Soil Health Beneath Amended Switchgrass: Effects of Biochar and Nitrogen on Active Carbon and Wet Aggregate Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Methods
2.2. Laboratory Methods
2.2.1. Biochar Analysis
2.2.2. Wet Aggregate Stability
2.2.3. Active Carbon
2.3. Statistical Analysis
3. Results
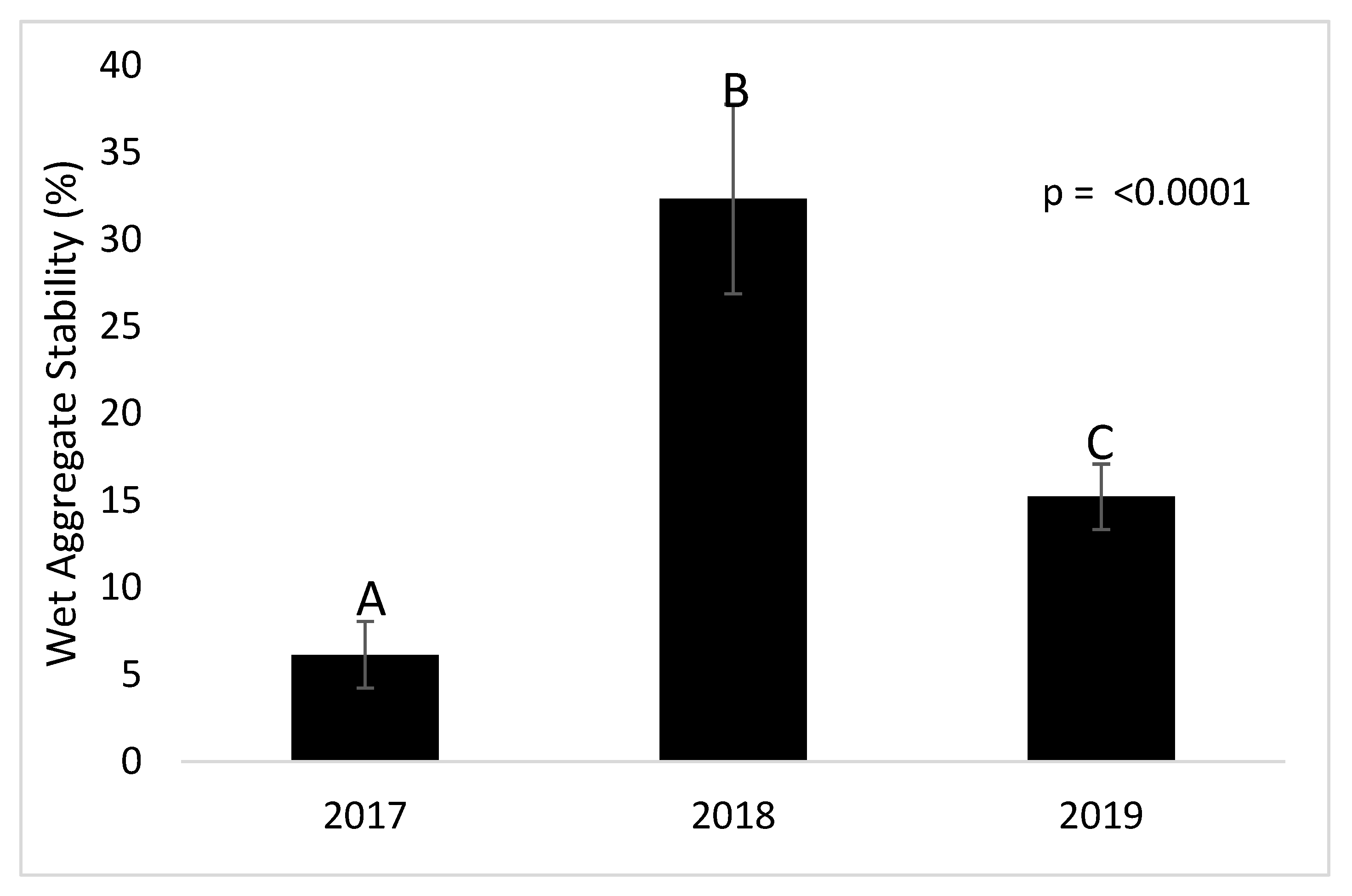
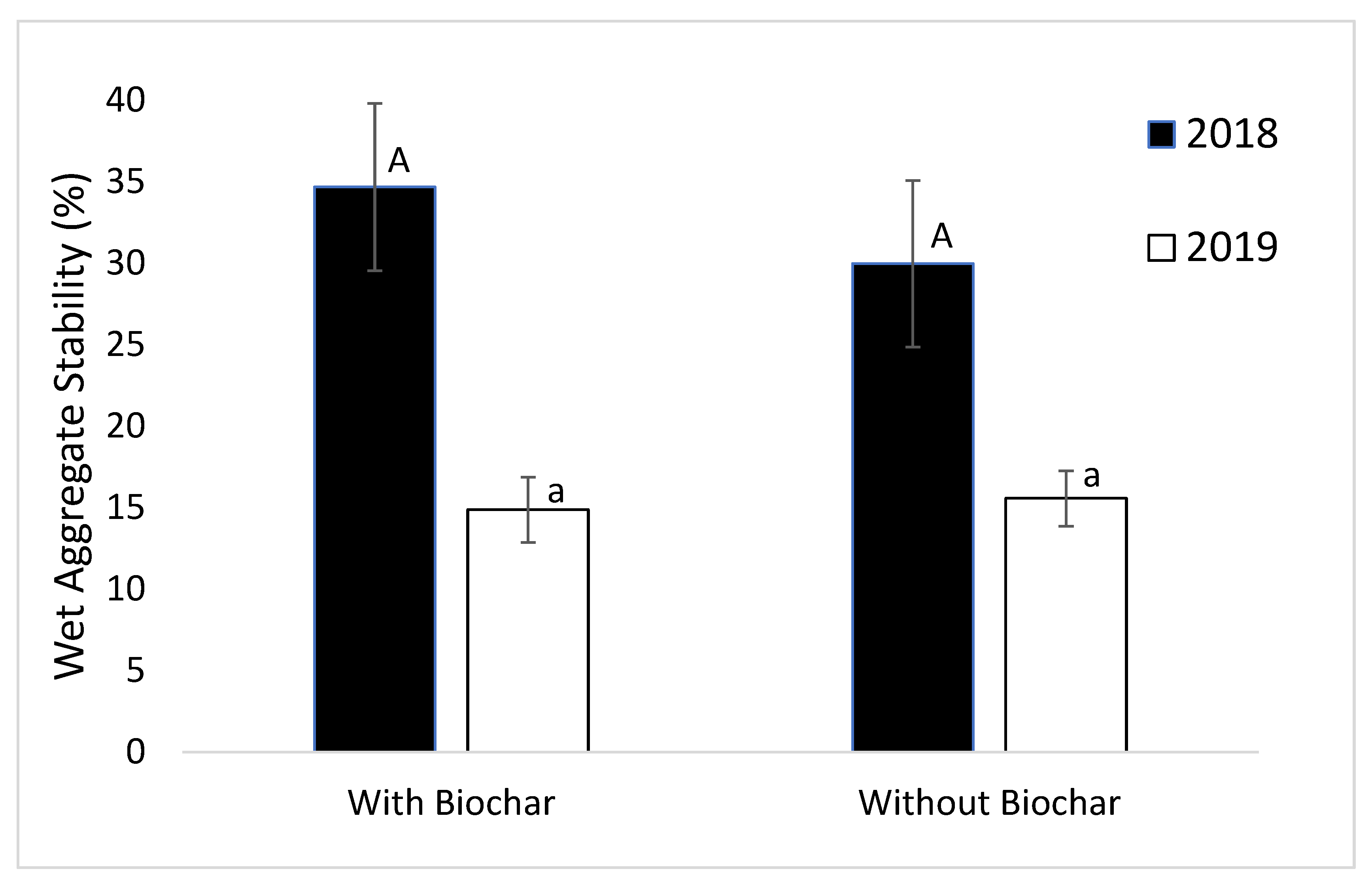
3.1. Wet Aggregate Stability (WAS)
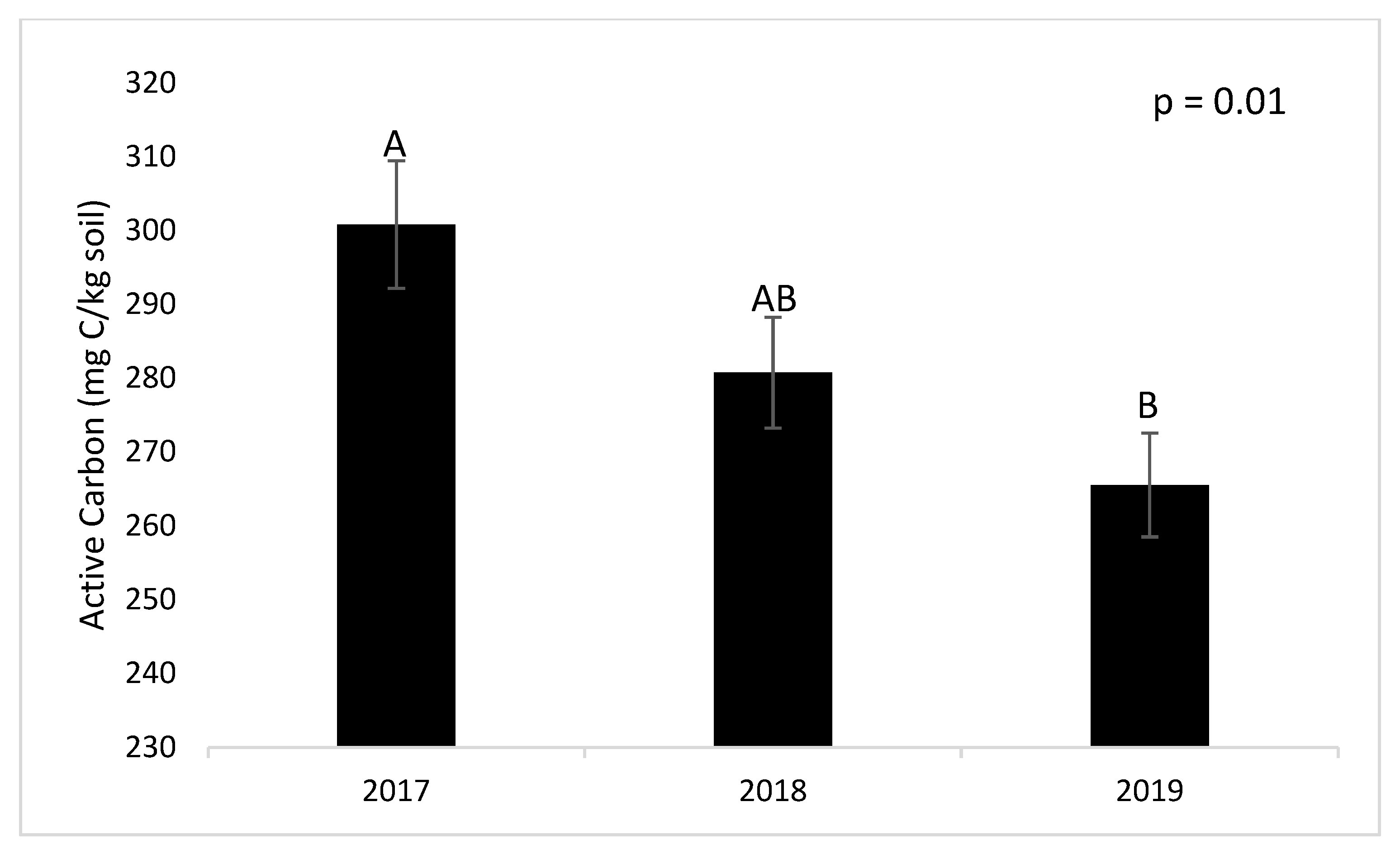
3.2. Active Carbon (AC)
4. Discussion
4.1. Aggregate Stability
4.2. Active Carbon (AC)
4.3. Biochar and Soil Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land clearing and the biofuel carbon debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, I.; Sahajpal, R.; Zhang, X.; Izaurralde, R.C.; Gross, K.L.; Robertson, G.P. Sustainable bioenergy production from marginal lands in the US Midwest. Nature 2013, 493, 514–517. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S. Mechanisms of soil carbon accrual and storage in bioenergy cropping systems. GCB Bioenergy 2015, 7, 161–174. [Google Scholar] [CrossRef]
- Office of Energy Efficiency and Renewable Energy; USDOE. Alternative fuel transportation program; replacement fuel goal modification. Fed. Regist. 2007, 72, 12041–12060. [Google Scholar]
- Follett, R.F.; Pruessner, E.G.; Samson-Liebig, S.E.; Kimble, J.M.; Waltman, S.W. Carbon sequestration under CRP in the historic grassland soils of the USA. In Soil Carbon Sequestration and the Greenhouse Effect; Lal, R., Ed.; Spec. Publ. No. 57; SSSA: Madison, WI, USA, 2001; pp. 27–40. [Google Scholar]
- Schmer, M.R.; Liebig, M.A.; Vogel, K.P.; Mitchell, R.B. Field-scale soil property changes under switchgrass managed for bioenergy. GCB Bioenergy 2011, 3, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Seifu, W.; Elias, E. Soil quality attributes and their role in sustainable agriculture: A review. Int. J. Plant Soil Sci. 2018, 26, 1–26. [Google Scholar] [CrossRef]
