Agroecological Strategies to Safeguard Insect Pollinators in Biodiversity Hotspots: Chile as a Case Study
Abstract
1. Introduction
2. Effects of IA on Pollinators
2.1. Landscape
2.2. External Inputs
2.2.1. Pesticides
2.2.2. Managed Pollinators
3. IA and AE in Biodiversity Hotspots: Chile, a Case Study
3.1. Pesticides
3.2. Managed Pollinators
4. Protecting Pollination: Strategies for the Future
4.1. Sharing, Restoring, and Protecting the Land
4.2. AE Internal Inputs for Sustainability and Pollinator Protection
4.3. Localized Research and Technology
4.4. Territorial Planning and AE Policies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Agroecology | Agronomic discipline focused on an environmental and socially responsible agricultural management. This is achieved through the study of ecological processes inside agroecosystems and the application of this knowledge to agricultural practices. |
| Agricultural intensification | Agricultural scheme that seeks to maximize crop yield per unit of area using external inputs. |
| Ecological intensification | Replacement of external inputs used in intensive agriculture (e.g., insecticides, fertilizers, and growth regulators) by ecosystems services to maximize crop yield with minimum environmental impacts. |
| Ecosystem services | Ecological functions that benefit and are essential for human beings. |
| Habitat | Environment inhabited by a particular species. |
| Landscape homogenization | Simplification and reduction of biotic components inside an area of land, which leads to a community of similar functional and structural traits. |
| Natural habitat | Pristine environment inhabited by native species. |
| Organic agriculture | agricultural scheme that does not use fertilizers and pesticides. |
| Patch | Area of land with the same characteristics, regardless of its size. |
| Seminatural habitat | A native environment partially modified by human activities. |
| Sustainable agriculture | Agricultural scheme that efficiently maximizes production while protecting the habitat and natural resources from which it depends, safeguarding biodiversity in the long term. |
Appendix A
| Use Classification in Chile 1 | Active Ingredient 2 | Pesticide Class | Effect 3 | Reference |
|---|---|---|---|---|
| I, R, A | Acephate | Organophosphate | Highly toxic to bees and other beneficial insects. | [298] |
| H | Atrazine | Triazine | Oxidative stress responses and alteration acetylcholinesterase activity in honeybees; pesticide detected in native bee tissue; found in stored pollen of honeybees; decreases survival, reduces food consumption, and negatively affects behavior in stingless bees. | [137,299,300,301,302] |
| H | Atrazine/S-metolachlor | Triazine/Chloroacetamide | Oxidative stress responses and alteration acetylcholinesterase activity in honeybees; pesticide detected in native bee tissue; found in stored pollen of honeybees; decreases survival, reduces food consumption, and negatively affects behavior in stingless bees. | [137,299,300,301,302] |
| F, B | Benomyl | Benzimidazole | Moderately toxic to honeybees | [303] |
| I, R, A | Cadusafos | Organophosphate | Highly toxic to bees | [304] |
| I, R, A | Carbaryl | Carbamate | Highly toxic to honeybees; found in stored pollen of honeybees | [299,305] |
| F, B | Carbendazim | Benzimidazole | May alter the immune response and P450-mediated detoxification of honeybees | [306] |
| F, B | Carbendazim/Epoxiconazole | Benzimidazole/Triazole | May alter the immune response and P450-mediated detoxification of honeybees; detected in corbicular pollen loads of honeybees | [306,307] |
| F, B | Carbendazim/Mancozeb | Benzimidazole/Carbamate | May alter the immune response and P450-mediated detoxification of honeybees | [306] |
| F, B | Tebuconazole/Carbendazim | Triazole/Benzimidazole | May alter the immune response and P450-mediated detoxification of honeybees; pesticide detected in native bee tissue | [137,306] |
| I, R, A | Cartap hydrochloride | Carbamate | Toxic to bumblebees | [308] |
| I, R, A | Cartap monohydrochloride | Carbamate | Highly toxic to insects | [309] |
| I, R, A | Chlorfenapyr | Pyrrole | Highly toxic to honeybees | [310] |
| F, B | Chlorothalonil/Carbendazim | Chloronitrile/Benzimidazole | May alter the immune response and P450-mediated detoxification of honeybees; found in stored pollen of honeybees | [299,306] |
| F, B | Copper 8-quinolinolate/Carbendazim | Organometallic compound/Benzimidazole | May alter the immune response and P450-mediated detoxification of honeybees | [306] |
| F, B | Copper oxychloride/Dibasic copper sulfate/Iprodione/Sulphur | Copper salt/Copper salt/Dicarboximide/Chalcogen | Decrease in honeybees’ forager survival; found in stored pollen of honeybees | [299,311] |
| I, R, A | Diazinon | Organophosphate | Precocious foraging in honeybees; Impaired olfactory learning in honeybees; found in stored pollen of honeybees | [299,312,313] |
| I, R, A | Fenpropathrin | Pyrethroid | Highly toxic to honeybees | [314] |
| I, R, A | Fenvalerate | Pyrethroid | Highly toxic to honeybees; hazardous to leafcutter bees | [315] |
| I, R, A | Fipronil | Phenylpyrazole | Highly toxic to honeybees; Impaired olfactory learning in honeybees; toxic to leafcutter bees; pesticide detected in native bee tissue; found in stored pollen of honeybees; causes lethargy, motor difficulty, paralysis and hyperexcitation in stingless bees | [137,299,316,317,318,319] |
| H | Glufosinate-ammonium | Phosphinic acid | Low toxicity in honeybees | [320] |
| H | Imazamox/Imazapyr | Imidazolinone/Imidazolinone | Low toxicity in honeybees | [321] |
| F, B | Iprodione | Dicarboximide | Decrease in honeybees’ forager survival; found in stored pollen of honeybees | [299,311] |
| F, B | Iprodione/Propiconazole | Dicarboximide/Triazole | Decrease in honeybees’ forager survival; pesticide detected in native bee tissue; detected in corbicular pollen loads of honeybees; found in stored pollen of honeybees | [137,299,307,311] |
| F, B | Iprodione/Sulphur | Dicarboximide/Chalcogen | Decrease in honeybees’ forager survival; found in stored pollen of honeybees | [299,311] |
| H | Isoproturon | Phenylurea | High mortality in honeybees; detected in corbicular pollen loads of honeybees | [307,322] |
| I, R, A | Methidathion | Organophosphate | Highly toxic to honeybees; found in beeswax of honeybees | [323,324] |
| I, R, A | Novaluron | Benzoylurea | Highly toxic to honeybees | [325] |
| H | Paraquat dichloride | Bipyridylium | Highly toxic to honeybees; changes the size of honeybee oenocytes | [326,327] |
| H | Paraquat dichloride/Diquat (dibromide) | Bipyridylium/Bipyridylium | Highly toxic to honeybees; changes the size of honeybee oenocytes | [326,327] |
| I, R, A | Permethrin | Pyrethroid | Highly toxic to honeybees; disorientation and disruption of normal behavior in honeybees; pesticide detected in native bee tissue | [137,328,329,330] |
| F, B | Tebuconazole/Propiconazole/Permethrin | Pyrethroid | Highly toxic to honeybees; disorientation and disruption of normal behavior in honeybees; pesticide detected in native bee tissue | [137,328,329,330] |
| F, B | Procymidone | Dicarboximide | Low toxicity to bees; found in stored pollen and beeswax of honeybees | [299,323,331] |
| I, R, A | Profenofos | Organophosphate | Highly toxic to honeybees; high mortality in honeybees | [332,333] |
| H | Saflufenacil | Pyrimidinedione | Low toxicity to honeybees | [334] |
| I, R, A | Thiocyclam hydrogen oxalate | Oxalate salt | Highly toxic to bees | [335] |
| I, R, A | Acetamiprid/Novaluron | Neonicotinoid/Benzoylurea | Highly toxic to honeybees; detected in corbicular pollen loads of honeybees; impaired long-term retention of olfactory learning and increased locomotor activity in honeybees; ataxia in bees; slow to no movements and ataxia in bumble bees and leafcutter bees; occur in sufficient quantities in natural bee food to have adverse effects on bees. | [307,325,336,337] |
| I, R, A | Dinotefuran | Neonicotinoid | Highly toxic to honeybees; higher number of bouts of behavior in honeybees | [338,339] |
| I, R, A | Fipronil/Imidacloprid | Phenylpyrazole/Neonicotinoid | Highly toxic to honeybees; impaired olfactory learning in honeybees; honeybees line up in perfect rows or clusters; pesticide detected in native bee tissue; found in stored pollen of honeybees; honeybees loose postural control and spent more time laying on their backs; inhibited grooming, reduced walking and lower righting reflex in honeybees; increased foraging and homing flight times in honeybees; detected in corbicular pollen loads of honeybees; trembling, excessive grooming, uncontrolled proboscis extension, slow to no movements, ataxia and reduced survival in bumble bees and leafcutter bees; toxic to leafcutter bees; occur in sufficient quantities in natural bee food to have adverse effects on bees. | [137,299,307,317,318,319,336,339,340] |
| I, R, A | Fipronil/Thiamethoxam | Phenylpyrazole/Neonicotinoid | Highly toxic to honeybees; Impaired olfactory learning in honeybees; toxic to leafcutter bees; pesticide detected in native bee tissue; found in stored pollen of honeybees; honeybees loss postural control and spent more time laying on their backs; honeybees spend more time grooming; impaired homing ability in honeybees; hyperactivity, ataxia, excessive grooming, permanent late-onset neuromuscular dysfunction and reduced survival in bumble bees and leafcutter bees; occur in sufficient quantities in natural bee food to have adverse effects on bees. | [137,299,317,318,319,336,339,341] |
| F, B | Orthoboric acid/Borax | Inorganic compound/Inorganic compound | Toxic to honeybees | [342] |
| F, B | Orthoboric acid/Fenpropimorph/Propiconazole | Inorganic compound/Morpholine/Triazole | Toxic to honeybees; detected in corbicular pollen loads of honeybees; found in stored pollen of honeybees | [299,307,342] |
| F, B | Picoxystrobin/Cyproconazole | Strobilurin/Triazole | Decreased survival, slight changes in pericardial cells and fat bodies in africanized honeybees; detected in corbicular pollen loads of honeybees | [307,343] |
| F, B | Tributyltin naphthenate/Permethrin | Organotin/Pyrethroid | Highly toxic to honeybees; found in honeybees and beeswax; associated with winter losses of honeybee colonies; disorientation and disruption of normal behavior in honeybees; pesticide detected in native bee tissue | [137,328,329,330,344] |
References
- Khush, G.S. Green Revolution: Preparing for the 21st Century. Genome 1999, 42, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.B.; Chappell, M.J.; Vandermeer, J.; Smith, G.; Quintero, E.; Bezner-Kerr, R.; Griffith, D.M.; Ketcham, S.; Latta, S.C.; McMichael, P.; et al. Effects of Industrial Agriculture on Climate Change and the Mitigation Potential of Small-Scale Agro-Ecological Farms. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–19. [Google Scholar] [CrossRef]
