Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review
Abstract
:1. Introduction
2. Conservation of Plant Genetic Resources: A Brief History
- (i)
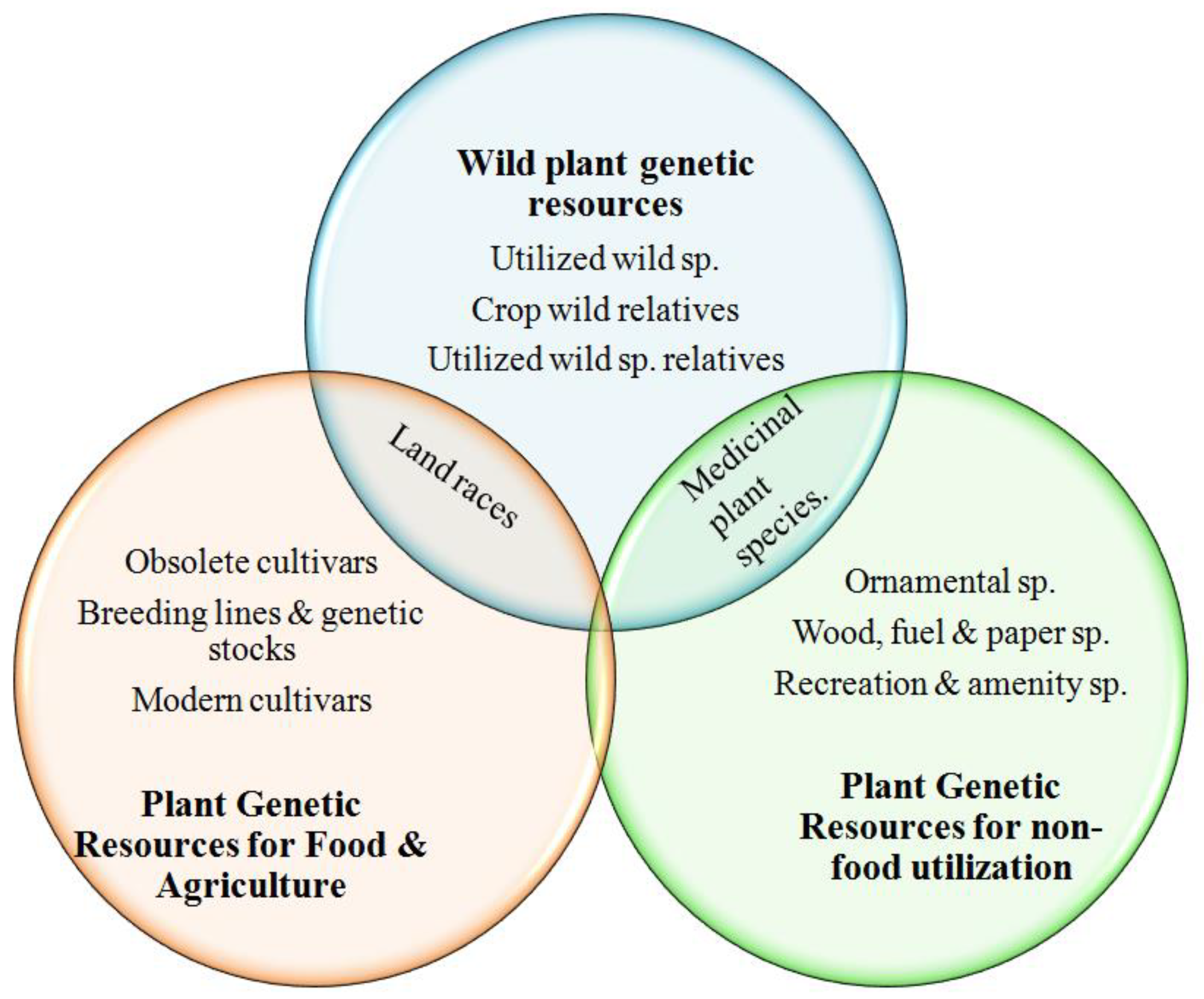
- The primary gene pool (GP1): Crossing among individuals is possible with normal seed set, segregation, and recombination such that gene transfer is possible through routine breeding. It includes both cultivated and wild races of a crop.
- (ii)
- Secondary gene pool (GP2): It includes biological species which have some barriers of crossability with the crop (GP1), resulting in sterile hybrids, as chromosome pairing is not normal; hence, transfer of genes is restricted. Overcoming barriers of crossability can lead to normal seed development
- (iii)
- Tertiary gene pool (GP3): More distant to GP2, crosses of GP3 with a crop (GP1) result in lethal or sterile hybrids due to abnormality in embryo development. Normal gene transfer is not possible but special tissue culture techniques can be deployed to produce hybrid embryos.
3. Need for Germplasm Conservation: Genetic Erosion and Genetic Vulnerability
Global Germplasm Conservation Programs
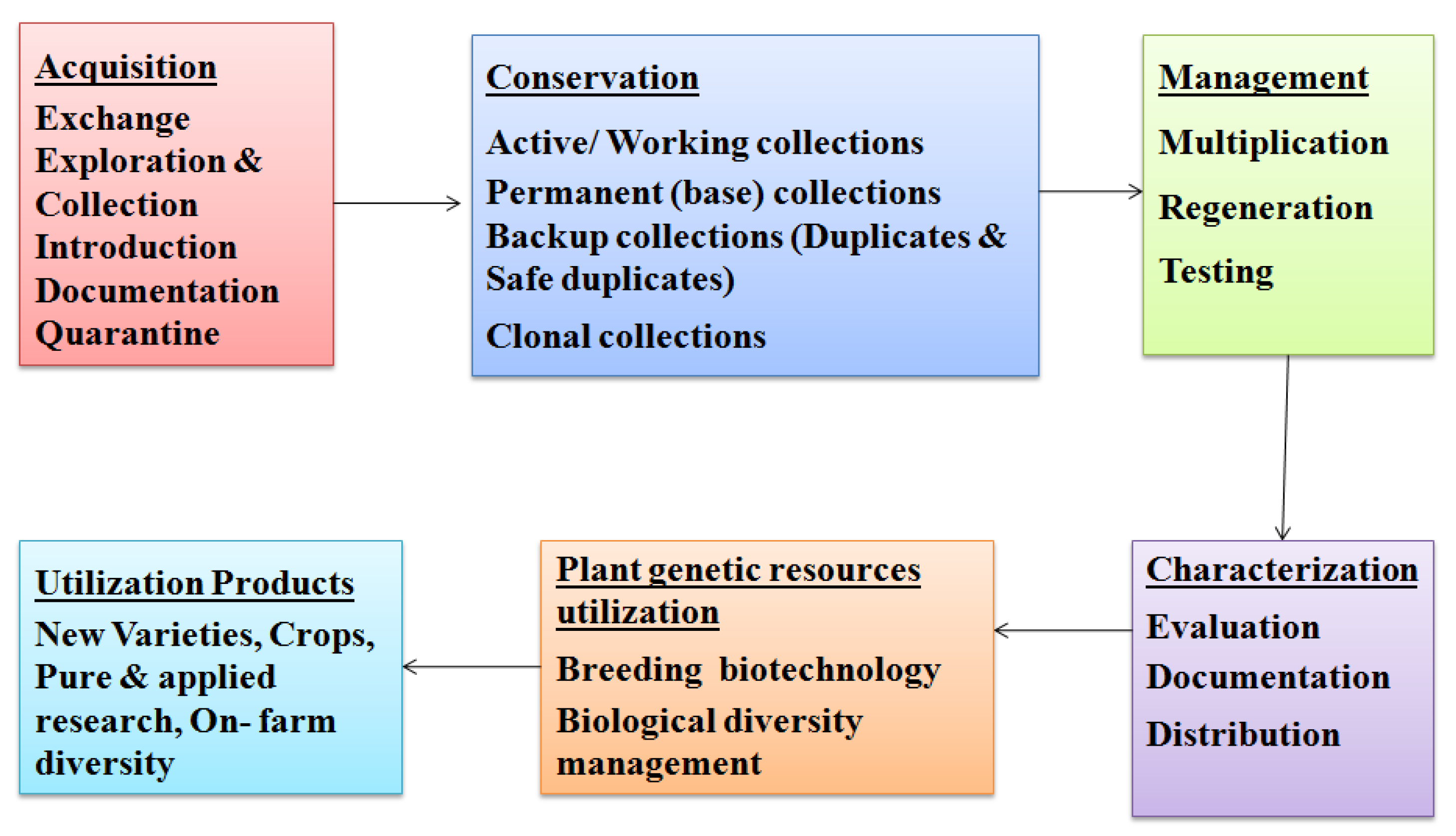
4. Methods of Germplasm Storage and Conservation
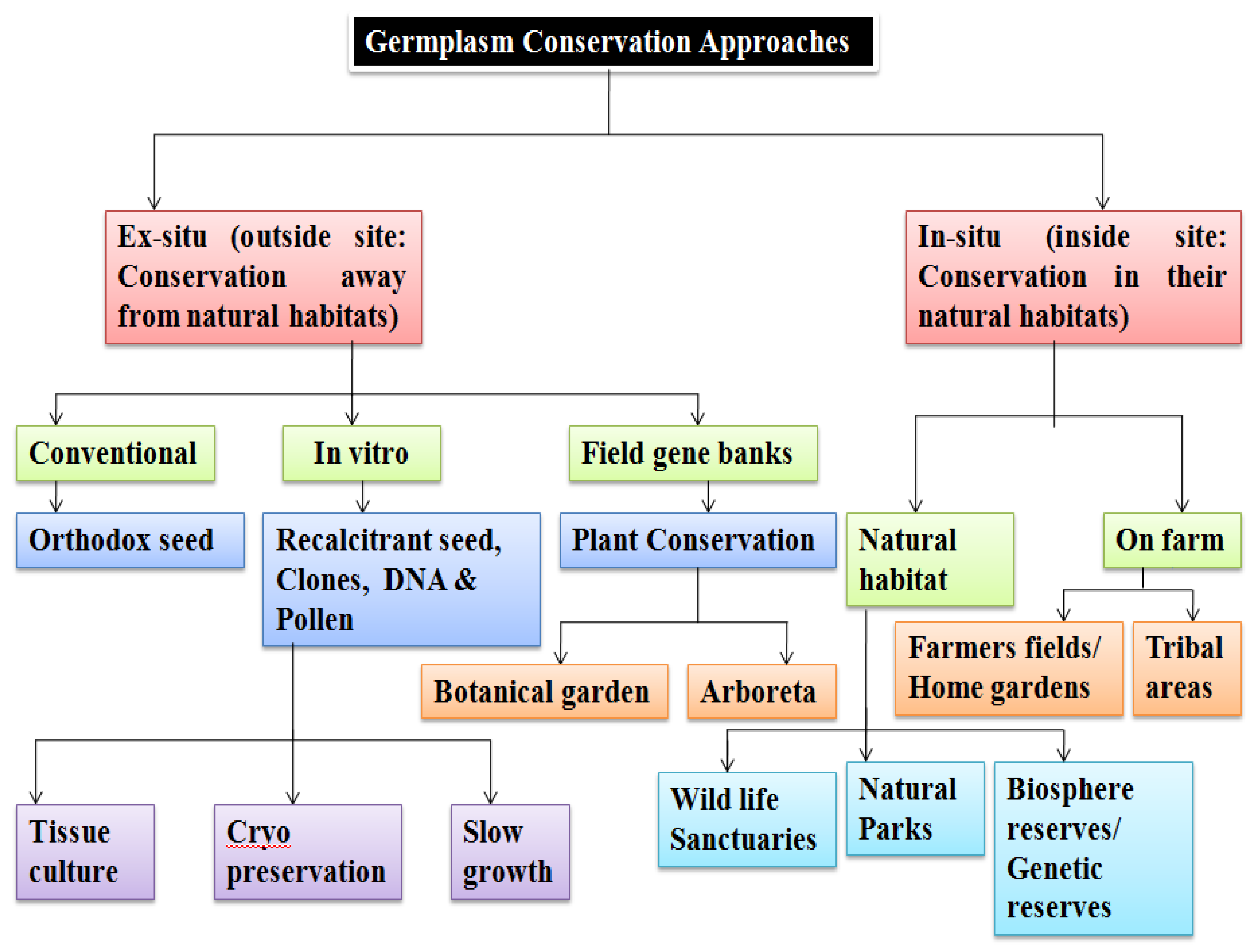
4.1. Ex Situ Conservation
- (i)
- Orthodox seeds: Such seeds can tolerate drying (5% RH) and freezing (very low temperatures) but remain viable. The vast majority of plants fall into this category whose seeds can thus be easily preserved for long periods of time [47]. Examples include citrus, guava, capsicum, cashew, and most grains and legumes.
- (ii)
- Recalcitrant seeds: Such seeds cannot tolerate drying and freezing. They lose viability significantly if the moisture content goes below 30–50%. Examples include a number of tropical trees and fruits, such as pineapples, cocoa, coffee, oil palm, mango, jackfruit, etc. Such seeds can be stored at temperatures of 0–10 °C briefly (1 to 5 years) while retaining their viability.
4.1.1. In Vitro Conservation
4.1.2. Methods Involved in the In Vitro Conservation of Germplasm
- (i)
- IVAG: In vitro active gene bank (in vitro conservation under slow growth) is widely used by a range of national and international research centers such as NBPGR, IITA (International Institute of Tropical Agriculture), and CIP (International Potato Centre) [60]. This technique can only be used as a short- to medium-term conservation strategy, meaning that it is impossible to preserve extensive collections using this process.
