Abstract
The increasing global population has led to an increase in food demand; consequently, aquaculture is one of the food production sectors that has offered opportunities to alleviate hunger, malnutrition, and poverty. However, the development of a sustainable aquaculture industry has been hindered by the limited availability of natural resources as well as its negative impact on the surrounding environment. Hence, there is an urgent need to search for better aquacultural production systems that, despite their high productivity and profitability, utilize fewer resources such as water, energy, land, and capital in conjunction with a negligible impact on the environment. Biofloc technology (BFT) is one of the most exciting and promising sustainable aquaculture systems; it takes into account the intensive culture of aquatic species, zero water exchange, and improved water quality as a result of beneficial microbial biomass activity, which, at the same time, can be utilized as a nutritious aquaculture feed, thus lowering the costs of production. Furthermore, BFT permits the installation of integrated multi-trophic aquaculture (IMTA) systems in which the wastes of one organism are utilized as feed by another organism, without a detrimental effect on co-cultured species. This review, therefore, highlights the basics of BFT, factors associated with BFT for the successful production of aquatic species, the significance of this food production system for the sustainable production of economically important aquatic species, its economic aspects, drawbacks, limitations, and recommended management aspects for sustainable aquaculture.
1. Introduction
According to the Food and Agriculture Organization, aquaculture is one of the food production sectors that offers a golden opportunity to alleviate hunger, malnutrition, and poverty through income generation and better use of natural resources [1]. With the increasing human population, projected to reach 9.6 billion by 2050 [2], the demand for food is escalating amidst limited natural resources such as water and land required for the continuous production of food. Nevertheless, aquaculture production is projected to rise from 40 million tons by 2008 to 82 million tons in 2050 [3]. This is probably due to the gradual adoption of semi-intensive and intensive aquacultural practices for the production of economically important aquatic species. However, intensive aquacultural practices are of great environmental concern due to the discharge of nutrient-rich wastewater into the environment. With all these constraints in mind, the development of sustainable aquaculture systems should focus more on system designs that permit not only the efficient use of fewer resources such as water, energy, land, and capital but also minimizing environmental pollution and maximizing production and profitability. This would, in the long run, lead to the fulfillment of the Sustainable Development Goals (SDGs), notably SDG 1 (end poverty), SDG 2 (zero hunger, achieve food security, improve nutrition, and promote sustainable agriculture), SDG 8 (promoting inclusive and sustainable economic growth, decent work for all), and SDG 14 (conservation and sustainable use of water bodies for sustainable development) [1].
Biofloc technology (BFT) is one of the most exciting food production alternatives that has attracted the attention of the scientific community and producers for sustainable aquaculture due to (i) zero water exchange, thus permitting efficient use of limited water resources and preventing the discharge of nutrient-rich wastewater into the environment; (ii) reduced artificial feed input (fishmeal), which reduces the costs of production while permitting the inclusion of alternatively cheaper and highly nutritious protein sources, and (iii) natural establishment of microbial biomass that not only purifies water but also enhances the growth, growth performance, and immunity of aquatic species reared in the system. The use of this system in farming practices for the production of crustaceans and some finfish species has been extensively studied [4,5,6,7,8,9,10,11,12]. The aim of this review, therefore, is to (i) give a brief overview of BFT systems, including operational parameters that affect their efficiency; (ii) review studies that have been conducted on the application of BFT systems for the sustainable production of economically important aquatic species; (iii) highlight the economic aspects of BFT systems, as well as their drawbacks and limitations, and recommend management aspects of BFT systems for sustainable aquaculture.
1.1. Biofloc Technology
According to the National Agricultural Library Glossary (United States Department of Agriculture, North Bend, WA, USA), BFT is defined as ‘the use of aggregates of bacteria, algae or protozoa, held together in a matrix along with particulate organic matter to improve water quality, waste treatment and disease prevention in intensive aquaculture systems’ [2]. In other words, BFT relies on the principle of nitrogenous waste recycling by several microbial species (bioflocs) in the system while improving water quality and the growth performance of the reared aquatic species. Heterotrophic bacteria within the system take up ammonium as a nitrogen (N) source for their biomass, thus resulting in a decrease in ammonium/ammonia in the water to non-toxic levels. This process is, however, faster than the nitrification process carried out by autotrophic nitrifying bacteria due to the faster growth rate of heterotrophic bacteria. Figure 1 shows a general overview of the BFT system. Certain factors that affect floc formation (mixture of microorganisms) and water quality in BFT systems are discussed below.
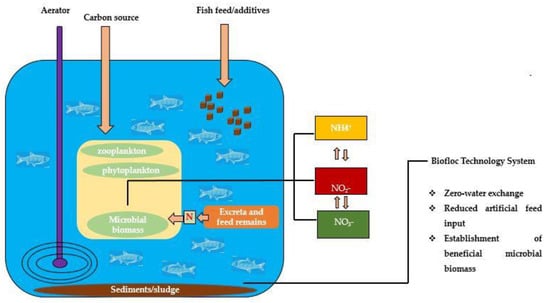
Figure 1.
Schematic diagram of a biofloc technology system.
1.1.1. Carbon–Nitrogen Ratio
In the aquatic environment, the carbon–nitrogen ratio (C/N) plays a vital role in the immobilization of toxic inorganic N compounds into useful microbial biomass that might act as a direct source of food for the reared aquatic species. Immobilization of inorganic N occurs at a C/N ratio of organic matter above 10 and, hence, any alteration in this ratio within the BFT system might result in a shift in microbial diversity, which might further affect the water quality. For example, De Schryver et al. [13] observed that a high C/N ratio favors the proliferation of heterotrophic bacteria, which leads to significant changes in water quality and biofloc composition. As such, manipulation of the C/N ratio can be achieved through modification of the carbohydrate content in the feed or the addition of an external carbon source in the rearing water so that microbes can assimilate waste ammonium for microbial biomass production. This will, in turn, decrease the concentrations of ammonium/ammonia to less toxic levels, thus making water exchange unnecessary [14]. Total suspended solids (TSS) is another important water quality parameter whose concentration in aquatic ecosystems depends on the C/N ratio. Xu et al. [15] observed that a high C/N ratio (15:1 and 18:1) rapidly increased the TSS concentrations in water, which negatively affected the growth performance of L. vannamei. Moreover, the authors anticipated that production costs would be reduced under the C/N ratio of 12:1 compared to 15:1 and 18:1 due to reduced utilization of organic carbon, saving approximately 20,000 L of molasses per hectare of shrimp production at the same stocking density. Pérez-Fuentes et al. [16] also found that, under high-density cultivation of O. niloticus in a BFT system, C/N ratios exceeding 15:1 promoted the production of dissolved salts and settled biomass, which affected the growth performance of fish. The authors recommended a C/N ratio of 10:1 as the optimum condition for the production of O. niloticus reared under similar conditions. In another study, Silva et al. [17] also observed poor water quality (high TSS, turbidity, alkalinity, and settleable solids) at a C/N ratio of 20:1, which affected the growth performance of O. niloticus. Similar results have been reported in Clarias gariepinus [18,19]. However, Yu et al. [20], Haghparast et al. [21], and Wang et al. [22] reported better growth performance and immune stimulation in carp at high C/N ratios (20:1 and/or 25:1) reared in BFT. The discrepancy in results could be attributed to the difference in species and the source of organic carbon.
1.1.2. Source of Organic Carbon
Different carbon sources, such as molasses, glucose, cassava starch, cornmeal, wheat flour, sorghum meal, sugar bagasse, sugar, rice bran, ground bread crumb, glycerol, and anhydrous glucose, are used to enhance nutrient dynamics through an altered C/N ratio as well as improving the production of crustaceans and certain finfish species [20,23,24,25,26,27,28,29]. The efficient establishment of flocs by different carbon sources mainly depends on their carbon content and speed of degradation, hence indicating that certain carbon sources are more efficient in promoting floc formation than others. Generally, simple sugars such as molasses are degraded faster than complex sugars such as cassava starch, leading to improved water quality, as indicated by lower concentrations of ammonia and a higher growth rate of beneficial microbial biomass [2]. Molasses are the most widely used carbon sources in BFT systems during larval, nursery, and grow-out phases due to their efficiency in improving water quality for the sustainable production of aquatic species [2,30].
