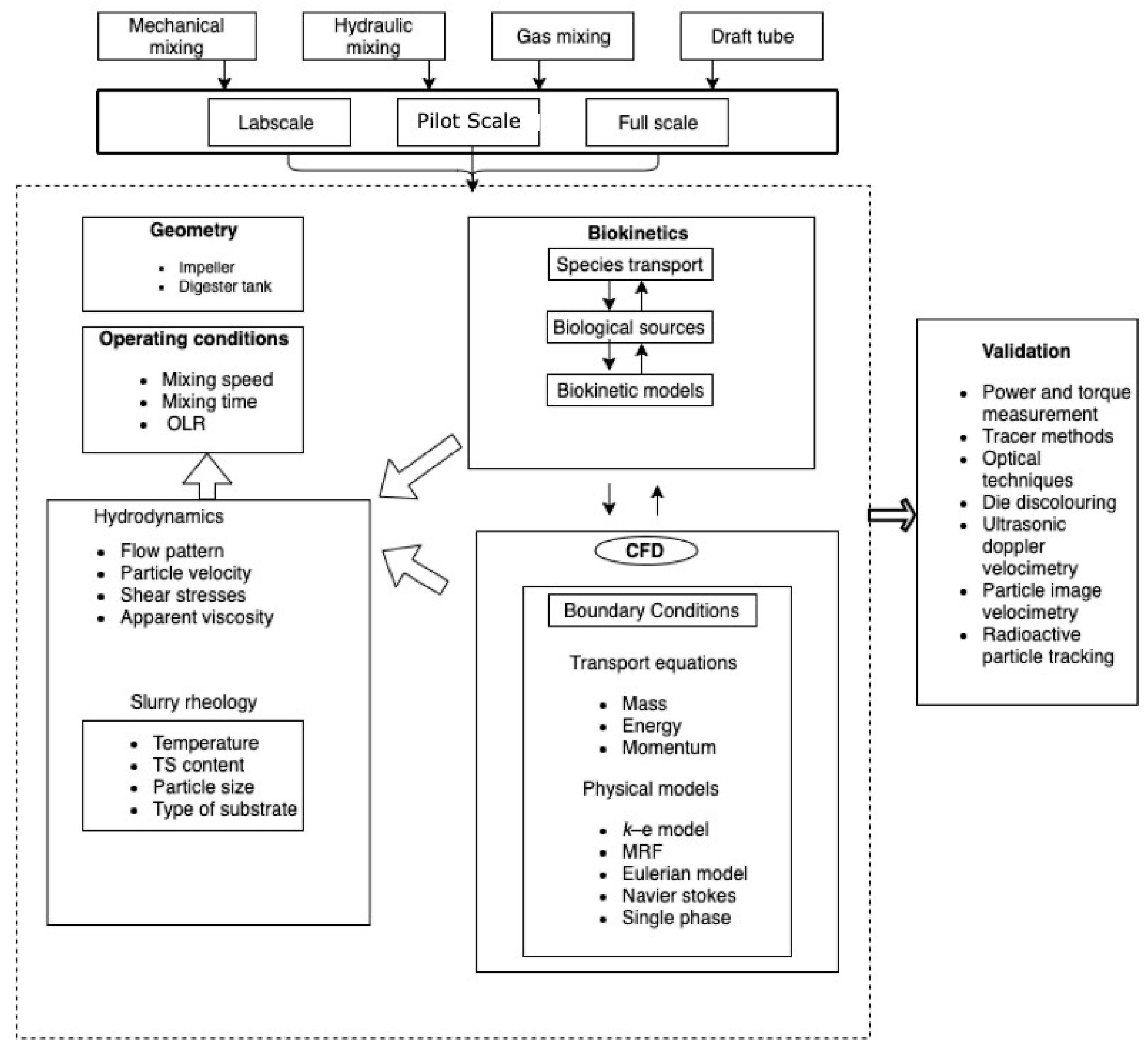
1. Introduction
Anaerobic digestion technology is one of the most trending sources of renewable energy resources with a very low carbon footprint [
1,
2,
3,
4]. AD is a complex multiphase biological-chemical-physical process that involves a series of biochemical steps through which the organic matter is broken down into simpler compounds and finally produces high-quality gaseous fuel (biogas), class-A manure. AD process has been studied meticulously over decades particularly focusing on the molecular level and operational parameters at both lab scale and large scale biogas plants [
5,
6,
7]. The operational optimization of a full biogas plant along with the biological process throughout the anaerobic digestion has great significance in enhancing the biogas production rates and conversion of waste to energy [
5,
8,
9,
10,
11,
12,
13]. The overall efficiency of a biogas plant can be determined in terms of process stability, methane production rates and internal power consumption by the plant [
14,
15,
16].
The performance of an anaerobic digester depends on the number of internal and external factors such as substrate, temperature, pH, HRT, mixing of slurry and C/N ratio [
17]. Mixing is one of the most prominent factors which determines the efficiency of the biogas plant in terms of overall power consumption by the biogas plant and biogas production rates [
14,
18,
19,
20,
21]. Numerous positive impacts of mixing have been noted in the literature that involve the uniform distribution of fresh substrate in the tank, avoiding temperature and pH gradient, foaming and scum formation, maintaining homogeneity of nutrients inside the entire volume of the digester [
22,
23,
24,
25]. Mixing is a very crucial abiotic factor that ensures the following:
The homogenization of nutrients within the entire volume of the digester and avoiding the accumulation of VFA’s and pH inhibitions.
Ensures biogas desorption by effective gas-liquid mass transfer and avoids H2 inhibition.
Uniform distribution of fresh substrate within the digester and increases the reaction time of microbes by ensuring continuous contact between substrate and microorganisms.
Homogeneous distribution of temperature in the digester.
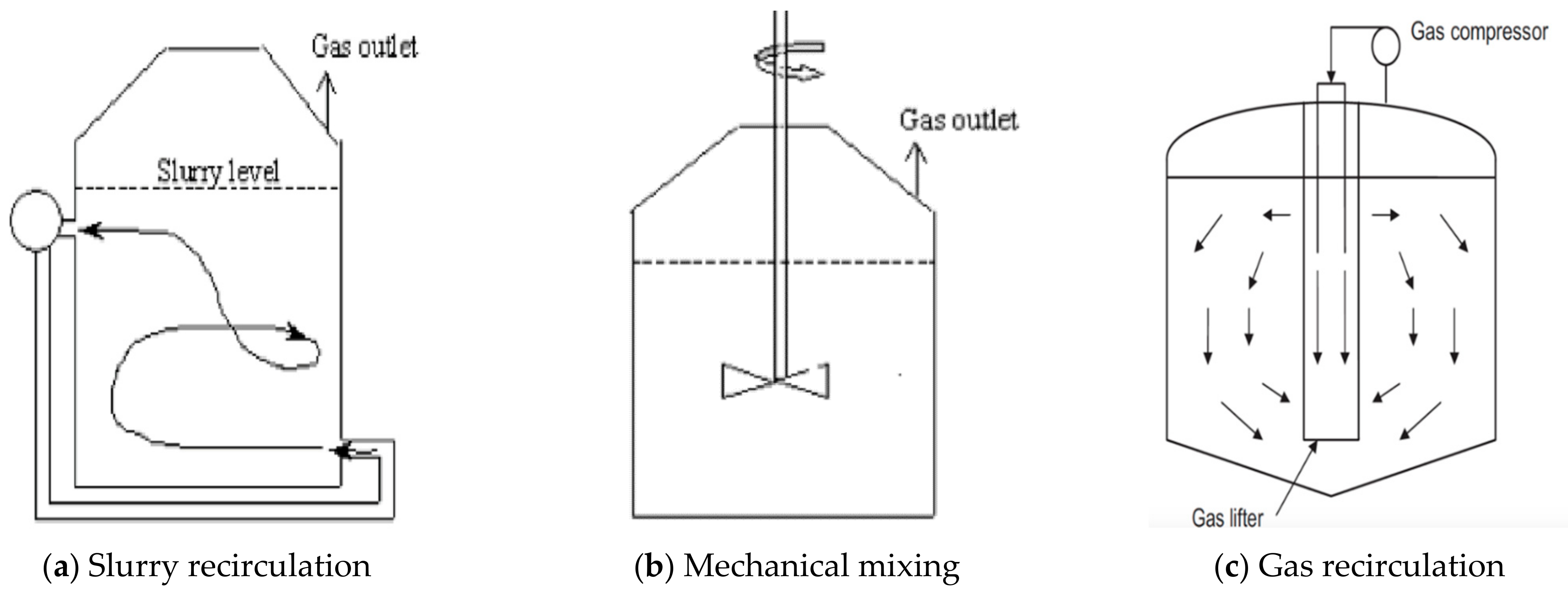
Mixing in an anaerobic digester can be attained by various modes (
Figure 1) such as slurry recirculation [
19,
23], impeller mixing [
21,
26,
27] and biogas recirculation [
28,
29]. From the above-mentioned mixing modes, mechanical mixing is considered one of the best ways to attain homogenization at lower power input and higher mixing efficiency [
30]. Scrupulous literature can be found focusing on the optimization of mixing in an anaerobic digester but still, it is debatable in the context of mixing regimes and the geometry of the mixing setup. Due to the complexity of the digestion process, the efficiency rests on a large number of factors such as the physical and chemical composition of the substrate, temperature, organic loading rate, pH and mixing of slurry, etc. [
31]. The evaluation of mechanical mixing requires the geometrical constraints of both the impeller and digester to predict the shear stresses and flow patterns and their effect on the microbiology and biogas production [
32]. On the other hand, most of the studies to evaluate mixing are just focused on lab-scale experiments whereas the mixing process should be scaled up to the large-scale biogas plants to achieve higher efficiency in a biogas plants in terms of internal energy consumption and biogas production rates. Many different types of impellers and their modified versions have been employed to enhance homogenization and uniform distribution of viscosity and velocity in digesters at lower rpm [
30,
33,
34]. Literature suggests that the intermittent mixing at impeller speed between 10–100 rpm and laminar flow regime is favorable in the context of increasing biogas production, but it is limited to the lab-scale digesters and can increase when scaled up to full scale digester.
In recent studies, it has been observed that the research in the field has shifted in the context of studying the effect of mixing on a microbial level [
28,
35,
36]. On other hand, some studies only focus on the flow pattern, particle velocity, velocity gradient and hydrodynamics of the impeller and digester [
37,
38,
39].
The hurdle in the optimization of mixing in a digester does not rest on the lack of available literature and technology but on the approach towards the evaluation and scaling up of the lab-scale setup to a full-scale biogas fermenter. This study emphasizes more on the mixing evaluation techniques rather than the results of the effects of mixing and optimization. The gap in research lies due to the scantiness of the correlation between the mechanical and biological aspects of agitation in an anaerobic digester. In this work, review of evaluation techniques is the primary goal rather than studying the effect of mixing on the results of anaerobic digester. The overall motive of this analysis is to highlight the recent numerical, analytical and experimental tools capable of evaluating the mixing in digesters, to summarize perspectives on process intensification and scale-up tools centered on mixing optimization, but also to explore issues that still need to be discussed, in particular the relationship between hydrodynamics and biokinetic models.
The present study has been considered with the following motives:
To determine the best combination of techniques and approaches towards the evaluation of mixing efficiency in anaerobic digesters.
To understand the importance of an interdisciplinary approach to the optimization of mixing in an anaerobic digester.
To discover the local and global parameters involved in the analysis of mixing in bioreactors.
To draw the directions for future research and scope in the field of optimization of mixing in digesters in terms of power consumption and biogas production rates.
2. Brief Overview of Results on Optimization of Mixing in Anaerobic Digester
Many reviews have been published that analyze previous research on the mixing in anaerobic digesters [
18,
40,
41]. Some similar conclusions have been drawn from reviewing the literature through these studies, but a general statement cannot be deduced. The foremost epilogue is that intermittent mixing at lower intensity is best to enhance biogas production. Therefore, there is no motivation to continue with the continuous stirring of slurry in an anaerobic digester because, in some cases, the biogas production rate was the same for both intermittent and continuous mixing, but the power consumption was higher in case of continuous mixing. It is concluded that continuous mixing is just a waste of energy as it has no positive impact on the overall efficiency of the anaerobic digester. Moreover, higher mixing intensities and continuous mixing disrupt the syntrophic relationships and the microbial flocs among the bacteria and methanogens [
42].
According to our last findings [
41], the impeller and digester design has a remarkable effect on the mixing efficiency and homogeneity of the slurry inside the tank. From the literature, it was deduced that the modification of impeller geometry and digester hydrodynamics can promote the homogeneous distribution of velocity and viscosity in the digester which is the best way to optimize the mixing in an anaerobic digester. Moreover, the total mixing time is also determined by the impeller geometry and rotational speed. Mixing time can be reduced by higher rpm of the impeller but on the other hand, it will generate high shear stresses, which will affect the microbial community and hence reduce biogas production rates.
Since mixing occurs as a function of flow patterns, hydrodynamic and mixing properties of anaerobic digesters can be determined using traditional chemical engineering and fluid dynamics techniques. This can be classified into two types: global techniques that calculate macroscopic parameters at the digester scale (referred to as global parameters) and local techniques that provide local data, such as the velocity field. Simultaneously, another classification can be described, particularly for local techniques: probing methods follow a local property at a fixed point in the reactor or in the influent/effluent streams, point-by-point methods allow sequential scanning of a digester region, and whole-field methods attempt to follow instantaneously local properties in a digester region or in the digester.