- Hubanks, H.; Deenik, J.; Crow, S. Getting the dirt on soil health and management. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Ontl, T.A.; Cambardella, C.A.; Schulte, L.A.; Kolka, R.K. Factors influencing soil aggregation and particulate organic matter response to bioenergy crops across a topographic gradient. Geoderma 2015, 255–256, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Moebius-Clune, B.N.; Moebius-Clune, D.J.; Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; Ristow, A.J.; van Es, H.M.; Thies, J.E.; Shayler, H.A.; McBride, M.B.; et al. Comprehensive Assessment of Soil Health: The Cornell Framework Manual, 3rd ed.; Cornell University: Ithaca, NY, USA, 2016; Available online: http://www.css.cornell.edu/extension/soil-health/manual.pdf (accessed on 24 June 2021).
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Kennedy, A.C.; Papendiek, R.I. Microbial characteristics of soil quality. J. Soil Water Conserv. 1995, 50, 243–248. [Google Scholar]
- Islam, K.R.; Weil, R.R. Soil quality indicator properties in mid-Atlantic soils as influenced by conservation management. J. Soil Water Conserv. 2000, 55, 69–78. [Google Scholar]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley-Smith, L. Light fraction organic matter in soils from long term crop rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Wander, M.M.; Bidart, M.G. Tillage practice influences on the physical protection, bioavailability and composition of particulate organic matter. Biol. Fert. Soils 2000, 32, 360–367. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Keeney, D.R. Soluble organics and extractable nitrogen in paired prairie and cultivated soil of central Iowa. Soil Sci. 1993, 155, 219–228. [Google Scholar] [CrossRef]
- Saviozzi, A.; Biasci, A.; Riffaldi, R.; Levi-Minzi, R. Long-term effects of farmyard manure and sewage sludge on some soil biochemical characteristics. Biol. Fert. Soils 1999, 30, 100–106. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction; in Biochar for environmental management. Sci. Technol. 2009, 1, 1–12. [Google Scholar]