- Pfister, S.; Bayer, P.; Koehler, A.; Hellweg, S. Projected Water Consumption in Future Global Agriculture: Scenarios and Related Impacts. Sci. Total Environ. 2011, 409, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Carvalheiro, L.G.; Aguirre-Gutiérrez, J.; Lucotte, M.; Guidoni-Martins, K.; Mertens, F. Virtual Pollination Trade Uncovers Global Dependence on Biodiversity of Developing Countries. AAAS Sci. Adv. 2021, 7, eabe6636. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global Food Security, Biodiversity Conservation and the Future of Agricultural Intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Wilting, H.C.; Schipper, A.M.; Bakkenes, M.; Meijer, J.R.; Huijbregts, M.A.J. Quantifying Biodiversity Losses Due to Human Consumption: A Global-Scale Footprint Analysis. Environ. Sci. Technol. 2017, 51, 3298–3306. [Google Scholar] [CrossRef]
- Green, J.M.H.; Croft, S.A.; Durán, A.P.; Balmford, A.P.; Burgess, N.D.; Fick, S.; Gardner, T.A.; Godar, J.; Suavet, C.; Virah-Sawmy, M.; et al. Linking Global Drivers of Agricultural Trade to On-the-Ground Impacts on Biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 26085–26086. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect Decline in the Anthropocene: Death by a Thousand Cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef]
- Freitas, B.M.; Imperatriz-Fonseca, V.L.; Medina, L.M.; Kleinert, A.D.M.P.; Galetto, L.; Nates-Parra, G.; Javier, J. Diversity, Threats and Conservation of Native Bees in the Neotropics. Apidologie 2009, 332–346. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape Perspectives on Agricultural Intensification and Biodiversity—Ecosystem Service Management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef]
- Medan, D.; Torretta, J.P.; Hodara, K.; de la Fuente, E.B.; Montaldo, N.H. Effects of Agriculture Expansion and Intensification on the Vertebrate and Invertebrate Diversity in the Pampas of Argentina. Biodivers. Conserv. 2011, 20, 3077–3100. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Muñoz-Sáez, A.; Perez-Quezada, J.F.; Estades, C.F. Agricultural Landscapes as Habitat for Birds in Central Chile. Rev. Chil. Hist. Nat. 2017, 90, 3. [Google Scholar] [CrossRef]
- Stanton, R.; Clark, R.G.; Morrissey, C.A. Intensive Agriculture and Insect Prey Availability Influence Oxidative Status and Return Rates of an Aerial Insectivore. Ecosphere 2017, 8, e01746. [Google Scholar] [CrossRef]
- Berka, C.; Schreier, H.; Hall, K. Linking Water Quality with Agricultural Intensification in a Rural Watershed. Water. Air. Soil Pollut. 2001, 127, 389–401. [Google Scholar] [CrossRef]
- Kennish, M.J. Environmental Threats and Environmental Future of Estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Rohr, J.R.; Schotthoefer, A.M.; Raffel, T.R.; Carrick, H.J.; Halstead, N.; Hoverman, J.T.; Johnson, C.M.; Johnson, L.B.; Lieske, C.; Piwoni, M.D.; et al. Agrochemicals Increase Trematode Infections in a Declining Amphibian Species. Nature 2008. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.J.; Campbell, B.; Ingram, J.S. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.C. Regionalizing Food Security? Imperatives, Intersections and Contestations in a Post-9/11 World. J. Rural Stud. 2013, 29, 7–18. [Google Scholar] [CrossRef]
- Melathopoulos, A.P.; Cutler, G.C.; Tyedmers, P. Where Is the Value in Valuing Pollination Ecosystem Services to Agriculture? Ecol. Econ. 2015, 109, 59–70. [Google Scholar] [CrossRef]
- Wezel, A.; Herren, B.G.; Kerr, R.B.; Barrios, E.; Gonçalves, A.L.R.; Sinclair, F. Agroecological Principles and Elements and Their Implications for Transitioning to Sustainable Food Systems. A Review. Agron. Sustain. Dev. 2020, 40. [Google Scholar] [CrossRef]
- HLPE. Agroecological and Other Innovative Approaches for Sustainable Agriculture and Food Systems That Enhance Food Security and Nutrition; HLPE: Rome, Italy, 2019; p. 163. [Google Scholar]
- Kluser, S.; Peduzzi, P. Global Pollinator Decline: A Literature Review; UNEP/GRID Europe: Geneva, Switzerland, 2007; p. 12. [Google Scholar]
- Merrill, M.C. Eco-Agriculture: A Review of Its History and Philosophy. Biol. Agric. Hortic. 1983, 1, 181–210. [Google Scholar] [CrossRef]
- Smith, L.; Williams, A.G.; Pearce, B.D. The Energy Efficiency of Organic Agriculture: A Review. Renew. Agric. Food Syst. 2014, 30, 1–22. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology: The Science of Sustainable Agriculture, 2nd ed.; CRC Press Taylor & Francis Group: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Kremen, C.; Iles, A.; Bacon, C. Diversified Farming Systems: An Agroecological, Systems-Based Alternative to Modern Industrial Agriculture. Ecol. Soc. 2012, 17. [Google Scholar] [CrossRef]
- Maran, A.M.; Weintraub, M.N.; Pelini, S.L. Does Stimulating Ground Arthropods Enhance Nutrient Cycling in Conventionally Managed Corn Fields? Agric. Ecosyst. Environ. 2020, 297, 106934. [Google Scholar] [CrossRef]
- Wezel, A.; Bellon, S.; Doré, T.; Francis, C.; Vallod, D.; David, C. Agroecology as a Science, a Movement and a Practice. Sustain. Agric. 2009, 2, 27–43. [Google Scholar] [CrossRef]
- Altieiri, M.A. Agroecology, Small Farms, and Food Sovereignty. Mon. Rev. 2009, 61. [Google Scholar] [CrossRef]
- Berkes, F.; Colding, J.; Folke, C. Rediscovery of Traditional Ecological Knowledge as Adaptive Management. Ecol. Appl. 2000, 10, 1251–1262. [Google Scholar] [CrossRef]
- Holdridge, G.A.; Sarmiento, F.O.; Birch, S.E.P.; Boley, B.; Reap, J.K.; Macdonald, E.A.; Navarro, M.; Hitchner, S.L.; Schelhas, J.W. Chapter 15: Feeding Futures Framed: Rediscovering Biocultural Diversity in Sustainable Foodscapes. In The Elgar Companion to Geography, Transdisciplinarity and Sustainability; Frolich, F.O.S., Ed.; Edwar Edgar Publishing: Cheltenham, UK, 2020; pp. 235–251. [Google Scholar] [CrossRef]
- Montalba, R.; Infante, A.; Contreras, A.; Vieli, L. Agroecology in Chile: Precursors, Pioneers, and Their Legacy. Agroecol. Sustain. Food Syst. 2017, 41, 416–428. [Google Scholar] [CrossRef]
- Gurr, G.; Wratten, S.; Altieri, M. Ecological Engineering for Pest Management. Advances in Habitat Manipulation for Arthropods; CSIRO: Collingwood, VIC, Canada, 2004. [Google Scholar] [CrossRef]
- González-Chang, M.; Dörner, J.; Zúñiga, F. Agroecología y Sistemas Agrícolas Sustentables. Agro Sur 2018, 46, 1–2. [Google Scholar] [CrossRef][Green Version]
- Southwood, R.E.; Way, M.J. Ecological Background to Pest Management. In Concepts of Pest Management; Rabb, R.C., Guthrie, F.E., Eds.; North Carolina State University: Raleigh, NC, USA, 1970; pp. 6–29. [Google Scholar]
- Altieri, M.A. The Ecological Role of Biodiversity in Agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological Intensification: Harnessing Ecosystem Services for Food Security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A. Biodiversity and Pest Management in Agroecosystems; Haworth Press: New York, NY, USA, 1994. [Google Scholar]
- Xin, C.; JianJun, T. Utilization of Biodiversity in Agriculture: Today and Tomorrow. Chin. J. Eco-Agric. 2013, 21, 54–60. [Google Scholar]
- Kremen, C.; Chaplin-Kramer, R. Insects as Providers of Ecosystem Services: Crop Pollination and Pest Control. In Insect Conservation BIology; Stewart, A.J.A., New, T.R., Lewis, O.T., Eds.; CABI Publishing: Cambridge, MA, USA, 2007; pp. 349–382. [Google Scholar]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-Bee Insects Are Important Contributors to Global Crop Pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef]
- Costanza, R.; D’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The Value of the World’s Ecosystem Services and Natural Capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; Dombeck, E.; Gerber, J.; Knuth, K.A.; Mueller, N.D.; Mueller, M.; Ziv, G.; Klein, A.M. Global Malnutrition Overlaps with Pollinator-Dependent Micronutrient Production. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic Valuation of the Vulnerability of World Agriculture Confronted with Pollinator Decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G. Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Bugg, R.L.; Nicola, N.; Smith, S.A.; Thorp, R.W.; Williams, N.M. Native Bees, Native Plants and Crop Pollination in California. Fremontia 2002, 30, 41–49. [Google Scholar]
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee Pollination Increases Yield Quantity and Quality of Cash Crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691. [Google Scholar] [CrossRef]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Bee Pollination and Fruit Set of Coffea Arabica and C. Canephora (Rubiaceae). Am. J. Bot. 2003, 90, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop Production in the USA Is Frequently Limited by a Lack of Pollinators. Proc. R. Soc. B 2020, 287, 2–9. [Google Scholar] [CrossRef]
- Venturini, E.M.; Drummond, F.A.; Hoshide, A.K.; Dibble, A.C.; Stack, L.B. Pollination Reservoirs for Wild Bee Habitat Enhancement in Cropping Systems: A Review. Agroecol. Sustain. Food Syst. 2017, 41, 101–142. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The Effectiveness of Flower Strips and Hedgerows on Pest Control, Pollination Services and Crop Yield: A Quantitative Synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Impact of Biotic and Abiotic Stressors on Managed and Feral Bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef]
- Sánchez-Bayoa, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 8–27. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Potts, S.G.; Packer, L.; Ghazoul, J. Pollinator Diversity and Crop Pollination Services Are at Risk [3] (Multiple Letters). Trends Ecol. Evol. 2005, 20, 651–652. [Google Scholar] [CrossRef]
- Ellis, A.M.; Myers, S.S.; Ricketts, T.H. Do Pollinators Contribute to Nutritional Health? PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluijs, J.P. Insect Decline, an Emerging Global Environmental Risk. Curr. Opin. Environ. Sustain. 2020. [Google Scholar] [CrossRef]
- Aizen, M.A.; Arbetman, M.P.; Chacoff, N.P.; Chalcoff, V.R.; Feinsinger, P.; Garibaldi, L.A.; Harder, L.D.; Morales, C.L.; Sáez, A.; Vanbergen, A.J. Invasive Bees and Their Impact on Agriculture, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Cock, M.J.W.; Biesmeijer, J.C.; Cannon, R.J.C.; Gerard, P.J.; Gillespie, D.; Jimenez, J.J.; Lavelle, P.M.; Raina, S.K. The Positive Contribution of Invertebrates to Sustainable Agriculture and Food Security. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2012, 7. [Google Scholar] [CrossRef]
- Cepeda-Valencia, J.; Gómez, P.D.; Nicholls, C. The Structure Matters: Bees Visitors of Coffee Flowers and Agroecological Main Structure (MAS). Rev. Colomb. Entomol. 2014, 40, 241–250. [Google Scholar]
- Garibaldi, L.A.; Pérez-Méndez, N.; Garratt, M.P.D.; Gemmill-Herren, B.; Miguez, F.E.; Dicks, L.V. Policies for Ecological Intensification of Crop Production. Trends Ecology Evolution. 2019, 282–286. [Google Scholar] [CrossRef]
- Orr, M.C.; Hughes, A.C.; Chesters, D.; Pickering, J.; Zhu, C.D.; Ascher, J.S. Global Patterns and Drivers of Bee Distribution. Curr. Biol. 2021, 31, 451–458.e4. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: http://www.fao.org/faostat/en/ (accessed on 1 June 2021).