For instance, Lebranchu et al. [
27] demonstrated that the overall biogas production in the digester equipped with DHR was 50% more than the digester equipped with RT. Moreover, for high mixing intensities, the methane content was lower as compared to mixing at slower rpm. The huge difference between the results was due to the lower percentage of dead volume and uniform distribution of velocity within the digester by helical ribbon. Similarly, Hashimoto et al. [
43] explored the effect of the operation time of mixing by a 3-B impeller at 220 rpm on biogas production rates. It was observed that the biogas production rate was highest in the case of intermittent mixing at 2 h/day as compared to continuous, 1 h/day and 3 h/day. The time of operation of mixing equipment and the amount of shear stresses developed by the impeller can be controlled by the optimization of the impeller and the speed of rotation.
Moreover, at different stages of AD, the microorganisms exhibit different behavior than the hydrodynamic shears [
36,
44]. For instance, Si-jia Ma et al. [
44] physically separated different phases of the digestion process. It was noted that mixing speeds of 90 and 120 rpm were appropriate for the acidification and hydrolysis phase as an abundance of proteobacteria, firmicutes, chloroflexi, actinobacteria and Bacteroidetes was found. Methanogens are highly sensitive to high shear stresses so the mixing intensity should be minimum as the process approaches methanogenesis.
Similarly, mixing in a digester is also depends on the rheological properties of the slurry. Mixing is significant only in the digesters operated at higher TS content. Various studies on the rheology of slurry demonstrate that the slurry viscosity rapidly increases with an increase in the TS content. Higher viscosity results in resistance to the particle flow and movement of the fluid. Hence the power consumption and mixing time increase.
Important variables affecting the mixing times and efficiency are:
Mixer type, configuration and proportions;
Digester tank size geometry;
Rotational speed of the impeller;
Physical and rheological properties of slurry such as viscosity, particle size and density;
Method and position of adding fresh substrate to the digester tank;
The criteria adopted for judging the mixing time.
Mixing has a significant effect on the efficiency of the anaerobic digester at higher solid content (above 4%), but at lower TS content (<4%), the operation of mixing is just an unnecessary wastage of energy as the results were found to be similar in mixed and unmixed digesters [
45,
46,
47]. Optimization of mixing is still a debatable subject because of the large number of factors that vary from one experiment to another.
3. Correlation between Lab-Scale and Large-Scale Mixing in Anaerobic Digesters
It has been observed that most of the studies on the evaluation of the effect of mixing regimes on biogas production are limited to lab-scale digesters [
27,
48,
49,
50,
51] and only a few represent the results regarding full-scale biogas plants [
35,
52,
53]. The major challenge in mixer setup design is to scale up from a laboratory or pilot scale to a full-scale unit. Effective design and scale-up from a laboratory to a large-scale bioreactor includes the optimization of design and operating parameters, including thorough knowledge of biokinetics and hydrodynamics.
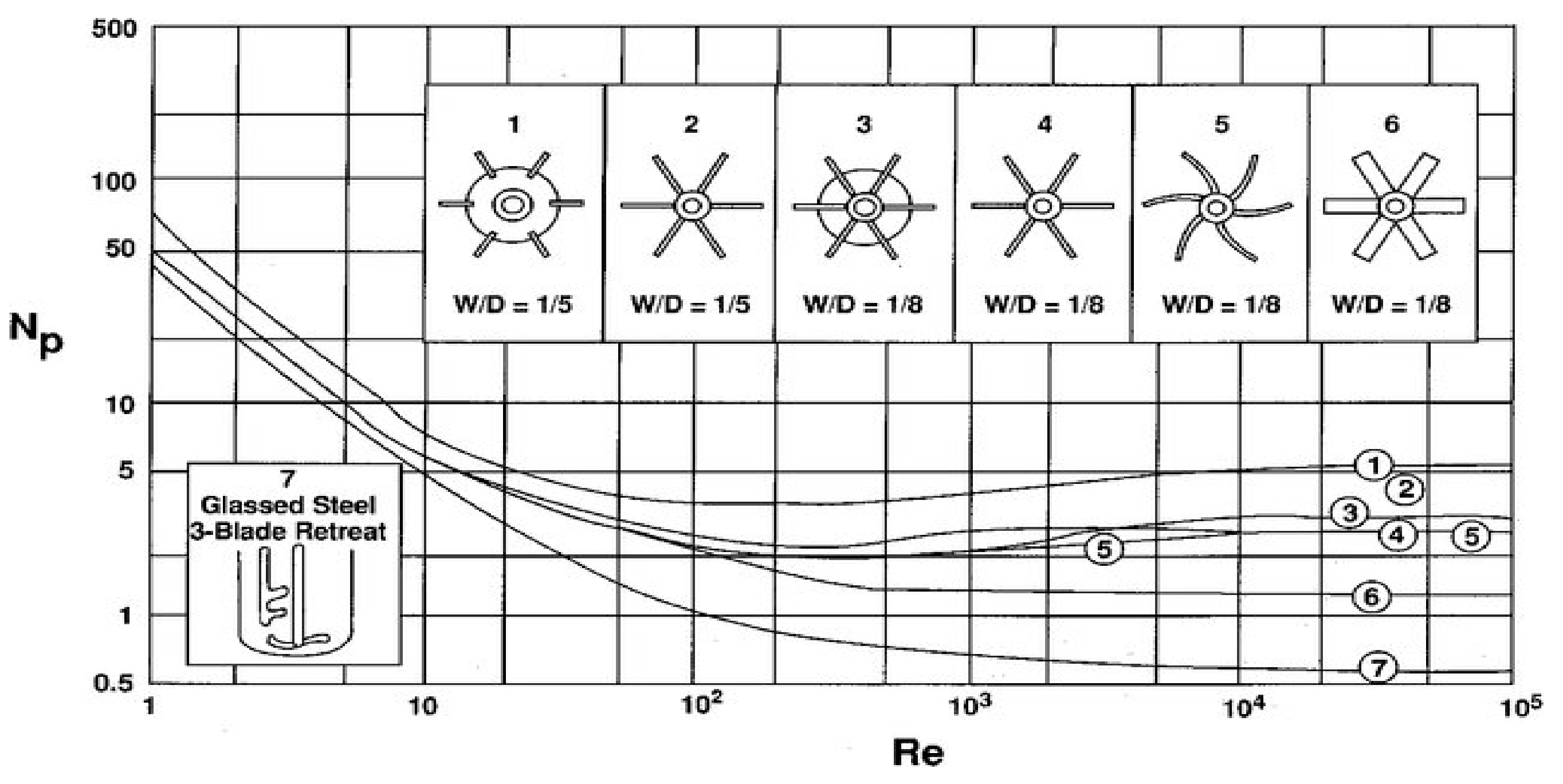
For some specific cases, the generalized power correlations between
Np versus Reynolds number for various impellers are available for scale-up as shown in
Figure 2. The Reynolds number is the ratio of inertial forces to viscous forces within a fluid, which is subjected to relative internal movement due to different fluid velocities. Another possible solution for scale-up is based on geometrical similarities between the laboratory and full-scale plant equipment. However, it is not always possible to have lab-scale and large-scale digesters that are geometrically similar. Furthermore, in some cases, it can be possible to obtain geometrical similarities, but it is very hard to have dynamic and kinematic similarities which will lead to divergence from the predicted results [
54].
Basically, the similarities between the different size of digester can be classified as:
Geometrical similarity.
Kinematic similarity.
Dynamic similarity.
Geometrical similarity refers to the similarity between the ratios of all corresponding dimensions in digesters of different sizes, this includes the diameter, off bottom and inter impeller clearance, tank shape, etc. The optimum ratio of vessel diameter to the impeller diameter for a given power input is a crucial factor in scale-up [
56]. This ratio is strongly influenced by the nature of the agitation problem. For a constant power input, the impeller speed will be higher if the impeller diameter is smaller. Correspondingly, for a lower speed of the impeller, the diameter of the impeller should be increased in the anaerobic digester because lower mixing intensity is preferred for maintaining a favorable environment for bacteria.
When constant power per unit volume and geometrical similarities are maintained in scaling up, the impeller speed changes with
Da−2/3 [
57]. The power per unit volume is:
The terms inside the bracket are constant so, must be constant.
During the scale-up of an anaerobic digester, in order to maintain
P/
V constant, reduction in impeller speed can lead to longer mixing time hence leading to higher power consumption. Dynamic similarity is attained when the ratio of all the corresponding forces is the same whereas kinematic similarity refers to the similarity in the ratio of the velocities at corresponding points. Additionally, these similarities have utmost importance, especially during the scale-up of an anaerobic digester and are presented together because they are interrelated in a fluid system. Equality of the groups in the following equation ensures the dynamic and kinematic similarities.
Power consumption in large-scale biogas plants can be accurately predicted from the curves of
Np versus
Re (
Figure 2). Usually, the power consumed by the impeller per unit volume of slurry is used as a measure of mixing effectiveness [
40]. The power use of the anaerobic digester impeller dynamically influences the characteristics of the device, such as the configuration, the geometry and the scale of the digester, the position of the impeller, the speed of the impeller and the rheological behavior of the substrate in the anaerobic digester. Precise measurement of the strength is important for the power unit selection for optimal mixing. Poor mixing units would contribute to excessive expenditure in equipment and higher energy use rates, which will reduce the productivity of the biogas project [
26].
Very few studies [
58,
59,
60,
61,
62,
63] have been noted in the literature that investigates hydrodynamics in an anaerobic digester under both scale-up and scale-down conditions of an actual biogas plant anaerobic digester. It is noted that the observation of mixing in digesters under real conditions is complex and cost-intensive. Fonti et al. [
59] analyzed the mixing in an anaerobic digester by scaling down the full-scale digester to 1:12. All the equipment in a real full-scale digester was scaled down and replicated.
Figure 3 represents the scale downsizing of mixers used in an actual large-scale digester. According to Fonti et al., the parameters derived from CFD simulations possessed good agreement with the experimental results derived from PIV and ADV techniques. In a real scenario of a biogas plant, the microbe composition will be different, the conditions will be anaerobic, the difference in temperature and various other factors will result in a totally different situation. There is a practical limit to biogas production at the industrial level. Deeper analysis of this interesting biological mechanism is essential. In terms of mechanical stirring, the researchers found a very simple and inexpensive technique that seems to work. Assumptions on the geometrical composition and substrate rheology will provide better understanding and optimization of mixing in the large-scale biogas plant digester.
4. Parameters for Evaluation of Mixing in Anaerobic Digester
Numerous parameters have been evaluated to study the mixing efficiency in an anaerobic digester. From the intensive literature review, it was observed that the approach to studying mixing efficiency depends on the scale of the biogas digester. For instance, in the lab-scale experiments, the general focus is on the determination of the amount of biogas production rate [
19,
64], methane content [
65,
66], the behavior of microorganisms [
36,
67,
68] and dead zones [
27,
51] by varying the rotational speed of the impeller. Each factor has its own significance and importance. Here it will not be erroneous to say that determination of biogas production rate should not be the only parameter to evaluate the efficiency of an impeller at various mixing speeds.
Another parameter to analyze the effect of mixing intensity on biogas production is the determination of velocity gradient. Various studies can be found dealing with the simulations and experimental calculation of average velocity [
69,
70], velocity gradient (G) [
19,
71] and mixing energy level (G
L) [
72] and their effect on biogas production rates and microbial flocs. These parameters directly depend on the impeller geometry, impeller speed, position and diameter along with the physical and rheological properties of slurry such as density and viscosity. According to US EPA recommendations, MEL of 5–8 W/m
3 and G of 50–80 s
−1 are favorable. Rivard et al. [
72] and Wu [
71] calculated the values of MEL above 8 Wm
−3 for slurries at a higher solid concentration, which resulted in higher power consumption per unit volume. The value of G and G
L is a valuable parameter for predicting the dead zones along with the positive and negative effects of mixing intensity on microorganisms.
Table 1 demonstrates the comparative analysis of different mixing modes on various influential parameters in an anaerobic digestion process. From the mentioned mixing modes, mechanical mixing can be considered the most favorable mixing method due to its positive response to all the influential parameters. Whereas, under the high TS content in the slurry the pneumatics mixing is ineffective to some extent due to an increase in viscosity of the fluid [
73]. Furthermore, in the case of hydraulic mixing, the chances of dead zones and unmixed regions are relatively higher under any conditions. Consequently, the rheology of the slurry is a key parameter that should be underlined during the analysis of mixing and designing of mixing equipment for an anaerobic digester [
74].
The geometry of the digester tank and the mechanical mixer is only considered in the lab-scale experiments which makes it easier to determine the hydrodynamic characteristics of digesters. Whereas for the full-scale biogas plant, the mixing is evaluated in terms of power consumed per unit volume and per unit fresh feedstock added to the digester [
14,
75,
76]. It is noted that the geometrical aspect is missing while studying the effect of mixing at large-scale biogas plants. Accordingly, the power consumption should not be the only parameter to study the effect of mixing because mixing is a physical process that is directly associated with the dimensions of the setup. Without knowing the exact geometrical configuration, it is impossible to determine the mixing time for slurry by the impeller and range of hydrodynamic stresses produced by the impeller blades.
5. Importance of Geometrical Constraints and Rheological Study of Slurry
Mixing is a physical process that produces the physical motion of the fluid between different parts of the whole volume. The mixing can be classified into various mechanisms such as bulk flow in laminar and turbulent regimes and both eddy and molecular diffusion. These classifications are determined by the physical and rheological properties of the fluid and the geometry of the impeller and the vessel tank. The design of the mixing equipment in an anaerobic digester involves the selection of type, size and operating conditions that can perform a desired service corresponding to the slurry rheology. The method of predicting the process performance characteristics of mixing equipment generally depends on the empirical methods involving the correlation of dimensionless groups and model relationships. Moreover, in the case of mixing in an anaerobic digester, it is even more complex to draw a correlation between microorganisms, mixing intensity and biogas production rates. In many studies dealing with the evaluation of mixing in a digester at the lab scale or large scale, the geometry of the mixers and the digesters is missing [
36,
66,
77]. It is very hard to determine the flow patterns, shear stresses and dead zones when geometrical characteristics are absent. Whereas some studies combine the two approaches of biogas production performance and hydrodynamics of digester [
44,
67,
78].
