Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions
Abstract
1. Introduction
2. Materials and Methods
| Global Climate Models | Regional Climate Models | ||
|---|---|---|---|
| CCLM44-8-17, CLM-Community [71] | RCA4, Rossby Centre, Norway [72] | REMO, GERICS, Germany [73] | |
| CNRM-CM5, CERFACS, France [74] | r1 (RCP 4.5/8.5) | r1 (RCP 4.5/8.5) | |
| EC-Earth, European consortium [75] | r12 (RCP 4.5/8.5) | r12 (RCP 4.5/8.5) | |
| HadGEM2-ES, Hadley Center, UK [76] | r1 (RCP 4.5/8.5) | r1 (RCP 4.5/8.5) | |
| MPI-ESM-LR, MPI-M, Germany [77] | r1 (RCP 4.5/8.5) | r1 (RCP 4.5/8.5) | r1 (RCP 4.5/8.5) r2 (RCP 4.5/8.5) |
- summer temperature: mean temperature from June to August;
- winter temperature: mean temperature from December to February;
- summer precipitation: precipitation sum from June to August.
3. Results
3.1. Twin Regions Map
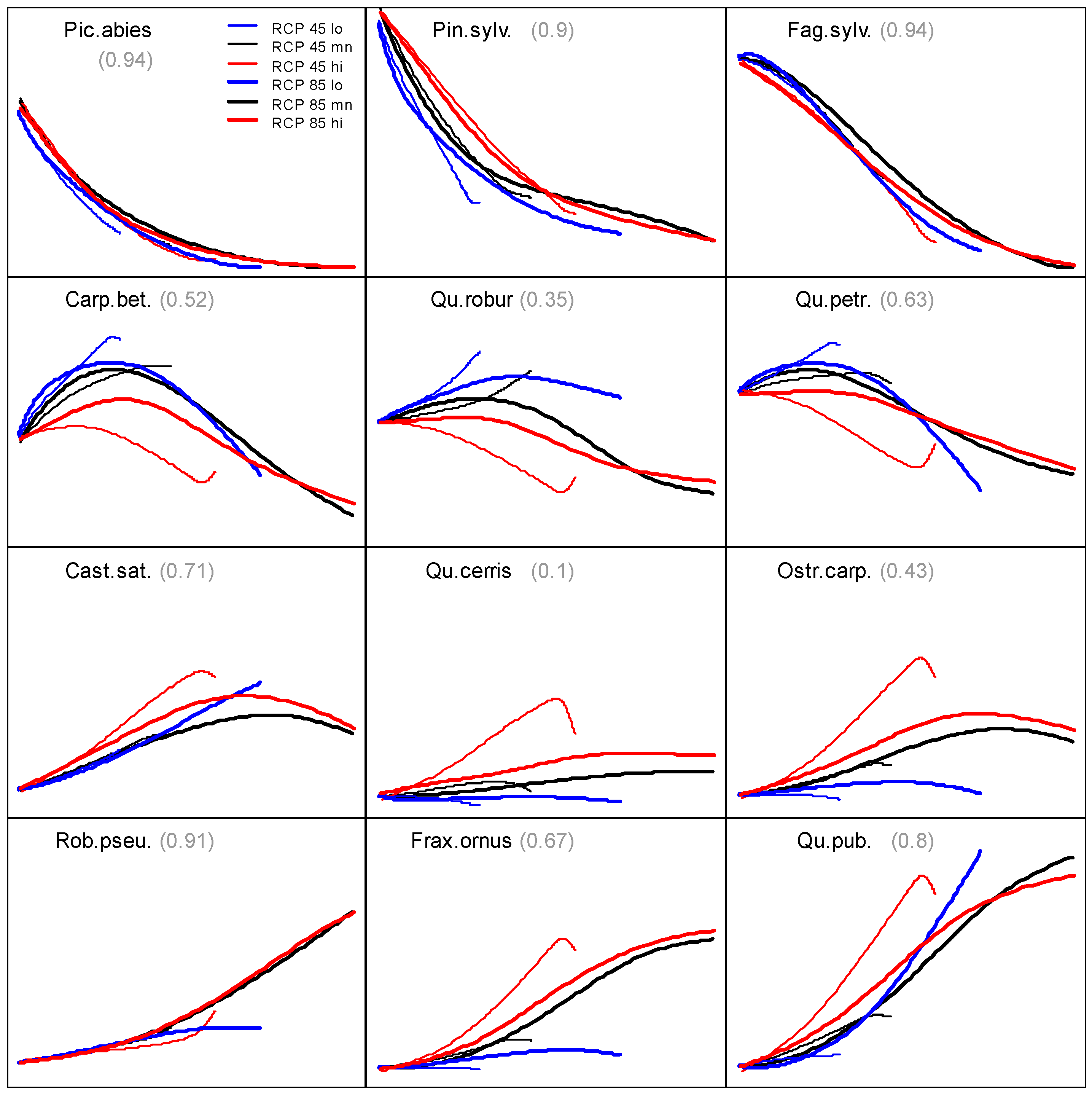
3.2. Prevalence Trajectory Graphics
| Species Name | Prevalence in Selected Countries’ NFIs (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| English | Scientific | Abbreviation | FIN | NOR | SWE | DEU | AUS | CHE | FRA | ITA | ESP |
| European larch | Larix decidua | Lar.decid. | 0 | 0 | 0.2 | 12.0 | 34.9 | 0 | 1.0 | 9.5 | 0 |
| Norway spruce | Picea abies | Pic.abies | 65.6 | 55.2 | 75.5 | 56.4 | 84.5 | 67.4 | 8.2 | 13.7 | 0 |
| Douglas fir | Pseudotsuga menziesii | Ps.menz. | 0 | 0 | 0 | 9.4 | 0.4 | 0.7 | 3.9 | 0.8 | 0.2 |
| Scots pine | Pinus sylvestris | Pin.sylv. | 84.3 | 47.2 | 75.9 | 39.1 | 24.3 | 8.8 | 12.6 | 6.3 | 14.3 |
| Black pine | Pinus nigra | Pin.nigra | 0 | 0 | 0 | 0.6 | 1.8 | 0.1 | 3.9 | 5.9 | 10.8 |
| maritime pine | Pinus pinaster | Pin.pinast. | 0 | 0 | 0 | 0 | 0 | 0 | 7.0 | 2.1 | 16.1 |
| common birch | Betula pendula | Bet.pend. | 27.4 | 3.5 | 5.7 | 23.0 | 9.5 | 5.3 | 9.3 | 2.8 | 0.2 |
| European beech | Fagus sylvatica | Fag.sylv. | 0 | 0.2 | 1.5 | 50.9 | 34.4 | 43.3 | 21.1 | 18.9 | 5.9 |
| mountain maple | Acer pseudoplatanus | Ac.pseu. | 0 | 0.1 | 0 | 16.0 | 14.7 | 19.1 | 4.5 | 6.9 | 0.2 |