- Chmidt, M.W.; Skjemstad, J.O.; Jager, C. Carbon isotope geochemistry and nanomorphology of soil black carbon: Black chernozemic soils in central Europe originate from ancient biomass burning. Glob. Biogeochem. Cycles 2002, 16, 1123. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Lone, A.H.; Najar, G.R.; Ganie, M.A.; Sofi, J.A.; Ali, T. Biochar for sustainable soil health: A review of prospects and concerns. Pedosphere 2015, 25, 639–653. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Zhang, L.; Zhang, Y.; Yang, L.; Yu, C.; Yin, G.; Doane, T.; Zhu, P.; Ma, X. Biochar improves soil aggregate stability and water availability in a mollisol after three years of field application. PLoS ONE 2016, 11, e0154091. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.A.S.; Ahmedna, M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Canqui, H.; Lal, R.; Lemus, R. Soil aggregate properties and organic carbon for switchgrass and traditional agricultural systems in the southeastern United States. Soil Sci. 2005, 170, 998–1012. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Energy crops and their implications on soil and environment. Agron. J. 2010, 102, 403–419. [Google Scholar] [CrossRef]
- Stewart, C.; Follet, R.; Pruessner, E.; Varvel, G.; Vogel, K.; Mitchell, R. Nitrogen and harvest effects on soil properties under rainfed switchgrass and no till corn over 9 years: Implications for soil quality. GCB Bioenergy 2015, 7, 288–301. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Idowu, O.J. Relationships between aggregate stability and selected soil properties in humid tropical environment. Commun. Soil Sci. Plant Anal. 2003, 34, 695–708. [Google Scholar] [CrossRef]
- Russell, A.E.; Kivlin, S.N.; Hawkes, C.V. Tropical tree species effects on soil pH and biotic factors and the consequences for macroaggregate dynamics. Forests 2018, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Amezketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.P.D.K. Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol. Biochem. 1990, 22, 570–584. [Google Scholar] [CrossRef]
