A Regenerative Business Model with Flexible, Modular and Scalable Processes in A Post-Covid Era: The Case of The Spinning Mesh Disc Reactor (SMDR)
Abstract
:1. Introduction
- Fast reaction rates and high selectivity to minimise by-products
- Suitable for shear sensitive catalysts (enzymes and microbes)
- Small and flexible design, easy scale-up and low capital investment cost
- Allow recovery and re-use of expensive catalysts
2. Current State of Catalytic Reactors
2.1. Manufacturing Processes
- Processing time for production at scale is longer compared to laboratory scale processes. This leads to catalyst degradation and deterioration of product quality over time resulting in reduced productivity of the process.
- Laboratory scale productivity is optimised in equipment with lower volume capacity. However, production at scale is carried out in large tanks or vessels which affects the homogeneity of the finished product and increases the energy cost required to mix the entirety of the reaction solution.
2.2. Socio-Ecological Challenges
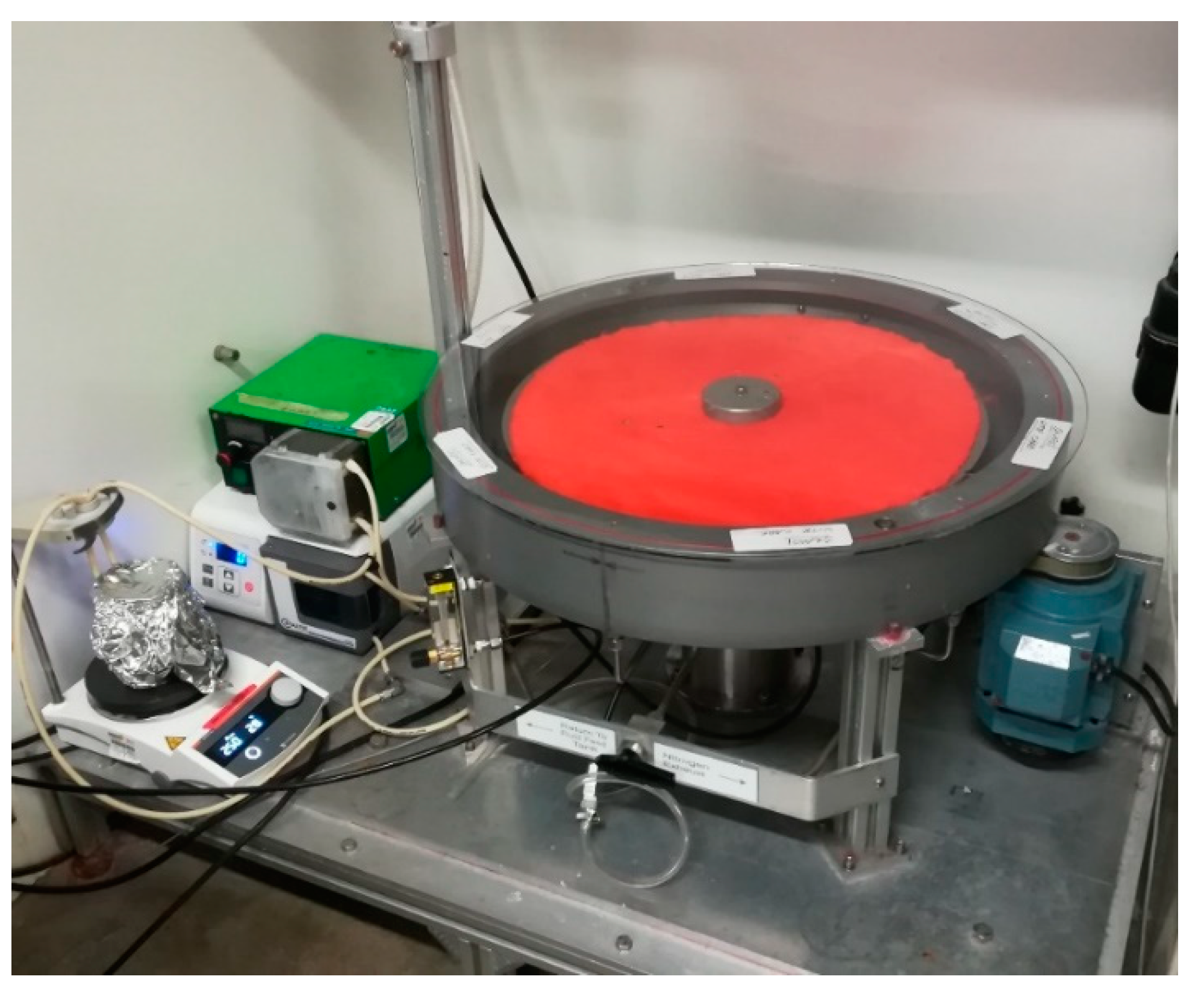
3. SMDR as a Case Study for Sustainable Reactors
3.1. The ICURe Journey: Global Market Research for the SMDR
- Cost of change: Chemical sectors such as pharmaceutical and cosmetic industries have to comply with stringent regulations and any change in the existing process requires lengthy and expensive regulatory approvals. The cost of production downtime often outweighs the benefit of higher process efficiency and product yield.