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.D.A.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Habitat Loss and Extinction in the Hotspots of Biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Altieri, M.A. Una Estrategia Agroecológica En Chile Como Base Para La Soberanía Alimentaria. Ambient. Desarro. CIPMA 2010, 24, 25–29. [Google Scholar]
- Dai, P.; Zhang, X.; Liu, Y. Conserving Pollinator Diversity and Improving Pollination Services in Agricultural Landscapes. Biodivers. Sci. 2015, 23, 408–418. [Google Scholar] [CrossRef]
- Seitz, N.; van Engelsdorp, D.; Leonhardt, S.D. Are Native and Non-Native Pollinator Friendly Plants Equally Valuable for Native Wild Bee Communities? Ecol. Evol. 2020, 10, 12838–12850. [Google Scholar] [CrossRef]
- Pérez-Méndez, N.; Andersson, G.K.S.; Requier, F.; Hipólito, J.; Aizen, M.A.; Morales, C.L.; García, N.; Gennari, G.P.; Garibaldi, L.A. The Economic Cost of Losing Native Pollinator Species for Orchard Production. J. Appl. Ecol. 2020, 57, 599–608. [Google Scholar] [CrossRef]
- Birch, K.; Levidow, L.; Papaioannou, T. Sustainable Capital? The Neoliberalization of Nature and Knowledge in the European “Knowledge-Based Bio-Economy”. Sustainability 2010, 2, 2898–2918. [Google Scholar] [CrossRef]
- Tognelli, M.F.; De Arellano, P.I.R.; Marquet, P.A. How Well Do the Existing and Proposed Reserve Networks Represent Vertebrate Species in Chile? Divers. Distrib. 2008, 14, 148–158. [Google Scholar] [CrossRef]
- Pliscoff, P.; Fuentes-Castillo, T. Representativeness of Terrestrial Ecosystems in Chile’s Protected Area System. Environ. Conserv. 2011, 38, 303–311. [Google Scholar] [CrossRef]
- Silva, E. Democracy, Market Economics, and Environmental Policy in Chile. J. Inter. Am. Stud. World Aff. 2007, 38, 1. [Google Scholar] [CrossRef]
- Ribbe, L.; Delgado, P.; Salgado, E.; Flügel, W.A. Nitrate Pollution of Surface Water Induced by Agricultural Non-Point Pollution in the Pocochay Watershed, Chile. Desalination 2008, 226, 13–20. [Google Scholar] [CrossRef]
- Crook, M.; Short, D.; South, N. Ecocide, Genocide, Capitalism and Colonialism: Consequences for Indigenous Peoples and Glocal Ecosystems Environments. Theor. Criminol. 2018, 22, 298–317. [Google Scholar] [CrossRef]
- Bambrick, H. Resource Extractivism, Health and Climate Change in Small Islands. Int. J. Clim. Chang. Strateg. Manag. 2018, 10. [Google Scholar] [CrossRef]
- Laterra, P.; Nahuelhual, L.; Vallejos, M.; Berrouet, L.; Arroyo Pérez, E.; Enrico, L.; Jiménez-Sierra, C.; Mejía, K.; Meli, P.; Rincón-Ruíz, A.; et al. Linking Inequalities and Ecosystem Services in Latin America. Ecosyst. Serv. 2019, 36, 100875. [Google Scholar] [CrossRef]
- DeFries, R.; Birkenholtz, T.; Uriarte, M.; Hecht, S.; Grau, R.; Lambin, E.F.; Baptista, S.; Schneider, L.; Lawrence, D.; Rudel, T.K.; et al. Agricultural Intensification and Changes in Cultivated Areas, 1970–2005. Proc. Natl. Acad. Sci. USA 2009, 106, 20675–20680. [Google Scholar] [CrossRef]
- Alexander, P.; Rounsevell, M.D.A.; Dislich, C.; Dodson, J.R.; Engström, K.; Moran, D. Drivers for Global Agricultural Land Use Change: The Nexus of Diet, Population, Yield and Bioenergy. Glob. Environ. Chang. 2015, 35, 138–147. [Google Scholar] [CrossRef]
- Gámez-Virués, S.; Perović, D.J.; Gossner, M.M.; Börschig, C.; Blüthgen, N.; De Jong, H.; Simons, N.K.; Klein, A.M.; Krauss, J.; Maier, G.; et al. Landscape Simplification Filters Species Traits and Drives Biotic Homogenization. Nat. Commun. 2015, 6, 8568. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of Functional Diversity under Land Use Intensification across Multiple Taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Reidsma, P.; Tekelenburg, T.; Van Den Berg, M.; Alkemade, R. Impacts of Land-Use Change on Biodiversity: An Assessment of Agricultural Biodiversity in the European Union. Agric. Ecosyst. Environ. 2006, 114, 86–102. [Google Scholar] [CrossRef]
- dos Santos, J.S.; Dodonov, P.; Oshima, J.E.F.; Martelloc, F.; Santos de Jesuse, A.; Ferreirae, M.E.; Silva-Netof, C.M.; Ribeiroc, M.C.; Garcia Collevatti, R. Landscape Ecology in the Anthropocene: An Overview for Integrating Agroecosystems and Biodiversity Conservation. Perspect. Ecol. Conserv. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and Ecosystem Services: A Multilayered Relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Hamilton, C.; Bonneuil, C.; Gemenne, F. The Anthropocene and the Global Environmental Crisis; Hamilton, C., Bonneuil, C., Gemenne, F., Eds.; Routledge, Taylor and Francis Group: London, UK; New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009, 14. [Google Scholar] [CrossRef]
- Karp, D.S.; Rominger, A.J.; Zook, J.; Ranganathan, J.; Ehrlich, P.R.; Daily, G.C. Intensive Agriculture Erodes β-Diversity at Large Scales. Ecol. Lett. 2012, 15, 963–970. [Google Scholar] [CrossRef]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How Sustainable Agriculture Can Address the Environmental and Human Health Harms of Industrial Agriculture. Environ. Health Perspect. 2002, 445–456. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil Compaction Effects on Soil Health and Cropproductivity: An Overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic Amendments as Sustainable Tool to Recovery Fertility in Intensive Agricultural Systems. J. Soil Sci. Plant Nutr. 2015, 333–352. [Google Scholar] [CrossRef]
- De Roos, A.J.; Blair, A.; Rusiecki, J.A.; Hoppin, J.A.; Svec, M.; Dosemeci, M.; Sandler, D.P.; Alavanja, M.C. Cancer Incidence among Glyphosate-Exposed Pesticide Applicators in the Agricultural Health Study. Environ. Health Perspect. 2005. [Google Scholar] [CrossRef]
- Lerro, C.C.; Beane Freeman, L.E.; DellaValle, C.T.; Andreotti, G.; Hofmann, J.N.; Koutros, S.; Parks, C.G.; Shrestha, S.; Alavanja, M.C.R.; Blair, A.; et al. Pesticide Exposure and Incident Thyroid Cancer among Male Pesticide Applicators in Agricultural Health Study. Environ. Int. 2021, 146, 106187. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barceló, D. Pharmaceuticals, Pesticides, Personal Care Products and Microplastics Contamination Assessment of Al-Hassa Irrigation Network (Saudi Arabia) and Its Shallow Lakes. Sci. Total Environ. 2020, 701, 135021. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Chapter 5 Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 102. [Google Scholar] [CrossRef]
- Hoshi, N. Chapter 12. Adverse Effects of Pesticides on Regional Biodiversity and Their Mechanisms. In Risks and Regulation of New Technologies; Matsuda, T., Wolff, J., Yanagawa, T., Eds.; Springer & Kobe University: Kobe, Japan, 2021; pp. 235–247. [Google Scholar]
- González-Varo, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan-Dewenter, I.; Szentgyörgyi, H.; Woyciechowski, M.; Vilà, M. Combined Effects of Global Change Pressures on Animal-Mediated Pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Bub, S.; Petschick, L.; Stehle, S.; Wolfram, J. Applied Pesticide Toxicity Shifts toward Plants and Invertebrates, Even in GM Crops. Science 2021, 372, 6537. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Plant Biodiversity Enhances Bees and Other Insect Pollinators in Agroecosystems. A Review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Stavert, J.R.; Pattemore, D.E.; Gaskett, A.C.; Beggs, J.R.; Bartomeus, I. Exotic Species Enhance Response Diversity to Land-Use Change but Modify Functional Composition. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Grixti, J.C.; Wong, L.T.; Cameron, S.A.; Favret, C. Decline of Bumble Bees (Bombus) in the North American Midwest. Biol. Conserv. 2009, 142, 75–84. [Google Scholar] [CrossRef]
- Benedek, P. Possible Indirect Effect of Weed Control on Popula- Tion Changes of Wild Bees Pollinating Lucerne. Acta Phytopathol. Acad. Sci. Hungaricae 1972, 7, 267–278. [Google Scholar]
- Kevan, P.G.; Viana, B.F. The Global Decline of Pollination Services. Biodiversity 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Eeraerts, M.; Meeus, I.; Van Den Berge, S.; Smagghe, G. Landscapes with High Intensive Fruit Cultivation Reduce Wild Pollinator Services to Sweet Cherry. Agric. Ecosyst. Environ. 2017, 239, 342–348. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop Pollination from Native Bees at Risk from Agricultural Intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape Effects on Crop Pollination Services: Are There General Patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Ockinger, E.; Smith, H.G. Seminatural Grasslands as Population Sources for Pollinating Insects in Agricultural Landscapes. J. Appl. Ecol. 2007, 44, 50–59. [Google Scholar] [CrossRef]
- Stuligross, C.; Williams, N.M. Pesticide and Resource Stressors Additively Impair Wild Bee Reproduction. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201390. [Google Scholar] [CrossRef]
- Hendrickx, F.; Maelfait, J.P.; Van Wingerden, W.; Schweiger, O.; Speelmans, M.; Aviron, S.; Augenstein, I.; Billeter, R.; Bailey, D.; Bukacek, R.; et al. How Landscape Structure, Land-Use Intensity and Habitat Diversity Affect Components of Total Arthropod Diversity in Agricultural Landscapes. J. Appl. Ecol. 2007, 44, 340–351. [Google Scholar] [CrossRef]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C.R. Landscape Impacts on Pollinator Communities in Temperate Systems: Evidence and Knowledge Gaps. Funct. Ecol. 2017, 31, 26–37. [Google Scholar] [CrossRef]
- Hopfenmüller, S.; Steffan-Dewenter, I.; Holzschuh, A. Trait-Specific Responses of Wild Bee Communities to Landscape Composition, Configuration and Local Factors. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Forrest, J.R.K.; Thorp, R.W.; Kremen, C.; Williams, N.M. Contrasting Patterns in Species and Functional-Trait Diversity of Bees in an Agricultural Landscape. J. Appl. Ecol. 2015, 52, 706–715. [Google Scholar] [CrossRef]
- Williams, N.M.; Crone, E.E.; Roulston, T.H.; Minckley, R.L.; Packer, L.; Potts, S.G. Ecological and Life-History Traits Predict Bee Species Responses to Environmental Disturbances. Biol. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- De Palma, A.; Kuhlmann, M.; Roberts, S.P.M.; Potts, S.G.; Börger, L.; Hudson, L.N.; Lysenko, I.; Newbold, T.; Purvis, A. Ecological Traits Affect the Sensitivity of Bees to Land-Use Pressures in European Agricultural Landscapes. J. Appl. Ecol. 2015, 52, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, Ú.; Murray, T.E.; Paxton, R.J.; Breen, J.; Cotton, D.; Santorum, V.; Brown, M.J.F. Rarity and Decline in Bumblebees—A Test of Causes and Correlates in the Irish Fauna. Biol. Conserv. 2007, 136, 185–194. [Google Scholar] [CrossRef]