- Jastrow, J.P.D.K.; Miller, R.M.; Lussenhop, J. Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol. Biochem. 1998, 30, 905–916. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Lentz, R.D.; Spokas, K.A.; Bolster, C.H.; Sistani, K.; Trippe, K.M.; Phillips, C.L.; Johnson, M.G. Soil health, crop productivity, microbial transport, and mine spoil response to biochars. Bioenerg. Res. 2016, 9, 454–464. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils: A Critical Scientific Review on Effects on Soil Properties, Processes and Functions; Office for the Official Publications of the European Communities: Luxemberg, 2010. [Google Scholar]
- O’Toole, A.; Moni, C.; Weldon, S.; Schols, N.; Carnol, M.; Bosman, B.; Rasse, D. Miscanthus biochar had limited effects on soil physical properties, microbial biomass, and grain yield in a four-year field experiment in Norway. Agriculture 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Crane-Droesch, A.; Abiven, S.; Jeffery, S.; Torn, M.S. Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environ. Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 2014, 123, 45–51. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Gajda, A.M.; Doran, J.W.; Weinhold, B.J.; Kettler, T.A. Estimation of particulate and total organic matter by weight loss-on-ignition. In Assessment Methods for Soil Carbon; Lal, R., Kimble, J.F., Follett, R.F., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 349–359. [Google Scholar]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H.; Gilley, J.; Eisenhauer, D.; Jasa, P.; Boldt, A. Soil carbon accumulation under switchgrass barriers. Agron. J. 2014, 106, 2185–2192. [Google Scholar] [CrossRef] [Green Version]
- Dou, F.G.; Hons, F.M.; Ocumpaugh, W.R.; Read, J.C.; Hussey, M.A.; Muir, J.P. Soil organic carbon pools under switchgrass grown as a bioenergy crop compared to other conventional crops. Pedosphere 2013, 23, 409–416. [Google Scholar] [CrossRef]