The rheological analysis of the digestate for the anaerobic digestion method is very relevant for the design of the digester and mixing equipment. As per the evidence from the literature, it is verified that if TS > 2.5% then the sludge has a non-Newtonian shear thinning behavior [
79,
80,
81,
82,
83]. In this scenario, a power law model may be proposed for the measurement of the apparent viscosity and shear rate.
For a non-Newtonian shear, the value of n is less than 1. For this case, the rheological data for wastewater sludge are taken from the literature [
50]. The average shear rate within the vessel can be determined according to the equation.
Here
is the Otto–Metzner constant which is directly associated with the impeller geometry.
Table 1. represents the value of the Otto–Metzner constant (
) for different types of impellers with respect to the
Dt/
Da ratio in the vessel. Equation (6) is used to calculate the mean shear rate under specific conditions of mixing. Apparently, it is very crucial to extract the volumetric curve (shear stress versus shear rate) for the slurry used in a digester experiment.
Table 2 shows value of Otto–Metzner constant (
ks) for different types of impellers for shear-thinning fluids (
n < 1).
The optimization of impeller geometry rests on achieving uniform distribution of velocity within minimum mixing time at low rpms. Impeller choice has high importance in the case of a bioreactor.
The impeller to be used for mixing the slurry in an anaerobic digester should have an almost constant pitch since it gives a consistent distribution of velocity at low shear speeds. Consequently, the scaling-up of pilot-scale mixing systems is a crucial feature for maximizing current mixing and flow processes while holding all measurements at a set ratio, known as the scale-up element.
6. Approaches towards Evaluation of Mixing Efficiency
6.1. CFD Analysis
CFD is a powerful tool for modeling and evaluating mixing operations in fluid dynamics. The mixing in anaerobic digesters has been modeled using CFD [
34,
85,
86]. CFD analysis in an anaerobic digester can be used to study the flow fields, velocity contours, movement of dissolved components, turbulence, particle trajectories and dead zones under different operating conditions. CFD provides the numerical simulation of viscosity distribution, flow pattern along with numerous output parameters such as turbulence levels, vorticity and particle velocity [
87]. Modeling by CFD can help to reduce the initial cost of the experimental setup and optimization of full-scale anaerobic digesters. After modeling, the CFD models can be validated against the experimental results. On the other side, the CFD focuses on fluid dynamics but not on kinematics, that is, anaerobic digestion.
The 3-D geometry is first constructed in computer-aided design software. The mesh is generated by dividing the entire volume of the geometry. The mesh is relatively fine near the impeller blades and it can be coarse far away from the impeller and size functions are used to control the mesh growth [
39,
71,
88,
89,
90]. The properties of different phases such as liquid, solid and gas are defined. Depending on the nature of the problem, that is, single-phase or multiphase, different turbulence models and solvers are selected to observe the effect of geometry and boundary conditions on mixing in an anaerobic digester.
Table 3 represents the summary of CFD simulation outcomes and methods used for solving governing equations and turbulence models.
Terashima et al. [
89] introduced a new parameter called uniformity index by numerically evaluating laminar flow. According to Mendoza et al. [
91] for the determination of dead zones, the flow inside the digester distribution of streamlines and velocities is very important. In another study by Manea et al. [
92], the optimum geometry and rotational speed were obtained by three-dimensional numerical simulation. Few studies have been reported dealing with simulations at large-scale digesters. Wu et al. [
37,
39,
71,
93,
94] predicted the flow pattern in large-scale digesters using large eddy simulations, turbulence models, Eulerian multiphase models and sliding mesh methods along with mixing characteristics of the impeller. However, the structural differences could preclude a clear application of operational information from laboratory-scale findings to full-scale designs.
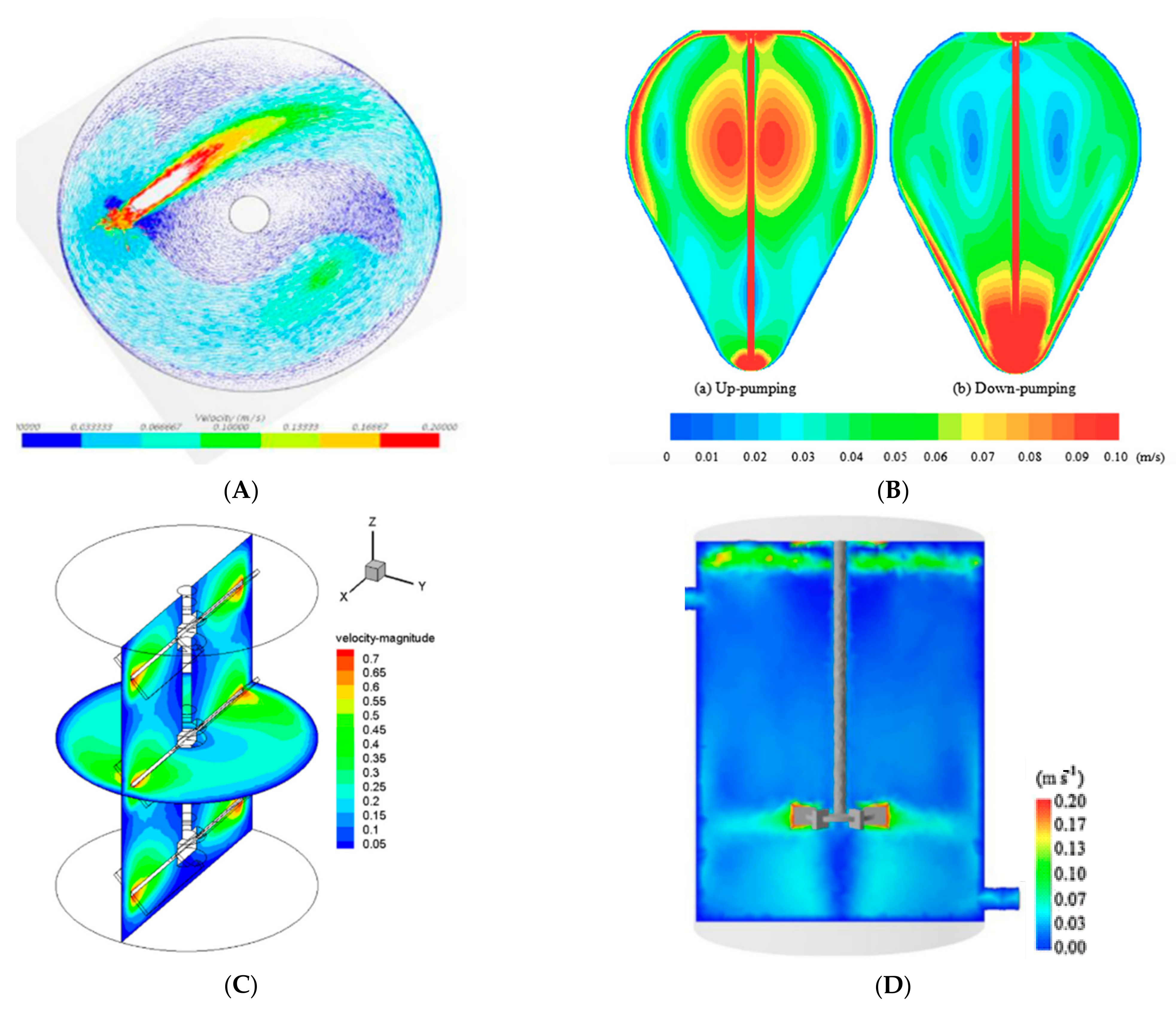
CFD analysis has been observed by many researchers at both lab-scale and full-scale digesters. In a study by Conti et al. [
62], mixing was modeled in a full-scale digester at various mixing speeds and the efficiency of mixing was studied at different impeller angles. The technological benefit was measured and tested for a total of 441 mixing setups. The investigation shows the favorable fluid mechanics in positions of rotors far from the bottom and high rotational angles (
Figure 4A). Similarly, Binxin observed that the digester shape has a significant influence on the mixing of slurry. In this research, the flow pattern of an egg-shaped (
Figure 4B) digester was tested by Computation Fluid Dynamics. It was observed that mixing in the egg-shaped digester is more uniform which leads to a reduction in power consumption, removal of dead zones, operational needs and energy demand to maintain the homogeneity of the digester and the amount of foam formation was reduced under various speeds and pumping modes.
Table 3.
Represents the various fluid models developed by using CFD in the context of evaluating mixing in anaerobic digesters on both lab-scale and large-scale biogas plants.
Table 3.
Represents the various fluid models developed by using CFD in the context of evaluating mixing in anaerobic digesters on both lab-scale and large-scale biogas plants.
| Study | Turbulence Model | Rheology of Slurry | CFD Software/Method | Results |
|---|
| Wu and Chen et al., 2008 [37] | k–ε turbulence model single equivalent phase | Non-Newtonian | ANSYS Fluent/finite volume | Flow pattern and dead zones |
| H. Caillet et al., 2018 [95] | LES- turbulence model | Newtonian and non-Newtonian | Open FOAM software/Finite volume | Temperature and velocity profile |
| Binxin Wu et al., 2010 [39] | k–ε turbulence model | Non-Newtonian | ANSYS Fluent/Multiple reference frame | Mixing energy levels and dead zones |
| J. Ding et al., 2010 [34] | k–ε turbulence model | Non-Newtonian | ANSYS CFX | Optimized impeller design/velocity distribution |
| R. Meroney et al., 2009 [96] | k–ε turbulence model | Newtonian | ANSYS Fluent/Finite volume | Digester volume turnover time/Mixture diffusion time |
| J. Bridgeman 2012 [45] | Standard k–ε (S k–ε), Realizable k–ε (R k–ε), | Non-Newtonian | FLUENT | Flow patterns combined with biogas yield |
| Vesvikar et al., 2005 [38] | k–ε turbulence model | Newtonian | CFX/Finite difference | Gas distribution and flow patterns |
| Dama et al., 2000 [97] | - | Newtonian | FLUENT/Finite difference | Velocity profile and flow patterns |
| B. Wu et al., 2010 [39] | - | Newtonian and non-Newtonian | FLUENT | Mixing energy levels |
| M. Terashima et al., 2009 [89] | Single phase/laminar | Non-Newtonian | ANSYS CFX/Finite element | Sludge distribution |
| Mohammadrezaei et al., 2017 [98] | k–ε turbulence model | Non-Newtonian | FLUENT/Multiple reference frame | Optimum mechanical stirrer and flow patterns |
| Li et al., 2004 [99] | K–ω and k–ε turbulence model | - | CFX | Flow and velocity prediction by impeller |
| Elena et al., 2012 [92] | Navier stokes equation | Newtonian | Mixsim | Nominal mixing speed and optimum geometry of impeller |
| Y. Zang et al., 2015 [20] | k–ε turbulence model | Non-Newtonian | FLUENT | Flow pattern |
| Fei Shen et al., 2013 [100] | Standard k–ε (S k–ε) | Non-Newtonian | Mixsim | Optimum mixing speed |
| Ahmed et al., 2009 [101] | k–ε turbulence model | Non-Newtonian | ANSYS CFX/Eulerian approach | Power consumption and mixing time |
| C. Maier et al., 2010 [102] | Euler-euler multiphase | Non-Newtonian | FLUENT/Finite volume | Scaleup process/mixing behavior |
| Huang et al., 2014 [103] | - | Non-Newtonian | FLUENT | |
| H.Azargoshasb et al., 2015 [104] | RNG k–ε | - | FLUENT | VFA’s concentration profiles |
| Karaeva et al., 2015 [105] | k–ε | Newtonian | COMSOLMultiphysics | Geometrical parameters in hydraulic mixing |
| Torotwa et al., 2018 [106] | k–ε model | Newtonian | COMSOL MultiphysicsEuler-Euler multiphase | Flow pattern in gas mixing |
| Leonzio et al., 2018 [107] | k–ε model | Non-Newtonian | COMSOL MultiphysicsEuler-Euler multiphase | Shear rate, velocity gradient and flow pattern |
| Manea et al., 2012 [92] | No turbulence | Non-Newtonian | Multiple reference frame | Impeller geometry and flow pattern |
It cannot be denied that CFD modeling is one of the best ways to analyze the mixing efficiency of the mixing equipment but, on the other hand, it is very important to link the CFD findings to the actual biogas production on all scales of anaerobic digestion. For example, studying the effect of shear stresses, mixing time, dead zones and flow regime on the microbes and biogas production at different phases of the anaerobic digestion process. The effect of mixing in the two-stage anaerobic digester is also an interesting fact to study. The effect of hydrodynamic shear rates on different categories of organisms can be better understood by the physical separation of hydrolysis and methanogenesis.
6.2. Mixing Time
The mixing period is one of the parameters used to describe the mixing of the liquid process in the stirred reactors. Mixing time is the time taken to reach a certain degree of homogeneity. Higher mixing time corresponds to higher power consumption by the mixing operation which will reduce the overall efficiency of the biogas plant.