- Basu, P.; Parui, A.K.; Chatterjee, S.; Dutta, A.; Chakraborty, P.; Roberts, S.; Smith, B. Scale Dependent Drivers of Wild Bee Diversity in Tropical Heterogeneous Agricultural Landscapes. Ecol. Evol. 2016, 6, 6983–6992. [Google Scholar] [CrossRef] [PubMed]
- Hass, A.L.; Liese, B.; Heong, K.L.; Settele, J.; Tscharntke, T.; Westphal, C. Plant-Pollinator Interactions and Bee Functional Diversity Are Driven by Agroforests in Rice-Dominated Landscapes. Agric. Ecosyst. Environ. 2018, 253, 140–147. [Google Scholar] [CrossRef]
- Ballantyne, G.; Baldock, K.C.R.; Rendell, L.; Willmer, P.G. Pollinator Importance Networks Illustrate the Crucial Value of Bees in a Highly Speciose Plant Community. Sci. Rep. 2017, 7, 8389. [Google Scholar] [CrossRef]
- Wood, T.; Holland, J.; Goulson, D. Diet Characterisation of Solitary Bees on Farmland: Dietary Specialisation Predicts Rarity. Biodivers. Conserv. 2016, 25, 2655–2671. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Roberts, S. Role of Nesting Resources in Organising Diverse Bee Communities in a Mediterranean Landscape. Ecol. Entomol. 2005, 30, 78–85. [Google Scholar] [CrossRef]
- Williams, N.M.; Kremen, C. Resource Distributions among Habitats Determine Solitary Bee Offspring Production in a Mosaic Landscape. Ecol. Appl. 2007, 17, 910–921. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A Global Quantitative Synthesis of Local and Landscape Effects on Wild Bee Pollinators in Agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Carré, G.; Roche, P.; Chifflet, R.; Morison, N.; Bommarco, R.; Harrison-Cripps, J.; Krewenka, K.; Potts, S.G.; Roberts, S.P.M.; Rodet, G.; et al. Landscape Context and Habitat Type as Drivers of Bee Diversity in European Annual Crops. Agric. Ecosyst. Environ. 2009, 133, 40–47. [Google Scholar] [CrossRef]
- Grab, H.; Branstetter, M.G.; Amon, N.; Urban-Mead, K.R.; Park, M.G.; Gibbs, J.; Blitzer, E.J.; Poveda, K.; Loeb, G.; Danforth, B.N. Agriculturally Dominated Landscapes Reduce Bee Phylogenetic Diversity and Pollination Services. Science 2019, 363, 282–284. [Google Scholar] [CrossRef]
- Hines, H.M.; Hendrix, S.D. Bumble Bee (Hymenoptera: Apidae) Diversity and Abundance in Tallgrass Prairie Patches: Effects of Local and Landscape Floral Resources. Environ. Entomol. 2005, 34, 1477–1484. [Google Scholar] [CrossRef]
- Giannini, T.C.; Tambosi, L.R.; Acosta, A.L.; Jaffé, R.; Saraiva, A.M.; Imperatriz-Fonseca, V.L.; Metzger, J.P. Safeguarding Ecosystem Services: A Methodological Framework to Buffer the Joint Effect of Habitat Configuration and Climate Change. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M.; Price, M.V. Tolerance of Pollination Networks to Species Extinctions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 2605–2611. [Google Scholar] [CrossRef]
- McKinney, M.; Lockwood, J. Biotic Homogenization: A Few Winners Replacing Many Losers in the next Mass Extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Martins, K.T.; Gonzalez, A.; Lechowicz, M.J. Pollination Services Are Mediated by Bee Functional Diversity and Landscape Context. Agric. Ecosyst. Environ. 2015, 200, 12–20. [Google Scholar] [CrossRef]
- Bennett, J.M.; Steets, J.A.; Burns, J.H.; Burkle, L.A.; Vamosi, J.C.; Wolowski, M.; Arceo-Gómez, G.; Burd, M.; Durka, W.; Ellis, A.G.; et al. Land Use and Pollinator Dependency Drives Global Patterns of Pollen Limitation in the Anthropocene. Nat. Commun. 2020, 11, 3999. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.M.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of Crop Pollination Services Is an Insufficient Argument for Wild Pollinator Conservation. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Cunningham, S.; Lindenmayer, D.; Young, A. Land Use Intensification: Effects on Agriculture, Biodiversity and Ecological Processes; CSIRO Publishing: Collingwood, VIC, Australia, 2012. [Google Scholar]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; Lebuhn, G.; Aizen, M.A. A Meta-Analysis of Bees’ Responses to Anthropogenic Disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef]
- Hladik, M.L.; Vandever, M.; Smalling, K.L. Exposure of Native Bees Foraging in an Agricultural Landscape to Current-Use Pesticides. Sci. Total Environ. 2016, 542, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Review Neonicotinoids—From Zero to Hero in Insecticide Chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- James, R.R.; Xu, J. Mechanisms by Which Pesticides Affect Insect Immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Martins, G.F.; Lima, M.A.P.; Campos, L.A.O.; Guedes, R.N.C. Imidacloprid-Induced Impairment of Mushroom Bodies and Behavior of the Native Stingless Bee Melipona Quadrifasciata Anthidioides. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Ken, T.; Chen, W.; Dong, S.; Liu, X.; Wang, Y.; Nieh, J.C. Imidacloprid Alters Foraging and Decreases Bee Avoidance of Predators. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Morandin, L.A.; Winston, M.L.; Franklin, M.T.; Abbott, V.A. Lethal and Sub-Lethal Effects of Spinosad on Bumble Bees (Bombus Impatiens Cresson). Pest Manag. Sci. 2005, 61, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Ken, T.; Chen, W.; Dong, S.; Liu, X.; Wang, Y.; Nieh, J.C. A Neonicotinoid Impairs Olfactory Learning in Asian Honey Bees (Apis Cerana) Exposed as Larvae or as Adults. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Crall, J.D.; Switzer, C.M.; Oppenheimer, R.L.; Ford Versypt, A.N.; Dey, B.; Brown, A.; Eyster, M.; Guérin, C.; Pierce, N.E.; Combes, S.A.; et al. Neonicotinoid Exposure Disrupts Bumblebee Nest Behavior, Social Networks, and Thermoregulation. Science 2018, 362, 683–686. [Google Scholar] [CrossRef]
- Sandrock, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal Neonicotinoid Insecticide Exposure Reduces Solitary Bee Reproductive Success. Agric. For. Entomol. 2014, 16, 119–128. [Google Scholar] [CrossRef]
- Gill, R.J.; Raine, N.E. Chronic Impairment of Bumblebee Natural Foraging Behaviour Induced by Sublethal Pesticide Exposure. Funct. Ecol. 2014, 28, 1459–1471. [Google Scholar] [CrossRef]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-Lethal Effects of Pesticide Residues in Brood Comb on Worker Honey Bee (Apis Mellifera) Development and Longevity. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Bebane, P.S.A.; Hunt, B.J.; Pegoraro, M.; Jones, A.R.C.; Marshall, H.; Rosato, E.; Mallon, E.B. The Effects of the Neonicotinoid Imidacloprid on Gene Expression and DNA Methylation in the Buff-Tailed Bumblebee Bombus terrestris. Proc. R. Soc. B Biol. Sci. 2019, 286. [Google Scholar] [CrossRef] [PubMed]
- Brevik, K.; Bueno, E.M.; McKay, S.; Schoville, S.D.; Chen, Y.H. Insecticide Exposure Affects Intergenerational Patterns of DNA Methylation in the Colorado Potato Beetle, Leptinotarsa Decemlineata. Evol. Appl. 2020. [Google Scholar] [CrossRef]
- Brevik, K.; Lindström, L.; McKay, S.D.; Chen, Y.H. Transgenerational Effects of Insecticides—Implications for Rapid Pest Evolution in Agroecosystems. Curr. Opin. Insect Sci. 2018, 26, 34–40. [Google Scholar] [CrossRef]
- Kevan, P.G. Forest Application of the Insecticide Fenitrothion and Its Effect on Wild Bee Pollinators (Hymenoptera: Apoidea) of Lowbush Blueberries (Vaccinium SPP.) in Southern New Brunswick, Canada. Biol. Conserv. 1975, 7, 301–309. [Google Scholar] [CrossRef]
- Park, M.G.; Blitzer, E.J.; Gibbs, J.; Losey, J.E.; Danforth, B.N. Negative Effects of Pesticides on Wild Bee Communities Can Be Buffered by Landscape Context. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, G.; Jeanneret, P.; Schneider, M.K.; Turnbull, L.A.; Arndorfer, M.; Balázs, K.; Báldi, A.; Bailey, D.; Bernhardt, K.G.; Choisis, J.P.; et al. Responses of Plants, Earthworms, Spiders and Bees to Geographic Location, Agricultural Management and Surrounding Landscape in European Arable Fields. Agric. Ecosyst. Environ. 2014, 186, 124–134. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of Neonicotinoid Use on Long-Term Population Changes in Wild Bees in England. Nat. Commun. 2016. [Google Scholar] [CrossRef]
- Tasei, J. Impact of Agrochemicals on Non-Apis Bees. In Honey Bees; Taylor & Francis Group: London, UK, 2010; pp. 101–131. [Google Scholar] [CrossRef]
- Kopit, A.M.; Pitts-Singer, T.L. Routes of Pesticide Exposure in Solitary, Cavity-Nesting Bees. Environ. Entomol. 2018, 47, 499–510. [Google Scholar] [CrossRef]
- Sgolastra, F.; Hinarejos, S.; Pitts-Singer, T.L.; Boyle, N.K.; Joseph, T.; Luckmann, J.; Raine, N.E.; Singh, R.; Williams, N.M.; Bosch, J. Pesticide Exposure Assessment Paradigm for Solitary Bees. Environ. Entomol. 2019, 48, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple Routes of Pesticide Exposure for Honey Bees Living near Agricultural Fields. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid Residues in Wildflowers, a Potential Route of Chronic Exposure for Bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Main, A.R.; Webb, E.B.; Goyne, K.W.; Mengel, D. Agriculture, Ecosystems and Environment Reduced Species Richness of Native Bees in Fi Eld Margins Associated with Neonicotinoid Concentrations in Non-Target Soils. Agric. Ecosyst. Environ. 2020, 287, 106693. [Google Scholar] [CrossRef]
- Bredeson, M.M.; Lundgren, J.G. Neonicotinoid Insecticidal Seed-Treatment on Corn Contaminates Interseeded Cover Crops Intended as Habitat for Beneficial Insects. Ecotoxicology 2019, 28, 222–228. [Google Scholar] [CrossRef]
- Stewart, S.D.; Lorenz, G.M.; Catchot, A.L.; Gore, J.; Cook, D.; Skinner, J.; Mueller, T.C.; Johnson, D.R.; Zawislak, J.; Barber, J. Potential Exposure of Pollinators to Neonicotinoid Insecticides from the Use of Insecticide Seed Treatments in the Mid-Southern United States. Environ. Sci. Technol. 2014, 48, 9762–9769. [Google Scholar] [CrossRef] [PubMed]