- (ii)
- Cryopreservation: This strategy makes use of solid carbon dioxide (−79 °C), minimum temperature deep freezers (−80 °C), vapor nitrogen (−150 °C), or liquid nitrogen (−196 °C) for preserving cells and tissues in a frozen state at extremely low temperatures. The cell can be preserved for a prolonged period of time when it is inactivated at such low temperatures. Plant tissues that can be cryopreserved include meristems, eggs, endosperms, ovules, plants, plant cells, plant protoplasts, and calli [61]. Two advanced cryopreservation methods are employed that focus on the mitigation of cell damage caused by the production of ice crystals. One approach includes vitrifying cellular water with cryoprotective products, whereas the other involves encasing specimens in alginate gel and then dehydrating them. When a specimen is vitrified, a cryoprotective fluid is infused, facilitating the conversion to a non-crystalline vitreous solid of mostly cellular water [62]. Encapsulation involves embedding the specimen into an alginate gel [63], either in the form of a shoot tip or somatic embryo, to provide an artificial seed that is dehydrated. This procedure involves many actions, of which freezing, thawing, and reculturing are the most significant. Shoots, leaves, floral parts, immature embryos, hypocotyl bits, or cotyledons can be used as explants [64], thus requiring the establishment of systematic protocols. The cryopreservation of dormant buds and in vitro shoot tips is an alternate solution for long-term protection [65,66]. Progress is now underway in the perception of best practices for the cryopreservation of a range of commercially significant plants, including apples, grapes, and citrus [67,68]. For example, in some cases, it provides alternate ways to save entire species. Second, the transfer of germplasm is facilitated. Third, approaches to molecular biology are used to address germplasm control and use-related issues. The fourth impact stems from the growing demands of biotechnologists for germplasm and conservation resources. Biotechnological techniques, including in vitro culture, cryopreservation, and molecular markers, would be beneficial to plant diversity research and genetic resource control studies and in turn eventual restoration [69]. However, because of their high susceptibility to desiccation, systemic sophistication, and heterogeneity, it is far less sophisticated for recalcitrant seed species. Many scientific barriers prevent the use of cryopreservation regularly for plant meristems, pollens, and plant cells. Although many scientific collections and germplasm banks conduct cryopreservation experiments, none use cryopreservation for the storage of non-seed germplasm.
- (i)
- Slow-growing cultures: This is a viable alternative to cryopreservation as it is cost-effective and straightforward and contamination through gene alterations are usually minimized [70]. Subculture cycles may be stretched up to 1 or 2 years, shortening the time, effort, and equipment needed to maintain them. Slower growth lessens the rate of cell division; thus, spontaneous mutation in culture is multiplied a number of times. Collections preserved under in vitro for slow growth are often susceptible to genetic instability and infection. All variables that affect culture development include temperature, nutritional constraint, growth regulation, and osmotic concentration. Other factors include oxygen concentration, the form of the propagation vessel used, and the light needed by cultures. In addition, stress variables may have different effects on the genotype of the population, some preferring somaclonal variants over others [71]. This could contribute to a cell population change and the genetic integrity of the original clonal material not being maintainable, as in shooting cultures of banana [72].
- (ii)
- DNA storage: Establishment of a DNA storage facility as a backup to traditional ex situ storage has been suggested [73] but is not widely used, since the use of stored genes for PGRFA is restricted because these need to be isolated, cloned, and then utilized through the production of a transgenic plant. DNA storage mainly complements germplasm conservation, as it forms a basis of genomic material that explains species origin or population diversity.
- (iii)
- Cold storage: This is a form of short-term storage, slow-growth preservation process where the germplasm is stored at a moderate, non-freezing temperature (1–9 °C). The prominent benefit of this method is that it accelerates plant growth in cold storage rather than stopping it during cryopreservation, so that plants are protected from cryogenic damage [74]. In addition, this technique is useful, inexpensive, and produces germplasm with higher rates of survival. Cold storage of the in vitro collection provides additional security while keeping the plants available for study or distribution. Many excellent reports exist on cold storage, such as virus-free strawberry plants that could be stored at 10 °C for around six years, certain grape plants that could be stored for about 15 years (by moving them to fresh medium every year) have recently been published [75].
- (iv)
- Low-pressure and low-oxygen storage: Under low-pressure storage (LPS), the atmospheric pressure surrounding the plant material is decreased which yields a partial reduction of the pressure exerted by the gases around the germplasm. The lowering of partial pressure decreases the in vitro growth of plants (of organized or unorganized tissues). Low-pressure storage systems are essential for both the short- and long-term storage of plant materials. The short-term storage is specifically useful to enhance the shelf life of many plant materials like fruits, vegetables, cut flowers, or plant cuttings. The storage of germplasm grown in cultures can be done long term under low pressure. In addition to germplasm preservation, LPS decreases the activity of pathogenic organisms and prevents spore germination in the plant culture systems. In low-oxygen storage, the oxygen concentration is decreased, but the atmospheric pressure (260 mm Hg) is maintained by the addition of inert gases (specifically nitrogen). There is a reduction in plant tissue growth if the partial pressure of oxygen is below 50 mm Hg. This is because, with less availability of O2, the production of CO2 is low. As a result, the photosynthetic activity is decreased, thereby halting the plant tissue growth and dimension.