The tracer technique is one of the very popular techniques for calculating the mixing time [
108,
109,
110] and the determination of hydrodynamics of the digester by RTD. It is calculated on basis of the time taken by the particle to enter and leave the digester. A known mass of chemical tracers such as lithium or fluoride is added through the inlet and concentration is detected and monitored at the outlet. RTD curves can be generated from the data obtained and are used to analyze the hydrodynamic characteristics of the digester in terms of dead zones, short-circuiting and breakthrough time [
108]. The significance of smooth exponential decay refers to a perfectly mixed digester. Typically, the tracer concentration at a point within the tank varies with time and the time taken by the variation to reduce below a certain level, say, within 5% of the fully mixed concentration, is taken as the mixing time. The same method can be used in a numerical calculation by injecting a neutrally buoyant virtual tracer at a given location. The concentration is governed by the following equation:
Some researchers (1963) [
111] suggested an equation for the determination of the dead zones.
where
and as
f → 0,
β → 0.
6.3. Optimize Impeller Design
Various variables that specifically influence the mixing period and the output rate of biogas in the digester include the design of the impeller, the bottom clearance of the impeller and the clearance of the impeller, the eccentricity of the impeller, the baffles and the location of the draft channel [
21,
26,
27,
112,
113,
114]. Various shapes and geometries have been tested, but there is a difference in the efficacy and performance outcomes of the various mixers owing to the different approaches utilized for the measurement of various substrates [
90]. Impeller choice is critical because its selection depends on different factors. The key goal of the impeller is to prevent dead areas, stratification and firm forming [
29,
31,
33,
101,
115,
116]. Coaxial impellers are used for small-scale digesters, whereas eccentric agitators may be utilized in large installations [
73]. Typically, the pumping action is created by the rotary movement, which allows the slurry to move in radial, axial and intermediate directions. However, the speed of slurry in the vessel is not usually a measure of the degree of mixing. The sludge may travel at a specific pace, but if all the sludge in the immediate vicinity travels at the same speed and in the same direction, the sludge is not mixed, rather the sludge is simply pushed inside the vessel [
56]. It has been found that the ideal behavior of tank mixing can deviate due to a variety of reasons associated with the location of inlets, exits, stratification and tank geometry. In addition, the existence of even a small variation in density between the mixing fluids can have a direct impact on the success of the mixing [
55].
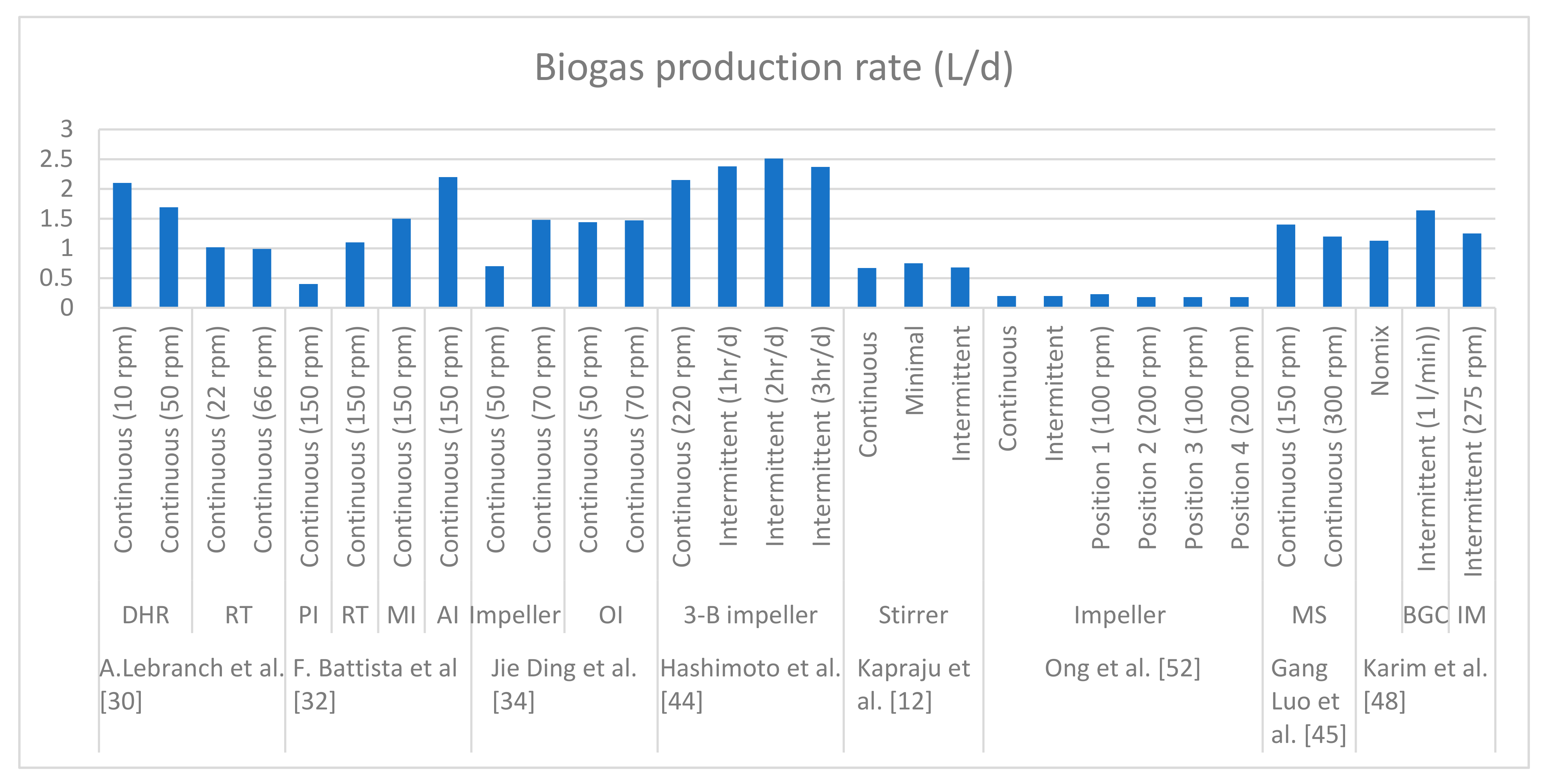
Figure 5 represents the data from the literature where various different types of geometries of impellers were compared and the outcomes in terms of flow patterns, particle velocity, dead zone volumes, shear stresses induced and biogas production rates were analyzed. It is clearly observed that the impellers with larger diameters such as the double helical ribbon impeller and the anchor impeller performed better compared to high shear impellers such as RT. The above literature rests on experimentation on lab-scale digesters, but optimization of mixing on a pilot scale and large scale is the prominent issue to be explored. The basic prerequisite for the scale-up of the pilot scale of the digester is a similarity among the geometric, dynamic and kinetic conditions of the lab-scale digester. Previous studies lack the scaling up of mixing equipment using the distinct forms of lab-scale mixer mentioned above [
29,
49,
67,
117].
It is clear that a lower mixing intensity and intermittent regime is favorable for enhancing biogas production, which is common in almost all the studies mentioned above, but the focus must be on the optimization of impeller geometry. It is observed that various research studies are in the direction of the optimization of impeller geometry to deliver a uniform distribution of velocity and viscosity.
6.4. Energy Consumption
The power consumption of the internal operation of a plant plays a significant role in the determination of plant efficiency. Energy consumption for agitation in large-scale biogas plants can be accurately predicted from the curves of
Np versus
Re number (
Figure 2). Usually, the power consumed by the impeller per unit volume of slurry is used as a measure of mixing effectiveness. As per US EPA, 5.8 Wm
−3 of bioreactor volume is recommended as power input for mixing in an anaerobic digester but it is still contentious [
40]. Power use of the anaerobic digester impeller dynamically influences the characteristics of the device, such as the configuration of the impeller, the geometry and the scale of the digester, the position of the impeller, the speed of the impeller and the rheological behavior of the substrate utilized in the digester. The exact measurement of the strength is important for the selection of the power unit in order to reach optimal mixing. Inadequate mixing units would contribute to excessive expenditure in equipment and higher energy use rates, which will reduce the productivity of the biogas project [
26].
Chen and Hashimoto [
75] reported that 7.3% of the overall energy production by a plant is used by mixers. However, as the volume of the digester increases the energy required for mixing decreases. The consumption of energy by the agitator may also be determined by the measurement of the torque with help of the strain gauge and the torque meter. The equations for the calculation of power input for mixing are as follows.
Biogas recirculation [
118]:
In addition, electrical power usage for agitation is often dependent on TS content, HRT and feed period [
4,
76]. In fact, owing to the variance in the geometrical parameters of the impellers and the rheological characteristics of the various substrates, the precise association of the basic electrical intake with the active tank volume and the moisture content is not known. The energy requirement may be improved by adjusting the mixing period and configuration. Frey et al. [
63] observed that the power usage for mixing declined by 50% by changing the agitator location without any mixing efficiency loss. Kress et al. [
47] observed an 85% reduction in electricity usage by increasing the resting time of the mixer.
6.5. Biogas Production Rates and Methane Content
The mixing effect at various mixing intensities and different geometries is also analyzed on the basis of biogas production rates and methane content. Biogas measurement is done either manometrically by keeping the volume constant and measuring the pressure increase, or volumetrically, by providing constant pressure conditions allowing measurement of the biogas volume. The rate and volume of biogas produced from anaerobic biodegradability assays include different techniques such as:
Lubricated syringes;
Volume displacement devices;
Pressure manometers or transducers;
Manometer-assisted syringes or low-pressure switch meters.
6.5.1. Volumetric and Manometric Gas Measurement
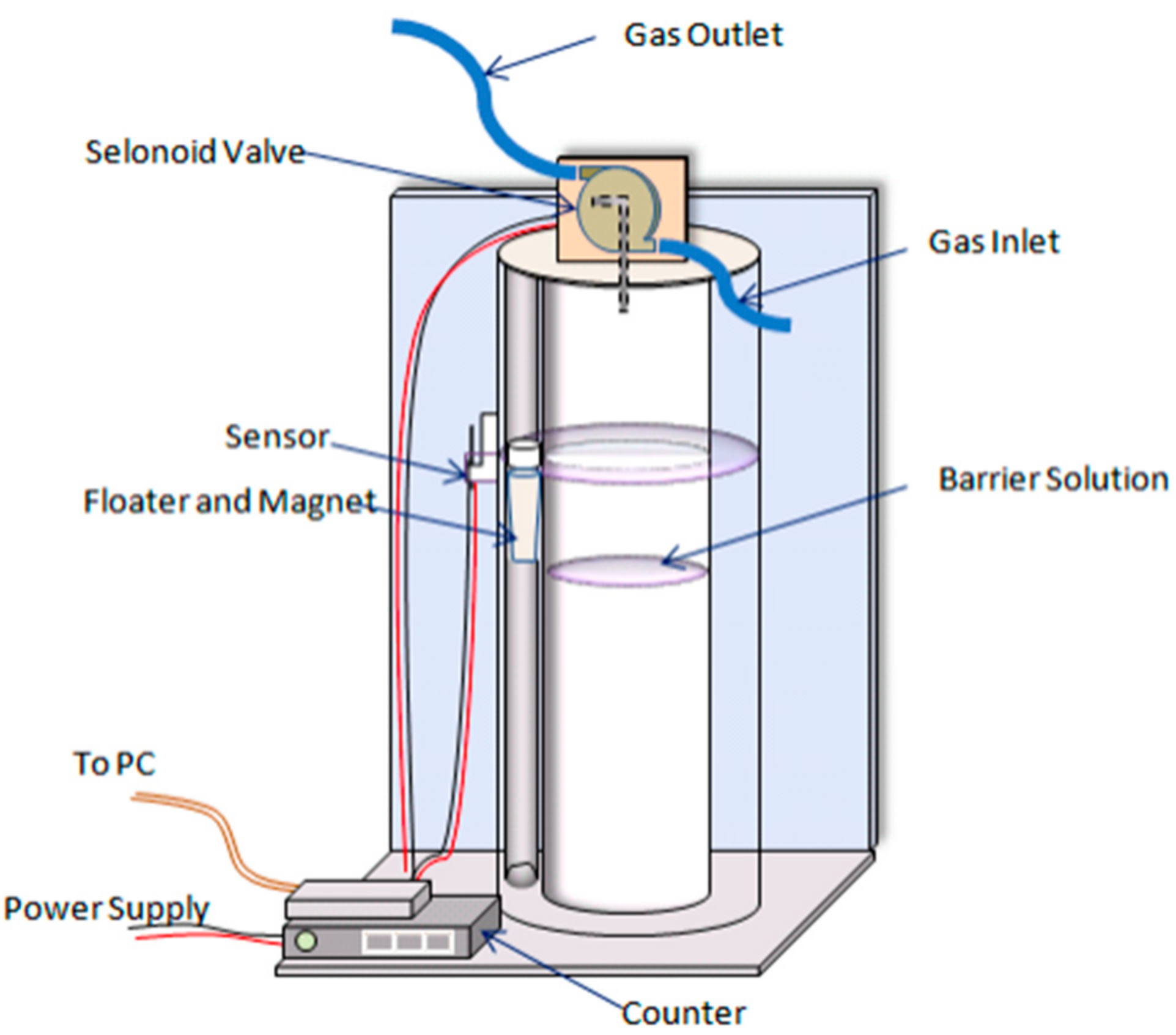
When it comes to displacement gas measuring equipment, different researchers have produced various devices that meet different study objectives [
119]. Gas displacement meters have a general operating principle based on the pressure differential between the meter’s input and exit. The difference in pressure produces the periodic filling and emptying of a predefined volume of gas in the measuring chamber. A sensor releases accumulated gas by opening and shutting a two-way or three-way solenoid valve, and the complete system is reset as a result. The total gas volume is equal to the number of fills or emptyings (recorded using a counter system) times the prescribed chamber volume [
120].