- Long, E.Y.; Krupke, C.H. Non-Cultivated Plants Present a Season-Long Route of Pesticide Exposure for Honey Bees. Nat. Commun. 2016, 7, 11629. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined Pesticide Exposure Severely Affects Individual- and Colony-Level Traits in Bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.A.; Garratt, M.P.D.; Wickens, J.B.; Wickens, V.J.; Potts, S.G.; Raine, N.E. Neonicotinoid Pesticide Exposure Impairs Crop Pollination Services Provided by Bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.; Park, K.; Goulson, D. Field Realistic Doses of Pesticide Imidacloprid Reduce Bumblebee Pollen Foraging Efficiency. Ecotoxicology 2014, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, D.J.; Robertson, J.L.; Mullin, C.; Frazier, J.; Ashcraft, S.A.; Rajotte, E.G.; Joshi, N.K.; Vaughn, M. Comparative Toxicities and Synergism of Apple Orchard Pesticides to Apis mellifera (L.) and Osmia Cornifrons (Radoszkowski). PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Ramos, G.S.; Araújo, M.F.; Santana, W.C.; Santos, G.R.; Guedes, R.N.C.; Maciel, C.D.; Newland, P.L.; Oliveira, E.E. Agrochemical Synergism Imposes Higher Risk to Neotropical Bees than to Honeybees. R. Soc. Open Sci. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, E.; Bosch, J.; Kemp, W.P.; Maini, S. Assessing Delayed and Acute Toxicity of Five Formulated Fungicides to Osmia Lignaria Say and Apis Mellifera. Apidologie 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Soares, H.M.; Jacob, C.R.O.; Carvalho, S.M.; Nocelli, R.C.F.; Malaspina, O. Toxicity of Imidacloprid to the Stingless Bee Scaptotrigona Postica Latreille, 1807 (Hymenoptera: Apidae). Bull. Environ. Contam. Toxicol. 2015, 94, 675–680. [Google Scholar] [CrossRef]
- Arena, M.; Sgolastra, F. A Meta-Analysis Comparing the Sensitivity of Bees to Pesticides. Ecotoxicology 2014, 23, 324–334. [Google Scholar] [CrossRef]
- Brittain, C.; Potts, S.G. The Potential Impacts of Insecticides on the Life-History Traits of Bees and the Consequences for Pollination. Basic Appl. Ecol. 2011, 12, 321–331. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do Managed Bees Have Negative Effects on Wild Bees?: A Systematic Review of the Literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef]
- Paini, D.R. Impact of the Introduced Honey Bee (Apis mellifera) (Hymenoptera: Apidae) on Native Bees: A Review. Austral Ecology 2004, 399–407. [Google Scholar] [CrossRef]
- Goulson, D. Effects of Introduced Bees on Native Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 1–26. [Google Scholar] [CrossRef]
- Thomson, D. Competitive Interactions between the Invasive European Honey Bee and Native Bumble Bees. Ecology 2004, 85, 458–470. [Google Scholar] [CrossRef]
- Kanbe, Y.; Okada, I.; Yoneda, M.; Goka, K.; Tsuchida, K. Interspecific Mating of the Introduced Bumblebee Bombus terrestris and the Native Japanese Bumblebee Bombus hypocrita sapporoensis Results in Inviable Hybrids. Naturwissenschaften 2008, 95, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.I.; Yamanaka, D.; Kanbe, Y.; Kunitake, Y.K.; Yoneda, M.; Tsuchida, K.; Goka, K. Reproductive Disturbance of Japanese Bumblebees by the Introduced European Bumblebee Bombus Terrestris. Naturwissenschaften 2009, 96, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Alomar, D.; González-Estévez, M.A.; Traveset, A.; Lázaro, A. The Intertwined Effects of Natural Vegetation, Local Flower Community, and Pollinator Diversity on the Production of Almond Trees. Agric. Ecosyst. Environ. 2018, 264, 34–43. [Google Scholar] [CrossRef]
- Matsumura, C.; Yokoyama, J.; Washitani, I. Invasion Status and Potential Ecological Impacts of an Invasive Alien Bumblebee, Bombus Terrestris L. (Hymenoptera: Apidae) Naturalized in Southern Hokkaido, Japan. Glob. Environ. Res. 2004, 8, 51–66. [Google Scholar]
- Inoue, M.N.; Yokoyama, J.; Washitani, I. Displacement of Japanese Native Bumblebees by the Recently Introduced Bombus Terrestris (L.) (Hymenoptera: Apidae). J. Insect Conserv. 2008, 12, 135–146. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Kingston, J.M.; Lee, A.; Holway, D.A.; Kohn, J.R. Non-Native Honey Bees Disproportionately Dominate the Most Abundant Floral Resources in a Biodiversity Hotspot. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182901. [Google Scholar] [CrossRef]
- Magrach, A.; González-Varo, J.P.; Boiffier, M.; Vilà, M.; Bartomeus, I. Honeybee Spillover Reshuffles Pollinator Diets and Affects Plant Reproductive Success. Nat. Ecol. Evol. 2017, 1, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H.; Tepedino, V.J. Gauging the Effect of Honey Bee Pollen Collection on Native Bee Communities. Conserv. Lett. 2017, 10, 205–210. [Google Scholar] [CrossRef]
- Carneiro, L.T.; Martins, C.F. Africanized Honey Bees Pollinate and Preempt the Pollen of Spondias mombin (Anacardiaceae) Flowers. Apidologie 2012, 43, 474–486. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.; Feldlaufer, M. Horizontal and Vertical Transmission of Viruses in the Honey Bee, Apis mellifera. J. Invertebr. Pathol. 2006, 92, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Alger, S.A.; Burnham, P.A.; Brody, A.K. Flowers as Viral Hot Spots: Honey Bees (Apis mellifera) Unevenly Deposit Viruses across Plant Species. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Purkiss, T.; Lach, L. Pathogen Spillover from Apis mellifera to a Stingless Bee. Proc. R. Soc. B Biol. Sci. 2019, 286. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Fürst, M.A.; Caspar, J.; Theodorou, P.; Brown, M.J.F.; Paxton, R.J. A Sting in the Spit: Widespread Cross-Infection of Multiple RNA Viruses across Wild and Managed Bees. J. Anim. Ecol. 2015, 84, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease Associations between Honeybees and Bumblebees as a Threat to Wild Pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.K.; Marquet, P.A.; Simonetti, J.A.; Cavieres, L.A. Chilean Winter Rainfall-Valdivian Forests. In Hotspots: Earth’s Biological Richest and most Endangered Terrestrial Ecoregions; Mittermeier, R.A., Robles, P., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C., et al., Eds.; CEMEX: Mexico City, Mexico, 2004; pp. 99–103. [Google Scholar]
- Harvey, C.A.; Komar, O.; Chazdon, R.; Ferguson, B.G.; Finegan, B.; Griffith, D.M.; Martínez-Ramos, M.; Morales, H.; Nigh, R.; Soto-Pinto, L.; et al. Integrating Agricultural Landscapes with Biodiversity Conservation in the Mesoamerican Hotspot. Conserv. Biol. 2008, 8–15. [Google Scholar] [CrossRef]
- Kehinde, T.; Samways, M.J. Endemic Pollinator Response to Organic vs. Conventional Farming and Landscape Context in the Cape Floristic Region Biodiversity Hotspot. Agric. Ecosyst. Environ. 2012, 146, 162–167. [Google Scholar] [CrossRef]
- Schutz, J. Creating an Integrated Protected Area Network in Chile: A GIS Assessment of Ecoregion Representation and the Role of Private Protected Areas. Environ. Conserv. 2018, 45, 269–277. [Google Scholar] [CrossRef]
- Montalva, J.; Ruz, L. Actualización de La Lista Sistemática de Las Abejas Chilenas (Hymenoptera: Apioidea). Rev. Chil. Entomol. 2010, 35, 15–52. [Google Scholar]
- United Nations. Paris Agreement (COP21). 2015. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 1 June 2021).
- NYDF Global Platform. New York Declaration on Forests. 2014. Available online: https://forestdeclaration.org/ (accessed on 1 June 2021).
- UN Convention on Biological Diversity. Aichi Biodiversity Targets. Chile National Targets. 2010. Available online: https://www.cbd.int/ (accessed on 1 June 2021).
- WRI. 20x20 Initiative. Healthy Lands for Food, Water and Climate. 2014. Available online: https://initiative20x20.org/es (accessed on 1 June 2021).
- Carmona, A.; Nahuelhual, L.; Echeverría, C.; Báez, A. Linking Farming Systems to Landscape Change: An Empirical and Spatially Explicit Study in Southern Chile. Agric. Ecosyst. Environ. 2010, 139, 40–50. [Google Scholar] [CrossRef]
- Del Pozo, A.; Lavin, A.; Etienne, M.; Ovalle, C.; Avendaño, J.; Aronson, J. Land Use Changes and Conflicts in Central Chile. In Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosystems; Rundel, P.W., Montenegro, G., Jaksic, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 155–168. [Google Scholar] [CrossRef]
- ODEPA. Panorama de La Agricultura Chilena (Chilean Agriculture Overview); A Impresores: Santiago, Chile, 2019; Available online: https://www.odepa.gob.cl/wp-content/uploads/2019/09/panorama2019Final.pdf (accessed on 1 June 2021).
- Ministerio del Medio Ambiente. Sexto Informe Nacional de Biodiversidad de Chile. Ministerio del Medio Ambiente. 2019, 220. Available online: https://mma.gob.cl/wp-content/uploads/2020/01/6NR_FINAL_ALTA-web.pdf (accessed on 1 June 2021).
- Gwynne, R.N. Globalisation, Commodity Chains and Fruit Exporting Regions in Chile. Tijdschr. Econ. Soc. Geogr. 1999. [Google Scholar] [CrossRef]
- Altieri, M.A.; Rojas, A. Ecological Impacts of Chile’s Neoliberal Policies, with Special Emphasis on Agroecosystems. Environ. Dev. Sustain. 1999, 1, 55–72. [Google Scholar] [CrossRef]
- Carruthers, D. Environmental Politics in Chile: Legacies of Dictatorship and Democracy. Third World Q. 2001, 22, 343–358. [Google Scholar] [CrossRef]
- Muñoz, O.; Ortega, H. Chilean Agriculture and Economic Policy. In Modernization and Stagnation: Latin American Agriculture into the 1990’s; Helwage, M.J.T., Ed.; Greenwood Press: New York, NY, USA, 1991; pp. 161–188. [Google Scholar]
- Clark, T.D. Putting the Market in Its Place: Food Security in Three Mapuche Communities in Southern Chile. Lat. Am. Res. Rev. 2011, 46, 154–179. [Google Scholar] [CrossRef]
- Kay, C. Chile’s Neoliberal Agrarian Transformation and the Peasantry. J. Agrar. Chang. 2002, 2, 464–501. [Google Scholar] [CrossRef]
- FAO. GIAHS: Globally Importan Agricultural Heritage Systems; Chiloé Agriculture, Chile. Available online: http://www.fao.org/3/bp773e/bp773e.pdf (accessed on 1 June 2021).
- Wratten, S.D.; Shields, M.W.; González-Chang, M. Prospects for Regenerative Agriculture in Chile. Agro Sur 2019, 47, 1–6. [Google Scholar] [CrossRef]
- SAG. Lista de Plaguicidas Autorizados. Available online: https://www.sag.gob.cl/ambitos-de-accion/plaguicidas-y-fertilizantes/78/registros (accessed on 1 June 2021).
- Samson-Robert, O.; Labrie, G.; Chagnon, M.; Fournier, V. Planting of Neonicotinoid-Coated Corn Raises Honey Bee Mortality and Sets Back Colony Development. PeerJ 2017, 5, e3670. [Google Scholar] [CrossRef]
- European Commission. Neonicotinoids. Available online: https://ec.europa.eu/food/plants/pesticides/approval-active-substances/renewal-approval/neonicotinoids_en (accessed on 1 June 2021).
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid Markedly Affects Hemolymph Proteolysis, Biomarkers, DNA Global Methylation, and the Cuticle Proteolytic Layer in Western Honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef]
- Colin, T.; Meikle, W.G.; Wu, X.; Barron, A.B. Traces of a Neonicotinoid Induce Precocious Foraging and Reduce Foraging Performance in Honey Bees. Environ. Sci. Technol. 2019, 53, 8252–8261. [Google Scholar] [CrossRef]
- Acta Reunión de Panel de Discusión de Expertos Proyecto Ley Apícola. Legislando Para La Protección de La Salud y Hábitat de Las Abejas, 2015; 1–6.