| hornbeam | Carpinus betulus | Carp.bet. | 0 | 0 | 0.3 | 13.6 | 7.7 | 2.1 | 17.3 | 4.4 | 0 |
| pedunculate oak | Quercus robur | Qu.robur | 0.1 | 0 | 5.3 | 24.9 | 8.2 | 3.4 | 26.0 | 1.8 | 5.4 |
| sessile oak | Quercus petraea | Qu.petr. | 0 | 0 | 0 | 21.1 | 7.4 | 4.5 | 20.6 | 4.6 | 2.0 |
| Common ash | Fraxinus excelsior | Frax.exc. | 0.1 | 1.0 | 1.2 | 16.8 | 14.8 | 16.0 | 12.1 | 6.0 | 0.9 |
| field maple | Acer campestre | Ac.camp. | 0 | 0 | 0 | 2.3 | 1.7 | 1.3 | 6.3 | 7.7 | 0.8 |
| wild service tree | Sorbus torminalis | Sor.torm. | 0 | 0 | 0 | 0.6 | 0.3 | 0.1 | 2.9 | 1.4 | 0.1 |
| sweet cherry | Prunus avium | Pr.avium | 0 | 0.1 | 0.3 | 5.0 | 3.0 | 3.2 | 6.1 | 7.9 | 0.2 |
| chestnut | Castanea sativa | Cast.sat. | 0 | 0 | 0 | 0.8 | 1.4 | 3.3 | 11.9 | 16.3 | 3.8 |
| Turkey oak | Quercus cerris | Qu.cerris | 0 | 0 | 0 | 0 | 1.8 | 0.1 | 0.2 | 21.2 | 0 |
| hop hornbeam | Ostrya carpinifolia | Ostr.carp. | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.2 | 20 | 0 |
| black locust | Robinia pseudoacacia | Rob.pseu. | 0 | 0 | 0 | 1.5 | 1.5 | 0.5 | 3.2 | 6.6 | 0.3 |
| Manna ash | Fraxinus ornus | Frax.ornus | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.2 | 21.5 | 0 |
| pubescent oak | Quercus pubescens | Qu.pub. | 0 | 0 | 0 | 0 | 0.1 | 0.8 | 12.7 | 30.2 | 2.8 |
| holm oak | Quercus ilex | Qu.ilex | 0 | 0 | 0 | 0 | 0 | 0 | 5.1 | 10.1 | 26.1 |
3.3. Tree Species Absolute Prevalence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovats, R.; Valentini, R.; Bouwer, L.M.; Georgopoulou, E.; Jacob, D.; Martin, E.; Rounsevell, M.; Soussana, J.-F. Europe: Chapter 23. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects: Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC WG2, Ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 1267–1326. ISBN 9781107415386. [Google Scholar]
- Forest Europe. State of Europe’s Forests 2020, Madrid. 2020. Available online: https://foresteurope.org/state-europes-forests-2020-report (accessed on 10 March 2021).