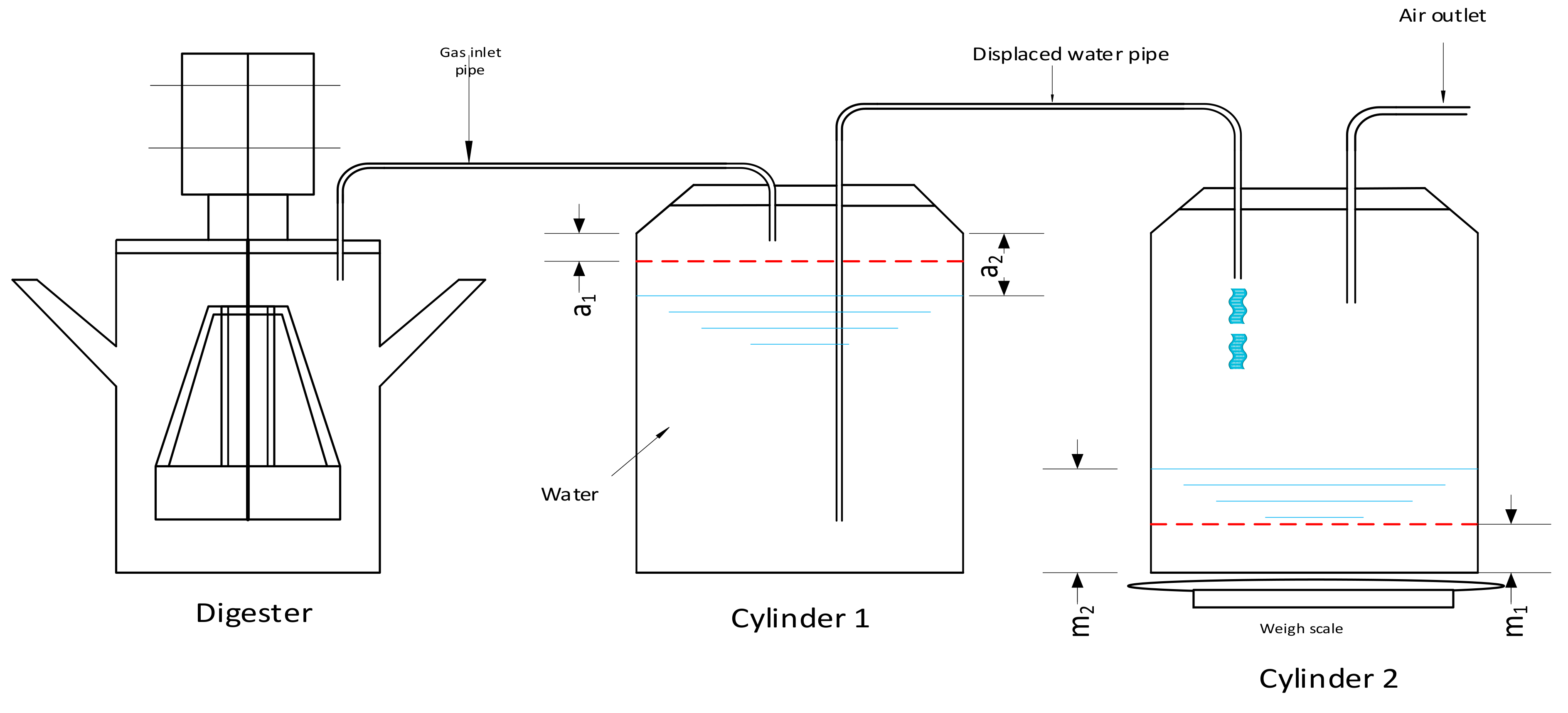
Biogas production is recorded continuously throughout the experiments. Because gasses have limited densities, it is generally not feasible to gather gas and determine its mass. In the case of gasses that are basically not soluble in water, it is necessary to extract the gas created by displacing the water from the bottle. This approach is quite straightforward in economic terms and operates over a longer period of time without maintenance. The process of displacement of water is one of the traditional methods of calculating regular gas output as shown in
Figure 6. In this process, the amount of water transported by gas implies the volume of biogas generated by the digester. Some mistakes can occur due to variations in ambient temperatures, so it is really important to report changes.
6.5.2. Gas Chromatography (GC)
GC has numerous benefits such as high resolution, fast speed, high sensitivity and quantitative findings. GC is often used in quality control applications. GC is a suitable approach since it is good for measuring gases that are in contact with their liquid phase [
121].
In measuring and analyzing the gas content of an anaerobic system, it is extremely important to analyze the significance of the liquid-to-gas ratio. The thermodynamic equilibrium of the system governs the solubility of the gas phase and the distribution of the analyte within the liquid phase. It is critical that temperature and pressure be consistent while producing headspace biogas since it will have a direct impact on the biogas concentration. Errors can develop in the measurement of the GC gas when the temperatures of samples and the calibration gas fluctuate considerably [
122]. The anaerobic microbiology could also be affected by a modest change in temperature when measuring gas carbon dioxide concentrations. GC may easily be used to aid the calculation of the total potential methane (VMT) and the rate of biodegradation for various compounds.
The composition of biogas is a very crucial parameter to determine the efficiency of the anaerobic digestion process. In many studies [
35,
42,
123], it is observed that mixing has a significant effect on methane content in biogas. Methane content in biogas not only depends on the substrate characteristics and other physical parameters (such as temperature, ph and ammonia concentration) but also on the quantity and homogeneity of methanogens within the active volume of the digester, which is determined by the mixing efficiency of the mixer in the digester. The overall focus of the optimization of mixing should not only be limited to an increase in biogas volume but should also be on the quality of the biogas in terms of higher methane content. As discussed in previous reviews, effective mixing can lead to a homogeneous distribution of both nutrients and substrate, it also leads to a uniform distribution of the major bacterial and archaeal classes. A high abundance of methanogens at the top could be partially attributable to the better methane production observed in the BMP test in various studies [
124,
125,
126,
127,
128,
129].
Optimization of biogas plants requires accurate estimates of substrate BMP and simple tools for estimating CH
4 potential. Methane content in biogas can be measured by online available software as discussed in recent studies [
130,
131] (
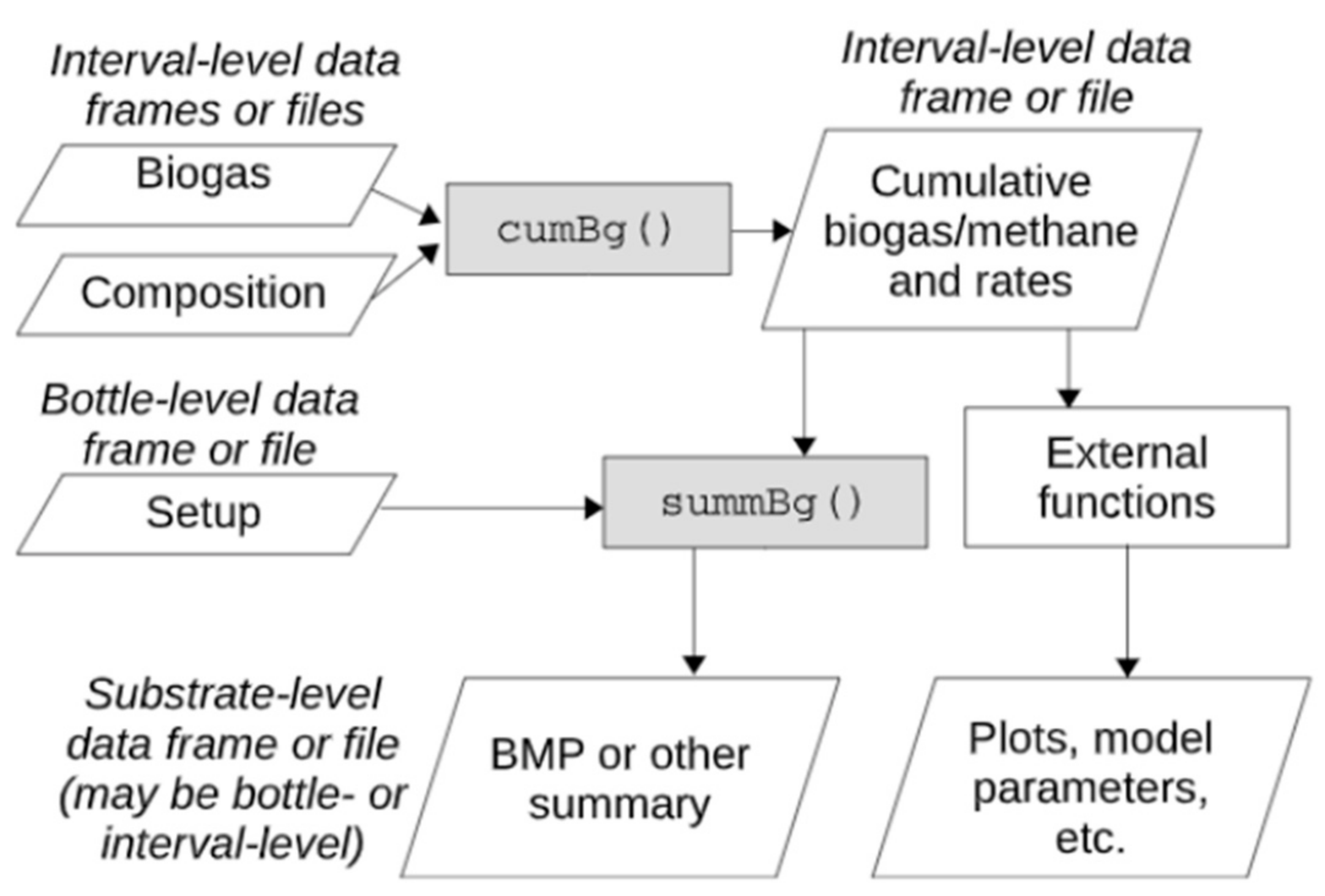
Figure 7). Links to the primary functions are provided by a web-based program. The program can be used to quantify the biochemical methane potential (BMP) reliably using a variety of different biogas calculation types as shown in
Figure 8. Additionally, the methane potential can be estimated based on the substrate structure, which simplifies experimental design and outcome analysis. By providing access to adaptable, reliable, structured and transparent algorithms, this software can improve the accuracy and efficiency of biogas research.
7. Present Scenario of Research and Future Directions for Analysis of Multi-Scale Mixing in an Anaerobic Digester
To proceed with research in any field, it is of utmost importance to consider the present scenario in that field. This section aims to categorize the literature on basis of the evaluation of mixing techniques implemented in the experiments on both lab-scale and large-scale digesters. It has been observed that anaerobic digestion is a biological and chemical process whereas mixing of slurry is the physical operation that makes the optimization of mixing in a digester an even harder challenge. For this purpose, it is very important to adopt an interdisciplinary approach to study the effect of mixing intensity, shear rate, shear stresses and flow patterns on microorganisms, bacteria and their syntrophic relationships. Optimization of mixing at a large scale will remain a challenge until the understanding of the actual description of the effect of mixing on the anaerobic digestion process. CFD provided the ultimate solution for mixing issues, but physical modeling still varies as far as literature is concerned, which has resulted in hurdles to accepting a general model in AD.
Optimization is not just about increasing the production of biogas. By reducing the mixing, there is also an ability to minimize power demand as well as maintenance and operational costs. It has been shown that the intermittent mixing approach can produce the same amount of biogas/methane and even increase the output of gas combined with a continuously mixed system, whereas reducing the maintenance and energy demand of the process. The digester architecture and the common use of continuous mixing are fair to question.
Several investigations were undergone to analyze various forms of mixer geometry and digester designs and other forms of bioreactors used in the AD process. This involves tracer method research, numerous lab experiments as well as modeling. To generate fundamental information on the effect of mixing on biogas production, the understanding of slurry flows in the digester needs to be enhanced. Optimization of the output of the process should be possible with a greater understanding of the relationship between mixing and biogas production.
Recently Leonzio [
107] studied the mixing properties in a digester using different geometric configurations. Results proposed an innovational mixing system consisting of an external recirculating pump. Accordingly, the dispersion of fluid tangents to the lateral surface resulted in lower dead zones and greater homogeneity of velocity distribution.
Table 4 summarizes a large number of studies in terms of multiscale analysis of mixing and their shortcomings. It can be clearly derived from the table that a lack of appropriate resources and analysis methods for the evaluation of mixing can lead to unnecessary confusion in this field. CFD modeling shows tremendous potential for biogas mixing, but it must be used to model the real output of, for example, shear forces and stationary zones for them to have the greatest impact. These sorts of long-term and preliminary simulations are particularly useful when developing a digester. Kinetic models have been used to some degree but are in the process of being studied and perfected, for the time being, they can only be approximations of the AD process.
Recent experimental and simulation results can help to better understand and improve the mixing properties in a bioreactor.
Figure 9 represents the correlations between mixing, biochemical processes, operating condition and setup geometry, including experimental and modeling tools for the analysis of mixing in an anaerobic digester.
Figure 9.
Hierarchy of interactions between reactor geometry, operating condition, mixing and biochemical processes including experimental and modeling tools for scale-up [
35,
59,
133].
Figure 9.
Hierarchy of interactions between reactor geometry, operating condition, mixing and biochemical processes including experimental and modeling tools for scale-up [
35,
59,
133].
Table 4.
Multi-scale analysis of mixing in various studies by various approaches adopted by researchers to evaluate the effect of mixing in an anaerobic digester.
Table 4.