- SAG. Informe de Venta de Plaguicidas de Uso Agrícola En Chile, Año 2012; Servicio Agrícola y Ganadero División Protección Agrícola y Forestal Subdepartamento de Plaguicidas y Fertilizantes, Gobierno de Chile: Chile, 2012.; Available online: http://www.sag.cl/sites/default/files/declaracion_de_venta_de_plaguicidas_ano_2012.pdf (accessed on 1 June 2021).
- Caceres-Jensen, L.; Rodriguez-Becerra, J.; Escudey, M.; Joo-Nagata, J.; Villagra, C.A.; Dominguez-Vera, V.; Neira-Albornoz, A.; Cornejo-Huentemilla, M. Nicosulfuron Sorption Kinetics and Sorption/Desorption on Volcanic Ash-Derived Soils: Proposal of Sorption and Transport Mechanisms. J. Hazard. Mater. 2020, 385, 121576. [Google Scholar] [CrossRef]
- Aparicio, V.C.; De Geronimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental Fate of Glyphosate and Aminomethylphosphonic Acid in Surface Waters and Soil of Agricultural Basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees the World, 2nd ed.; The Johns Hopkins Univertity Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Ministerio de Salud; Subsecretaría de Salud Pública. Decreto 157. Reglamento de Pesticidas de Uso Sanitario y Doméstico; Biblioteca del Congreso Nacional de Chile: Santiago de Chile, Chile, 2007; p. 19. [Google Scholar]
- Linnaeus, C. Systema Naturae, 10th ed.; Salvii: Stockholm, Sweden, 1758; Volume 1. [Google Scholar]
- Fabricius, J.C. Systema Entomologiae, Sistens Insectorum Classes, Ordines, Genera, Species, Adjectis Synonymis, Locis, Descriptionibus, Observationibus, Flensbvrgi et Lipsiae: In Officina Libraria Kortii. 1775.
- Guérin-Méneville, F.-É. Bombus Dahlbomii Pp. Pl. 75, Fig. 3. In Iconographie du Règne Animal de G. Cuvier, ou Représentation D’après Nature de l’une des Espèces les Plus Remarquables, et Souvent non Encore Figurées, de Chaque Genre D’animaux; Pouvant Servir D’atlas à Tous les Traités de Zoologie; Guérin-Méneville, F.E., Ed.; Baillière: Paris, France, 1835; p. 576. [Google Scholar]
- Madjidian, J.A.; Morales, C.L.; Smith, H.G. Displacement of a Native by an Alien Bumblebee: Lower Pollinator Efficiency Overcome by Overwhelmingly Higher Visitation Frequency. Oecologia 2008, 156, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.L.; Arbetman, M.P.; Cameron, S.A.; Aizen, M.A. Rapid Ecological Replacement of a Native Bumble Bee by Invasive Species. Front. Ecol. Environ. 2013, 11, 529–534. [Google Scholar] [CrossRef]
- Montalva, J.; Dudley, L.S.; Sepúlveda, J.E.; Smith-Ramírez, C. The Giant Bumble Bee (Bombus dahlbomii) in Mapuche Cosmovision. Ethnoentomology 2020, 4, 1–11. [Google Scholar]
- Smith-Ramírez, C.; Vieli, L.; Barahona-Segovia, R.M.; Montalva, J.; Cianferoni, F.; Ruz, L.; Fontúrbel, F.E.; Valdivia, C.E.; Medel, R.; Pauchard, A.; et al. Las Razones de Por Qué Chile Debe Detener La Importación Del Abejorro Comercial Bombus terrestris (Linnaeus) y Comenzar a Controlarlo. Gayana (Concepción) 2018, 82, 118–127. [Google Scholar] [CrossRef]
- Geslin, B.; Morales, C.L. New Records Reveal Rapid Geographic Expansion of Bombus terrestris Linnaeus, 1758 (Hymenoptera: Apidae), an Invasive Species in Argentina. Check List 2015, 11, 1620. [Google Scholar] [CrossRef]
- Monzón, V.H.; Avendaño-Soto, P.; Araujo, R.O.; Garrido, R.; Mesquita-Neto, J.N. Avocado Crops as a Floral Resource for Native Bees of Chile. Rev. Chil. Hist. Nat. 2020, 93. [Google Scholar] [CrossRef]
- Morales, C.; Montalva, J.; Arbetman, M.; Aizen, M.; Smith-Ramírez, C.; Vieli, L.; Hatfield, R. Bombus dahlbomii. The IUCN Red List of Threatened Species 2016. [Google Scholar] [CrossRef]
- Estay, P.; Wagner, A.; Escaff, M. Evaluación DE Bombus dahlbomii (GUÉR.) como agente polinizador de flores de tomate (lycopersicon esculentum (mill)), bajo condiciones de invernadero. Agric. Técnica Scielocl. 2001, 113–119. [Google Scholar] [CrossRef]
- Santos, E.; Daners, G.; Morelli, E.; Galván, G.A. Diversity of Bee Assemblage (Family Apidae) in Natural and Agriculturally Intensified Ecosystems in Uruguay. Environ. Entomol. 2020, 49, 1232–1241. [Google Scholar] [CrossRef]
- Le Féon, V.; Poggio, S.L.; Torretta, J.P.; Bertrand, C.; Molina, G.A.R.; Burel, F.; Baudry, J.; Ghersa, C.M. Diversity and Life-History Traits of Wild Bees (Insecta: Hymenoptera) in Intensive Agricultural Landscapes in the Rolling Pampa, Argentina. J. Nat. Hist. 2016, 50, 1175–1196. [Google Scholar] [CrossRef]
- Rosanigo, M.P.; Marrero, H.J.; Torretta, J.P. Limiting Resources on the Reproductive Success of a Cavity-Nesting Bee Species in a Grassland Agroecosystem. J. Apic. Res. 2020, 59, 583–591. [Google Scholar] [CrossRef]
- Allendes, J.L.; Montalva, J.; Castro, B. Las Abejas (Hymenoptera: Apoidea) Del Jardín Botánico Chagual. Estudio de Caso de Abejas Nativas En Zonas Urbanas de Santiago de Chile. Rev. Chagual 2010, 8, 13–23. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary Boundaries: Guiding Human Development on a Changing Planet. Science 2015, 347. [Google Scholar] [CrossRef]
- Desing, H.; Brunner, D.; Takacs, F.; Nahrath, S.; Frankenberger, K.; Hischier, R. A Circular Economy within the Planetary Boundaries: Towards a Resource-Based, Systemic Approach. Resour. Conserv. Recycl. 2020, 155, 104673. [Google Scholar] [CrossRef]
- Heck, V.; Hoff, H.; Wirsenius, S.; Meyer, C.; Kreft, H. Land Use Options for Staying within the Planetary Boundaries—Synergies and Trade-Offs between Global and Local Sustainability Goals. Glob. Environ. Chang. 2018, 49, 73–84. [Google Scholar] [CrossRef]
- Parraguez-Vergara, E.; Contreras, B.; Clavijo, N.; Villegas, V.; Paucar, N.; Ther, F. Does Indigenous and Campesino Traditional Agriculture Have Anything to Contribute to Food Sovereignty in Latin America? Evidence from Chile, Peru, Ecuador, Colombia, Guatemala and Mexico. Int. J. Agric. Sustain. 2018, 16, 326–341. [Google Scholar] [CrossRef]
- Mickey, S. Learning Native Wisdom: What Traditional Cultures Teach Us About Subsistence, Sustainability, and Spirituality. Worldviews Glob. Relig. Cult. Ecol. 2013, 13, 136–139. [Google Scholar] [CrossRef]
- Grey, S.; Patel, R. Food Sovereignty as Decolonization: Some Contributions from Indigenous Movements to Food System and Development Politics. Agric. Human Values 2015, 32, 431–444. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Minden, V.; Klein, A.; Leonhardt, S.D. Social Bees Are Fitter in More Biodiverse Environments. Sci. Rep. 2018, 8, 12353. [Google Scholar] [CrossRef]
- Kremen, C. Reframing the Land-sparing/Land-sharing Debate for Biodiversity Conservation. Ann. N. Y. Acad. Sci. 2015, 1355, 52–76. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Bugg, R.L.; Fay, J.P.; Thorp, R.W. The Area Requirements of an Ecosystem Service: Crop Pollination by Native Bee Communities in California. Ecol. Lett. 2004, 7, 1109–1119. [Google Scholar] [CrossRef]
- Bailey, S.; Requier, F.; Nusillard, B.; Roberts, S.P.M.; Potts, S.G.; Bouget, C. Distance from Forest Edge Affects Bee Pollinators in Oilseed Rape Fields. Ecol. Evol. 2014, 4, 370–380. [Google Scholar] [CrossRef]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Boïrger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local Biodiversity Is Higher inside than Outside Terrestrial Protected Areas Worldwide. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Felipe Viana, B. How Well Do We Understand Landscape Effects on Pollinators and Pollination Services? J. Pollinat. Ecol. 2012, 7, 31–40. [Google Scholar] [CrossRef]
- Sobreiro, A.I. Recover and They’ll Come: Flower Visiting Bees Benefit from the Continuous of Micro- Environments Set by Regenerating Forest Fragments. Sociobiology 2021, 68, 1–17. [Google Scholar] [CrossRef]
- Chape, S.; Harrison, J.; Spalding, M.; Lysenko, I. Measuring the Extent and Effectiveness of Protected Areas as an Indicator for Meeting Global Biodiversity Targets. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 443–455. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Effects of Habitat Isolation on Pollinator Communities and Seed Set. Oecologia 1999, 121, 432–440. [Google Scholar] [CrossRef]
- Carrié, R.; Andrieu, E.; Ouin, A.; Steffan-Dewenter, I. Interactive Effects of Landscape-Wide Intensity of Farming Practices and Landscape Complexity on Wild Bee Diversity. Landsc. Ecol. 2017, 32, 1631–1642. [Google Scholar] [CrossRef]
- Morandin, L.A.; Kremen, C. Hedgerow Restoration Promotes Pollinator Populations and Exports Native Bees to Adjacent Fields. Ecol. Appl. 2013, 23, 829–839. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger Patches of Diverse Floral Resources Increase Insect Pollinator Density, Diversity, and Their Pollination of Native Wildflowers. Basic Appl. Ecol. 2014, 15, 701–711. [Google Scholar] [CrossRef]
- Riojas-López, M.E.; Díaz-Herrera, I.A.; Fierros-López, H.E.; Mellink, E. The Effect of Adjacent Habitat on Native Bee Assemblages in a Perennial Low-Input Agroecosystem in a Semiarid Anthropized Landscape. Agric. Ecosyst. Environ. 2019, 272, 199–205. [Google Scholar] [CrossRef]
- Burkle, L.A.; Delphia, C.M.; O’Neill, K.M. A Dual Role for Farmlands: Food Security and Pollinator Conservation. J. Ecol. 2017, 105, 890–899. [Google Scholar] [CrossRef]
- Krewenka, K.M.; Holzschuh, A.; Tscharntke, T.; Dormann, C.F. Landscape Elements as Potential Barriers and Corridors for Bees, Wasps and Parasitoids. Biol. Conserv. 2011, 144, 1816–1825. [Google Scholar] [CrossRef]