- Initial capital expenditure (CAPex): The current batch reactor technology for manufacturing in fine chemical industries has been in use for decades and is well established. Adopting new reactor technology would require capital investment for procuring and installation of these reactors to replace the existing reactors. The reactors themselves are depreciating assets and they have no resale value. Medium scale fine chemical companies in the US and India stated that it was not beneficial for the company to invest in a low TRL technology with high uncertainty when they were able to generate profits with their existing reactor capabilities. These companies are improving the efficiency of the batch reactors by investing in improved impeller designs as they can be retrofitted to the batch reactor with minimum disruption to the process. However, most companies in Europe and large-scale companies in the US and India were investing in new reactor technologies despite a significant capital investment. The reactors were being employed for either newly commissioned processes (with no existing production facility) and/or for niche chemicals of high value with lower production demands. Most companies acknowledged the potential of these reactors for the production of high value chemicals as they improve product yield, while also reducing the operating costs for the process.
- Knowledge gap: PI reactors have shown great potential to be implemented on a commercial scale owing to their success in laboratory scale operations. Some PI reactors like the micro-channel reactor are increasingly being used for commercial operations but are not often publicised due to competition and confidentiality. PI reactors are known to be inherently safer than batch reactors due to lower reaction volumes. However, conversations with companies showed that there have been safety related incidents with PI reactors due to the lack of specialist knowledge required to optimise and operate these reactors. These factors have affected the commercial success of PI reactors.
- Motivation for change: This was one of the major challenges identified by chemical companies for replacing batch reactors. Companies involved in manufacturing new reactor technologies mentioned that it was hard to spark enthusiasm among the top management in chemical companies as they are the decision makers. The interactions also showed that the US was a tough market for new reactors as there is no incentive or drive for a process change. On the other hand, the Asian–Pacific region is a fast-growing market for emerging reactor technologies due to large manufacturing facilities and most reactor manufacturers have found traction through clients from this region. The European market was found to be equally responsive to change as it is an active research hub for emerging reactor technologies incentivised by government and company policies for sustainable manufacturing.
3.2. Sustainable manufacturing: Opportunities for the SMDR
3.2.1. Affordability and Market Outreach: Low Capital Investment
3.2.2. High Productivity and Scaling Up
3.2.3. Inherent Safety
3.3. A Regenerative Approach for Resilient Manufacturing
- (1)
- Sustainability: The resilience of a successful SBM comes from its ability to make strategic business decisions in reaction to social, economic and environmental shock to sustain the innovation in the long-run [1]. Here, SMDR provides a modular and regenerative approach to fine chemical production. SMDR is able to fulfil its long-term goal of producing on-demand, at the point of resource extraction, for immediate consumption. Such context-based technology means that, in the event of external shocks on the supply chain as for example experienced with the Covid crisis, such shocks will be absorbed by the regenerative supply chain, able to answer quickly to supply changes by scale-up or down with limited costs.
- (2)
- Circular economy: Reducing waste and managing resources as efficiently as possible to narrow the energy and material loops is key to a successful SMDR [1]. Raworth [29] has explained the ways in which a circular economy enhances industrial manufacturing with a regenerative system that minimises the loss of biological and technical nutrients. The SMDR includes the use of biological components and processes that are inherently safe, as guaranteed by the application of the 12 principles of green chemistry (see point c above). The resulting effect on the circular system is that human interactions with the production process are safe, thus minimising the negative impact of chemical production on human health and biodiversity. The technical nutrients within the SMDR process include the catalyst, tailor-made to production requirements, and the cloth on top of the reactor that can be re-used in subsequent production cycles. The resulting effect on the circular system is the reuse of the cloth, which can also be adjusted to the scale of production.