- European Environmental Agency (EEA). Indicator Assessment. Global and European Temperatures. Available online: https://www.eea.europa.eu/ds_resolveuid/35d56669c1274e179b08dff5de5694a7 (accessed on 10 March 2021).
- Forzieri, G.; Girardello, M.; Ceccherini, G.; Spinoni, J.; Feyen, L.; Hartmann, H.; Beck, P.S.A.; Camps-Valls, G.; Chirici, G.; Mauri, A.; et al. Emergent vulnerability to climate-driven disturbances in European forests. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate Extremes: Observations, Modeling, and Impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Thom, D.; Rammer, W.; Seidl, R. Disturbances catalyze the adaptation of forest ecosystems to changing climate conditions. Glob. Chang. Biol. 2017, 23, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Buras, A.; Rammig, A.; Zang, C.S. Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences 2020, 17, 1655–1672. [Google Scholar] [CrossRef]
- Rigling, A.; Etzold, S.; Bebi, P.; Brang, P.; Ferretti, M.; Forrester, D.; Gärtner, H. Wie viel Trockenheit ertragen unsere Wälder? Lehren aus extremen Trockenjahren. Forum Wissen WSL-Ber. 2019, 78, 39–51. [Google Scholar]
- Walthert, L.; Ganthaler, A.; Mayr, S.; Saurer, M.; Waldner, P.; Walser, M.; Zweifel, R.; von Arx, G. From the comfort zone to crown dieback: Sequence of physiological stress thresholds in mature European beech trees across progressive drought. Sci. Total Environ. 2021, 753, 141792. [Google Scholar] [CrossRef]
- Mette, T.; Falk, W. Extreme Trockenheit—Wie sie auf Vitalität und Anbaurisiko von Waldbäumen wirkt: Was passiert, wenn Witterungsextreme den Toleranzbereich von Waldbäumen überschreiten? LWF Aktuell 2020, 126, 30–34. [Google Scholar]
- The Greenhouse Effect, Climatic Change, and Ecosystems; Bolin, B., Doos, B.R., Jager, J., Warrick, R.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1986; ISBN 978-0471910121. [Google Scholar]
- Climate Change. The IPCC Impacts Assessment: Working Group 2 Contribution to the First Assessment Report of the Intergovern-Mental Panel on Climate Change; IPCC WG2, Ed.; Australian Government Publishing Service: Canberra, Australia, 1990. [Google Scholar]
- Dale, V. Forests and Climate Change (S.I.). Sci. Total Environ. 2000, 262, 201–324. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Blennow, K.; Persson, J.; Tomé, M.; Hanewinkel, M. Climate Change: Believing and Seeing Implies Adapting. PLoS ONE 2012, 7, e50182. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Bentz, J.; Silva, J.M.; Fonseca, A.L.; Swart, R.; Santos, F.D.; Penha-Lopes, G. Adaptation to climate change at local level in Europe: An overview. Environ. Sci. Policy 2018, 86, 38–63. [Google Scholar] [CrossRef]
- Sousa-Silva, R.; Verbist, B.; Lomba, Â.; Valent, P.; Suškevičs, M.; Picard, O.; Hoogstra-Klein, M.A.; Cosofret, V.-C.; Bouriaud, L.; Ponette, Q.; et al. Adapting forest management to climate change in Europe: Linking perceptions to adaptive responses. For. Policy Econ. 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Coll, L.; Ameztegui, A.; Collet, C.; Löf, M.; Mason, B.; Pach, M.; Verheyen, K.; Abrudan, I.V.; Barbati, A.; Barreiro, S.; et al. Knowledge gaps about mixed forests: What do European forest managers want to know and what answers can science provide? For. Ecol. Manag. 2018, 407, 106–115. [Google Scholar] [CrossRef]
- Howe, G.M. Agro-Climatic Analogues. Nature 1948, 161, 983–984. [Google Scholar] [CrossRef]
- Boshell, F.; Neild, R. A computer-statistical procedure to determine agroclimatic analogues for tea production in Colombia. Agric. Meteorol. 1975, 15, 221–230. [Google Scholar] [CrossRef]
- Glantz, M. Societal Responses to Climate Change: Forecasting by Analogy; Westview Press: Boulder, CO, USA, 1988. [Google Scholar]
- Hogg, T.; Hurdle, P.A. The aspen parkland in western Canada: A dry-climate analogue for the future boreal forest? Water Air Soil Pollut. 1995, 82, 391–400. [Google Scholar] [CrossRef]
- Hallegatte, S.; Hourcade, J.-C.; Ambrosi, P. Using climate analogues for assessing climate change economic impacts in urban areas. Clim. Chang. 2007, 82, 47–60. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 2007, 5, 475–482. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Loarie, S.R.; Cornwell, W.; Weiss, S.B.; Hamilton, H.; Branciforte, R.; Kraft, N.J.B. The geography of climate change: Implications for conservation biogeography. Divers. Distrib. 2010, 16, 476–487. [Google Scholar] [CrossRef]
- Bergmann, J.; Pompe, S.; Ohlemüller, R.; Freiberg, M.; Klotz, S.; Kühn, I. The Iberian Peninsula as a potential source for the plant species pool in Germany under projected climate change. Plant Ecol. 2010, 207, 191–201. [Google Scholar] [CrossRef]
- Ford, J.D.; Keskitalo, E.C.H.; Smith, T.; Pearce, T.; Berrang-Ford, L.; Duerden, F.; Smit, B. Case study and analogue methodologies in climate change vulnerability research. Wiley Interdiscip. Rev. Clim. Chang. 2010, 1, 374–392. [Google Scholar] [CrossRef]
- Ramírez-Villegas, J.; Lau, C.; Köhler, A.-K.; Signer, J.; Jarvis, A.; Arnell, N.; Osborne, T.; Hooker, J. Climate Analogues: Finding Tomorrow’s Agriculture Today: Working Paper No. 12, Cali, Colombia, 2011. Available online: www.ccafs.cgiar.org (accessed on 10 March 2021).