4.1.3. Ex Situ Conservation in Field Gene Banks, Botanic Gardens, and Arboreta
4.2. In Situ Conservation
4.2.1. Natural Reserves or Genetic Reserves
4.2.2. On-Farm and Home Garden Conservation
5. Status of Germplasm Conservation
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkes, G. In Situ Conservation of Agricultural Systems. In Biodiversity; Culture, Conservation and Eco Development; Oldfield, M., Alcorn, J., Eds.; West View Press: Boulder, CO, USA, 1991; pp. 86–101. [Google Scholar]
- Rao, N.K. Plant genetic resources: Advancing conservation and use through biotechnology. Afr. J. Biotechnol. 2004, 3, 136–145. [Google Scholar] [CrossRef]
- Baute, G.J.; Dempewolf, H.; Rieseberg, L.H. Using genomic approaches to unlock the potential of CWR for crop adaptation to climate change. In Crop Wild Relatives and Climate Change; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kell, S.; Marino, M.; Maxted, N. Bottlenecks in the PGRFA use system: Stakeholders’ perspectives. Euphytica 2017, 213, 170. [Google Scholar] [CrossRef] [Green Version]
- Fao/Ipgri/Onu. Genebank standards for Plant Genetic Resources for Food and Agriculture, Rev. ed.; FAO: Rome, Italy, 2014; p. 182. ISBN 9290432365. [Google Scholar]
- Gosal, S.S.; Wani, S.H.; Kang, M.S. Biotechnology and crop improvement. J. Crop. Improv. 2010, 24, 153–217. [Google Scholar] [CrossRef]
- De Wet, J.M.J. Cereals for the semi-arid tropics. In Plant Domestication by Induced Mutation, Proceedings of the Advisory Group Meeting on The Possible Use of Mutation Breeding for Rapid Domestication of New Crop Plants, Vienna, Austria, 17–21 November 1986; Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture: Rome, Italy, 1989; pp. 79–88. [Google Scholar]
- Wright, B.D. Crop genetic resource policy: The role of ex situ gene banks. Aust. J. Agric. Resour. Econ. 1997, 41, 81–115. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Gowda, C.L.L.; Buhariwalla, H.K.; Crouch, J.H. Efficient use of crop germplasm resources: Identifying useful germplasm for crop improvement through core and mini-core collections and molecular marker approaches. Plant Genet. Resour. 2006, 4, 25–35. [Google Scholar] [CrossRef]
- Debouck, D.G. Managing Plant Genetic Diversity. Crop Sci. 2003, 43, 749–750. [Google Scholar] [CrossRef]
- Warschefsky, E.; Penmetsa, R.V.; Cook, D.R.; Von Wettberg, E.J.B. Back to the wilds: Tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. Am. J. Bot. 2014, 101, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.M.; Aranzana, M.J. Attention sports fans! the far-reaching contributions of bud sport mutants to horticulture and plant biology. Hortic. Res. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vavilov, N.I. Origin and Geography of Cultivated Plant; Cambridge University Press: Cambridge, UK, 1992; ISBN 0521404274. [Google Scholar]
- Zhukovsky, P.M. Main gene centres of cultivated plants and their wild relatives within the territory of the U.S.S.R. Euphytica 1965, 14, 177–188. [Google Scholar] [CrossRef]
- Harlan, J.R.; De Wet, J.M.J. Toward a Rational Classification of Cultivated Plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Haussmann, B.I.G.; Parzies, H.K.; Presterl, T.; Miedaner, T. Plant genetic resources in crop improvement. Plant Genet. Resour. Charact. Util. 2006, 2, 3–21. [Google Scholar] [CrossRef]
- IUCN RED LIST 2019-20. Source-IUCN Official Website. Available online: https://www.iucnredlist.org/ (accessed on 15 March 2021).
- Offord, C.A. Germplasm Conservation. In Encyclopedia of Applied Plant Science, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 2, pp. 281–288. ISBN 9780123948083. [Google Scholar] [CrossRef]
- Crop Trust. Securing Crop Diversity for Sustainable Development; Global Crop Diversity Trust: Bonn, Germany, 2015. [Google Scholar]
- Offord, C.; Makinson, R. Options and major considerations for plant germplasm conservation. In Guidelines for Germplasm Conservation in Australia: Strategies and Guidelines for Developing, Managing and Utilizing Ex Situ Conservation Collections; Offord, C.A., Meagher, P.F., Eds.; Australian Network for Plant Conservation: Canberra, Australia, 2009; pp. 11–34. [Google Scholar]
- Malhotra, N.; Panatu, S.; Singh, B.; Negi, N.; Singh, D.; Singh, M.; Chandora, R. Genetic Resources: Collection, Conservation, Characterization and Maintenance; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135228. [Google Scholar]
- Harlan, H.V. The Origin of Hooded Barley. J. Hered. 1931, 22, 265–272. [Google Scholar] [CrossRef]
- Martos, V.; Royo, C.; Rharrabti, Y.; Garcia Del Moral, L.F. Using AFLPs to determine phylogenetic relationships and genetic erosion in durum wheat cultivars released in Italy and Spain throughout the 20th century. Fields Crop. Res. 2005, 91, 107–116. [Google Scholar] [CrossRef]
- Rocha, F.; Bettencourt, E.; Gaspar, C. Genetic erosion assessment through the re-collecting of crop germplasm. Counties of ArcodeValdevez, Melgaço, Montalegre, Ponte da Barca and Terras de Bouro (Portugal). Plant Genet. Resour. Newsl. 2008, 154, 6–13. [Google Scholar]
- Ruiz, M.; Rodriguez-Quizano, M.; Metakovsky, E.V.; Vazquez, J.F.; Carrillo, J.M. Polymorphism, variation and genetic identity of Spanish common wheat germplasm based on gliadins alleles. Field Crops Res. 2002, 79, 185–196. [Google Scholar] [CrossRef]
- Ross-Ibarra, J.; Morrell, P.L.; Gaut, B.S. Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proc. Acad. Natl. Sci. USA 2007, 104 (Suppl. 1), 8641–8648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, L. Approaches to measuring genetic erosion. PGR Documentation and Information in Europe—Towards a sustainable and user-oriented information infrastructure. In Proceedings of the EPGRIS Final Conference Combined with a Meeting of the ECP/GR Information and Documentation Network, Prague, Czech Republic, 11–13 September 2003. [Google Scholar]
- Upadhyaya, H.D.; Gowda, C.L.L. Managing and Enhancing the Use of Germplasm—Strategies and Methodologies; Technical Manual No. 10; ICRISAT: Patancheru, India, 2009. [Google Scholar]
- Brodie, J.F.; Aslan, C.E.; Rogers, H.S.; Redford, K.H.; Maron, J.L.; Bronstein, J.L.; Groves, C.R. Secondary Extinctions of Biodiversity. Trends Ecol. Evol. 2014, 29, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.P.; Fox, P.N. Impact of CIMMYT varieties on the genetic diversity of wheat in Australia. Aust. J. Agric. Res. 1993, 49, 175–178. [Google Scholar] [CrossRef]
- Kolodinska, B.A.; Von Bothmer, R.; Dayeg, C.; Rashal, I.; Tuvesson, S.; Weibull, J. Inter simple sequence repeat analysis of genetic diversity and relationships in cultivated barley of Nordic and Baltic region. Hereditas 2004, 14, 186–192. [Google Scholar] [CrossRef]
- Castaneda-Alvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. The Convention on Biological Diversity Plant Conservation Report: A Review of Progress in Implementing the Global Strategy of Plant Conservation (GSPC); Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009. [Google Scholar]
- Aravind, J.; Radhamani, J.; Srinivasan, K.; Subhash, B.A.; Tyagi, R.K.; Aubrey, M.; Atkinson, K.; Philips, L. Package ‘PGRdup’ Title Discover Probable Duplicates in Plant Genetic Resources Collections. 2017. Available online: https://cran.rproject.org/package=PGRdup (accessed on 15 March 2021).