One of the most elegant flexibilities of BFT systems is the capability of reusing water-containing flocs for the production of certain detritivorous species under intensive cultivation. This practice aims to prevent the discharge of nutrient-rich wastewater into the environment, which might result in pollution. Liu et al. [31] conducted a 56-day experiment to elucidate the effect of no carbohydrate addition applied to control water quality in water-reusing BFT systems for tilapia (GIFT Oreochromis niloticus, 99.62 ± 7.34 g). Results indicated no significant difference in growth performance between fish culture in tanks with or without carbohydrate (glucose) addition, hence indicating the feasibility of no carbohydrate addition in water-reusing BFT systems for tilapia. Similar results have been obtained in L. vannamei juveniles (3.5 g) reared in a BFT system for 30 days [32].
2. Bioflocs as a Nutritious Food Source, for Dietary Protein Reduction, Compensatory Growth, and Productivity of Economically Important Aquatic Species
Bioflocs as a Nutritious Feed Source
One of the major challenges facing aquaculture producers is the high cost of aquaculture feeds. Protein levels and adequate amino acid balance are critical in aquaculture feeds due to their essential role in maintaining the growth and the general wellbeing of aquatic organisms. However, these nutrients are an expensive component of the feeds and hence influence their market price [33]. In tilapia, for example, feeding can account for 50% of the operational costs and could even reach higher levels with high-protein diets and/or inadequate protein [33,34]. However, this could be mitigated by feeding tilapia on alternative feed sources such as phytoplankton, zooplankton, and algae, whose nutritive content would enhance the growth, survival, and production of fish [35]. Avnimelech and Kochba [36] found that tilapia can uptake 240 mg N kg−1 of biofloc, which is equivalent to 25% of the protein in fish diets. Moreover, bioflocs can contain 20%–<40% crude protein, <1%–>8% lipids, <1%–>15% fiber, <18–>35% total carbohydrates, and <15%–>60% ash, thus providing an alternative feed source to the reared aquatic species [2].
It is worth noting that the nutritional value of bioflocs is highly dependent on the microbial community that encompasses it and, as mentioned in the previous section, certain factors such as carbon sources and C/N ratio influence the biochemical composition of bioflocs. For example, Moreno-Arias et al. [37] reported that the fatty acid and amino acid composition of both biofloc and shrimp cultivated in BFT systems depends on the composition of the aquaculture feed used. The use of plant-based protein sources in the feed is more favorable for biofloc systems and is considered to be more eco-friendly and sustainable. This is because their use reduces the release of phosphorous and nitrogenous wastes in the aquatic ecosystem as well as the dependency on overexploited marine sources [14,38]. The effect of biofloc feed on the general wellbeing and sustainable production of aquatic species is discussed below.
Emerenciano et al. [39] investigated the influence of BFT as a food source in a limited water exchange nursery system on the growth performance of pink shrimp (Farfantepenaeus brasiliensis) post-larvae. The authors reported that rearing post-larvae in the BFT system without commercial food supply did not affect the growth performance of the animals. Moreover, no significant differences in final biomass and weight gain were noted between shrimp reared in BFT with or without commercial diet supplementation. The good growth performance of the larvae was attributed to the diverse microbial community that consisted of protozoa grazers, rotifers, cyanobacteria, and diatoms, which were utilized as a food source. In another study, Emerenciano et al. [40] found no significant differences in the final biomass and survival of early post-larvae pink shrimp (Farfantepenaeus paulensis) reared in BFT with or without commercial feed supplementation. Emerenciano et al. [11] also observed no significant difference in spawning performance among females reared in BFT with or without feed supplementation. Zhang et al. [10] found that culturing gibel carp (C. auratus gibelio ♀ × C. carpio ♂, 6.4 ± 0.5 g) in BFT without feed addition for 30 days did not affect the growth performance (weight gain, specific growth, and survival) of fish. The fish were able to utilize the bioflocs as a feed, with increased digestive enzyme activity of pepsin and amylase noted in fish reared in water containing high TSS (300, 600, 800, and 1000 mg L−1 TSS). Furthermore, bioflocs enhanced the fish’s innate immunity, as indicated by increased superoxide dismutase (SOD) and total antioxidant capacity (TAOC) activity in the skin and mucus. Upregulated immune-related genes included intelectin (ITLN), dual-specificity phosphatase 1 (DUSP 1), keratin 8 (KRT 8), myeloid-specific-peroxidase (MPO), c-type lysozyme (c-lys), and interleukin-11 (IL-11).
The nutritive content and quality of bioflocs are rich and, as such, bioflocs have been used as a cheaper and sustainable alternative to the highly expensive fishmeal. For example, in shrimp culture, 15% to 30% of conventional protein sources can be replaced by biofloc meal without negatively affecting the general wellbeing of the species [2]. The incorporation of biofloc meal in aquaculture indeed reduces the costs of production whilst permitting an intensive culture of species, hence maximizing profits. Several studies have shown that replacing fishmeal with biofloc meal alone or in combination with certain dietary sources such as lysine, soy protein concentrate, and protein hydrolysate improves the growth performance, survival, digestive enzyme activity, and immunity of the reared aquatic species [41,42,43,44,45,46,47,48].
Currently, more research studies in the field of pro- and prebiotic bioflocs are ongoing. Probiotics are beneficial microbes that are either added or naturally developed in the BFT system to stimulate the immune system for the reared aquatic species against biotic and abiotic stress. Several beneficial microorganisms, such as those from the Bacillaceae family, have been previously identified and isolated from the shrimp culture BFT system [49]. These bacteria have been used in the biocontrol of disease outbreaks caused by pathogenic microbes as well as immunostimulants for enhancing the general wellbeing of aquatic species. Table 1 shows some of the conducted studies on probiotics in BFT systems included in animal diets or added directly into the rearing water for enhancement of the general wellbeing of the reared aquatic species.

Table 1.
Some of the conducted studies on probiotics in BFT systems included in animal diets or added directly into the rearing water for enhancement of the general wellbeing of the reared aquatic species.
3. Dietary Protein Reduction
Dietary protein (DP) is the most expensive ingredient in aquaculture feeds. It is used for tissue and body maintenance as well as sustaining the growth of aquatic organisms. Using high-protein diets above the recommended range not only pollutes the aquatic ecosystem via nitrogenous waste excretion but also increases the costs of production [60]. For example, in Clarias sp. production, farmers in the hatchery generally feed the fry on high-protein diets in the range of 38–40% to reach the maximum output. This is costly to the farmer and does not match the low selling prices of the fish fry [8]. Since bioflocs can serve as alternative nutritive sources for the reared aquatic species, Tacon et al. [61] suggested that bioflocs might also permit the use of lower feed rations and a reduction in the use of costly feed ingredients. Moreover, several studies have indicated that the protein content of bioflocs ranges between 12% and 50% and this depends on the type of organic carbon source used [39,62,63,64,65].