- Ungar, J.; Peters-Anders, J.; Loibl, W. Climate Twins—An Attempt to Quantify Climatological Similarities. In Environmental Software Systems. Frameworks of eEnvironment. 9th IFIP WG 5.11 International Symposium, ISESS 2011, Brno, CZ, 27.-29.6; Hřebíček, J., Schimak, G., Denzer, R., Eds.; Springer: Berlin, Gernamy, 2011; pp. 428–436. [Google Scholar]
- Veloz, S.; Williams, J.W.; Lorenz, D.; Notaro, M.; Vavrus, S.; Vimont, D.J. Identifying climatic analogs for Wisconsin under 21st-century climate-change scenarios. Clim. Chang. 2011, 112, 1037–1058. [Google Scholar] [CrossRef]
- Leibing, C.; Signer, J.; Van Zonneveld, M.; Jarvis, A.; Dvorak, W. Selection of Provenances to Adapt Tropical Pine Forestry to Climate Change on the Basis of Climate Analogs. Forest 2013, 4, 155–178. [Google Scholar] [CrossRef]
- Kölling, C.; Zimmermann, L. Klimawandel gestern und morgen: Neue Argumente können die Motivation zum Waldumbau erhöhen. LWF Aktuell 2014, 99, 27–31. [Google Scholar]
- Ozolincius, R.; Lekevičius, E.; Stakėnas, V.; Galvonaitė, A.; Samas, A.; Valiukas, D. Lithuanian forests and climate change: Possible effects on tree species composition. Eur. J. For. Res. 2013, 133, 51–60. [Google Scholar] [CrossRef]
- Sybertz, J.; Reich, M. Assessing Climate Change Induced Turnover in Bird Communities Using Climatically Analogous Regions. Diversity 2015, 7, 36–59. [Google Scholar] [CrossRef]
- Rohat, G.; Goyette, S.; Flacke, J. Twin climate cities—An exploratory study of their potential use for awareness-raising and urban adaptation. Mitig. Adapt. Strat. Glob. Chang. 2016, 22, 929–945. [Google Scholar] [CrossRef]
- Buras, A.; Menzel, A. Projecting Tree Species Composition Changes of European Forests for 2061–2090 Under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Dunn, R.R. Contemporary climatic analogs for 540 North American urban areas in the late 21st century. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Mayr, H. Fremdländische Wald- und Parkbäume für Europa; Verlagsbuchhandlung Paul Parey: Berlin, Germany, 1906; ISBN 3968787730. [Google Scholar]
- Grenier, P.; Parent, A.-C.; Huard, D.; Anctil, F.; Chaumont, D. An Assessment of Six Dissimilarity Metrics for Climate Analogs. J. Appl. Meteorol. Clim. 2013, 52, 733–752. [Google Scholar] [CrossRef]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J., Jr.; Johns, T.; Krinner, G.; et al. Chapter 12: Long-term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013: The Physical Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC; Cambridge University Press: Cambridge, UK, 2014; pp. 1029–1136. ISBN 978-1-107-66182-0. [Google Scholar]
- Iverson, L.R.; Schwartz, M.W.; Prasad, A.M. How fast and far might tree species migrate in the eastern United States due to climate change? Glob. Ecol. Biogeogr. 2004, 13, 209–219. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2011, 18, 1042–1052. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for Climate Change: Forestry and Assisted Migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- McLachlan, J.S.; Hellmann, J.J.; Schwartz, M.W. A Framework for Debate of Assisted Migration in an Era of Climate Change. Conserv. Biol. 2007, 21, 297–302. [Google Scholar] [CrossRef]
- Kreyling, J.; Bittner, T.; Jaeschke, A.; Jentsch, A.; Steinbauer, M.; Thiel, D.; Beierkuhnlein, C. Assisted Colonization: A Question of Focal Units and Recipient Localities. Restor. Ecol. 2011, 19, 433–440. [Google Scholar] [CrossRef]
- Pedlar, J.H.; McKenney, D.W.; Aubin, I.; Beardmore, T.; Beaulieu, J.; Iverson, L.R.; O’Neill, G.A.; Winder, R.S.; Ste-Marie, C. Placing Forestry in the Assisted Migration Debate. BioScience 2012, 62, 835–842. [Google Scholar] [CrossRef]
- Iverson, L.R.; Prasad, A.M.; Peters, M.P.; Matthews, S.N. Facilitating Adaptive Forest Management under Climate Change: A Spatially Specific Synthesis of 125 Species for Habitat Changes and Assisted Migration over the Eastern United States. Forests 2019, 10, 989. [Google Scholar] [CrossRef]
- Gömöry, D.; Krajmerová, D.; Hrivnák, M.; Longauer, R. Assisted migration vs. close-to-nature forestry: What are the prospects for tree populations under climate change? Cent. Eur. For. J. 2020, 66, 63–70. [Google Scholar] [CrossRef]
- Brandl, S. ANALOG—Waldzukunft zum Anfassen (Projekt C 43): Klimaanalogien als Informations- und Kommunikationsmittel in der Klimawandelanpassung der Wälder um Nürnberg. Available online: https://www.lwf.bayern.de/boden-klima/baumartenwahl/263859/index.php (accessed on 10 March 2021).