Multi-scale analysis of mixing in various studies by various approaches adopted by researchers to evaluate the effect of mixing in an anaerobic digester.
| Reference | Digester Scale | V | Numerical Appr. | Empirical Approach | Digester Geometry | Mixer Geometry | Microbial Analysis | Biogas Yield Analysis | CH4 Yield Analysis |
|---|
| Robert et al. [96] | LS | 10,000 m3 | CFD | o | ♦ | ♦ | o | o | o |
| Subramanian et al. [77] | LS | 2911 m3 | o | ♦ | o | o | ♦ | ♦ | o |
| Ghanimeh et al. [36] | Lab-S | 9 L | o | ♦ | o | o | ♦ | ♦ | ♦ |
| Ratanatasmskul et al. [19] | PS | 12 m3 | o | ♦ | o | o | o | ♦ | ♦ |
| L. Yu et al. [64] | Lab-S
PS | 1 L
70 L | CFD
CFD | ♦
♦ | ♦
♦ | ♦
♦ | o
o | ♦
♦ | o
o |
| Kshirsagar et al. [85] | PS | n.a. | CFD | o | ♦ | n.a. | o | o | o |
| Mohammadrezaei et al. [134] | Lab-S | 1.2 m3 | CFD | ♦ | ♦ | ♦ | o | ♦ | o |
| R. Sindall et al. [67] | Lab-S | 6 L | CFD | ♦ | ♦ | ♦ | ♦ | ♦ | o |
| Gang Luo et al. [68] | Lab-S | 1 L | o | ♦ | o | o | ♦ | ♦ | ♦ |
| Rico et al. [23] | PS | 1.5 m3 | o | ♦ | o | o | o | ♦ | ♦ |
| Z. Tian et al. [124] | Lab-S | 5 L | o | ♦ | o | ♦ | ♦ | o | ♦ |
| R. Bello et al. [135] | n.a. | n.a. | MATLAB | ♦ | o | o | o | o | o |
| N. Stalin et al. [136] | PS | 168 L | o | ♦ | ♦ | n.a. | o | ♦ | o |
| Andrew G. et al. [43] | Lab-S | 4 L | o | ♦ | o | ♦ | o | ♦ | ♦ |
| H. K Ong et al. [115] | Lab-S | 10 L | o | ♦ | o | o | o | ♦ | ♦ |
| K. C. Lin et al. [65] | Lab-S | 7 L | o | ♦ | ♦ | ♦ | o | ♦ | ♦ |
| X. Zhai et al. [48] | PS | 1.6 m3 | CFD | ♦ | ♦ | ♦ | o | o | ♦ |
| A. Noorpoor et al. [137] | LS | 30 m3 | CFD | o | ♦ | ♦ | o | o | o |
| V. A. Vanlin et al. [78] | Lab-S | 1 L | CFD | ♦ | o | o | ♦ | o | ♦ |
| A. Sulaiman et al. [52] | PS | 500 m3 | o | ♦ | o | o | ♦ | ♦ | ♦ |
| Mohammad et at. [98] | LS | 1200 L | CFD | o | ♦ | ♦ | o | o | o |
| H. Caillet et al. [95] | LS | n.a. | CFD | ♦ | ♦ | ♦ | o | ♦ | ♦ |
| F. Battista et al. [33] | Lab-S | 2 L | o | ♦ | o | o | o | ♦ | ♦ |
| Terashima et al. [89] | LS | n.a. | CFD | o | ♦ | ♦ | o | o | o |
| Lebranch et al. [27] | Lab-S | 2 L | CFD | ♦ | ♦ | ♦ | o | ♦ | ♦ |
| Hughes [138] | Lab-S | 1 L | o | ♦ | o | o | o | ♦ | ♦ |
| Bridgn [45] | Lab-S | 6 L | ♦ | ♦ | ♦ | ♦ | o | ♦ | o |
| James et al. [139] | Lab-S | 9 L | o | ♦ | o | ♦ | o | ♦ | o |
| K. Latha et al. [28] | Lab-S | 4.5 L | o | ♦ | ♦ | ♦ | ♦ | ♦ | o |
| J. Jiang et al. [94] | Lab-S | 4.1 L | o | ♦ | ♦ | ♦ | o | ♦ | ♦ |
| Ismail et al. [140] | Lab-S | 4.5 L | o | ♦ | ♦ | ♦ | o | ♦ | ♦ |
| Hoffman et al. [69] | Lab-S | 4.5 L | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Suliaman et al. [52] | PS | 500 m3 | o | ♦ | o | o | ♦ | ♦ | ♦ |
| Stroot et al. [49] | Lab-S | 2 L | o | ♦ | o | o | o | ♦ | ♦ |
| M. Kim et al. [141] | Lab-S | 4 L | o | ♦ | o | o | o | ♦ | ♦ |
| B. Wang et al. [123] | Lab-S | 0.5 L | ♦ | ♦ | o | o | o | o | ♦ |
| Lindmark et al. [142] | Lab-S | 1 L | o | ♦ | o | o | o | ♦ | ♦ |
| Semen et al. [143] | LS | 2659 m3 | o | ♦ | o | ♦ | o | ♦ | ♦ |
| Peng Wei et al. [144] | Lab-S | 7.2 L | CFD | o | ♦ | ♦ | o | o | o |
| Fei Shen et al. [100] | Lab-S | 8.0 L | CFD | ♦ | ♦ | ♦ | o | ♦ | o |
| C. Maier et al. [102] | LS | n.a | CFD | ♦ | ♦ | ♦ | o | o | o |
8. Discussion and Conclusions
After careful analysis of the literature on the optimization of mixing it is concluded that due to the involvement of microbiology, chemical aspects and hydrodynamics, mixing efficiency is considered a complex subject in terms of optimizing and unfurling various facts related to it. Intensive literature was reviewed, which provided evidence of both positive and negative effects of mixing on biogas production under various operating conditions. Each study describes the outcome of results in a different manner due to the reason that the mixing evaluation technique and the setup of the experiments are totally different from each other. CFD provided the ultimate solution to mixing issues but physical modeling still varies as far as the literature is concerned, which has resulted in hurdles to accepting a general model in AD. The optimized multi-scale modeling approach that applies prototypes to various scales tends to provide the best balance in terms of efficiency, robustness and precision for technology and scale-up applications. Further research should be focused on analyzing the effect of mixing by physical separation of different steps of AD. Moreover, the design of the impeller and the digester tank can be changed according to mixing requirements in the slurry. Here, the following conclusions can be drawn:
A multidisciplinary approach is very important to evaluate the effect of impeller and digester geometry on biogas production rates.
The evaluation of mixing in digesters without disclosing the geometry of the digester and impeller is not valuable enough unless the amount of shear stresses produced and the time taken to mix is known.
Scaling up of lab-scale digesters and scaling down of full-scale digesters should be focused on to optimize the mixing intensity and time of operation.
Results can vary due to a lot of factors intertwined in the AD process so it is very important to focus on the parameters of the actual workings of a full-scale biogas plant.
The impact of agitation on microbial populations is only marginally discussed in AD and we believe that this study will prompt future work in this area.
Author Contributions
Data curation, B.S., Z.Č., and N.S.; formal analysis, B.S., M.K., Z.P., and N.S.; funding acquisition, Z.Č. and M.K.; investigation, B.S., Z.S. (Zoltán Siménfalvi), Z.S. (Zoltán Szamosi) and Z.P.; methodology, B.S., Z.Č., Z.S. (Zoltán Siménfalvi) and Z.S. (Zoltán Szamosi); project administration, Z.Č. and M.K.; resources, B.S., N.S.; validation, Z.P.; visualization, Z.P.; writing—original draft, B.S. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovak Research and Development Agency: APVV-19-0576 and the Ministry of Education, Science, Research and Support of the Slovak Republic and the Slovak Academy of Sciences: VEGA 1/0757/21. This research was supported by the European Union and the Hungarian State, co-financed by the European Regional Development Fund in the framework of the GINOP-2.3.4-15-2016-00004 project, aimed to promote the cooperation between higher education and the industry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Apparent viscosity (Pa s) |
| K | Consistency index |
| Average shear rate (s−1) |
| N | Revolution per minute (s−1) |
| Density (kg m−3) |
| Reynolds number |
| Np | Power number |
| P | Power consumption (kWh) |
| Source |
| C(t) | Residence time function |
| r | Residence time correction factor |
| t | Time |
| p | Fraction of material that moves with infinite velocity |
| Especi. | Specific power consumption for stirring (kWhel) |
| Especi./100 m3 active digester volume (kWhel/100 m3active digester.d) |
| Daily total stirring electric energy consumption (kWhel d−1) |
| Daily added feedstock |
| TS | Total Solids |
| AD | Anaerobic digestion |
| HRT | Hydraulic retention time |
| OI | Optimized impeller |
| 3-B | 3 blade impeller |
| V | Active volume (m3) |
| Dt | Diameter of digester tank (m) |
| Da | Impeller diameter (m) |
| H | Liquid dept in vessel (m) |
| n | Power law index |
| Fr | Froude number |
| u | Fluid velocity |
| Tracers diffusion coefficient |
| Co | Concentration at outlet |
| a | Perfectly mixed effective system volume |
| f | Fraction of material |
| L | Lag phase |
| THRT | Average residence time |
| VActive digester | Active tank/digester volume (m3) |
| Especi./tonne added substrate (kWhel tFM−1) |
| Stirring power consumption (kWhel d−1) |
| MEL | Mixing energy level |
| DHR | Double helical ribbon |
| RT | Rushton turbine |
| PI | Pelton impeller |
| MI | Marine impeller |
References
- Holm-Nielsen, J.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z.; Rosas-Casals, M. Decentralized biomass for biogas production. Evaluation and potential assessment in Punjab (India). Energy Rep. 2020, 6, 1702–1714. [Google Scholar] [CrossRef]
- Lantz, M. The economic performance of combined heat and power from biogas produced from manure in Sweden—A comparison of different CHP technologies. Appl. Energy 2012, 98, 502–511. [Google Scholar] [CrossRef]
- Frey, J.; Grüssing, F.; Nägele, H.-J.; Oechsner, H. Cutting the electric power consumption of biogas plants: The impact of new technologies. Landtechnik 2013, 68, 58–63. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2012, 32, 172–186. [Google Scholar] [CrossRef]
- Suryawanshi, P.C.; Chaudhari, A.B.; Kothari, R.M. Mesophilic anaerobic digestion: First option for waste treatment in tropical regions. Crit. Rev. Biotechnol. 2010, 30, 259–282. [Google Scholar] [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125, 344–355. [Google Scholar] [CrossRef]
- Sonnleitner, M. Ecological and Economic Optimisation of Biogas Plants. Ph.D. Thesis, De Montfort University Leicester, Leicester, UK, 2012. [Google Scholar]
- Banks, C.J.; Zhang, Y. Optimising Inputs and Outputs from Anaerobic Digestion Processes; University of Southampto: Southampton, UK, 2010. [Google Scholar]
- Suryawanshi, P.C.; Chaudhari, A.B.; Kothari, R.M. Thermophilic anaerobic digestion: The best option for waste treatment. Crit. Rev. Biotechnol. 2010, 30, 31–40. [Google Scholar] [CrossRef] [PubMed]
- De La Rubia, M.A.; Riau, V.; Raposo, F.; Borja, R. Thermophilic anaerobic digestion of sewage sludge: Focus on the influence of the start-up. A review. Crit. Rev. Biotechnol. 2012, 33, 448–460. [Google Scholar] [CrossRef]
- Usman, M.; Zha, L.; Abomohra, A.E.-F.; Li, X.; Zhang, C.; Salama, E.-S. Evaluation of animal- and plant-based lipidic waste in anaerobic digestion: Kinetics of long-chain fatty acids degradation. Crit. Rev. Biotechnol. 2020, 40, 733–749. [Google Scholar] [CrossRef]
- Naegele, H.-J.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Electric Energy Consumption of the Full Scale Research Biogas Plant “Unterer Lindenhof”: Results of Longterm and Full Detail Measurements. Energies 2012, 5, 5198–5214. [Google Scholar] [CrossRef]
- Berglund, M.; Börjesson, P. Assessment of energy performance in the life-cycle of biogas production. Biomass-Bioenergy 2006, 30, 254–266. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. Impact of mixing intensity and duration on biogas production in an anaerobic digester: A review. Crit. Rev. Biotechnol. 2020, 40, 508–521. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Lindmark, J.; Thorin, E.; Fdhila, R.B.; Dahlquist, E. Effects of mixing on the result of anaerobic digestion: Review. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Ratanatamskul, C.; Saleart, T. Effects of sludge recirculation rate and mixing time on performance of a prototype single-stage anaerobic digester for conversion of food wastes to biogas and energy recovery. Environ. Sci. Pollut. Res. 2015, 23, 7092–7098. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, G.; Yu, L.; Siddhu, M.A.H.; Gao, M.; Abdeltawab, A.A.; Al-Deyab, S.S.; Chen, X. Computational fluid dynamics study on mixing mode and power consumption in anaerobic mono- and co-digestion. Bioresour. Technol. 2016, 203, 166–172. [Google Scholar] [CrossRef]
- Chen, Y.R. Impeller power consumption in mixing livestock manure slurries. Trans. ASAE 1981, 24, 0187–0192. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Rico, C.; Rico, J.L.; Muñoz, N.; Gómez, B.; Tejero, I. Effect of mixing on biogas production during mesophilic anaerobic digestion of screened dairy manure in a pilot plant. Eng. Life Sci. 2011, 11, 476–481. [Google Scholar] [CrossRef]
- Jobard, M.; Pessiot, J.; Nouaille, R.; Fonty, G.; Sime-Ngando, T. Microbial diversity in support of anaerobic biomass valorization. Crit. Rev. Biotechnol. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- He, Q.; Xu, P.; Zhang, C.; Zeng, G.; Liu, Z.; Wang, D.; Tang, W.; Dong, H.; Tan, X.; Duan, A. Influence of surfactants on anaerobic digestion of waste activated sludge: Acid and methane production and pollution removal. Crit. Rev. Biotechnol. 2019, 39, 746–757. [Google Scholar] [CrossRef]