- (3)
- Value chains: A successful SBM nurtures the value chain starting with resources, suppliers, customers, support activities, and input activities [1]. A main ecological challenge with traditional batch reactors is that by-product formation often exceeds the production of the target chemical. However, with the SMDR process, production can be made on site and on-demand, shortening the supply chain to its direct output requirements, thus cutting down on transportation costs and on the storage of chemicals with its potential environmental damages.
- (4)
- Value creation: The concept of “value” in SBM here relies on the link between corporate strategy and intangible assets that create value in the long-run [1]. Such intangible assets include finance, manufacturing, intellectual property, human capabilities, social and natural relationships. Impact research would be needed here to assess the impact of SMDR on value creation at the implementation stage, but feature (1) to (3) provides a sound theoretical grounding for value creation in the long-run.
- (5)
- Information technology: Innovation in IT to support the manufacturing innovation in modelling, managing, and controlling its impact on ecosystem processes [1]. The SMDR aligns with “Industry 4.0” to support smart manufacturing through reactor automation, providing improved control on product quality.
- (6)
- Core values: Interdependence, reliability, loyalty, commitment, consistency, efficiency, creativity, inclusivity, respect and positivity are core values of a successful SBM [1]. Inclusivity of all potential producers is possible with a subscription system allowing the SMDR to overcome the main entry barriers of the initial investment and the knowledge transfer necessary to make the SMDR operational on a small and large scale. Local producers in the Global South, often overpowered by the interests of transnational corporations and governmental pressures could therefore have easy access to such technology. For example, in the Democratic Republic of the Congo, a coalition between transnational capital and the Congolese state has held back locally led processes of mining mechanisation [30]. Access to simple, modular, and regenerative technology such as SMDR could empower local producers to process and scale-up mining output locally.
- (7)
- Organisational values: Employee, social and environmental safety, responsibility, profitability and drive for results are key organisational values of an SBM [1]. Such values will depend on the organisation in which SMDR is implemented, but the inherent safety (c) and high-productivity (b) of SMDR is conducive to enhance an SBM.
- (8)
- Performance management: A successful SBM needs to incorporate performance measurement into all aspects of industrial processes including service, business management, quality, productivity and efficiency [1]. Performance management will again depend on the organisation in which SMDR is implemented, but with a lease-system allowing to monitor its implementation and efficiency, there is again scope for SMDR to enhance an SBM.
- (9)