- Kölling, C.; Mette, T. Wälder im Klimawandel—Neues Klima erfordert neue Baumarten. In Wald in der Vielfalt Möglicher Perspektiven: Von der Pluralität Lebensweltlicher Bezüge und Wissenschaftlichen Thematisierungen; Jenal, C., Berr, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; in press. [Google Scholar]
- Brandl, S.; Mette, T.; Kölling, C. ANALOG—Waldzukunft zum Anfassen. Available online: https://www.waldwissen.net/de/waldwirtschaft/waldbau/forstliche-planung/analog-waldzukunft-zum-anfassen (accessed on 10 March 2021).
- Mette, T.; Kölling, C. Die Zukunft der Kiefer in Franken: Eine Zeitreise in den Klimawandel. LWF Aktuell 2020, 2, 14–17. [Google Scholar]
- Kölling, C.; Mette, T.; Brandl, S.; Walter, K.; Körner, A.; Dauer, S.; Stapff, M. Klima-Atlas Roth—Waldzukunft zum Anfassen; AELF Roth: Roth, Germany, 2021. [Google Scholar]
- Mauri, A.; Strona, G.; San-Miguel-Ayanz, J. EU-Forest, a high-resolution tree occurrence dataset for Europe. Sci. Data 2017, 4, 160123. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.N.; Conrad, O.; Boehner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- EURO-CORDEX. EURO-CORDEX—Coordinated Downscaling Experiment—European Domain. Available online: https://www.euro-cordex.net/ (accessed on 10 March 2021).
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Grenier, P.; Firlej, A.; Blondlot, A.; Logan, T.; Ricard, M.-P. The issue of properly ordering climate indices calculation and bias correction before identifying spatial analogs for agricultural applications. Clim. Serv. 2019, 16, 100122. [Google Scholar] [CrossRef]
- Wilcke, R.; Bärring, L.; Dobler, A.; Nikulin, G.; Vautard, R.; Vrac, M.; Braconnot, P.; Otto, J.; Erik, J. Climate Model Data for Europe: CLIPC DELIVERABLE (D-N°: 6.1). 2014. Available online: http://www.clipc.eu/content/content.php?htm=45 (accessed on 10 March 2021).