Braga et al. [66] evaluated the effect of feeds with different protein levels (BFT + FF: 68.48% dietary protein; BFT + BF: 52.51%; BFT + JF: 39.91%) on the spermatophore and sperm quality of Litopenaeus vannamei males (36.40 ± 3.13 g) cultured for 30 days under the BFT system during the pre-maturation period. Compared to shrimp cultured under a clear water system and fed on a mixture of fresh food, higher sperm quality (survival %, spermatophore weight, sperm count %, normal sperm rate %, and dead sperm rate %) was recorded in shrimp fed on BFT + JF compared to other protein levels and a mixture of fresh food. Generally, better sperm and spermatophore quality of shrimp cultured in BFT system were noted compared to those reared under a clear water system, hence indicating the superiority of a BFT-dominated zero exchange system in maintaining better reproductive performance. Xia et al. [60] investigated the influence of different DP levels (31%, 35%, 39%, 43%, and 47%) on the growth, digestibility, digestive enzyme activity, and stress tolerance of Litopenaeus vannamei (Boone, 1931) (6.2 ± 0.2 g) reared under a BFT system for 60 days. The authors observed increasing weight gain with increasing DP levels up to 43% DP, whereas the lowest feed conversion ratio and protein digestibility were recorded at 43% DP. Moreover, higher digestive enzyme activity and tolerance in a sudden decline in salinity were noted at 43% DP. Using spoilage date extract (SDE) as a carbon source for the BFT system, Abbaszadeh et al. [67] found that feeding Litopenaeus vannamei (5.4 ± 0.3 g) on diets containing different protein levels (P15 and P25%) for 35 days led to improved growth performance (protein efficiency ratio and protein productive value). Moreover, lower total ammonia nitrogen (TAN) was observed in the SDE BFT system, indicating better water quality. Under a super-intensive biofloc-dominated system for the culture of Litopenaeus vannamei, Prangnell et al. [65] have recently shown that feeding shrimp (4.70 ± 0.66 g) on commercial feeds of different protein content (35% and 40% DP) for 77 days enhanced the final weight and water quality parameters (35% DP), hence indicating that lower DP levels can support shrimp growth performance and maintain good water properties under intensive cultivation. Similar results have previously been reported by Xu et al. [68], Pinho et al. [69], Kumar et al. [70], and Brito et al. [71]. Xu and Pan [62] reported that manipulating C/N ratios in the zero exchange culture of Litopenaeus vannamei (6.95 ± 0.22 g) fed on different DP levels (P25 and P35) influences the growth performance and water quality of shrimp. The authors found no significant difference in the growth performance of shrimp fed on P25 and P35, except for the FCR, where shrimp fed on P35 had a lower FCR. This could be attributed to the low protein content of the bioflocs and difficulty in ingesting bioflocs under experimental conditions. C/N ratios or their interaction with DP levels did not affect the growth performance of animals.
In Nile tilapia (O. niloticus), several studies have indicated the maintenance of good water properties and growth performance of fish cultured in BFT systems and fed on lower levels of crude protein in the range of 20–31% [33,72,73,74,75,76].
In Juvenile common carp (Cyprinus carpio), Aalimahmoudi et al. [77] found that rearing fish in a BFT system for 8 weeks with a C/N ratio of 15% and 25% DP level improved growth and feeding parameters, body composition, blood biochemical parameters, and water quality suitable for common carp. Zhao et al. [78] also found that a 20% decrease in DP did not negatively affect the growth performance of mirror cap (Cyprinus carpio specularis) reared in a BFT polyculture system.
Khasanah et al. [8] demonstrated that 34% DP (C/N ratio 15) can replace 38% DP in catfish (Clarias sp. 4–5 cm) fry reared in a BFT system for 35 days. Sawant et al. [79] evaluated the effect of different DP levels (15%, 20%, 25%, 30%, 35%, and 40%) on the growth and survival of Labeo rohita (Hamilton, 1822) reared in a BFT system for 80 days and found 25% DP (C/N ratio 19:1) to be superior in terms of the improvement in growth performance (average weight, weight gain, length gain, specific growth rate, and survival) compared to other DP levels. Yu et al. [80] also found that decreasing the DP levels of Rhynchocypris lagowski cultured in the BFT system (8 weeks) from 37% to 29% slightly decreased the growth performance, digestive enzyme activity, and immune response in fish, but no noticeable difference was noted with those fed on a control diet (clear water condition, 37% DP).
4. Compensatory Growth and Productivity
Throughout their life cycle, aquatic organisms experience a period of fasting, probably due to certain factors such as a periodically inadequate food supply, poor water quality, and the presence of pathogens in their environment, which causes stress. In aquaculture, food restriction is considered stressful but this technique has been used to reduce operational costs as well as the concentration of organic matter, nitrogenous, and phosphorus wastes in effluent water [81,82]. Furthermore, when food is restored after a period of starvation, aquatic organisms exhibit a phase of accelerated growth, also known as compensatory growth, which is usually associated with increased weight gain [82]. It is worth noting that animals use tissue energy reserves such as lipids for the induction of compensatory growth and this depends on several factors such as the type of species, development stage, body size, food quality, and the duration of starvation [82,83]. Hence, compensatory growth is a form of an internal adjustment mechanism that assists animals in adjusting to environmental stress [82,84,85]. Studies on feed restriction in BFT systems and its effect on the growth performance and productivity of crustaceans and fish have been conducted, and, below, we present research findings obtained from some of these studies.
Kaya et al. [9] found high productivity indices in the establishment of speckled shrimp (Metapenaeus Monoceros, Fabricus) in a BFT system during 30 days of cultivation. The study was carried out with four different biofloc treatments and a control with a stocking density of 12 shrimp/0.24 m2, using feed restriction regimes that consisted of one day of starvation and varying days of feeding. Generally, all BFT treatments exhibited improved growth performance (final weight, weight gain, daily weight gain, specific growth rate, protein efficiency ratio, survival rate, and food conversion ratio), whole-body crude protein ratios, and ash contents. Likewise, histopathological examinations did not indicate any pathological findings. In another study, Lara et al. [86] investigated L. vannamei juveniles (1.14 ± 0.38 g) cultured in a BFT system with 21 days of artificial feed restriction and with 29 days of artificial feedback and found partial compensatory growth in the second period and enhanced survival (>95%), resulting in 24.79% savings on artificial feed application. Rocha et al. [87] found that 1-day repetitive feed restriction and 1-day feeding in L. vannamei (0.46 ± 0.18 g) juveniles led to partial compensatory growth as a result of enhanced feed conversion efficiency driven by increased enzyme activity.
Correa et al. [88] also found 70% survival in Nile tilapia (O. niloticus, 4.78 ± 0.13 g) juveniles reared in a BFT system subjected to four days of feeding and three days of feed deprivation. Moreover, the reduction in feed costs was 46.7% and the authors anticipated that this would result in a 3% increase in the farmer’s partial profit. Likewise, the authors observed that two days of feed deprivation and 4 days of refeeding resulted in a high feed consumption ratio, feed efficiency ratio, and protein efficiency ratio, hence indicating that feed deprivation in tilapia does not affect the growth performance of fish.
Gallardo-Collí et al. [5] found that cyclic feeding based on 12 days of feed restriction and 36 days of feeding triggered a complete compensation in weight and restoration of energy reserves, with similar measures of productive performance observed compared to the control. Moreover, feed restriction did not affect the proximal composition.
5. Biofloc-Based Integrated Multi-Trophic Aquaculture
Much as BFT systems facilitate the maintenance of good water quality suitable for the survival and general wellbeing of reared aquatic species without the use of high water volumes and exchange rate, the accumulation of total suspended solids (TSS) and organic matter in the rearing units might result in several ecological problems in the surrounding environment. Therefore, there is an urgent need for sustainable utilization of the accumulated substances, which might pose a danger to the aquatic ecosystems and surrounding environment.
BFT systems permit the installation of integrated multi-trophic aquaculture (IMTA) systems in which the wastes of one organism are utilized as feed by another organism [89,90,91,92,93,94]. In other words, filter-feeding organisms such as Oreochromis sp. and Mugil liza are utilized for the assimilation of suspended solids and organic matter that accumulate at the bottom of rearing units. However, the choice of these organisms will depend on certain factors, as reported by Borges et al. [89]. These include:
- The absence of competition for food between the co-cultured species;
- The filter feeders should be able to consume the suspended solids and organic matter without detrimental effects on their general wellbeing;
- The filter feeders should not negatively affect the growth performance and general wellbeing of a co-cultured species in the rearing unit.
Previous studies on the integration of shrimp and Nile tilapia (Oreochromis niloticus) have reported increased N and P recovery, yields, growth, growth performance, and immunity in shrimp or tilapia under different stocking densities [4,90,91,93].