- Stakeholder engagement: Partnership, participation, communication, and consultation are key elements for a successful SBM. As such, the key features of SMDR (a) to (c) are well-aligned with the current Sustainable Development Goals [31], especially Goal 9 Industry, Innovation and Infrastructure; Goal 11 Sustainable Cities and Communities, and Goal 12, Responsible Consumption and Production. There is therefore scope to engage governmental and international support to implement SMDR in both the Global South, such as sub-Saharan Africa [32] and the Global North.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goni, F.A.; Chofreh, A.G.; Orakani, Z.E.; Klemeš, J.J.; Davoudi, M.; Mardani, A. Sustainable business model: A review and framework development. Clean Technol. Environ. Policy 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Barthelemy, P.; Agyeman-Budu, E. European chemical industry’s contribution to sustainable development. Curr. Opin. Green Sustain. Chem. 2016, 1, 28–32. [Google Scholar] [CrossRef]
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- Bieringer, T.; Buchholz, S.; Kockmann, N. Future production concepts in the chemical industry: Modular–small-scale–continuous. Chem. Eng. Technol. 2013, 36, 900–910. [Google Scholar] [CrossRef]
- Gorak, A.; Stankiewicz, A. Intensified reaction and separation systems. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 431–451. [Google Scholar] [CrossRef]
- Van Gerven, T.; Stankiewicz, A. Structure, energy, synergy, time—The fundamentals of process intensification. Ind. Eng. Chem. Res. 2009, 48, 2465–2474. [Google Scholar] [CrossRef]
- Miyazaki, M.; Maeda, H. Microchannel enzyme reactors and their applications for processing. Trends Biotechnol. 2006, 24, 463–470. [Google Scholar] [CrossRef]
- Chung, B.H.; Chang, H.; Han, M.H. Enzymatic conversion of rifamycin B in a rotating packed disk reactor. J. Ferment. Technol. 1986, 64, 343–345. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, Y.-H.; Lin, R.-H.; Shang, N.-C. A continuous-flow biodiesel production process using a rotating packed bed. Bioresour. Technol. 2010, 101, 668–673. [Google Scholar] [CrossRef]
- Visscher, F.; Van der Schaaf, J.; Nijhuis, T.; Schouten, J. Rotating reactors—A review. Chem. Eng. Res. Des. 2013, 91, 1923–1940. [Google Scholar] [CrossRef]
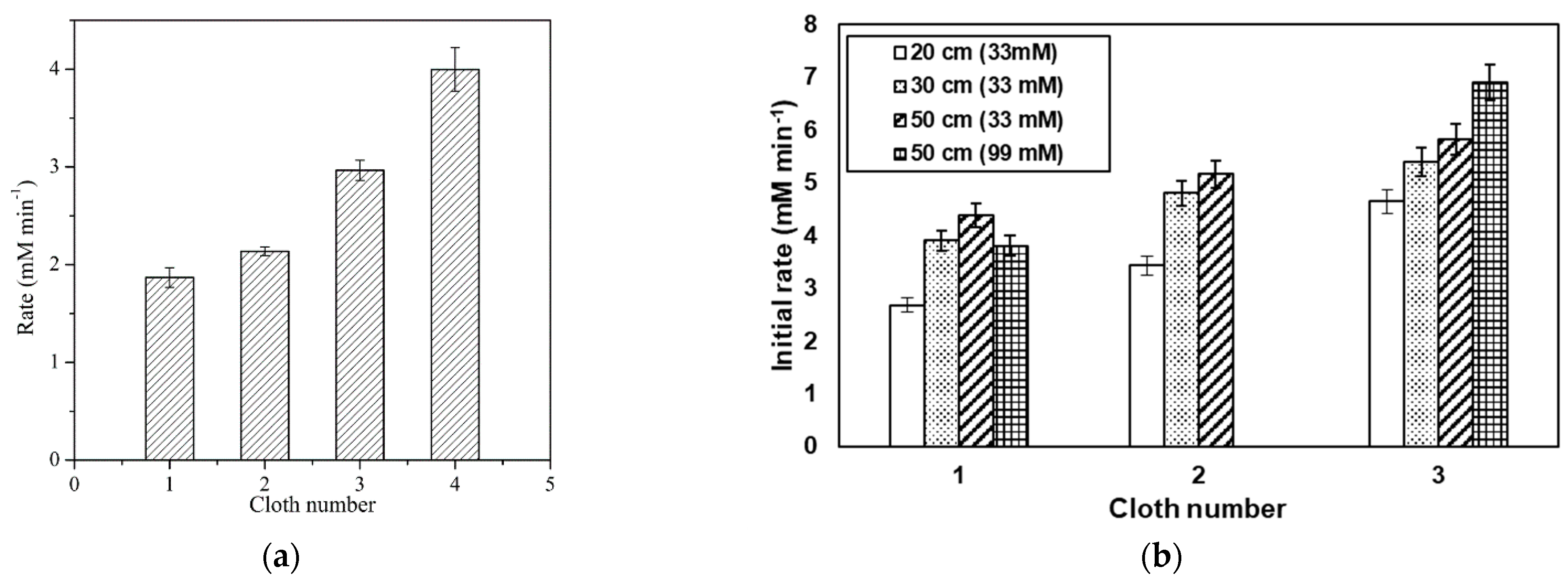
- Shivaprasad, P.; Jones, M.D.; Frith, P.; Emanuelsson, E.A.C. Investigating the effect of increasing cloth size and cloth number in a spinning mesh disc reactor (SMDR): A study on the reactor performance. Chem. Eng. Process. 2020, 147, 107780. [Google Scholar] [CrossRef]
- Shivaprasad, P.; Jones, M.D.; Patterson, D.A.; Emanuelsson, E.A.C. Kinetic resolution of 1-phenylethanol in the spinning mesh disc reactor: Investigating the reactor performance using immobilised lipase catalyst. Chem. Eng. Process. -Process Intensif. 2018, 132, 56–64. [Google Scholar] [CrossRef]
- Shivaprasad, P.; Jones, M.D.; Patterson, D.A.; Emanuelsson, E.A.C. Process intensification of catalysed henry reaction using copper-wool catalyst in a spinning mesh disc reactor. Chem. Eng. Process. Process Intensif. 2017, 122, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Patterson, D.A.; Balaban, M.; Fauconnier, G.; Emanuelsson, E.A.C. The spinning cloth disc reactor for immobilized enzymes: A new process intensification technology for enzymatic reactions. Chem. Eng. J. 2013, 221, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Garside, M. Total Revenue of the Global Chemical Industry from 2005 to 2019. Available online: https://www.statista.com/statistics/302081/revenue-of-global-chemical-industry/ (accessed on 1 March 2021).