- Cumby, T. Slurry mixing with impellers: Part 1, theory and previous research. J. Agric. Eng. Res. 1990, 45, 157–173. [Google Scholar] [CrossRef]
- Lebranchu, A.; Delaunay, S.; Marchal, P.; Blanchard, F.; Pacaud, S.; Fick, M.; Olmos, E. Impact of shear stress and impeller design on the production of biogas in anaerobic digesters. Bioresour. Technol. 2017, 245, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J. Clean. Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Waste strength versus impact of mixing. Bioresour. Technol. 2005, 96, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Pagilla, K.R.; Craney, K.C.; Kido, W.H. Causes and effects of foaming in anaerobic sludge digesters. Water Sci. Technol. 1997, 36, 463–470. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. Comparison of mixing efficiency of different impellers for agitation of slurry in anaerobic digester. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2273, p. 050004. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. Hydrodynamic factors in an anaerobic digester. In The Publications of the MultiScience—XXXII. MicroCAD International Scientific Conference; University of Miskolc: Miskolc, Hungary, 2018; Volume 2018, pp. 5–6. [Google Scholar]
- Battista, F.; Fino, D.; Mancini, G.; Ruggeri, B. Mixing in digesters used to treat high viscosity substrates: The case of olive oil production wastes. J. Environ. Chem. Eng. 2016, 4, 915–923. [Google Scholar] [CrossRef]
- Ding, J.; Wang, X.; Zhou, X.-F.; Ren, N.-Q.; Guo, W.-Q. CFD optimization of continuous stirred-tank (CSTR) reactor for biohydrogen production. Bioresour. Technol. 2010, 101, 7005–7013. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Johir, A.H.; Commault, A.; Bustamante, H.; Aurisch, R.; Lowrie, R.; Nghiem, L.D. Impacts of mixing on foaming, methane production, stratification and microbial community in full-scale anaerobic co-digestion process. Bioresour. Technol. 2019, 281, 226–233. [Google Scholar] [CrossRef]
- Ghanimeh, S.A.; Al-Sanioura, D.N.; Saikaly, P.E.; El-Fadel, M. Correlation between system performance and bacterial composition under varied mixing intensity in thermophilic anaerobic digestion of food waste. J. Environ. Manag. 2018, 206, 472–481. [Google Scholar] [CrossRef]
- Wu, B.; Chen, S. CFD simulation of non-Newtonian fluid flow in anaerobic digesters. Biotechnol. Bioeng. 2008, 99, 700–711. [Google Scholar] [CrossRef]
- Vesvikar, M.S.; Al-Dahhan, M. Flow pattern visualization in a mimic anaerobic digester using CFD. Biotechnol. Bioeng. 2005, 89, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Wu, B. CFD simulation of mixing in egg-shaped anaerobic digesters. Water Res. 2010, 44, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, I.D.; Zhai, X.; Wu, B. Influence of mixing on anaerobic digestion efficiency in stirred tank digesters: A review. Water Res. 2018, 143, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. State of the art on mixing in an anaerobic digester: A review. Renew. Energy 2019, 141, 922–936. [Google Scholar] [CrossRef]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef]
- Hashimoto, A.G. Effect of mixing duration and vacuum on methane production rate from beef cattle waste. Biotechnol. Bioeng. 1982, 24, 9–23. [Google Scholar] [CrossRef]
- Ma, S.-J.; Ma, H.-J.; Hu, H.; Ren, H.-Q. Effect of mixing intensity on hydrolysis and acidification of sewage sludge in two-stage anaerobic digestion: Characteristics of dissolved organic matter and the key microorganisms. Water Res. 2019, 148, 359–367. [Google Scholar] [CrossRef]
- Bridgeman, J. Computational fluid dynamics modelling of sewage sludge mixing in an anaerobic digester. Adv. Eng. Softw. 2012, 44, 54–62. [Google Scholar] [CrossRef]
- Benbelkacem, H.; Garcia-Bernet, D.; Bollon, J.; Loisel, D.; Bayard, R.; Steyer, J.-P.; Gourdon, R.; Buffière, P.; Escudié, R. Liquid mixing and solid segregation in high-solid anaerobic digesters. Bioresour. Technol. 2013, 147, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Baroutian, S.; Eshtiaghi, N.; Gapes, D.J. Rheology of a primary and secondary sewage sludge mixture: Dependency on temperature and solid concentration. Bioresour. Technol. 2013, 140, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Kariyama, I.D.; Wu, B. Investigation of the effect of intermittent minimal mixing intensity on methane production during anaerobic digestion of dairy manure. Comput. Electron. Agric. 2018, 155, 121–129. [Google Scholar] [CrossRef]
- Stroot, P.G. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—I. digester performance. Water Res. 2001, 35, 1804–1816. [Google Scholar] [CrossRef]
- Clark, I.C.; Zhang, R.H.; Upadhyaya, S.K. The effect of low pressure and mixing on biological hydrogen production via anaerobic fermentation. Int. J. Hydrog. Energy 2012, 37, 11504–11513. [Google Scholar] [CrossRef]
- Trad, Z.; Vial, C.; Fontaine, J.-P.; Larroche, C. Mixing and liquid-to-gas mass transfer under digester operating conditions. Chem. Eng. Sci. 2017, 170, 606–627. [Google Scholar] [CrossRef]
- Sulaiman, A.; Hassan, M.A.; Shirai, Y.; Abdaziz, S. The effect of mixing on methane production in a semi-commercial closed digester tank treating palm oil mill effluent. Aust. J. Basic Appl. Sci. 2009, 3, 1577–1583. [Google Scholar]
- Elnekave, M.; Tüfekçİ, N.; Kİmchİe, S.; Shelef, G. Tracing the mixing efficiency of a primary mesophilic anaerobic digester in a municipal wastewater treatment plant. In Proceedings of the 13th International Symposium on Environmental Pollution and its Impact on Life in the Mediterranean Region (MESAEP), Thessaloniki, Greece, 8–12 October 2005; pp. 1098–1105. [Google Scholar]
- Chandrasekharan, K.; Calderbank, P. Further observations on the scale-up of aerated mixing vessels. Chem. Eng. Sci. 1981, 36, 818–823. [Google Scholar] [CrossRef]
- Mesa, D.; Brito-Parada, P.R. Scale-up in froth flotation: A state-of-the-art review. Sep. Purif. Technol. 2019, 210, 950–962. [Google Scholar] [CrossRef]
- Pohar, A.; Naneh, O.; Bajec, D.; Likozar, B. Chemical reactor/compounding vessel fingerprinting: Scale-up/down considerations for homogeneous and heterogeneous mixing using computational fluid dynamics. Chem. Eng. Res. Des. 2020, 163, 125–137. [Google Scholar] [CrossRef]
- Uhl, V. Mixing V1: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Montgomery, L.; Schoepp, T.; Fuchs, W.; Bochmann, G. Design, calibration and validation of a large lab-scale system for measuring viscosity in fermenting substrate from agricultural anaerobic digesters. Biochem. Eng. J. 2016, 115, 72–79. [Google Scholar] [CrossRef]
- Conti, F.; Wiedemann, L.; Sonnleitner, M.; Saidi, A.; Goldbrunner, M. Monitoring the mixing of an artificial model substrate in a scale-down laboratory digester. Renew. Energy 2019, 132, 351–362. [Google Scholar] [CrossRef]
- Conti, F.; Saidi, A.; Goldbrunner, I.M. Numeric Simulation-Based Analysis of the Mixing Process in Anaerobic Digesters of Biogas Plants. Chem. Eng. Technol. 2020, 43, 1522–1529. [Google Scholar] [CrossRef]
- Conti, F.; Wiedemann, L.; Saidi, A.; Goldbrunner, M. Effect of mixing of waste biomass in anaerobic digesters for production of biogas. IOP Conf. Series: Mater. Sci. Eng. 2018, 446, 012011. [Google Scholar] [CrossRef]
- Conti, F.; Saidi, A.; Goldbrunner, M. CFD modelling of biomass mixing in anaerobic digesters of biogas plants. Environ. Clim. Technol. 2019, 23, 57–69. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Schwede, S.; Gerber, M.; Span, R. Scale up of laboratory scale to industrial scale biogas plants. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011; Volume 57, pp. 48–55. [Google Scholar]
- Yu, L.; Ma, J.; Frear, C.; Zhao, Q.; Dillon, R.; Li, X.; Chen, S. Multiphase modeling of settling and suspension in anaeorobic digester. Appl. Energy 2013, 111, 28–39. [Google Scholar] [CrossRef]
- Lin, K.C.; Pearce, M.E.J. Effects of mixing on anaerobic treatment of potato-processing wastewater. Can. J. Civ. Eng. 1991, 18, 504–514. [Google Scholar] [CrossRef]
- Ghanimeh, S.; El Fadel, M.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012, 117, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sindall, R.; Bridgeman, J.; Carliell-Marquet, C. Velocity gradient as a tool to characterise the link between mixing and biogas production in anaerobic waste digesters. Water Sci. Technol. 2013, 67, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: Process performance and microbial insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef]
- Hoffmann, R.A.; Garcia, M.L.; Veskivar, M.; Karim, K.; Al-Dahhan, M.H.; Angenent, L.T. Effect of shear on performance and microbial ecology of continuously stirred anaerobic digesters treating animal manure. Biotechnol. Bioeng. 2008, 100, 38–48. [Google Scholar] [CrossRef]
- Wu, B. CFD investigation of turbulence models for mechanical agitation of non-Newtonian fluids in anaerobic digesters. Water Res. 2011, 45, 2082–2094. [Google Scholar] [CrossRef]
- Wu, B. CFD simulation of gas and non-Newtonian fluid two-phase flow in anaerobic digesters. Water Res. 2010, 44, 3861–3874. [Google Scholar] [CrossRef]
- Rivard, C.J.; Kay, B.D.; Kerbaugh, D.H.; Nagle, N.J.; Himmel, M.E. Horsepower requirements for high-solids anaerobic digestion. Appl. Biochem. Biotechnol. 1995, 51, 155–162. [Google Scholar] [CrossRef]
- Benbelkacem HBayard, R.; Escudié, R.; Buffière, P.B.J. Towards optimization of the total solid content in high-solid (dry) municipal solid waste digestion. Chem. Eng. J. 2015, 273, 261–267. [Google Scholar] [CrossRef]
- Tixier, N.; Guibaud, G.; Baudu, M. Determination of some rheological parameters for the characterization of activated sludge. Bioresour. Technol. 2003, 90, 215–220. [Google Scholar] [CrossRef]
- Chen, Y.R.; Hashimoto, A.G. Energy Requirements for Anaerobic Fermentation of Livestock Wastes. Livest. Waste a Renew Resourous. American Society of Agricultural Engineers: Saint Joseph, MI, USA, 1980. [Google Scholar]
- Kress, P.; Nägele, H.-J.; Oechsner, H.; Ruile, S. Effect of agitation time on nutrient distribution in full-scale CSTR biogas digesters. Bioresour. Technol. 2018, 247, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Pagilla, K.R. Anaerobic digester foaming in full-scale cylindrical digesters—Effects of organic loading rate, feed characteristics, and mixing. Bioresour. Technol. 2014, 159, 182–192. [Google Scholar] [CrossRef]
- Vavilin, V.; Lokshina, L.; Flotats, X.; Angelidaki, I. Anaerobic digestion of solid material: Multidimensional modeling of continuous-flow reactor with non-uniform influent concentration distributions. Biotechnol. Bioeng. 2007, 97, 354–366. [Google Scholar] [CrossRef]
- Aranowski, R.; Hupka, J.; Jungnickel, C. Changes in rheological properties during anaerobic digestion of activated sludge. Physicochem. Probl. Miner. Process 2010, 44, 13–22. [Google Scholar]
- Mu, Y.; Chen, X.-H.; Yu, H.-Q. Rheological properties of anaerobic hydrogen-producing flocs. Biochem. Eng. J. 2007, 34, 87–91. [Google Scholar] [CrossRef]
- Seyssiecq, I.; Ferrasse, J.-H.; Roche, N. State-of-the-art: Rheological characterisation of wastewater treatment sludge. Biochem. Eng. J. 2003, 16, 41–56. [Google Scholar] [CrossRef]
- Brambilla, M.; Romano, E.; Cutini, M.; Bisaglia, C.; Pari, L. Rheological properties of manure/biomass mixtures and pumping strategies to improve ingestate formulation: A Review. Trans. ASABE 2013, 56, 1905–1920. [Google Scholar] [CrossRef]
- Hreiz, R.; Adouani, N.; Fünfschilling, D.; Marchal, P.; Pons, M.-N. Rheological characterization of raw and anaerobically digested cow slurry. Chem. Eng. Res. Des. 2017, 119, 47–57. [Google Scholar] [CrossRef]
- Vincent, W.U.; Joseph, B.G. Mixing Theory and Practice, 1st Edit.; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Kshirsagar, V.S.; Pawar, P.M. Mixing performance improvement by passive modifications in an anaerobic digester design. Mater. Today Proc. 2018, 5, 20600–20607. [Google Scholar] [CrossRef]
- Ford, C.; Ein-Mozaffari, F.; Bennington, C.P.J.; Taghipour, F. Simulation of mixing dynamics in agitated pulp stock chests using CFD. AIChE J. 2006, 52, 3562–3569. [Google Scholar] [CrossRef]