- Engels, J.; Visser, L. A Guide to Effective Management of Germplasm Collections; Bioversity International: Maccarese-Stazione, Italy, 2003; Volume 59, ISBN 9290435828. [Google Scholar]
- Frankel, O.; Brown, A. Current Plant Genetic Resources: A Critical Appraisal; Oxford & IBH Publ. Co.: New Delhi, India, 1984. [Google Scholar]
- Upadhyaya, H.D.; Ortiz, R. A mini-core collection for capturing diversity and promoting utilization of chickpea genetic resources in crop improvement. Theor. Appl. Genet. 2001, 102, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Haupt, M.; Schmid, K. Combining focused identification of germplasm and core collection strategies to identify genebank accessions for central European soybean breeding. Plant Cell Environ. 2020, 43, 1421–1436. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.J. Methodology to establish a composite collection: Case study in lentil. Plant Genet. Resour. 2006, 4, 2–12. [Google Scholar] [CrossRef]
- Azough, Z.; Kehel, Z.; Benomar, A.; Bellafkih, M.; Amri, A. Predictive Characterization of ICARDA Gene bank Barley Accessions Using FIGS and Machine Learning. Intell. Environ. 2019, 121–129. [Google Scholar]
- Maxted, N. Ex Situ, In Situ Conservation. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 683–695. ISBN 9780122268656. [Google Scholar] [CrossRef]
- Ifeanyi, N.; Okorie, N.; Marshall, U.E. Germplasm Preservation and Propagation; The Foundation of Agricultural Development—A Review. IOSR J. Pharm. Biol. Sci. 2016, 11, 70–73. [Google Scholar] [CrossRef]
- International Seed Testing Association. International Rules for Seed Testing 2018; ISTA: Bassersdorf, Switzerland, 2018. [Google Scholar]
- Roberts, E.H. Predicting the storage life of seeds. Proceedings 1973, 1, 499–514. [Google Scholar]
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The Ex Situ Conservation of Plant Genetic Resources; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Harrington, J.F. Thumb Rules of Drying Seed. Crops Soils 1960, 13, 16–17. [Google Scholar]
- Merritt, D.; Dixon, K. Restoration Seed Banks—A Matter of Scale. Science 2011, 332, 424–425. [Google Scholar] [CrossRef]
- Kholina, A.B.; Voronkova, N.M. Seed Cryopreservation of Some Medicinal Legumes. J. Bot. 2012, 2012, 186891. [Google Scholar] [CrossRef] [Green Version]
- Horna, D.; Smale, M. Evaluating Cost-Effectiveness of Collection Management: A Methodological Framework. 2010. Available online: http://cropgenebank.sgrp.cgiar.org/images/file/management/DST/framework_dst.pdf (accessed on 14 April 2021).
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Hay, F.R.; Chen, W.Y.; Zhou, Z.K.; Pritchard, H.W. A comparative study of desiccation responses of seeds of Asian Evergreen Oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. S. Afr. J. Bot. 2012, 78, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Han, C.Y.; Sun, W.B. Seed storage behaviour of Magnolia odoratissima. Seed Sci. Technol. 2013, 41, 143–147. [Google Scholar] [CrossRef]
- Jayasuriya, K.M.G.G.; Wijetunga, A.S.T.B.; Baskin, J.M.; Baskin, C.C. Seed dormancy and storage behaviour in tropical Fabaceae: A study of 100 species from Sri Lanka. Seed Sci. Res. 2013, 23, 257–269. [Google Scholar] [CrossRef]
- Henshaw, G.G. Technical aspects of tissue culture storage for genetic conservation. In Crop Genetic Resources for Today and Tomorrow; Cambridge University Press: Cambridge, UK, 1975; pp. 349–358. [Google Scholar]
- Morel, G. Meristem culture techniques for the long-term storage of cultivated plants. In Crop Genetic Resources for Today and Tomorrow; Frankel, O.H., Hawkes, J.G., Eds.; Cambridge University Press: Cambridge, UK, 1975; pp. 327–332. [Google Scholar]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Ding, F.; Jin, S.; Hong, N.; Zhong, Y.; Cao, Q.; Yi, G.; Wang, G. Vitrification-cryopreservation, an efficient method for eliminating Candidatus Liberobacter asiaticus, the citrus Huanglongbing pathogen, from in vitro adult shoot tips. Plant Cell Rep. 2008, 27, 241–250. [Google Scholar] [CrossRef] [PubMed]
- El-Dougdoug, K.A.; El-Shamy, M.M. Plant Tissue Culture: Current Status and Opportunities. Afr. J. Microbiol. 2011. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Costa, M.D.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A new technique to obtain grapevine plants free of viruses. Rev. Bras. Frutic. 2016, 38, 833. [Google Scholar] [CrossRef] [Green Version]
- Valerie, C.P.; Jorge, A.S.; Victor, M.V.A.; Florent, E. In Vitro Collecting Techniques for Germplasm Conservation; IPGRI Technical Bulletin No. 7; International Plant Genetic Resource Institute: Rome, Italy, 2002; p. 250. [Google Scholar]
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop Sci. 2011, 5, 778–800. [Google Scholar]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- West, T.P.; Preece, J.E. Use of acephate, benomyl and alginate encapsulation for eliminating culture mites and fungal contamination from in vitro cultures of hardy hibiscus (Hibiscus moscheutos L.). In Vitro Cell. Dev. Biol. Plant 2006, 42, 301–304. [Google Scholar] [CrossRef]
- Paunescu, A. Biotechnology for Endangered Plant Conservation: A Critical Overview. Rom. Biotechnol. Lett. 2009, 14, 4095–4103. [Google Scholar]
- Wang, M.-R.; Chen, L.; da Silva, J.A.T.; Volk, G.M.; Wang, Q.-C. Cryobiotechnology of apple (Malus spp.): Development, progress and future prospects. Plant Cell Rep. 2018, 37, 689–709. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Cryopreservation of grapevine (Vitis spp.) shoot tips from growth chamber-sourced plants and histological observations. Vitis 2019, 78, 71–78. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Reed, B.M. Cryopreserved storage of clonal germplasm in the USDA National Plant Germplasm System. In Vitro Cell. Dev. Biol. Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Normah, M.N.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Dixit, S.; Mandal, B.B.; Ahuja, S.; Srivastava, P.S. Genetic stability assessment of plants regenerated from cryopreserved embryogenic tissues of Dioscoreabulbifera Using RAPD, biochemical and morphological analysis. Cryoletters 2003, 24, 77–84. [Google Scholar]