Borges et al. [89] demonstrated that the integrated culture of mullet (Mugil liza) and white shrimp (Litopenaeus vannamei) in the same rearing unit or two separate units for 41 days resulted in a reduction in sludge, thus indicating the utilization of organic matter in the rearing units by mullet. Holanda et al. [94] also showed that integrating mullet and shrimp culture not only reduces the concentration of TSS in rearing water but also improves the water quality and growth performance of shrimp. However, Hoang et al. [95] found that gray mullet (Mugil cephalus) is not an efficient biofloc consumer under the shrimp polyculture BFT system. The discrepancy in results could be attributed to differences in mullet species and rearing conditions. Furthermore, de Oliveira Costa et al. [12] have recently shown that the integration of oyster (Crassostrea gasar) and shrimp (Litopenaeus vannamei) in BFT systems did not influence the concentrations of TSS in the rearing units. The authors hence suggested that larger stocking densities could result in more noticeable changes in TSS concentrations. Therefore, the choice of species and rearing conditions is vital for the maintenance of good water quality and to increase crop production.
Sometimes, photoautotrophs (halophytes) are used for the absorption of N and P, all of which lead to the maintenance of good water quality and the improved survival and growth performance of the reared species [92]. For example, Legarda et al. [96] found that an integrated BFT system (L. vannamei and Ulva fasciata) facilitated N and P recovery at 5.5% and 7.6%, respectively, compared to the control (without Ulva fasciata). Moreover, shrimp yields were 2.91 kg m−3 with a survival rate of 90.6% and FCR of 1.84. Likewise, Pinheiro et al. [97] observed that integrating L. vannamei and Sarcocornia ambigua at different water salinities (16 and 24 psu) favored the elimination of N and phosphate compounds and did not negatively affect the growth of shrimp or plant growth.
6. Economic Aspects of BFT Systems
In comparison to the conventional aquaculture systems, BFT systems generally improve the growth performance (growth, specific growth rate, survival rate, and FCR) of the reared aquatic species and water quality and, as such, these parameters play a vital role in determining the aquaculture management costs. Moreover, reducing production costs while optimizing profits is the major strategic goal driving the aquaculture industry [98]. Certain growth performance parameters such as survival rate influence cost returns and profitability. For example, a 20% increase in stocking density and growth rate increases profitability by 57% and 45%, respectively [99]. Likewise, reducing feeding costs by 20% can significantly impact profitability [98]. As mentioned earlier, bioflocs can replace commercial feeds without negatively affecting the survival, growth performance, and production of aquatic species. The efficiency of protein utilization is two times higher in BFT systems compared to conventional systems. Production of one kilogram of tilapia and green tiger shrimp (Penaeus semisulcatus) in BFT is associated with a 10% and 33% reduction in costs, respectively, and this is dependent on the species, aquaculture feed, the quantity of consumed biofloc, and the price of carbohydrates [98,100,101]. It is worth noting that in BFT, generally, carbon source costs are incurred, thus eliminating the costs of organic and inorganic fertilizer input. Likewise, BFT systems facilitate zero water exchange, permit the reduction of water treatment costs by 30%, reduce the cultivation period, and increase the survival and growth rate of aquatic species compared to the conventional systems; hence, they are sustainable systems for aquaculture production [7,13,98].
7. Drawbacks, Limitations, and Management Aspects of BFT Systems
Despite their use in commercial applications since the mid-1990s, BFT systems are still facing serious drawbacks and operational difficulties. For example, the dependency of outdoor systems on prevailing weather conditions often results in fluctuations in water quality due to changes in microalgae bloom. Therefore, for sustainable aquaculture production, certain factors such as site location, light intensity, and season of the year should be taken into consideration when setting up outdoor BFT systems. Furthermore, the concentration of total suspended solids (TSS) should be carefully monitored. The desirable concentration of TSS for aquaculture ranges from 500 to 1000 mg L−1, above which turbidity, visibility, and FCR are increased, thus leading to poor growth performance and poor productivity [98].
According to Avnimelech et al. [36], monitoring the following water quality parameters is vital:
- NO2. Nitrite is highly toxic to fish if present in levels above 1 mg L−1. This means the presence of anaerobic regions, which lead to the accumulation of sludge. This will therefore necessitate the changing of aerators to increase levels of dissolved oxygen required by aerobic microbes to convert nitrite to nitrates.
- TAN. Total ammonia nitrogen below 0.5 mg L−1 indicates that the system is working properly. An increase in TAN above this level warrants the addition of carbon into the system.
- DO. Dissolved oxygen should not fall below 5 mg L−1. Below this level, more aerators should be added to the system to provide more oxygen.
- Floc volume (FV) should be in the range of 5 to 50 mL L−1 and this can be monitored using Imhoff cones. When FV concentrations are above 50 mL L−1, sludge should be removed, and if below 5 mL L−1, carbohydrates should be added.
The slow establishment of nitrifying bacteria within the BFT system is also one of the drawbacks of this technology. It takes more than one month for the initial bioflocs to develop, which might affect aquatic life at sensitive stages of their life cycle. Luo et al. [102] have recently shown that a strategy of a one-time carbohydrate addition at a C/N ratio maintained at 20:1 has a good nitrification performance. However, the authors did not show how fast the nitrifying bacteria were established; thus, more research is needed. De Morais et al. [103] have also shown that increasing the aeration at a rate of 33.75 L/min in Litopenaeus vannamei (Boone, 1931) biofloc culture speeded up the establishment of nitrifying bacteria, whereas a low aeration rate slowed down the process. Jiménez-Ordaz et al. [104] have recently demonstrated that the addition of Schizochytrium sp., L. fermentum (TD19), and two diatoms (Grammatophora sp. and Navicula sp.) can induce the formation of bioflocs in a hyper-intensive culture of P. vannamei reared in a BFT system. Therefore, more research is needed to optimize the conditions necessary for the fast establishment of slow-growing nitrifying bacteria in BFT systems.
Another serious limitation of BFT is high energy costs. Aerators and pumps require energy for their normal operation and any incident of power failure can cause huge economic losses. Likewise, high energy costs (hydroelectricity) make BFT systems less feasible to small-scale farmers and those from developing countries. Hence, there is an urgent need to search for cheaper and environmentally cleaner sources of energy that would sustainably permit intensive cultivation and result in maximum profits. Another important concern of the BFT system is the development of off-flavors (geosmin and 2-methylisoborneol) that lower the quality and market cost of BFT-produced fish and shrimps. These off-flavors develop as a result of high turbidity, filamentous cyanobacteria, and Actinomycetes. Transferring fish and shrimps to clean running water before harvest would alleviate this problem but this is costly and unsustainable. However, Schrader et al. [105] suggested that lowering the feeding application rates could decrease the production of off-flavors, particularly 2-methylisoborneol (MIB) in channel catfish (Ictalurus punctatus). Another strategy is to introduce certain microorganisms, such as those from the Bacillaceae family, into the BFT system designs as bioreactors. These have been reported to play a vital role in the degradation of geosmin and MIB [106,107].
8. Conclusions
The increasing food demand amidst a growing population, water scarcity, and limited land for the expansion of aquacultural practices have become major constraints on a global scale. To satisfy the growing demand for animal protein, intensive aquaculture is one of the promising alternatives. However, this system of food production comes with its demerits, one of which is its pollution of the surrounding ecosystem due to the discharge of nutrient-rich effluents. Likewise, intensive aquacultural practices have led to increased disease outbreaks, poor growth performance, and increased dependency on fishmeal, which is costly and scarce. BFT is, however, a sustainable and more environmentally friendly food production system characterized by zero water exchange and intensive culture of aquatic species. Moreover, this technology permits the utilization of bioflocs as a nutritious feed source, hence lowering feeding and production costs as well as dependency on fishmeal. Bioflocs also stimulate the immune system of the animals, thus lowering the risk of pathogen-associated disease outbreaks in the system. Likewise, several studies have indicated that probiotic addition in BFT systems and/or their dietary administration improves the immunity of cultured fishes and crustaceans. Despite the increasing popularity of BFT systems, more research is needed to optimize the operational parameters (such as energy needs) with lower costs of production for the system in order to ensure that they are acceptable and feasible for small-scale farmers and those from developing countries. Energy alternatives such as solar power, gas, and wind turbines should be considered. Furthermore, for this system to create a more sustainable impact in aquaculture production, more studies on tolerance levels regarding the water quality of aquatic species reared in BFT under commercial settings are needed since reference levels used for the production of certain species are derived from clear water or water exchange systems. Moreover, most of these studies are conducted on a small scale or under laboratory settings, which are far from commercial conditions. Lastly, there is an urgent need to disseminate research findings on BFT systems to farmers as this will help them in acquiring the skills required for better management of the system and, in turn, generate more profit.