- Cebeci, T.; Shao, J.P.; Kafyeke, F.; Laurendeau, E. Computational Fluid Dynamics for Engineers; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Devals, C.; Heniche, M.; Takenaka, K.; Tanguy, P. CFD analysis of several design parameters affecting the performance of the Maxblend impeller. Comput. Chem. Eng. 2008, 32, 1831–1841. [Google Scholar] [CrossRef]
- Terashima, M.; Goel, R.; Komatsu, K.; Yasui, H.; Takahashi, H.; Li, Y.; Noike, T. CFD simulation of mixing in anaerobic digesters. Bioresour. Technol. 2009, 100, 2228–2233. [Google Scholar] [CrossRef]
- Leonzio, G. Studies of Mixing Systems in Anaerobic Digesters using CFD and the Future Applications of Nanotechnologies. Waste Biomass-Valoriz. 2019, 11, 5925–5955. [Google Scholar] [CrossRef]
- Jiménez, P.A.L. Applocation of CFD methods to an anaerobic digester: The case of Ontinyent WWTP, Valencia, Spain. Int. J. Tech. 2011, 2, 963–974. [Google Scholar]
- Manea, E.; Robescu, D. Simulation of mechanical mixing in anaerobic digesters. UPB Sci. Bull. Ser. D 2012, 74, 235–242. [Google Scholar]
- Wu, B. Large eddy simulation of mechanical mixing in anaerobic digesters. Biotechnol. Bioeng. 2012, 109, 804–812. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, J.; Poncin, S.; Li, H.Z. Effect of hydrodynamic shear on biogas production and granule characteristics in a continuous stirred tank reactor. Process. Biochem. 2016, 51, 345–351. [Google Scholar] [CrossRef]
- Caillet, H.; Bastide, A.; Chuppa-Tostain, G.; Petit, T. Anaerobic Digestion of Vinasse and CFD Modelling Approach; HalArchives-OuvertesFr. Proceedings of WasteEng2018—7th International Conference on Engeneering for Waste and Biomass Valorization, Prague, Czech Republic, 2–5 July 2018. [Google Scholar]
- Meroney, R.N.; Colorado, P. CFD simulation of mechanical draft tube mixing in anaerobic digester tanks. Water Res. 2009, 43, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Dama, P.; Bell, J.; Brouckaert, C.J.; Buckley, C.; Stuckey, D.C. Computational fluid dynamics: Application to the design of the anaerobic baffled reactor. In Proceedings of the WISA 2000 Biennial Conference, Sun City, South Africa, 28 May–1 June 2000; pp. 1–7. [Google Scholar]
- Mohammadrezaei, R.; Zareei, S.; Behroozi-Khazaei, N. Improving the performance of mechanical stirring in biogas plant by computational fluid dynamics (CFD). Agric. Eng. Int. CIGR J. 2017, 19, 91–97. [Google Scholar]
- Li, M.; White, G.; Wilkinson, D.; Roberts, K.J. LDA Measurements and CFD Modeling of a Stirred Vessel with a Retreat Curve Impeller. Ind. Eng. Chem. Res. 2004, 43, 6534–6547. [Google Scholar] [CrossRef]
- Shen, F.; Tian, L.; Yuan, H.; Pang, Y.; Chen, S.; Zou, D.; Zhu, B.; Liu, Y.; Li, X. Improving the Mixing Performances of Rice Straw Anaerobic Digestion for Higher Biogas Production by Computational Fluid Dynamics (CFD) Simulation. Appl. Biochem. Biotechnol. 2013, 171, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.U.; Ranganathan, P.; Pandey, A.; Sivaraman, S. Computational fluid dynamics modeling of gas dispersion in multi impeller bioreactor. J. Biosci. Bioeng. 2010, 109, 588–597. [Google Scholar] [CrossRef]
- Maier, C.; Weichselbaum, W.; Schlerka, M.; Harasek, M. Development of Agitation Systems in Biogas Plants: Investigation of Mixing Characteristics, Improvement of Energy Efficiency and Scale-Up Using Computational Fluid Dynamics; Chemical Engineering Transactions: Milano, Italy, 2010; Volume 21, pp. 1195–2000. [Google Scholar] [CrossRef]
- Huang, R.; Long, Y.; Luo, T.; Mei, Z.; Wang, J.; Long, E. The Research on Optimization of the Multiphase Flow Field of Biogas Plant by Using CFD Software. J. Energy Power Eng. 2014, 8. [Google Scholar] [CrossRef]
- Azargoshasb, H.; Mousavi, S.; Amani, T.; Jafari, A.; Nosrati, M. Three-phase CFD simulation coupled with population balance equations of anaerobic syntrophic acidogenesis and methanogenesis reactions in a continuous stirred bioreactor. J. Ind. Eng. Chem. 2015, 27, 207–217. [Google Scholar] [CrossRef]
- Karaeva, J.V.; Khalitova, G.R.; Kovalev, D.A.; Trakhunova, I.A. Study of the Process of Hydraulic Mixing in Anaerobic Digester of Biogas Plant. Chem. Process. Eng. 2015, 36, 101–112. [Google Scholar] [CrossRef]
- Torotwa, I.; Ji, C. A Study of the Mixing Performance of Different Impeller Designs in Stirred Vessels Using Computational Fluid Dynamics. Designs 2018, 2, 10. [Google Scholar] [CrossRef]
- Leonzio, G. Study of mixing systems and geometric configurations for anaerobic digesters using CFD analysis. Renew. Energy 2018, 123, 578–589. [Google Scholar] [CrossRef]
- Capela, I.; Bilé, M.J.; Silva, F.; Nadais, H.; Prates, A.; Arroja, L. Hydrodynamic behaviour of a full-scale anaerobic contact reactor using residence time distribution technique. J. Chem. Technol. Biotechnol. 2009, 84, 716–724. [Google Scholar] [CrossRef]
- Séguret, F.; Racault, Y. Hydrodynamic behaviour of a full-scale submerged biofilter and its possible influence on performances. Water Sci. Technol. 1998, 38, 249–256. [Google Scholar] [CrossRef]
- Kjellstrand, R.; Mattsson, A.; Niklasson, C.; Taherzadeh, M. Short circuiting in a denitrifying activated sludge tank. Water Sci. Technol. 2005, 52, 79–87. [Google Scholar] [CrossRef]
- Wolf, D.; Resnick, W. Residence Time Distribution in Real Systems. Ind. Eng. Chem. Fundam. 1963, 2, 287–293. [Google Scholar] [CrossRef]
- Cui, M.-H.; Zheng, Z.-Y.; Yang, M.; Sangeetha, T.; Zhang, Y.; Liu, H.-B.; Fu, B.; Liu, H.; Chen, C.-J. Revealing hydrodynamics and energy efficiency of mixing for high-solid anaerobic digestion of waste activated sludge. Waste Manag. 2021, 121, 1–10. [Google Scholar] [CrossRef]
- Gogate, P.R.; Beenackers, A.A.; Pandit, A.B. Multiple-impeller systems with a special emphasis on bioreactors: A critical review. Biochem. Eng. J. 2000, 6, 109–144. [Google Scholar] [CrossRef]
- Meister, M.; Rezavand, M.; Ebner, C.; Pümpel, T.; Rauch, W. Mixing non-Newtonian flows in anaerobic digesters by impellers and pumped recirculation. Adv. Eng. Softw. 2018, 115, 194–203. [Google Scholar] [CrossRef]
- Ong, H.K.; Greenfield, P.F.; Pullammanappallil, P.C. Effect of mixing on Biomethanation of cattle-manure slurry. Environ. Technol. 2002, 23, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, U.; Viccione, G.; Coppola, S.; Landi, A.; Meda, A.; Gualtieri, C. Analysis of anaerobic digester mixing: Comparison of long shafted paddle mixing vs. gas mixing. Water Sci. Technol. 2020, 81, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.J. Requirements and methods for mixing in anaerobic digesters. In Proceedings of the Seminar Organized by the Commission of the European Communities, Athens, Greece, 14–15 May 1984; pp. 90–103. [Google Scholar]
- Hafner, S.D.; Astals, S. Systematic error in manometric measurement of biochemical methane potential: Sources and solutions. Waste Manag. 2019, 91, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Montes, J.A.; Rico, J.L.; Rico, C. Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests. Waste Manag. 2016, 48, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- McNair, H.M.; Miller, J.M.; Snow, N.H. Basic Gas Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Wang, B.; Björn, A.; Strömberg, S.; Nges, I.A.; Nistor, M.; Liu, J. Evaluating the influences of mixing strategies on the Biochemical Methane Potential test. J. Environ. Manag. 2017, 185, 54–59. [Google Scholar] [CrossRef]
- Tian, Z.; Cabrol, L.; Ruiz-Filippi, G.; Pullammanappallil, P. Microbial ecology in anaerobic digestion at agitated and non-agitated conditions. PLoS ONE 2014, 9, e109769. [Google Scholar] [CrossRef]
- Leite, W.; Magnus, B.S.; Guimarães, L.B.; Gottardo, M.; Filho, P.B. Feasibility of thermophilic anaerobic processes for treating waste activated sludge under low HRT and intermittent mixing. J. Environ. Manag. 2017, 201, 335–344. [Google Scholar] [CrossRef]
- Tian, Z.; Chauliac, D.; Pullammanappallil, P. Comparison of non-agitated and agitated batch, thermophilic anaerobic digestion of sugarbeet tailings. Bioresour. Technol. 2013, 129, 411–420. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Dokianakis, S.N.; Stamatelatou, K.; Zafiri, C.; Kornaros, M. Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 2009, 248, 891–906. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Harnisch, E.; Schwede, S.; Gerber, M.; Span, R. Different mixing modes for biogas plants using energy crops. Appl. Energy 2013, 112, 465–472. [Google Scholar] [CrossRef]
- Al Mamun, M.R.; Torii, S. Enhancement of methane concentration by removing contaminants from biogas mixtures using combined method of absorption and adsorption. Int. J. Chem. Eng. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Pham, C.H.; Triolo, J.M.; Cu, T.T.T.; Pedersen, L.; Sommer, S.G. Validation and recommendation of methods to measure biogas production potential of animal manure. Asian-Australas. J. Anim. Sci. 2013, 26, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.D.; Koch, K.; Carrere, H.; Astals, S.; Weinrich, S.; Rennuit, C. Software for biogas research: Tools for measurement and prediction of methane production. SoftwareX 2018, 7, 205–210. [Google Scholar] [CrossRef]
- Cinar, S.; Cinar, S.O.; Wieczorek, N.; Sohoo, I.; Kuchta, K. Integration of artificial intelligence into biogas plant operation. Processes 2021, 9, 85. [Google Scholar] [CrossRef]
- Christopher, J.; Rivard, M.E.; Himmel, T.B.; Vinzant, W.S.; Adney, C.E.; Wyman, K.G. Anaerobic digestion of processed municipal solid waste using a novel high solids reactor: Maximum solids levels and mixing requirements. Biotechnol. Lett. 1990, 12, 235–240. [Google Scholar]
- Mohammadrezaei, R.; Zareei, S.; Khazaei, N.B. Optimum mixing rate in biogas reactors: Energy balance calculations and computational fluid dynamics simulation. Energy 2018, 159, 54–60. [Google Scholar] [CrossRef]
- Bello-Mendoza, R.; Sharratt, P.N. Modelling the effects of imperfect mixing on the performance of anaerobic reactors for sewage sludge treatment. J. Chem. Technol. Biotechnol. 1998, 71, 121–130. [Google Scholar] [CrossRef]
- Stalin, N.; Prabhu, H.J. Performance evaluation of partial mixing anaerobic digester. ARPN J. Eng. Appl. Sci. 2007, 2, 1–6. [Google Scholar]
- Noorpoor, A.; Dabiri, S. Sedimentation and mixing analysis in cattle manure feedstock in a stirred tank of anaerobic digestion Sedimentation and mixing analysis in cattle manure feedstock in a stirred tank of anaerobic digestion. In Proceedings of the 4th Edition of International Conference on Environmental Science & Technology, Vienna, Austria, 29–31 March 2018. [Google Scholar]
- Lewis, K.; Hughes, W. Optimisation of Methane Production from Anaerobically Digested Cow Slurry Using Mixing Regime and Hydraulic Retention Time; University of Exeter: Exeter, UK, 2015. [Google Scholar]
- McLeod, J.D.; Othman, M.Z.; Parthasarathy, R. Process intensification of anaerobic digestion: Influence on mixing and process performance. Bioresour. Technol. 2019, 274, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.M.; Idaty, T.; Ghazi, M.; Omar, R.; Azlina, W.; Ghani, W.A.K. Anaerobic batch digestion of cattle manure under various oscillatory flow mixing. Pertanika J. Sci. Technol. 2017, 25, 161–170. [Google Scholar]
- Kim, M.; Kim, B.-C.; Choi, Y.; Nam, K. Minimizing mixing intensity to improve the performance of rice straw anaerobic digestion via enhanced development of microbe-substrate aggregates. Bioresour. Technol. 2017, 245, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, J.; Eriksson, P.; Thorin, E. The effects of different mixing intensities during anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2014, 34, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Önen, S.; Nsair, A.; Kuchta, K. Innovative operational strategies for biogas plant including temperature and stirring management. Waste Manag. Res. 2019, 37, 237–246. [Google Scholar] [CrossRef]
- Wei, P.; Mudde, R.F.; Uijttewaal, W.; Spanjers, H.; Van Lier, J.B.; de Kreuk, M. Characterising the two-phase flow and mixing performance in a gas-mixed anaerobic digester: Importance for scaled-up applications. Water Res. 2019, 149, 86–97. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).