- Yang, W.; Andréasson, J.; Graham, L.P.; Olsson, J.; Rosberg, J.; Wetterhall, F. Distribution-based scaling to improve usability of regional climate model projections for hydrological climate change impacts studies. Hydrol. Res. 2010, 41, 211–229. [Google Scholar] [CrossRef]
- Häggmark, L.; Ivarsson, K.-I.; Gollvik, S.; Olofsson, P.-O. Mesan, an operational mesoscale analysis system. Tellus A Dyn. Meteorol. Oceanogr. 2000, 52, 2–20. [Google Scholar] [CrossRef]
- Moreno, A.; Hasenauer, H. Spatial downscaling of European climate data. Int. J. Clim. 2015, 36, 1444–1458. [Google Scholar] [CrossRef]
- Keuler, K.; Radtke, K.; Kotlarski, S.; Lüthi, D. Regional climate change over Europe in COSMO-CLM: Influence of emission scenario and driving global model. Meteorol. Z. 2016, 25, 121–136. [Google Scholar] [CrossRef]
- Strandberg, G.; Bärring, L.; Hansson, U.; Jansson, C.; Jones, C.; Kjellström, E.; Kolax, M.; Kupiainen, M.; Nikulin, G.; Samuelsson, P.; et al. CORDEX Scenarios for Europe from the Rossby Centre Regional Climate Model RCA4; RMK Report Meteorology and Climatology No. 116; SMHI: Norrköping, Sweden, 2014. [Google Scholar]
- Teichmann, C.; Eggert, B.; Elizalde, A.; Haensler, A.; Jacob, D.; Kumar, P.; Moseley, C.; Pfeifer, S.; Rechid, D.; Remedio, A.R.; et al. How Does a Regional Climate Model Modify the Projected Climate Change Signal of the Driving GCM: A Study over Different CORDEX Regions Using REMO. Atmosphere 2013, 4, 214–236. [Google Scholar] [CrossRef]
- Voldoire, A.; Sanchezgomez, E.; Mélia, D.S.; Decharme, B.; Cassou, C.; Senesi, S.; Valcke, S.; Beau, I.; Alias, A.; Chevallier, M.; et al. The CNRM-CM5.1 global climate model: Description and basic evaluation. Clim. Dyn. 2013, 40, 2091–2121. [Google Scholar] [CrossRef]
- Hazeleger, W.; Severijns, C.; Semmler, T.; Ştefănescu, S.; Yang, S.; Wang, X.; Wyser, K.; Dutra, E.; Baldasano, J.M.; Bintanja, R.; et al. EC-Earth. Bull. Am. Meteorol. Soc. 2010, 91, 1357–1364. [Google Scholar] [CrossRef]
- Collins, W.J.; Bellouin, N.; Doutriaux-Boucher, M.; Gedney, N.; Halloran, P.; Hinton, T.; Hughes, J.; Jones, C.D.; Joshi, M.; Liddicoat, S.; et al. Development and evaluation of an Earth-System model—HadGEM2. Geosci. Model Dev. 2011, 4, 1051–1075. [Google Scholar] [CrossRef]
- Popke, D.; Stevens, B.; Voigt, A. Climate and climate change in a radiative-convective equilibrium version of ECHAM6. J. Adv. Model. Earth Syst. 2013, 5, 1–14. [Google Scholar] [CrossRef]
- Schultz, J. Die Ökozonen der Erde, 5, Vollständig Überarbeitete Auflage; Verlag Eugen Ulmer: Stuttgart, Germany, 2016; ISBN 9783825246280. [Google Scholar]
- Brandl, S.; Mette, T.; Falk, W.; Vallet, P.; Rötzer, T.; Pretzsch, H. Static site indices from different national forest inventories: Harmonization and prediction from site conditions. Ann. For. Sci. 2018, 75, 56. [Google Scholar] [CrossRef]
- Kölling, C.; Mette, T.; Knoke, T. Waldertrag und Anbaurisiko in einer unsicheren Klimazukunft. Schweiz. Z. Forstwes. 2016, 167, 29–38. [Google Scholar] [CrossRef][Green Version]
- BMEL—Bundesministerium für Ernährung und Landwirtschaft. Der Wald in Deutschland—Ausgewählte Ergebnisse der Dritten Bundeswaldinventur. Berlin, 2014. Available online: www.bundeswaldinventur.de (accessed on 10 May 2021).
- DWD Climate Data Center (CDC). Raster der Monatsmittel der Lufttemperatur (2 m) für Deutschland, Version 1.0; Raster der Monatssumme der Niederschlagshöhe für Deutschland, Version v1.0. Available online: https://www.dwd.de/DE/leistungen/cdc/climate-data-center.html (accessed on 10 March 2021).
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Falk, W.; Mellert, K.H. Species distribution models as a tool for forest management planning under climate change: Risk evaluation of Abies alba in Bavaria. J. Veg. Sci. 2011, 22, 621–634. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Tomppo, E.; Gschwantner, T.; Lawrence, M.; McRoberts, R.E. National Forest Inventories; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-3232-4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth and Yield; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-88306-7. [Google Scholar]
- Semerci, A.; Semerci, H.; Çalişkan, B.; Çiçek, N.; Ekmekçi, Y.; Mencuccini, M. Morphological and physiological responses to drought stress of European provenances of Scots pine. Eur. J. For. Res. 2017, 136, 91–104. [Google Scholar] [CrossRef]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Matias, L.; Gonzalez-Díaz, P.; Jump, A.S. Larger investment in roots in southern range-edge populations of Scots pine is associated with increased growth and seedling resistance to extreme drought in response to simulated climate change. Environ. Exp. Bot. 2014, 105, 32–38. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of Birch (Betula pendula Roth and B. pubescens Ehrh.) for Forestry and Forest-Based Industry Sector within the Changing Climatic and Socio-Economic Context of Western Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Mette, T.; Dolos, K.; Meinardus, C.; Bräuning, A.; Reineking, B.; Blaschke, M.; Pretzsch, H.; Beierkuhnlein, C.; Gohlke, A.; Wellstein, C. Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Hohnwald, S.; Indreica, A.; Walentowski, H.; Leuschner, C. Microclimatic Tipping Points at the Beech–Oak Ecotone in the Western Romanian Carpathians. Forests 2020, 11, 919. [Google Scholar] [CrossRef]
- Stimm, K.; Heym, M.; Uhl, E.; Tretter, S.; Pretzsch, H. Height growth-related competitiveness of oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) under climate change in Central Europe. Is silvicultural assistance still required in mixed-species stands? For. Ecol. Manag. 2021, 482, 118780. [Google Scholar] [CrossRef]
- StMELF. Baumarten für den Klimawald. Leitlinien, München, 2020. Available online: http://www.waldbesitzer-portal.bayern.de/klimawald-baumarten (accessed on 10 March 2021).