Author Contributions
Conceptualization, M.M., M.A.O.D., F.K. and H.S.; writing—original draft preparation, M.M., M.A.O.D., F.K. and H.S.; writing—review and editing, M.M., M.A.O.D., F.K. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hambrey, J. The 2030 Agenda and the Sustainable Development Goals: The challenge for aquaculture development and management. In FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2017; p. 1141. [Google Scholar]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; Fisheries Department. Total World Fisheries. In The State of World Fisheries and Aquaculture; Food & Agriculture Org.: Rome, Italy, 2010; Volume 3, p. 10. [Google Scholar]
- Martins, M.A.; Poli, M.A.; Legarda, E.C.; Pinheiro, I.C.; Carneiro, R.F.S.; Pereira, S.A.; Martins, M.L.; Gonçalves, P.; Schleder, D.D.; do Nascimento Vieira, F. Heterotrophic and mature biofloc systems in the integrated culture of Pacific white shrimp and Nile tilapia. Aquaculture 2020, 514, 734517. [Google Scholar] [CrossRef]
- Gallardo-Collí, A.; Pérez-Fuentes, M.; Pérez-Rostro, C.I.; Hernández-Vergara, M. Compensatory growth of Nile tilapia Oreochromis niloticus, L. subjected to cyclic periods of feed restriction and feeding in a biofloc system. Aquac. Res. 2020, 51, 1813–1823. [Google Scholar] [CrossRef]
- Fauzi, M.; Putra, I.; Rusliadi, R.; Tang, U.M.; Muchlisin, Z.A. Growth performance and feed utilization of African catfish Clarias gariepinus fed a commercial diet and reared in the biofloc system enhanced with probiotic. F1000Research 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Sontakke, R.; Haridas, H. Economic Viability of Biofloc Based System for the Nursery Rearing of Milkfish (Chanos chanos). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2960–2970. [Google Scholar] [CrossRef]
- Khasanah, N.R.; Utomo, N.B.P.; Setiawati, M.; Yuhana, M. The evaluation of different levels diets protein for growth performance of Clarias sp. fry cultured in biofloc-based system. J. Akuakultur Indones. 2017, 16, 136. [Google Scholar] [CrossRef] [Green Version]
- Kaya, D.; Genc, M.A.; Aktas, M.; Yavuzcan, H.; Ozmen, O.; Genc, E. Effect of biofloc technology on growth of speckled shrimp, Metapenaeus monoceros (Fabricus) in different feeding regimes. Aquac. Res. 2019, 50, 2760–2768. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Xu, D.H.; Qiao, G.; Zhang, J.; Qi, Z.; Li, Q. Effect of different water biofloc contents on the growth and immune response of gibel carp cultured in zero water exchange and no feed addition system. Aquac. Res. 2018, 49, 1647–1656. [Google Scholar] [CrossRef]
- Emerenciano, M.; Cuzon, G.; Arévalo, M.; Gaxiola, G. Biofloc technology in intensive broodstock farming of the pink shrimp Farfantepenaeus duorarum: Spawning performance, biochemical composition and fatty acid profile of eggs. Aquac. Res. 2014, 45, 1713–1726. [Google Scholar] [CrossRef]
- Costa, L.C.d.; Poersch, L.H.d.; Abreu, C. Biofloc removal by the oyster Crassostrea gasar as a candidate species to an Integrated Multi-Trophic Aquaculture (IMTA) system with the marine shrimp Litopenaeus vannamei. Aquaculture 2021, 540, 736731. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Bossier, P.; Ekasari, J. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol. 2017, 10, 1012–1016. [Google Scholar] [CrossRef]
- Xu, W.J.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Pérez-Fuentes, J.A.; Hernández-Vergara, M.; Pérez-Rostro, C.I.; Fogel, I. C:N ratios affect nitrogen removal and production of Nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 2016, 452, 247–251. [Google Scholar] [CrossRef]
- Silva, U.L.; Falcon, D.R.; Pessôa, M.N.D.C.; Correia, E.D.S. Carbon sources and C:N ratios on water quality for Nile tilapia farming in biofloc system. Rev. Caatinga 2017, 30, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Dauda, A.B.; Romano, N.; Ebrahimi, M.; Teh, J.C.; Ajadi, A.; Chong, C.M.; Karim, M.; Natrah, I.; Kamarudin, M.S. Influence of carbon/nitrogen ratios on biofloc production and biochemical composition and subsequent effects on the growth, physiological status and disease resistance of African catfish (Clarias gariepinus) cultured in glycerol-based biofloc systems. Aquaculture 2018, 483, 120–130. [Google Scholar] [CrossRef]
- Bakar, N.S.A.; Nasir, N.M.; Lananan, F.; Hamid, S.H.A.; Lam, S.S.; Jusoh, A. Optimization of C/N ratios for nutrient removal in aquaculture system culturing African catfish, (Clarias gariepinus) utilizing Bioflocs Technology. Int. Biodeterior. Biodegrad. 2015, 102, 100–106. [Google Scholar] [CrossRef]
- Yu, Z.; Li, L.; Zhu, R.; Li, M.; Duan, J.; Wang, J.Y.; Liu, Y.H.; Wu, L.F. Monitoring of growth, digestive enzyme activity, immune response and water quality parameters of Golden crucian carp (Carassius auratus) in zero-water exchange tanks of biofloc systems. Aquac. Rep. 2020, 16, 100283. [Google Scholar] [CrossRef]
- Haghparast, M.M.; Alishahi, M.; Ghorbanpour, M.; Shahriari, A. Evaluation of hemato-immunological parameters and stress indicators of common carp (Cyprinus carpio) in different C/N ratio of biofloc system. Aquac. Int. 2020, 28, 2191–2206. [Google Scholar] [CrossRef]
- Wang, G.; Yu, E.; Xie, J.; Yu, D.; Li, Z.; Luo, W.; Qiu, L.; Zheng, Z. Effect of C/N ratio on water quality in zero-water exchange tanks and the biofloc supplementation in feed on the growth performance of crucian carp, Carassius auratus. Aquaculture 2015, 443, 98–104. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tokmechi, A.; Farah, K.R.; Jalali, A.S. Growth performance, haematology, antioxidant status, immune response and histology of common carp (Cyprinus carpio L.) fed biofloc grown on different carbon sources. Aquac. Res. 2018, 49, 393–403. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tukmechi, A.; Farah, K.R. Use of different carbon sources for the biofloc system during the grow-out culture of common carp (Cyprinus carpio L.) fingerlings. Aquaculture 2018, 484, 259–267. [Google Scholar] [CrossRef]
- Romano, N.; Dauda, A.B.; Ikhsan, N.; Karim, M.; Kamarudin, M.S. Fermenting rice bran as a carbon source for biofloc technology improved the water quality, growth, feeding efficiencies, and biochemical composition of African catfish Clarias gariepinus juveniles. Aquac. Res. 2018, 49, 3691–3701. [Google Scholar] [CrossRef]
- Dauda, A.B.; Romano, N.; Chen, W.W.; Natrah, I.; Kamarudin, M.S. Differences in feeding habits influence the growth performance and feeding efficiencies of African catfish (Clarias gariepinus) and lemon fin barb hybrid (Hypsibarbus wetmorei ♂ × Barboides gonionotus ♀) in a glycerol-based biofloc technology system versu. Aquac. Eng. 2018, 82, 31–37. [Google Scholar] [CrossRef]
- Dauda, A.B.; Romano, N.; Ebrahimi, M.; Karim, M.; Natrah, I.; Kamarudin, M.S.; Ekasari, J. Different carbon sources affects biofloc volume, water quality and the survival and physiology of African catfish Clarias gariepinus fingerlings reared in an intensive biofloc technology system. Fish. Sci. 2017, 83, 1037–1048. [Google Scholar] [CrossRef]