- Marquez, C.O.; Victor, G.J.; Schultz, R.C.; Isenhart, T.M. Assessment of soil aggradation through soil aggregation and particulate organic matter by riparian switchgrass buffers. Agronomy 2017, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J.E.; Zink, E. Length of life of roots of ten species of perennial range and pasture grasses. Plant Physiol. 1946, 21, 201–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetrick, B.; Kitt, D.G.; Wilson, G. Mycorrhizal dependence and growth habitat of warm-season and cool-season grass prairie plants. Can. J. Bot. 1988, 66, 1376–1380. [Google Scholar] [CrossRef]
- Tufekcioglu, A.; Raich, J.; Isenhart, T.; Schultz, R. Fine root dynamics, coarse root biomass, root distribution, and soil respiration in a multispecies riparian buffer in Central Iowa, USA. Agrofor. Syst. 1999, 44, 163–174. [Google Scholar] [CrossRef]
- Liang, F.; Li, G.; Lin, Q.; Zhao, X. Crop yields and soil properties in the first 3 years after biochar application to a calcareous soil. J. Integr. Agric. 2014, 13, 525–532. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 2013, 193, 122–130. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef] [Green Version]

| Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| Nutrient | Control | 0 N + Bio | N1 | N2 | N3 | N1 + Bio | N2 + Bio | N3 + Bio |
| Potassium (K) mg kg−1 | 107 | 73.8 | 61.5 | 64.8 | 64.5 | 67.0 | 69.8 | 68.8 |
| Calcium (Ca) mg kg−1 | 3132 | 2780 | 2758 | 2862 | 2858 | 2880 | 2819 | 2964 |
| Magnesium (Mg) mg kg−1 | 202 | 192 | 185 | 199 | 195 | 196 | 196 | 208 |
| Boron (B) mg kg−1 | 0.60 | 0.48 | 0.50 | 0.53 | 0.53 | 0.53 | 0.50 | 0.53 |
| Iron (Fe) mg kg−1 | 37.3 | 40.5 | 35.8 | 37.3 | 40.0 | 38.5 | 38.3 | 38.8 |
| Manganese (Mn) mg kg−1 | 276 | 239 | 247 | 272 | 259 | 297 | 263 | 269 |
| Sodium (Na) mg kg−1 | 17.5 | 17.0 | 18.3 | 17.0 | 21.3 | 17.0 | 20.3 | 18.0 |
| Zinc (Zn) mg kg−1 | 6.38 | 5.53 | 3.58 | 4.10 | 5.08 | 3.98 | 5.08 | 4.85 |
| Nitrate (NO3−) mg kg−1 | 1.50 | 1.50 | 1.25 | 1.25 | 1.25 | 1.00 | 1.50 | 1.25 |
| Ammonium (NH4+) mg kg−1 | 21.5 | 15.0 | 16.3 | 17.3 | 14.5 | 16.8 | 16.0 | 15.0 |
| Total Carbon (C) % | 1.19 | 1.07 | 1.14 | 1.09 | 1.09 | 1.12 | 1.09 | 1.14 |
| Total Nitrogen (N) % | 0.12 | 0.10 | 0.10 | 0.11 | 0.10 | 0.11 | 0.10 | 0.10 |