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef]
- Kumaresan, T.; Joshi, J.B. Effect of impeller design on the flow pattern and mixing in stirred tanks. Chem. Eng. J. 2006, 115, 173–193. [Google Scholar] [CrossRef]
- Stitt, E. Alternative multiphase reactors for fine chemicals: A world beyond stirred tanks? Chem. Eng. J. 2002, 90, 47–60. [Google Scholar] [CrossRef]
- Eder, P.; Sotoudeh, M. Innovation and Cleaner Technologies as a Key to Sustainable Development: The CASE of the chemical Industry; European Commission, Joint Research Centre, Institute for Prospective, European Commission: Brussles, Belgium, 2000. [Google Scholar]
- Song, J.; Han, B. Green chemistry: A tool for the sustainable development of the chemical industry. Natl. Sci. Rev. 2015, 2, 255–256. [Google Scholar] [CrossRef] [Green Version]
- Shivaprasad, P.; Emanuelsson, E.A.C. Process Intensification of Immobilized Enzyme Reactors. In Intensification of Biobased Processes; Górak, A., Stankiewicz, A., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 249–267. [Google Scholar]
- Béfort, N.; de Fouchécour, F.d.S.; de Rouffignac, A.; Holt, C.A.; Leclère, M.; Loth, T.; Moscoviz, R.; Pion, F.; Ruault, J.-F.; Thierry, M. Toward a European bioeconomic transition: Is a soft shift enough to challenge hard socio-ecological issues? In European Workshop on Bioeconomy; HAL: Paris, France, 2017. [Google Scholar]
- Global Generics; Marketline: London, UK, 2020.
- Kiran Pulidindi, H.P. Flow Chemistry Market Size. Available online: https://www.gminsights.com/industry-analysis/flow-chemistry-market (accessed on 26 March 2021).
- Generics in China; Marketline: London, UK, 2018.
- Generics in India; Marketline: London, UK, 2020.
- Seifert, T.; Sievers, S.; Bramsiepe, C.; Schembecker, G. Small scale, modular and continuous: A new approach in plant design. Chem. Eng. Process. 2012, 52, 140–150. [Google Scholar] [CrossRef]
- Boons, F.; Lüdeke-Freund, F. Business models for sustainable innovation: State-of-the-art and steps towards a research agenda. J. Clean. Prod. 2013, 45, 9–19. [Google Scholar] [CrossRef]
- Raworth, K. Doughnut Economics: Seven Ways to Think Like a 21st-Century Economist; Chelsea Green Publishing: London, UK, 2017. [Google Scholar]
- Radley, B.; Geenen, S. Struggles over value: Corporate–state suppression of locally led mining mechanisation in the Democratic Republic of the Congo. Rev. Afr. Polit. Econ. 2021, 1–17. [Google Scholar]
- Nations, U. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations, Department of Economic and Social Affairs: New York, NY, USA, 2015. [Google Scholar]
- Hilson, G.; Maconachie, R. Artisanal and small-scale mining and the Sustainable Development Goals: Opportunities and new directions for sub-Saharan Africa. Geoforum 2020, 111, 125–141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emanuelsson, E.A.C.; Charles, A.; Shivaprasad, P. A Regenerative Business Model with Flexible, Modular and Scalable Processes in A Post-Covid Era: The Case of The Spinning Mesh Disc Reactor (SMDR). Sustainability 2021, 13, 6944. https://doi.org/10.3390/su13126944
Emanuelsson EAC, Charles A, Shivaprasad P. A Regenerative Business Model with Flexible, Modular and Scalable Processes in A Post-Covid Era: The Case of The Spinning Mesh Disc Reactor (SMDR). Sustainability. 2021; 13(12):6944. https://doi.org/10.3390/su13126944
Chicago/Turabian StyleEmanuelsson, Emma Anna Carolina, Aurelie Charles, and Parimala Shivaprasad. 2021. "A Regenerative Business Model with Flexible, Modular and Scalable Processes in A Post-Covid Era: The Case of The Spinning Mesh Disc Reactor (SMDR)" Sustainability 13, no. 12: 6944. https://doi.org/10.3390/su13126944
APA StyleEmanuelsson, E. A. C., Charles, A., & Shivaprasad, P. (2021). A Regenerative Business Model with Flexible, Modular and Scalable Processes in A Post-Covid Era: The Case of The Spinning Mesh Disc Reactor (SMDR). Sustainability, 13(12), 6944. https://doi.org/10.3390/su13126944