- De Lima, E.C.R.; de Souza, R.L.; Girao, J.M.; Braga, Í.F.M.; Correia, E.D.S. Culture of Nile tilapia in a biofloc system with different sources of carbon. Rev. Cienc. Agron. 2018, 49, 458–466. [Google Scholar] [CrossRef]
- García-Ríos, L.; Miranda-Baeza, A.; Coelho-Emerenciano, M.G.; Huerta-Rábago, J.A.; Osuna-Amarillas, P. Biofloc technology (BFT) applied to tilapia fingerlings production using different carbon sources: Emphasis on commercial applications. Aquaculture 2019, 502, 26–31. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Speed, M.; Ali, A.M.; Burger, J.M.; Almeida, R.V.; Ayub, Z.; Harisanto, M.; Horowitz, A.; Brock, D.L. Use of molasses as carbon source in limited discharge nursery and grow-out systems for Litopenaeus vannamei. Aquac. Eng. 2007, 36, 184–191. [Google Scholar] [CrossRef]
- Liu, W.; Luo, G.; Chen, W.; Tan, H.; Wu, S.; Zhang, N.; Yu, Y. Effect of no carbohydrate addition on water quality, growth performance and microbial community in water-reusing biofloc systems for tilapia production under high-density cultivation. Aquac. Res. 2018, 49, 2446–2454. [Google Scholar] [CrossRef]
- Krummenauer, D.; Samocha, T.; Poersch, L.; Lara, G.; Wasielesky, W. The reuse of water on the culture of Pacific white shrimp, Litopenaeus vannamei, in BFT system. J. World Aquac. Soc. 2014, 45, 3–14. [Google Scholar] [CrossRef]
- Hisano, H.; Parisi, J.; Cardoso, I.L.; Ferri, G.H.; Ferreira, M.F. Dietary protein reduction for Nile tilapia fingerlings reared in biofloc technology. J. World Aquac. Soc. 2020, 51, 452–462. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Alternative dietary protein sources for farmed. Aquaculture 1999, 179, 149–168. [Google Scholar] [CrossRef]
- Narimbi, J.; Mazumder, D.; Sammut, J. Stable isotope analysis to quantify contributions of supplementary feed in Nile Tilapia Oreochromis niloticus (GIFT strain) aquaculture. Aquac. Res. 2018, 49, 1866–1874. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Kochba, M. Evaluation of nitrogen uptake and excretion by tilapia in bio floc tanks, using 15N tracing. Aquaculture 2009, 287, 163–168. [Google Scholar] [CrossRef]
- Moreno-Arias, A.; López-Elías, J.A.; Martínez-Córdova, L.R.; Ramírez-Suárez, J.C.; Carvallo-Ruiz, M.G.; García-Sánchez, G.; Lugo-Sánchez, M.E.; Miranda-Baeza, A. Effect of fishmeal replacement with a vegetable protein mixture on the amino acid and fatty acid profiles of diets, biofloc and shrimp cultured in BFT system. Aquaculture 2018, 483, 53–62. [Google Scholar] [CrossRef]
- Ray, A.J.; Lewis, B.L.; Browdy, C.L.; Leffler, J.W. Suspended solids removal to improve shrimp (Litopenaeus vannamei) production and an evaluation of a plant-based feed in minimal-exchange, superintensive culture systems. Aquaculture 2010, 299, 89–98. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.C.; Cavalli, R.O.; Wasielesky, W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac. Res. 2012, 43, 447–457. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.C.; Cavalli, R.O.; Wasielesky, W. Effect of biofloc technology (BFT) on the early postlarval stage of pink shrimp Farfantepenaeus paulensis: Growth performance, floc composition and salinity stress tolerance. Aquac. Int. 2011, 19, 891–901. [Google Scholar] [CrossRef]
- Promthale, P.; Pongtippatee, P.; Withyachumnarnkul, B.; Wongprasert, K. Bioflocs substituted fishmeal feed stimulates immune response and protects shrimp from Vibrio parahaemolyticus infection. Fish. Shellfish Immunol. 2019, 93, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, Y.; Wang, G.; Xia, B.; Li, Y. Dietary supplementation of biofloc influences growth performance, physiological stress, antioxidant status and immune response of juvenile sea cucumber Apostichopus japonicus (Selenka). Fish. Shellfish Immunol. 2018, 72, 143–152. [Google Scholar] [CrossRef]
- Bauer, W.; Prentice-Hernandez, C.; Tesser, M.B.; Wasielesky, W.; Poersch, L.H.S. Substitution of fishmeal with microbial floc meal and soy protein concentrate in diets for the Pacific white shrimp Litopenaeus vannamei. Aquaculture 2012, 342–343, 112–116. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.B.T.; Ahilan, B.; Santhakumar, R.; Jemila, A. Influence of Biofloc meal and Lysine supplementation on the growth performances of GIFT tilapia. J. Entomol. Zool. Stud. 2017, 5, 35–39. [Google Scholar]
- Megahed, M.E.; Mohamed, K. Sustainable Growth of Shrimp Aquaculture through Biofloc Production as Alternative to Fishmeal in Shrimp Feeds. J. Agric. Sci. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Valle, B.C.S.; Dantas, E.M., Jr.; Silva, J.F.X.; Bezerra, R.S.; Correia, E.S.; Peixoto, S.R.M.; Soares, R.B. Replacement of fishmeal by fish protein hydrolysate and biofloc in the diets of Litopenaeus vannamei postlarvae. Aquac. Nutr. 2015, 21, 105–112. [Google Scholar] [CrossRef]
- Shao, J.; Liu, M.; Wang, B.; Jiang, K.; Wang, M.; Wang, L. Evaluation of biofloc meal as an ingredient in diets for white shrimp Litopenaeus vannamei under practical conditions: Effect on growth performance, digestive enzymes and TOR signaling pathway. Aquaculture 2017, 479, 516–521. [Google Scholar] [CrossRef]
- Dantas, E.M.; Valle, B.C.S.; Brito, C.M.S.; Calazans, N.K.F.; Peixoto, S.R.M.; Soares, R.B. Partial replacement of fishmeal with biofloc meal in the diet of postlarvae of the Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2016, 22, 335–342. [Google Scholar] [CrossRef]
- Ferreira, M.G.; Melo, F.; Lima, J.V.; Andrade, H.A.; Severi, W.; Correia, E.S. Bioremediation and biocontrol of commercial probiotic in marine shrimp culture with biofloc. Lat. Am. J. Aquat. Res. 2017, 45, 167–176. [Google Scholar] [CrossRef]
- Aguilera-Rivera, D.; Prieto-Davó, A.; Escalante, K.; Chávez, C.; Cuzon, G.; Gaxiola, G. Probiotic effect of FLOC on Vibrios in the pacific white shrimp Litopenaeus vannamei. Aquaculture 2014, 424–425, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Panigrahi, A.; Das, R.R.; Sivakumar, M.R.; Saravanan, A.; Saranya, C.; Sudheer, N.S.; Vasagam, K.K.; Mahalakshmi, P.; Kannappan, S.; Gopikrishna, G. Bio-augmentation of heterotrophic bacteria in biofloc system improves growth, survival, and immunity of Indian white shrimp Penaeus indicus. Fish. Shellfish Immunol. 2020, 98, 477–487. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Bolívar, N.C.; Pereira, S.A.; Guertler, C.; do Nascimento Vieira, F.; Mouriño, J.L.P.; Seiffert, W.Q. Microbial biofloc as source of probiotic bacteria for the culture of Litopenaeus vannamei. Aquaculture 2015, 448, 273–279. [Google Scholar] [CrossRef]
- Hu, X.; Cao, Y.; Wen, G.; Zhang, X.; Xu, Y.; Xu, W.; Xu, Y.; Li, Z. Effect of combined use of Bacillus and molasses on microbial communities in shrimp cultural enclosure systems. Aquac. Res. 2017, 48, 2691–2705. [Google Scholar] [CrossRef]
- Hapsari, F. The effect of fermented and non fermented biofloc inoculated with bacterium Bacillus cereus for catfish (Clarias gariepinus) juveniles. AACL Bioflux 2016, 9, 334–339. [Google Scholar]
- Cienfugos, K.; Dosta, M.C.M.; Hamdan, A.; Aguirre, F. Aquat. Stud. 2018, 6, 525–533. Available online: www.fisheriesjournal.com (accessed on 18 May 2021).
