: Location | NP | 32.4d | 17.8ab | 0.53a |
| | VAL | 8.2b | 19.2b | 0.89b |
| | IRI | 20.3c | 17.5ab | 0.70ab |
| | LC | 2.3a | 14.5a | 0.13a |
| | F | 209.89 | 2.98 | 5.35 |
| | (p) | (<0.001) | (0.032) | (0.001) |
| C: Type | BIG | 18.4b | 20.7b | 0.56 |
| | SMA | 13.2a | 13.9a | 0.57 |
| | F | 32.49 | 58.94 | 0.01 |
| | (p) | (<0.001) | (<0.001) | (0.921) |
| D: Time | DEC | 22.6c | 18.8b | 0.12a |
| | JAN | 20.2c | 18.3ab | 0.14ab |
| | FEB | 14.6b | 17.6ab | 0.55bc |
| | MAR | 11.6ab | 16.4ab | 0.72c |
| | APR | 10.0a | 15.1a | 1.28d |
| | F | 27.88 | 2.65 | 17.60 |
| | (p) | (<0.001) | (0.033) | (<0.001) |
| AxBxCxD | F | 0.17 | 1.26 | 3.16 |
| | (p) | (0.999) | (0.240) | (0.001) |