| Feedstock | Pinewood |
|---|---|
| Pyrolysis temp | 500 °C |
| pH | 6.13 |
| CEC | 4.73 cmolc kg−1 |
| Total Carbon | 63.50% |
| Moisture | 6.58% |
| Phosphorus (P) | 26.67 mg kg−1 |
| Potassium (K) | 292 mg kg−1 |
| Magnesium (Mg) | 49.33 mg kg−1 |
| Calcium (Ca) | 478.67 mg kg−1 |
| Biochar (Yes or No) | N Rate (kg ha−1) | 2018 LSmean WAS | 2019 LSmean WAS |
|---|---|---|---|
| Yes | 0 | 38.7 (A) | 16.0 (a) |
| No | 0 | 33.6 (AB) | 16.0 (a) |
| Yes | 17 | 34.2 (AB) | 14.8 (a) |
| No | 17 | 29.7 (AB) | 16.5 (a) |
| Yes | 34 | 32.2 (AB) | 15.0 (a) |
| No | 34 | 28.5 (B) | 15.1 (a) |
| Yes | 67 | 33.6 (AB) | 13.8 (a) |
| No | 67 | 28.1 (B) | 14.8 (a) |
| Biochar (Yes or No) | N Rate (kg ha−1) | 2018 LSmean AC | 2019 LSmean AC |
|---|---|---|---|
| Yes | 0 | 272 (AB) | 266 (ab) |
| No | 0 | 276 (AB) | 267 (ab) |
| Yes | 17 | 300 (AB) | 264 (ab) |
| No | 17 | 261 (B) | 226 (b) |
| Yes | 34 | 268 (AB) | 267 (ab) |
| No | 34 | 308 (A) | 277 (ab) |
| Yes | 67 | 275 (AB) | 286 (a) |
| No | 67 | 257 (B) | 280 (a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, P.; de Koff, J.P.; Link, R.; Robbins, C. Soil Health Beneath Amended Switchgrass: Effects of Biochar and Nitrogen on Active Carbon and Wet Aggregate Stability. Sustainability 2021, 13, 7176. https://doi.org/10.3390/su13137176
Saini P, de Koff JP, Link R, Robbins C. Soil Health Beneath Amended Switchgrass: Effects of Biochar and Nitrogen on Active Carbon and Wet Aggregate Stability. Sustainability. 2021; 13(13):7176. https://doi.org/10.3390/su13137176
Chicago/Turabian StyleSaini, Priya, Jason P. de Koff, Richard Link, and Chris Robbins. 2021. "Soil Health Beneath Amended Switchgrass: Effects of Biochar and Nitrogen on Active Carbon and Wet Aggregate Stability" Sustainability 13, no. 13: 7176. https://doi.org/10.3390/su13137176
APA StyleSaini, P., de Koff, J. P., Link, R., & Robbins, C. (2021). Soil Health Beneath Amended Switchgrass: Effects of Biochar and Nitrogen on Active Carbon and Wet Aggregate Stability. Sustainability, 13(13), 7176. https://doi.org/10.3390/su13137176