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.R.; Dawood, M.A.O. The enrichment of diet with beneficial bacteria (single- or multi- strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
- Aly, S.M.; Ahmed, Y.A.G.; Ghareeb, A.A.A.; Mohamed, M.F. Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish. Shellfish Immunol. 2008, 25, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Damusaru, J.H.; Park, Y.; Kim, K.; Seong, M.; Je, H.W.; Kim, S.; Bai, S.C. Autotrophic biofloc technology system (ABFT) using Chlorella vulgaris and Scenedesmus obliquus positively affects performance of Nile tilapia (Oreochromis niloticus). Algal Res. 2017, 27, 259–264. [Google Scholar] [CrossRef]
- Dash, P.; Tandel, R.S.; Bhat, R.A.H.; Mallik, S.; Pandey, N.N.; Singh, A.K.; Sarma, D. The addition of probiotic bacteria to microbial floc: Water quality, growth, non-specific immune response and disease resistance of Cyprinus carpio in mid-Himalayan altitude. Aquaculture 2018, 495, 961–969. [Google Scholar] [CrossRef]
- Xia, S.; Li, Y.; Wang, W.; Rajkumar, M.; Vasagam, K.K.; Wang, H. Influence of dietary protein levels on growth, digestibility, digestive enzyme activity and stress tolerance in white-leg shrimp, Litopenaeus vannamei (Boone, 1931), reared in high-density tank trials. Aquac. Res. 2010, 41, 1845–1854. [Google Scholar] [CrossRef]
- ATacon, G.J.; Cody, J.J.; Conquest, L.D.; Divakaran, S.; Forster, I.; Decamp, O.E. Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac. Nutr. 2002, 8, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.J.; Pan, L.Q. Dietary protein level and C/N ratio manipulation in zero-exchange culture of Litopenaeus vannamei: Evaluation of inorganic nitrogen control, biofloc composition and shrimp performance. Aquac. Res. 2014, 45, 1842–1851. [Google Scholar] [CrossRef]
- Ogello, E.O.; Musa, S.M.; Aura, C.M.; Abwao, J.O. An Appraisal of the Feasibility of Tilapia Production in Ponds Using Biofloc Technology: A review. Int. J. Aquat. Sci. 2014, 5, 21–39. [Google Scholar]
- Ekasari, J.; Angela, D.; Waluyo, S.H.; Bachtiar, T.; Surawidjaja, E.H.; Bossier, P.; de Schryver, P. The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 2014, 426–427, 105–111. [Google Scholar] [CrossRef]
- Prangnell, D.I.; Castro, L.F.; Ali, A.S.; Browdy, C.L.; Samocha, T.M. The performance of juvenile Litopenaeus vannamei fed commercial diets of differing protein content, in a super-intensive biofloc-dominated system. J. Appl. Aquac. 2020, 1–22. [Google Scholar] [CrossRef]
- Braga, A.; Lopes, D.L.A.; Magalhães, V.; Poersch, L.H.; Wasielesky, W. Use of biofloc technology during the pre-maturation period of Litopenaeus vannamei males: Effect of feeds with different protein levels on the spermatophore and sperm quality. Aquac. Res. 2015, 46, 1965–1973. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Yavari, V.; Hoseini, S.J.; Nafisi, M.; Mozanzadeh, M.T. Effects of different carbon sources and dietary protein levels in a biofloc system on growth performance, immune response against white spot syndrome virus infection and cathepsin L gene expression of Litopenaeus vannamei. Aquac. Res. 2019, 50, 1162–1176. [Google Scholar] [CrossRef]
- WXu, J.; Pan, L.Q.; Zhao, D.H.; Huang, J. Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture 2012, 350–353, 147–153. [Google Scholar] [CrossRef]
- SPinho, M.; Emerenciano, M.G.C. Sensorial attributes and growth performance of whiteleg shrimp (Litopenaeus vannamei) cultured in biofloc technology with varying water salinity and dietary protein content. Aquaculture 2021, 540, 736727. [Google Scholar] [CrossRef]
- Kumar, S.; Anand, P.S.S.; De, D.; Deo, A.D.; Ghoshal, T.K.; Sundaray, J.K.; Ponniah, A.G.; Jithendran, K.P.; Raja, R.A.; Biswas, G.; et al. Effects of biofloc under different carbon sources and protein levels on water quality, growth performance and immune responses in black tiger shrimp Penaeus monodon (Fabricius, 1978). Aquac. Res. 2017, 48, 1168–1182. [Google Scholar] [CrossRef]
- Brito, L.O.; Junior, L.C.; Abreu, J.L.; Severi, W.; Moraes, L.B.S.; Galvez, A.O. Effects of two commercial feeds with high and low crude protein content on the performance of white shrimp Litopenaeus vannamei raised in an integrated biofloc system with the seaweed Gracilaria birdiae. Span. J. Agric. Res. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Da Silva, M.A.; de Alvarenga, É.R.; Alves, G.F.D.O.; Manduca, L.G.; Turra, E.M.; de Brito, T.S.; de Sales, S.C.M.; da Silva Junior, A.F.; Borges, W.J.; Teixeira, E.D.A. Crude protein levels in diets for two growth stages of Nile tilapia (Oreochromis niloticus) in a biofloc system. Aquac. Res. 2018, 49, 2693–2703. [Google Scholar] [CrossRef]
- Amany, A.G.; Elnady, M.A.; Salem, M.A.I.; Metwally, N.E. Influence of dietary protein level and feed inputs on growth and feeding performance of the Nile tilapia under biofloc conditions. Egypt. J. Aquat. Biol. Fish. 2019, 23, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Nguyen, H.Y.N.; Trinh, T.L.; Baruah, K.; Lundh, T.; Kiessling, A. Growth and feed utilisation of Nile tilapia (Oreochromis niloticus) fed different protein levels in a clear-water or biofloc-RAS system. Aquaculture 2021, 536, 736404. [Google Scholar] [CrossRef]
- Mansour, A.T.; Esteban, M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef]
- Aalimahmoudi, M.; Mohammadiazarm, H. Dietary protein level and carbon/nitrogen ratio manipulation in bioflocs rearing of Cyprinus carpio juvenile: Evaluation of growth performance, some blood biochemical and water parameters. Aquaculture 2019, 513, 734408. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Q.; Luo, L.; Qiao, G.; Wang, L.; Li, J.; Wang, C. Effect of bio-floc on water quality and the production performance of bottom and filter feeder carp fed with different protein levels in a pond polyculture system. Aquaculture 2021, 531, 735906. [Google Scholar] [CrossRef]
- Sawant, K.; Meshram, S.; Dhamagaye, H.; Chavan, B.R. Growth and Survival of Labeo rohita (HAMILTON, 1822) fry in biofloc system using various dietary protein levels. J. Exp. Zool. India 2020, 23, 765–769. [Google Scholar]
- Yu, Z.; Huang, Z.Q.; Du, H.L.; Li, H.J.; Wu, L.F. Influence of differential protein levels of feed on growth, copper-induced immune response and oxidative stress of Rhynchocypris lagowski in a biofloc-based system. Aquac. Nutr. 2020, 26, 2211–2224. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Lin, X.T.; Pan, J.X.; Xu, Z.N. Effect of cyclical feeding on compensatory growth, nitrogen and phosphorus budgets in juvenile Litopenaeus vannamei. Aquac. Res. 2016, 47, 283–289. [Google Scholar] [CrossRef]