- Chauhan, R.; Singh, V.; Quareshi, A. In Vitro Conservation Through Slow-Growth Storage. Synth. Seeds Germplasm Regen. Preserv. Prospect. 2019, 1, 482. [Google Scholar] [CrossRef]
- Silva, R.L.; Ferreira, C.F.; Ledo, C.A.S.; Souza, E.H.; Silva, P.H.; Costa, M.A.P.C.; Souza, F.V.D. Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell. Tissue Organ Cult. 2016, 127, 123–133. [Google Scholar] [CrossRef]
- Kulkarni, V.M.; Ganapathi, T.R. A simple procedure for slow growth maintenance of banana (Musa spp.) embryogenic cell suspension cultures at low temperature. Curr Sci. 2009, 96, 1372–1377. [Google Scholar]
- Dulloo, M.E.; Hunter, D.; Borelli, T. Ex situ and in situ conservation of agricultural biodiversity: Major advances and research needs. Not. Bot. Horti Agrobot. Cluj Napoca 2010, 38, 123–135. [Google Scholar] [CrossRef]
- West, T.P.; Ravindra, M.B.; Preece, J.E. Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell. Tissue Organ Cult. 2006, 87, 223–231. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Lyudvikova, Y.; Volgina, M.; Reed, B.M. Medium, container and genotype all influence in vitro cold storage of apple germplasm. Plant Cell Tissue Organ Cult. 2009, 96, 127–136. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef] [Green Version]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Bernstein, M.E.; Carroll, G.C. Internal fungi in old-growth Douglas fir foliage. Can. J. Bot. 1977, 55, 644–653. [Google Scholar] [CrossRef]
- Smith, R.H.; Burrows, J.; Kurten, K. Challenges associated with micropropagation of Zephyranthes and Hippeastrum sp. (Amaryllidaceae). In Vitro Cell. Devel. Biol. Plant 1999, 35, 281–282. [Google Scholar] [CrossRef]
- Merkle, S.A.; Bailey, R.L.; Pauley, B.A.; Neu, K.A.; Kim, M.K.; Rugh, C.L. Somatic embryogenesis from tissues of mature sweetgum trees. Can. J. For. Resour. 1997, 27, 959–964. [Google Scholar] [CrossRef]
- Pence, V.C. In vitro collection (IVCN). In A Colour Atlas of Plant Propagation and Conservation; Bowes, B., Ed.; CRC Press: Boca Raton, FL, USA, 1999; ISBN 1840765410. [Google Scholar]
- MacKay, W.A. Micropropagation systems for the Mexican red bud (Cercis canadensisvar.mexicana L.) and other woody plants of the Chichuahuan desert. In Vitro Cell. Devel. Biol. Plant 1999, 35, 283–284. [Google Scholar] [CrossRef]
- De, M. Field Gene Banks: The Living Repositories of Plant Genetic Resources. Harvest 2017, 2, 21. [Google Scholar]
- O’Donnell, K.; Sharrock, S. Botanic gardens complement agricultural gene bank in collecting and conserving plant genetic diversity. Biopreserv. Biobank. 2018, 16, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Cruz, C.A.; Gonzalez-Arnao, M.T.; Engelman, F. Biotechnology and conservation of plant biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Hoyt, E. Conserving the Wild Relatives of Crops; International Board for Plant Genetic Resources: Rome, Italy, 1988. [Google Scholar]
- IBPGR. Ecogeographical Surveying and In Situ Conservation of Crop Relatives; International Board for Plant Genetic Resources: Rome, Italy, 1985. [Google Scholar]
- Meir, N.; Anikster, Y.; Waldman, M.; Ashri, A. Population dynamics research for in situ conservation: Wild wheat in Israel. Plant Genet. Resour. Newsl. 1989, 75/76, 9–11. [Google Scholar]
- Plucknett, D.L.; Smith, N.J.H. Gene Banks and The World’s Food; Princeton University Press: Princeton, NJ, USA, 2014; ISBN 1400858119. [Google Scholar]
- Kaya, Z.; Kün, E.; Güner, A. National Plan for In Situ Conservation of Plant Genetic Diversity in Turkey; Ministry of Environment, Ministry of Agriculture and Rural Affairs and Ministry of Forestry: Ankara, Turkey, 1998; pp. 98–113. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2015046805 (accessed on 25 March 2021).
- Maxted, N.; Guarino, L.; Myer, L.; Chiwona, E.A. Towards a methodology for on-farm conservation of plant genetic resources. Genet. Resour. Crop Evol. 2002, 49, 31–46. [Google Scholar] [CrossRef]
- Swaminathan, M.S. The past, present and future contributions of farmers to the conservation and development of genetic diversity. In Managing Plant Genetic Diversity, Proceedings of an International Conference, Kuala Lumpur, Malaysia, 12–16 June 2000; CABI: Wallingford, UK; pp. 23–31. [CrossRef]
- Halewood, M.; Jamora, N.; Noriega, I.L.; Anglin, N.L.; Wenzl, P.; Payne, T.; Ndjiondjop, M.N.; Guarino, L.; Lava Kumar, P.; Yazbek, M. Germplasm acquisition and distribution by cigar gen ebanks. Plants 2020, 9, 1296. [Google Scholar] [CrossRef] [PubMed]
- FAO. WIEWS: World Information and Early Warning System on Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/wiews (accessed on 10 April 2021).
- FAO. 2020. Available online: http://www.fao.org/sustainable-development-goals/indicators/251a/en/2020 (accessed on 13 April 2021).
- Byrne, P.F.; Volk, G.M.; Gardner, C.; Gore, M.A.; Simon, P.W.; Smith, S. Sustaining the Future of Plant Breeding: The Critical Role of the USDA-ARS National Plant Germplasm System. Crop Sci. 2018, 468, 451–468. [Google Scholar] [CrossRef] [Green Version]
- GRIN-Global. U.S. National Plant Germplasm System. GRIN-Glob. Web Version 11040. 2019. Available online: https://npgsweb.ars-grin.gov/gringlobal/query/summary.aspx (accessed on 2 April 2021).
- Migicovsky, Z.; Warschefsky, E.; Klein, L.L.; Miller, A.J. Using living germplasm collections to characterize, improve, and conserve woody perennials. Crop Sci. 2019, 59, 2365–2380. [Google Scholar] [CrossRef] [Green Version]
- National Fruit Collection in the United Kingdom. Available online: http://www.nationalfruitcollection.org.uk/ (accessed on 20 March 2021).
- N.I. Vavilov All-Russian Science Research Institute of Plant Industry’s Fruit Collection. Available online: http://www.vir.nw.ru/unu-kollektsiya-vir/ (accessed on 15 March 2021).
- Foreign Centre for Research in Agronomy. Available online: http://www.vir.nw.ru/unu-kollekts (accessed on 10 April 2021).