- Boscolo, D.; Tokumoto, P.M.; Ferreira, P.A.; Ribeiro, J.W.; Santos, J.S. dos. Positive Responses of Flower Visiting Bees to Landscape Heterogeneity Depend on Functional Connectivity Levels. Perspect. Ecol. Conserv. 2017, 15, 18–24. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wojtjowsjki, P.A. Agroecological Economics. Sustainability and Biodiversity; Elsevier: London, UK, 2008. [Google Scholar]
- Nicholls, C.I.; Altieri, M.A.; Vazquez, L. Agroecology: Principles for the Conversion and Redesign of Farming Systems. J. Ecosyst. Ecography 2016, 01. [Google Scholar] [CrossRef]
- Altieri, M.A.; Toledo, V.M. The Agroecological Revolution in Latin America: Rescuing Nature, Ensuring Food Sovereignty and Empowering Peasants. J. Peasant Stud. 2011, 38, 587–612. [Google Scholar] [CrossRef]
- Altieri, M.A.; Rosset, P. Agroecology and the Conversion of Large-Scale Conventional Systems to Sustainable Management. Int. J. Environ. Stud. 1996, 50, 165–185. [Google Scholar] [CrossRef]
- Jacobi, J.; Mathez-Stiefel, S.L.; Gambon, H.; Rist, S.; Altieri, M. Whose Knowledge, Whose Development? Use and Role of Local and External Knowledge in Agroforestry Projects in Bolivia. Environ. Manage. 2017, 59, 464–476. [Google Scholar] [CrossRef]
- Holt-Giménez, E.; Altieri, M.A. Agroecology, Food Sovereignty, and the New Green Revolution. Agroecol. Sustain. Food Syst. 2013, 37, 90–102. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Altieri, M.A. Pathways for the Amplification of Agroecology. Agroecol. Sustain. Food Syst. 2018, 42, 1170–1193. [Google Scholar] [CrossRef]
- Landaverde-González, P.; Quezada-Euán, J.J.G.; Theodorou, P.; Murray, T.E.; Husemann, M.; Ayala, R.; Moo-Valle, H.; Vandame, R.; Paxton, R.J. Sweat Bees on Hot Chillies: Provision of Pollination Services by Native Bees in Traditional Slash-and-Burn Agriculture in the Yucatán Peninsula of Tropical Mexico. J. Appl. Ecol. 2017, 54, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Catacora-Vargas, G.; Piepenstock, A.; Sotomayor, C.; Cuentas, D.; Cruz, A.; Delgado, F. Brief Historical Review of Agroecology in Bolivia. Agroecol. Sustain. Food Syst. 2017, 41, 429–447. [Google Scholar] [CrossRef]
- Pinstrup-Andersen, P. Food Security: Definition and Measurement. Food Secur. 2009, 1, 5–7. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Agroecology: Challenges and Opportunities for Farming in the Anthropocene. Int. J. Agric. Nat. Resour. 2020, 47, 204–215. [Google Scholar] [CrossRef]
- Matsuda, T.; Wolff, J.; Yanagawa, T. Risk and the Regulation of New Technologies; Unive, K., Yanagawa, T., Eds.; Springer & Kobe University: Kobe, Japan, 2021. [Google Scholar] [CrossRef]
- Shaw, A.; Wilson, K. The Bill and Melinda Gates Foundation and the Necro-Populationism of ‘Climate-Smart’ Agriculture. Gend. Place Cult. 2020, 27, 370–393. [Google Scholar] [CrossRef]
- Shields, M.W.; Johnson, A.C.; Pandey, S.; Cullen, R.; González-Chang, M.; Wratten, S.D.; Gurr, G.M. History, Current Situation and Challenges for Conservation Biological Control. Biol. Control. 2019, 25–35. [Google Scholar] [CrossRef]
- Laterra, P.; Barral, P.; Carmona, A.; Nahuelhual, L. Focusing Conservation Efforts on Ecosystem Service Supply May Increase Vulnerability of Socio-Ecological Systems. PLoS ONE 2016, 11, 100875. [Google Scholar] [CrossRef]
- Cock, M.J.W. Strategic Entry Points for Funding Taxonomic Support to Agriculture in Developing Countries. CABI Working Paper 3; CABI: Wallingford, UK, 2011; p. 32. [Google Scholar]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How Many Species Are There on Earth and in the Ocean? PLoS Biol. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V.; New, T.R. The Seven Impediments in Invertebrate Conservation and How to Overcome Them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Clark, J.A.; May, R.M. Taxonomic Bias in Conservation Research. Science 2002, 297, 191–192. [Google Scholar] [CrossRef]
- Wilson, E.O. The Diversity of Life; Harvard University Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242. [Google Scholar] [CrossRef]
- Dewalt, B. Using Indigenous Knowledge to Improve Agricul- Ture and Natural Resource Management. Hum. Organ. 1994, 53, 123–131. [Google Scholar] [CrossRef]
- Johnson, J.T.; Howitt, R.; Cajete, G.; Berkes, F.; Louis, R.P.; Kliskey, A. Weaving Indigenous and Sustainability Sciences to Diversify Our Methods. Sustain. Sci. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Gemmill-Herren, B.; Garibaldi, L.A.; Kremen, C.; Ngo, H.T. Building Effective Policies to Conserve Pollinators: Translating Knowledge into Policy. Curr. Opin. Insect Sci. 2021, 46, 64–71. [Google Scholar] [CrossRef]
- Lyver, P.; Perez, E.; Carneiro da Cunha, M.; Roué, M. Indigenous and Local Knowledge about Pollination and Pollinators Associated with Food Production: Outcomes from the Global Dialogue Workshop. In Outcomes from the Global Dialogue Workshop USDA IPBBES UNEP UNESCO FAO UNDP; Lyver, R., Perez, E., Carneiro da Cunha, M., Roué, M., Eds.; United Nations Educational, Scientic and Cultural Organization: Panama City, Panama, 2014; p. 106. [Google Scholar]
- Mikkelson, G.M.; Gonzalez, A.; Peterson, G.D. Economic Inequality Predicts Biodiversity Loss. PLoS ONE 2007, 2. [Google Scholar] [CrossRef]
- Altieri, M.A. Linking Ecologists and Traditional Farmers in the Search for Sustainable Agriculture. Front. Ecol. Environ. 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Richard, E. Bioregionalism and Global Ethics: A Transactional Approach to Achieving Ecological Sustainability, Social Justice, and Human Well-Being; Routledge, Taylor and Francis Group: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Aitken, D.; Rivera, D.; Godoy-Faúndez, A.; Holzapfel, E. Water Scarcity and the Impact of the Mining and Agricultural Sectors in Chile. Sustainability 2016, 8, 128. [Google Scholar] [CrossRef]
- Hipólito, J.; Coutinho, J.; Mahlmann, T.; Santana, T.B.R.; Magnusson, W.E. Legislation and Pollination: Recommendations for Policymakers and Scientists. Perspect. Ecol. Conserv. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Wittman, H. Food Sovereignty: A New Rights Framework for Food and Nature? Environ. Soc. 2012, 2, 87–105. [Google Scholar] [CrossRef]
- Seminar, A.; Sarwoprasodjo, S.; Santosa, D.; Rilus, A. Agroecological Education Aimed at Achieving Food Sovereignty. J. Dev. Sustain. Agric. 2017, 12, 34–44. [Google Scholar] [CrossRef]
- Baker, A.M.; Redmond, C.T.; Malcolm, S.B.; Potter, D.A. Suitability of Native Milkweed (Asclepias) Species versus Cultivars for Supporting Monarch Butterflies and Bees in Urban Gardens. PeerJ 2020, 8, e9823. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.; Brown, C.; Rounsevell, M. Europe’s Green Deal Offshores Environmental Damage to Other Nations; Nature Publishing Group: London, UK, 2020; Volume 586, pp. 671–673. [Google Scholar] [CrossRef]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable Intensification in Agriculture: Premises and Policies. Science 2013, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Gervais, J.; Buhl, K.; Stone, D. Acephate General Fact Sheet. 2011. Available online: http://npic.orst.edu/factsheets/acephagen.html (accessed on 16 September 2018).
- Bernal, J.L.; Garrido-Bailon, E.; Del Nozal, J.B.; González-Porto, A.V.; Hernández, R.M.; Diego, J.C.; Jimenez, J.J.; Higes, M. Overview of Pesticide Residues in Stored Pollen and Their Potential Effect on Bee Colony (Apis mellifera) Losses in Spain. J. Econ. Èntomol. 2010, 103, 1964–1971. [Google Scholar] [CrossRef]
- Boily, M.; Sarrasin, B.; DeBlois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef]
- Araújo, R.D.S.; Bernardes, R.C.; Martins, G.F. A mixture containing the herbicides Mesotrione and Atrazine imposes toxicological risks on workers of Partamona helleri. Sci. Total. Environ. 2021, 763, 142980. [Google Scholar] [CrossRef]
- Williams, J.R. Biomarkers of Oxidative Stress in Atrazine-Treated Honey Bees: A Laboratory and In-Hive Study. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2016; p. 78. Available online: http://hdl.handle.net/10919/72949 (accessed on 1 June 2021).
- North Carolina Department of Agriculture & Consumer Services. Pesticide Toxicity to Bees “Traffic Light”. 2016. Available online: http://www.ncagr.gov/pollinators/documents/BeePesticideRiskTrafficLight3-2-17.pdf (accessed on 1 June 2021).
- European Food Safety Authority. Conclusion regarding the peer review of the pesticide risk assessment of the active substance cadusafos. EFSA Sci. Rep. 2006, 1–70. [Google Scholar]
- Bond, C.; Hallman, A.; Buhl, K.; Stone, D. Carbaryl General Fact Sheet. 2016. Available online: http://npic.orst.edu/factsheets/carbarylgen.html (accessed on 16 September 2018).
- Shi, T.; Burton, S.; Zhu, Y.; Wang, Y.; Xu, S.; Yu, L. Effects of Field-Realistic Concentrations of Carbendazim on Survival and Physiology in Forager Honey Bees (Hymenoptera: Apidae). J. Insect Sci. 2018, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Böhme, F.; Bischoff, G.; Zebitz, C.P.W.; Rosenkranz, P.; Wallner, K. Pesticide residue survey of pollen loads collected by honeybees (Apis mellifera) in daily intervals at three agricultural sites in South Germany. PLoS ONE 2018, 13, e0199995. [Google Scholar] [CrossRef] [PubMed]
- Marletto, F.; Patetta, A.; Manino, A. Laboratory assessment of pesticide toxicity to bumblebees. Bull. Insectology 2003, 56, 155–158. [Google Scholar]
- Kegley, S.E.; Hill, B.R.; Orme, S.; Choi, A.H. Cartap Monohydrochloride. 2016. Available online: http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC38464 (accessed on 16 September 2018).