- Maciel, J.C.; Francisco, C.J.; Miranda-Filho, K.C. Compensatory growth and feed restriction in marine shrimp production, with emphasis on biofloc technology. Aquac. Int. 2018, 26, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cui, Y.; Yang, Y.; Cai, F. Compensatory growth in hybrid tilapia, Oreochromis mossambicus x O. niloticus, reared in seawater. Aquaculture 2000, 189, 101–108. [Google Scholar] [CrossRef]
- Wasielesky, W.; Froes, C.; Fóes, G.; Krummenauer, D.; Lara, G.; Poersch, L. Nursery of Litopenaeus vannamei reared in a biofloc system: The effect of stocking densities and compensatory growth. J. Shellfish Res. 2013, 32, 799–806. [Google Scholar] [CrossRef]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish. Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Lara, G.; Hostins, B.; Bezerra, A.; Poersch, L.; Wasielesky, W. The effects of different feeding rates and re-feeding of Litopenaeus vannamei in a biofloc culture system. Aquac. Eng. 2017, 77, 20–26. [Google Scholar] [CrossRef]
- Rocha, J.V.; Silva, J.F.; Barros, C.; Peixoto, S.; Soares, R. Compensatory growth and digestive enzyme activity of Litopenaeus vannamei submitted to feeding restriction in a biofloc system. Aquac. Res. 2019, 50, 3653–3662. [Google Scholar] [CrossRef]
- Correa, A.D.S.; Pinho, S.M.; Molinari, D.; Pereira, K.D.R.; Gutiérrez, S.M.; Monroy-Dosta, M.D.C.; Emerenciano, M.G.C. Rearing of Nile tilapia (Oreochromis niloticus) juveniles in a biofloc system employing periods of feed deprivation. J. Appl. Aquac. 2020, 32, 139–156. [Google Scholar] [CrossRef]
- Borges, B.A.A.; Rocha, J.L.; Pinto, P.H.O.; Zacheu, T.; Chede, A.C.; Magnotti, C.C.F.; Cerqueira, V.R.; Arana, L.A.V. Integrated culture of white shrimp Litopenaeus vannamei and mullet Mugil liza on biofloc technology: Zootechnical performance, sludge generation, and Vibrio sp. reduction. Aquaculture 2020, 524, 735234. [Google Scholar] [CrossRef]
- Poli, M.A.; Martins, M.A.; Pereira, S.A.; Jesus, G.F.A.; Martins, M.L.; Mouriño, J.L.P.; do Nascimento Vieira, F. Increasing stocking densities affect hemato-immunological parameters of Nile tilapia reared in an integrated system with Pacific white shrimp using biofloc technology. Aquaculture 2021, 536, 736497. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Martins, M.A.; Vieira, F.D.N. Pacific white shrimp and Nile tilapia integrated in a biofloc system under different fish-stocking densities. Aquaculture 2019, 498, 83–89. [Google Scholar] [CrossRef]
- Pinheiro, I.; Arantes, R.; do Espírito Santo, C.M.; do Nascimento Vieira, F.; Lapa, K.R.; Gonzaga, L.V.; Fett, R.; Barcelos-Oliveira, J.L.; Seiffert, W.Q. Production of the halophyte Sarcocornia ambigua and Pacific white shrimp in an aquaponic system with biofloc technology. Ecol. Eng. 2017, 100, 261–267. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Holanda, M.; Santana, G.; Furtado, P.; Rodrigues, R.V.; Cerqueira, V.R.; Sampaio, L.A.; Wasielesky, W., Jr.; Poersch, L.H. Evidence of total suspended solids control by Mugil liza reared in an integrated system with Pacific white shrimp Litopenaeus vannamei using biofloc technology. Aquac. Rep. 2020, 18, 100479. [Google Scholar] [CrossRef]
- Hoang, M.N.; Nguyen, P.N.; Bossier, P. Water quality, animal performance, nutrient budgets and microbial community in the biofloc-based polyculture system of white shrimp, Litopenaeus vannamei and gray mullet, Mugil cephalus. Aquaculture 2020, 515, 734610. [Google Scholar] [CrossRef]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; de Lorenzo, M.A.; Hayashi, L.; do Nascimento Vieira, F. Sea lettuce integrated with Pacific white shrimp and mullet cultivation in biofloc impact system performance and the sea lettuce nutritional composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- Pinheiro, I.; Carneiro, R.F.S.; do Nascimento Vieira, F.; Gonzaga, L.V.; Fett, R.; de Oliveira Costa, A.C.; Magallon-Barajas, F.J.; Seiffert, W.Q. Aquaponic production of Sarcocornia ambigua and Pacific white shrimp in biofloc system at different salinities. Aquaculture 2020, 519, 734918. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Browdy, C.L.; Bratvold, D.; Stokes, A.D.; McIntosh, R. Perspectives on the application of closed shrimp culture systems. In The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture 2001; Browdy, C.L., Jory, D.E., Eds.; World Aquaculture Society: Baton Rouge, LA, USA, 2001; pp. 20–34. [Google Scholar]
- Megahed, M.E. The Effect of Microbial Biofloc on Water Quality, Survival and Growth of the Green Tiger Shrimp (Penaeus Semisulcatus) Fed with Different crude Protein Levels. I: Sustainable Solution to the Dependency on Fish Oil, Fishmeal and Environmental Problems. J. Arab. Aquac. Soc. 2010, 5, 119–142. [Google Scholar]
- De Schryver, P.; Verstraete, W. Nitrogen removal from aquaculture pond water by heterotrophic nitrogen assimilation in lab-scale sequencing batch reactors. Bioresour. Technol. 2009, 100, 1162–1167. [Google Scholar] [CrossRef]
- Luo, G.; Chen, X.; Tan, J.; Abakari, G.; Tan, H. Effects of carbohydrate addition strategy and biofloc levels on the establishment of nitrification in biofloc technology aquaculture systems. Aquaculture 2020, 514, 734441. [Google Scholar] [CrossRef]
- Paula, A.; de Morais, M.; Cesar, P.; Wasielesky, W. Effect of aeration intensity on the biofilm nitrification process during the production of the white shrimp Litopenaeus vannamei (Boone, 1931) in Biofloc and clear water systems. Aquaculture 2020, 514, 734516. [Google Scholar] [CrossRef]
- Jiménez-Ordaz, F.J.; Cadena-Roa, M.A.; Pacheco-Vega, J.M.; Rojas-Contreras, M.; Tovar-Ramírez, D.; Arce-Amezquita, M. Microalgae and probiotic bacteria as biofloc inducers in a hyper-intensive Pacific white shrimp (Penaeus vannamei) culture. Lat. Am. J. Aquat. Res. 2021, 49, 155–168. [Google Scholar] [CrossRef]
- Schrader, K.K.; Green, B.W.; Perschbacher, W. Development of phytoplankton communities and common off-flavors in a biofloc technology system used for the culture of channel catfish (Ictalurus punctatus). Aquac. Eng. 2011, 45, 118–126. [Google Scholar] [CrossRef]
- Lauderdale, C.V.; Aldrich, H.C.; Lindner, A.S. Isolation and characterization of a bacterium capable of removing taste- and odor-causing 2-methylisoborneol from water. Water Res. 2004, 38, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Guttman, L.; van Rijn, J. Isolation of bacteria capable of growth with 2-methylisoborneol and geosmin as the sole carbon and energy sources. Appl. Environ. Microbiol. 2012, 78, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).