- CGIAR Genebank Platform. 2019 Genebank Platform Annual Report; Global Crop Diversity Trust: Bonn, Germany, 2020. [Google Scholar]
- CWR Project. Annual Report; Global Crop Diversity Trust: Bonn, Germany, 2019. [Google Scholar]
- Miller, A.J.; Novy, A.; Glover, J.; Kellogg, E.A.; Maul, J.E.; Raven, P.; Jackson, P.W. Expanding the role of botanical gardens in the future of food. Nat. Plants. 2015, 1, 15078. [Google Scholar] [CrossRef]
- Cibrian-Jaramillo, A.; Hird, A.; Oleas, N.; Ma, H.; Meerow, A.W.; Francisco-Ortega, J.; Griffith, M.P. What is the conservation value of a plant in a botanic garden. Using indicators to improve management of ex Situ collections. Bot. Rev. 2013, 79, 559–577. [Google Scholar] [CrossRef]
- Jackson, P.W.; Kennedy, K. The global strategy for plant conservation: A challenge and opportunity for the international community. Trends Plant Sci. 2009, 14, 578–580. [Google Scholar] [CrossRef]
- Genesys Database. Available online: www.genesys-pgr.org (accessed on 22 March 2021).
- BGCI’s Plant Search. Available online: https://tools.bgci.org/plant_search.php (accessed on 22 March 2021).
- Available online: http://www.fao.org/home/en/ (accessed on 26 March 2021).
- Hanson, J.; Ellis, R.H. Progress and challenges in ex situ conservation of forage germplasm: Grasses, herbaceous legumes and fodder trees. Plants 2020, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, X.; Guo, T.; Zhu, C.; Wu, Y.; Mitchell, S.E.; Roozeboom, K.L.; Wang, D.; Wang, M.L.; Pederson, G.A.; et al. Genomic prediction contributing to a promising global strategy to turbo charge gene banks. Nat. Plants. 2016, 2, 16150. [Google Scholar] [CrossRef]
- IITA-International Institute of Tropical Agriculture. Available online: https://www.genebanks.org/genebanks/iita/ (accessed on 9 April 2021).
- CIAT-International Centre for Tropical Agriculture. Available online: https://www.genebanks.org/genebanks/ciat/ (accessed on 9 April 2021).
- CIMMYT-International Maize and Wheat Improvement Centre. Available online: https://www.genebanks.org/genebanks/cimmyt/ (accessed on 3 April 2021).
- CIP-International Potato Centre. Available online: https://www.genebanks.org/genebanks/international-potato-centre/ (accessed on 9 April 2021).
- ICARDA-International Centre for Agricultural Research in the Dry Areas. Available online: https://www.genebanks.org/genebanks/icarda/ (accessed on 3 April 2021).
- ICRISAT-International Crops Research Institute for the Semi-Arid Tropics. Available online: https://www.genebanks.org/genebanks/icrisat/ (accessed on 3 April 2021).
- AfricaRice-Africa Rice Centre. Available online: https://www.genebanks.org/genebanks/africarice/ (accessed on 9 April 2021).
- Bioversity International. Available online: https://www.genebanks.org/genebanks/biodiversity-international/ (accessed on 9 April 2021).
- ICRAF-World Agro Forestry. Available online: https://www.genebanks.org/genebanks/icraf/ (accessed on 3 April 2021).
- ILRI-International Livestock Research Institute. Available online: https://www.genebanks.org/genebanks/ilri/ (accessed on 9 April 2021).
- IRRI-International Rice Research Institute. Available online: https://www.genebanks.org/genebanks/irri/ (accessed on 3 April 2021).
- National Bureau of Plant Genetic Resources (NBPGR). Available online: http://www.nbpgr.ernet.in/Research_Projects/Base_Collection_in_NGB.aspx (accessed on 13 April 2021).
- Acuna, C.A.; Martínez, E.J.; Zilli, A.L.; Brugnoli, E.A.; Espinoza, F.; Marcon, F.; Urbani, M.H.; Quarin, C.L. Reproductive Systems in Paspalum: Relevance for Germplasm Collection and Conservation, Breeding Techniques, and Adoption of Released Cultivars. Front. Plant Sci. 2019, 10, 1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nass, L.L.; Sigrist, M.S.; Ribeiro, C.S.C.; Reifschneider, F.J.B. Genetic resources: The basis for sustainable and competitive plant breeding. Crop Breed. Appl. Biotechnol. 2012, 12, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.R.; Pinto, A.C.Q. Germplasm Conservation and Use of Genebanks for Research Purposes of Tropical and Subtropical Fruits in Brazil. Acta Hortic. 2010, 864, 21–28. [Google Scholar] [CrossRef]
- Halewood, M.; Chiurugwi, T.; Sackville Hamilton, R.; Kurtz, B.; Marden, E.; Welch, E.; Michiels, F.; Mozafari, J.; Sabran, M.; Patron, N.; et al. Plant genetic resources for food and agriculture: Opportunities and challenges emerging from the science and information technology revolution. New Phytol. 2018, 217, 1407–1419. [Google Scholar] [CrossRef]