- Rhodes, J.; Scott, M. Pesticides: A Guide to Their Effects on Honey Bees; New South Wales Department of Primary Industries: Orange, Australia, 2006. [Google Scholar]
- Fisher, A., II; Coleman, C.; Hoffmann, C.; Fritz, B.; Rangel, J. Apiculture & Social Insects the Synergistic Effects of Almond Protection Fungicides on Honey Bee (Hymenoptera: Apidae) Forager Survival. J. Econ. Entomol. 2017, 110, 802–808. [Google Scholar] [PubMed]
- Mackenzie, K.A.E.; Winston, A.L. Effects of Sublethal Exposure to Diazinon on Longevity and Temporal Division of Labor in the Honey Bee (Hymenoptera: Apidae). J. Econ. Entomol. 1989, 82, 75–82. [Google Scholar] [CrossRef]
- Weick, J.; Thorn, R.S. Effects of acute sublethal exposure to coumaphos or diazinon on acquisition and discrimination of odor stimuli in the honey bee (Hymenoptera: Apidae). J. Econ. Èntomol. 2002, 95, 227–236. [Google Scholar] [CrossRef]
- Gromisz, M. Gastric toxicity to bees of fenropatrin—The active ingredient of Danitol and Danirun formulas. J. Apic. Sci. 2001, 45, 51–60. [Google Scholar]
- National Research Council of Canada, Associate Committee on Scientific Criteria for Environmental Quality. Pesticide–pollinator interactions. NCCC, Publication Nº 18477 of the Environmental Secretariat; Subcommitte on Pesticides and Industrial Organic Chemicals: Ottawa, Canada, 1981; p. 190. [Google Scholar]
- De Morais, C.R.; Travençolo, B.A.N.; Carvalho, S.M.; Beletti, M.; Santos, V.S.V.; Campos, E.; Júnior, E.O.D.C.; Pereira, B.B.; Naves, M.P.C.; De Rezende, A.A.A.; et al. Ecotoxicological effects of the insecticide fipronil in Brazilian native stingless bees Melipona scutellaris (Apidae: Meliponini). Chemosphere 2018, 206, 632–642. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gauthier, M.; Armengaud, C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol. Biochem. Behav. 2005, 82, 30–39. [Google Scholar] [CrossRef]
- Mayer, D.F.; Lunden, J.D. Effects of imidacloprid insecticide on three bee pollinators. Hortic Sci 1997, 29, 93–97. [Google Scholar]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion regarding the peer review of the pesticide risk assessment of the active substance glufosinate finalized: 14 March 2005. Eur. Food Safety Auth. J. 2005. [Google Scholar] [CrossRef]
- European Food Safety Authority. Peer Review of the Pesticide Risk Assessment of the Active Substance Imazamox. European Food Safety Authority Journal 14:n/a-n/a. 2016. Available online: http://10.0.11.87/j.efsa.2016.4432%5Cnhttp://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,url,cookie,uid&db=aph&AN=115160811&site=ehost-live&scope=site) (accessed on 1 June 2021).
- Abrol, D.P.; Andotra, R.S. Field Toxicity of Pesticides to Honeybee, Apis mellifera L., Foragers. Journal of Apiculture 2001, 1, 19–26. Available online: http://dev02.dbpia.co.kr/0/95/59/955925.pdf?article=NODE00955926 (accessed on 1 June 2021).
- Chauzat, M.; Faucon, J. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest. Manag. Sci. 2007, 1106, 1100–1106. [Google Scholar] [CrossRef]
- EPA. Reregistration Eligibility Decision for Methidathion. United States Environmental Protection Agency: Washington, DC, USA, 2006. Available online: http://www.epa.gov/opp00001/methods/atmpmethods/QC-23-01.pdf (accessed on 1 June 2021).
- Fine, J.D.; Mullin, C.A.; Frazier, M.T.; Reynolds, R.D. Field Residues and Effects of the Insect Growth Regulator Novaluron and Its Major Co-Formulant N -Methyl-2-Pyrrolidone on Honey Bee Reproduction and Development. J. Econ. Entomol. 2017, 110, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Cousin, M.; Silva-Zacarin, E.; Kretzschmar, A.; El Maataoui, M.; Brunet, J.L.; Belzunces, L.P. Size Changes in Honey Bee Larvae Oenocytes Induced by Exposure to Paraquat at Very Low Concentrations. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.O.; Morton, H.L.; Macdonald, R.H. Toxicity of Herbicides to Newly Emerged Honey Bees. Environ. Entomol. 1972, 1, 102–104. [Google Scholar] [CrossRef]
- Cox, R.L.; Wilson, W.T. Effects of Permethrin on the Behavior of Individually Tagged Honey Bees, Apis mellifera L. (Hymenoptera: Apidae). Environ. Èntomol. 1984, 13, 375–378. [Google Scholar] [CrossRef]
- Hagler, J.R.; Waller, G.D.; Lewis, B.E. Mortality of honeybees (Hymenoptera: Apidae) exposed to permethrin and combinations of permethrin with piperonyl butoxide. J. Apic. Res. 1989, 28, 208–211. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef]
- FAO. PROCYMIDONE. FAO Specifications for Plant. Protection Products; 2001; p. 97. Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/procymid.pdf (accessed on 1 June 2021).
- Melisie, D.; Damte, T.; Thakur, A.K. Effects of some insecticidal chemicals under laboratory condition on honeybees [Apis mellifera L. (Hymenoptera: Apidae)] that forage on onion flowers. Environ. Sci. Pollut. Res. 2015, 10, 1295–1300. [Google Scholar]
- Stanley, J.; Sah, K.; Jain, S.; Bhatt, J.; Sushil, S. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere 2015, 119, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Australian Pesticides and Veterinary Medicines Authority. Australian Government 2012. Public Release Summary on the Evaluation of the New Active Saflufenacil in the Product SHARPEN WG HERBICIDE (Previously Heat Herbicide); APVMA Product Number 62853; Australian Pesticides and Veterinary Medicines Authority: Armidale, NSW, Australia, 2012; p. 66. [Google Scholar]
- Jimenez, D.R. Efecto letal agudo de los insecticidas en formulación comercial Imidacloprid, Spinosad y Thiocyclam hidrogenoxalato en obreras de Bombus atratus (Hymenoptera: Apidae). Rev. Biol. Trop. 2016, 64, 1737–1745. [Google Scholar] [CrossRef][Green Version]
- Baines, D.; Wilton, E.; Pawluk, A.; De Gorter, M.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gary, V.; Lambin, M.; Gauthier, M.; Armengaud, C. Effects of Sublethal Doses of Acetamiprid and Thiamethoxam on the Behavior of the Honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 2007, 54, 653–661. [Google Scholar] [CrossRef]
- EPA. Pesticide Fact Sheet: Dinotefuran. United States Environmental Protection Agency: Washington, DC, USA. 2004. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-044312_01-Sep-04.pdf (accessed on 1 June 2021).
- Williamson, S.M.; Willis, S.J.; Wright, G.A. Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology 2014, 23, 1409–1418. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. RFID Tracking of Sublethal Effects of Two Neonicotinoid Insecticides on the Foraging Behavior of Apis mellifera. PLoS ONE 2012, 7, e30023. [Google Scholar] [CrossRef]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Taylor, M.A.; Goodwin, M.; McBrydie, H.; Cox, H.M. Destroying managed and feral honey bee (Apis mellifera) colonies to eradicate honey bee pests. N. Z. J. Crop. Hortic. Sci. 2007, 35, 313–323. [Google Scholar] [CrossRef]
- Domingues, C.E.; Abdalla, F.C.; Balsamo, P.J.; Pereira, B.V.; Hausen, M.D.A.; Costa, M.J.; Silva-Zacarin, E.C. Thiamethoxam and picoxystrobin reduce the survival and overload the hepato-nephrocitic system of the Africanized honeybee. Chemosphere 2017, 186, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Kalnins, M.A.; Detroy, B.F. The effect of wood preservative treatment of beehives on honey bees and hive products. J. Agric. Food Chem. 1984, 32, 1176–1180. [Google Scholar] [CrossRef]
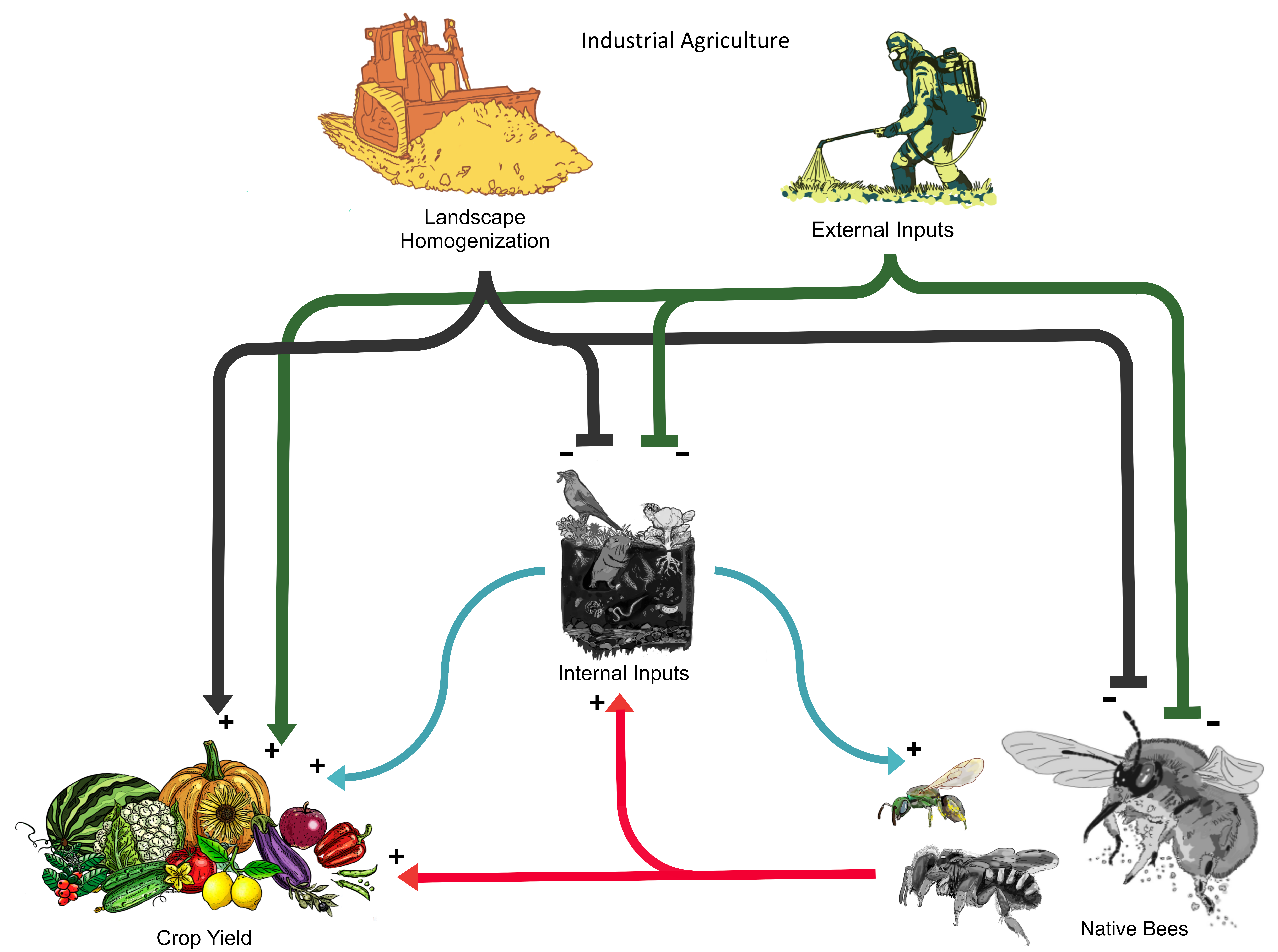


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henríquez-Piskulich, P.A.; Schapheer, C.; Vereecken, N.J.; Villagra, C. Agroecological Strategies to Safeguard Insect Pollinators in Biodiversity Hotspots: Chile as a Case Study. Sustainability 2021, 13, 6728. https://doi.org/10.3390/su13126728
Henríquez-Piskulich PA, Schapheer C, Vereecken NJ, Villagra C. Agroecological Strategies to Safeguard Insect Pollinators in Biodiversity Hotspots: Chile as a Case Study. Sustainability. 2021; 13(12):6728. https://doi.org/10.3390/su13126728
Chicago/Turabian StyleHenríquez-Piskulich, Patricia A., Constanza Schapheer, Nicolas J. Vereecken, and Cristian Villagra. 2021. "Agroecological Strategies to Safeguard Insect Pollinators in Biodiversity Hotspots: Chile as a Case Study" Sustainability 13, no. 12: 6728. https://doi.org/10.3390/su13126728
APA StyleHenríquez-Piskulich, P. A., Schapheer, C., Vereecken, N. J., & Villagra, C. (2021). Agroecological Strategies to Safeguard Insect Pollinators in Biodiversity Hotspots: Chile as a Case Study. Sustainability, 13(12), 6728. https://doi.org/10.3390/su13126728