- Smale, M.; Jamora, N. Valuing genebanks. Food Sec. 2020, 12, 905–918. [Google Scholar] [CrossRef]
| Category | Status |
|---|---|
| EX—Extinct | 122 |
| EW—Extinct in the wild | 42 |
| CR—Critically endangered | 4674 |
| EN—Endangered | 8593 |
| VU—Vulnerable | 8459 |
| LR/cd—Lower risk: Conservation dependent | 157 |
| NT or LR/nt—Near threatened | 3181 |
| LC or LR/lc—Least concern | 24,810 |
| DD—Data deficient | 4090 |
| Crop | Number of Accessions | Major Gene Bank | Country | Percentage of World Germplasm Represented |
|---|---|---|---|---|
| Wheat (Triticum) | 856,168 | CIMMYT | Mexico | 13 |
| Rice (Oryza) | 773,948 | IRRI | Philippines | 14 |
| Maize (Zea) | 327,932 | CIMMYT | Mexico | 8 |
| Bean (Phaseolus) | 261,963 | CIAT | Colombia | 14 |
| Apple (Malus) | 59,922 | GEN (USA167) | USA | 12 |
| Palm (Elaeis) | 21,103 | INFRA | D.R. Congo | 84 |
| Medicago | 91,922 | AMGRC (AUS006) | Australia | 30 |
| Cacao (Theobroma) | 12,373 | ICGT | Trinidad | 19 |
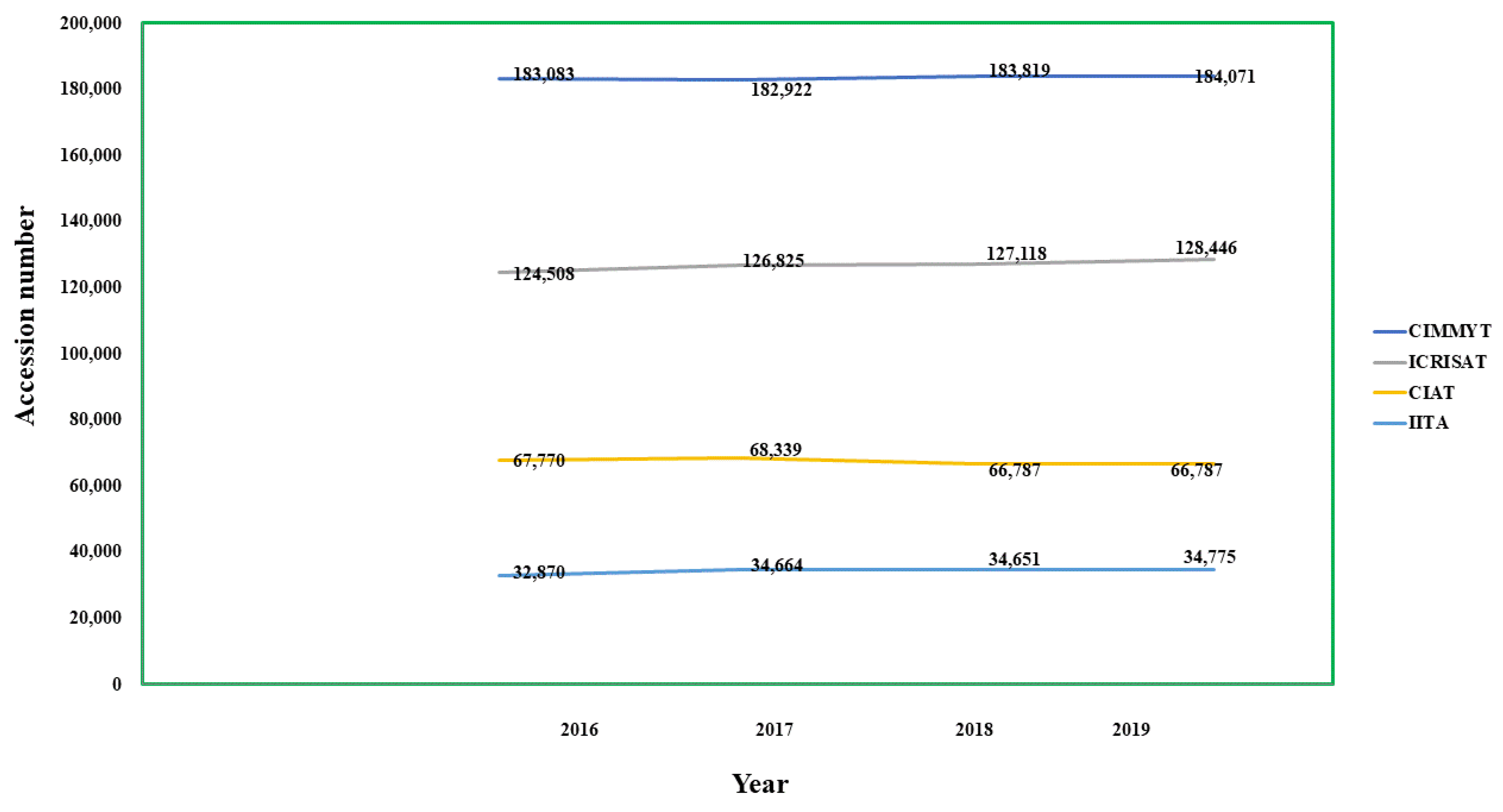
| International Institutes | Number of Accessions under Corresponding Crops as Per 2019–2020 |
|---|---|
| IITA- International Institute of Tropical Agriculture, Ibadan, Nigeria (my.iita.org/accession2/ (accessed on 15 March 2021)) (https://www.genebanks.org/genebanks/iita/(accessed on 15 March 2021)) [112] | African Yam Bean-324, Groundnut-1890, Cassava-3184, Cowpea-15923, Maize-1561, Banana & Plantain-393, Soyabean-1575, Vigna-1878, Yam-5839 |
| CIAT- International Centre for Tropical Agriculture, Cali, Colombia (https://ciat.cgiar.org/ (accessed on 15 March 2021)) (https://www.genebanks.org/genebanks/ciat/(accessed on 15 March 2021)) [113] | Bean-37938, Cassava-6155, Forage-22694 |
| CIMMYT- International Maize and Wheat Improvement Centre, Mexico City, Mexico (https://www.genebanks.org/genebanks/cimmyt/(accessed on 15 March 2021) ) [114] | Maize-28746, Wheat-155325 |
| CIP- International Potato Centre, Lima, Peru (https://www.genebanks.org/genebanks/international-potato-centre/ (accessed on 15 March 2021)) [115] | Andean roots and tubers-2526, Potato-7224, Sweet potato-8080 |
| ICARDA- International Centre for Agricultural Research in the Dry Areas, Aleppo, Syria (https://www.genebanks.org/genebanks/icarda/(accessed on 15 March 2021) [116] | Barley-31392, Chickpea-13299, Fababean-8736, Forages-24632, Grasspea-3992, Lentil-13128, Pea-4159, Wheat-40,843 |
| ICRISAT- International Crops Research Institute for the Semi-Arid Tropics, Patancheru, Hyderabad (https://www.genebanks.org/genebanks/icrisat/(accessed on 15 March 2021)) [117] | Chickpea-20764, Groundnut-15699, Pearl millet-24514, Pigeon pea-13783, Small millets-11797, Sorghum-41889 |
| AfricaRice- Africa Rice Centre, Abidjan, Côte d’Ivoire (https://www.genebanks.org/genebanks/africarice/ (accessed on 15 March 2021)) [118] | Rice- 21300 |
| Bioversity International, Rome, Italy (https://www.genebanks.org/genebanks/biodiversity-international/(accessed on 15 March 2021)) [119] | Musa-1617 |
| ICRAF- World Agro forestry, Nairobi, Kenya (https://www.genebanks.org/genebanks/icraf/(accessed on 15 March 2021)) [120] | Fruits-8246, Multipurpose trees-6456 |
| ILRI- International Livestock Research Institute, Nairobi, Kenya (https://www.genebanks.org/genebanks/ilri/(accessed on 15 March 2021)) [121] | Forage grasses and legumes-18662 |
| IRRI- International Rice Research Institute, Los Baños, Philippines (https://www.genebanks.org/genebanks/irri/(accessed on 15 March 2021)) [122] | Rice-132661 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyanka, V.; Kumar, R.; Dhaliwal, I.; Kaushik, P. Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review. Sustainability 2021, 13, 6743. https://doi.org/10.3390/su13126743
Priyanka V, Kumar R, Dhaliwal I, Kaushik P. Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review. Sustainability. 2021; 13(12):6743. https://doi.org/10.3390/su13126743
Chicago/Turabian StylePriyanka, Veerala, Rahul Kumar, Inderpreet Dhaliwal, and Prashant Kaushik. 2021. "Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review" Sustainability 13, no. 12: 6743. https://doi.org/10.3390/su13126743
APA StylePriyanka, V., Kumar, R., Dhaliwal, I., & Kaushik, P. (2021). Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review. Sustainability, 13(12), 6743. https://doi.org/10.3390/su13126743