- Francis, J.; Vavrus, S.J. Evidence for a wavier jet stream in response to rapid Arctic warming. Environ. Res. Lett. 2015, 10, 14005. [Google Scholar] [CrossRef]
- De Vries, H.; Haarsma, R.J.; Hazeleger, W. Western European cold spells in current and future climate. Geophys. Res. Lett. 2012, 39, L04706. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Jandl, R.; Spathelf, P.; Bolte, A.; Prescott, C.E. Forest adaptation to climate change—Is non-management an option? Ann. For. Sci. 2019, 76, 48. [Google Scholar] [CrossRef]
- Paul, C.; Brandl, S.; Friedrich, S.; Falk, W.; Härtl, F.; Knoke, T. Climate change and mixed forests: How do altered survival probabilities impact economically desirable species proportions of Norway spruce and European beech? Ann. For. Sci. 2019, 76, 14. [Google Scholar] [CrossRef]
- Kölling, C.; Beinhofer, B.; Hahn, A.; Knoke, T. Wie soll die Forstwirtschaft auf neue Risiken im Klimawandel reagieren? Allg. Forst. Z. Waldwirtsch. Umweltvorsorge 2010, 5, 18–22. [Google Scholar]
- Kölling, C.; Rothkegel, W.; Rupert, O. Das Nelderrad als sparsames und wirksames Pflanzschema. AFZ/DerWald 2020, 75, 42–46. [Google Scholar]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.; Harvey, B.J.; Higuera, P.; Mack, M.C.; Meentemeyer, R.; Metz, M.R.; Perry, G.L.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Woodall, C.; Oswalt, C.; Westfall, J.; Perry, C.; Nelson, M.; Finley, A. An indicator of tree migration in forests of the eastern United States. For. Ecol. Manag. 2009, 257, 1434–1444. [Google Scholar] [CrossRef]
- Renwick, K.M.; Rocca, M.E. Temporal context affects the observed rate of climate-driven range shifts in tree species. Glob. Ecol. Biogeogr. 2015, 24, 44–51. [Google Scholar] [CrossRef]
- Iverson, L.; Prasad, A.; Matthews, S. Modeling potential climate change impacts on the trees of the northeastern United States. Mitig. Adapt. Strat. Glob. Chang. 2008, 13, 487–516. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.-C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Talluto, M.V.; Boulangeat, I.; Vissault, S.; Thuiller, W.; Gravel, D. Extinction debt and colonization credit delay range shifts of eastern North American trees. Nat. Ecol. Evol. 2017, 1, 637. [Google Scholar] [CrossRef]

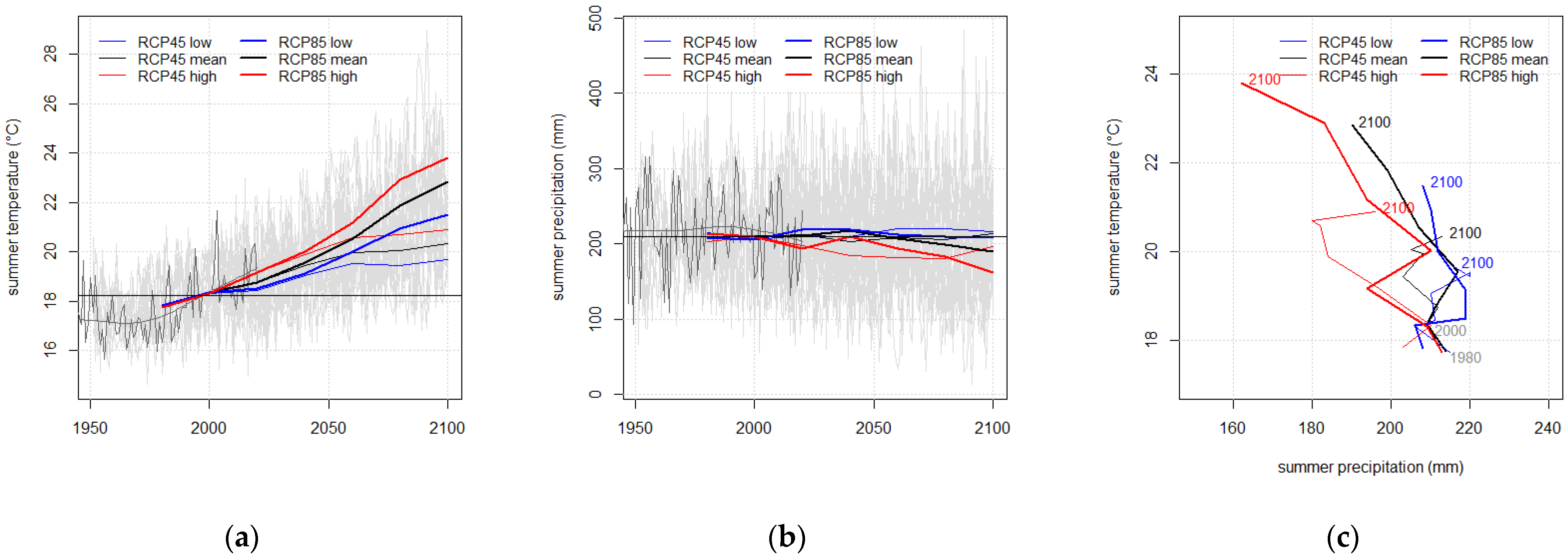
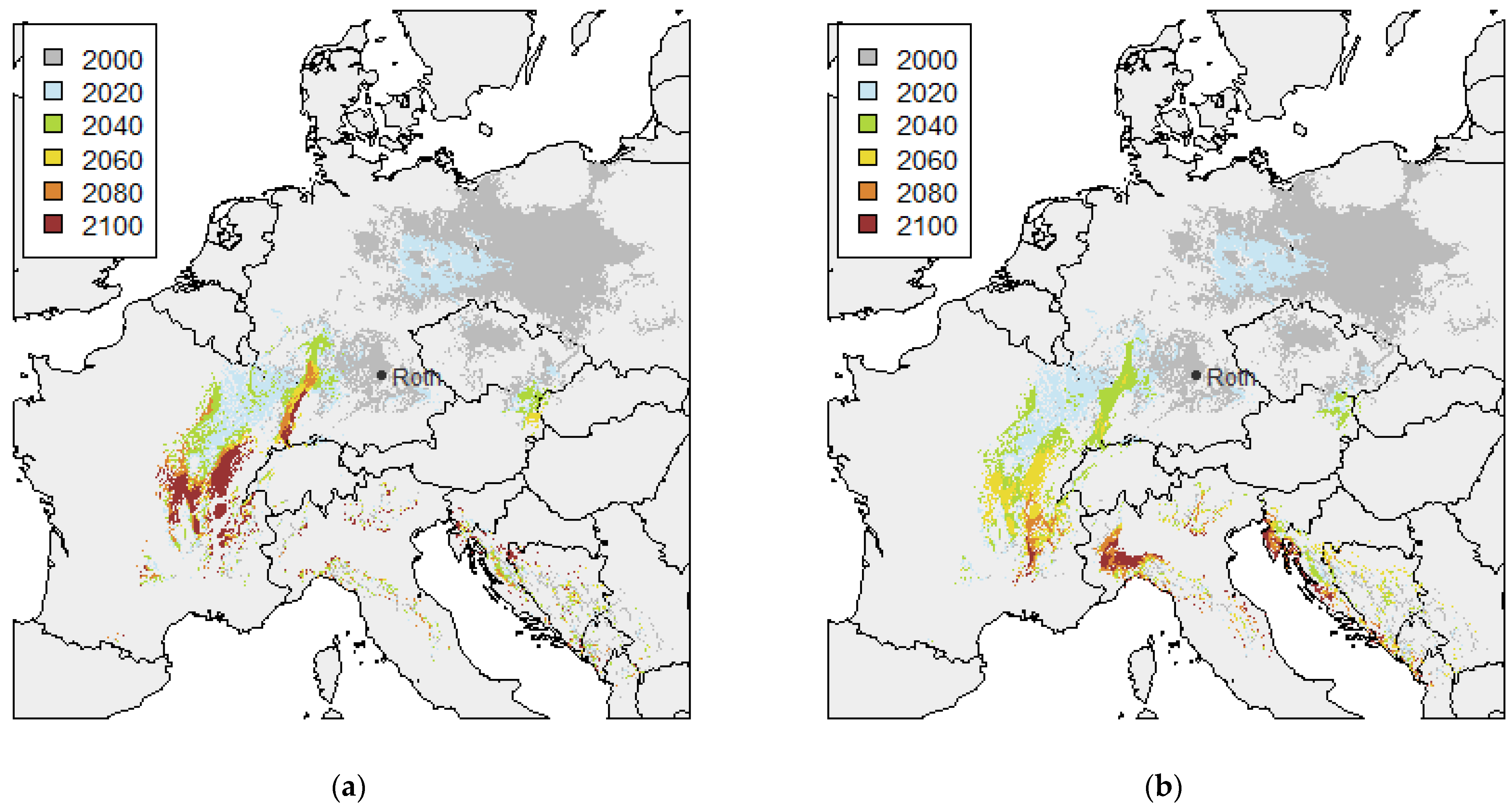

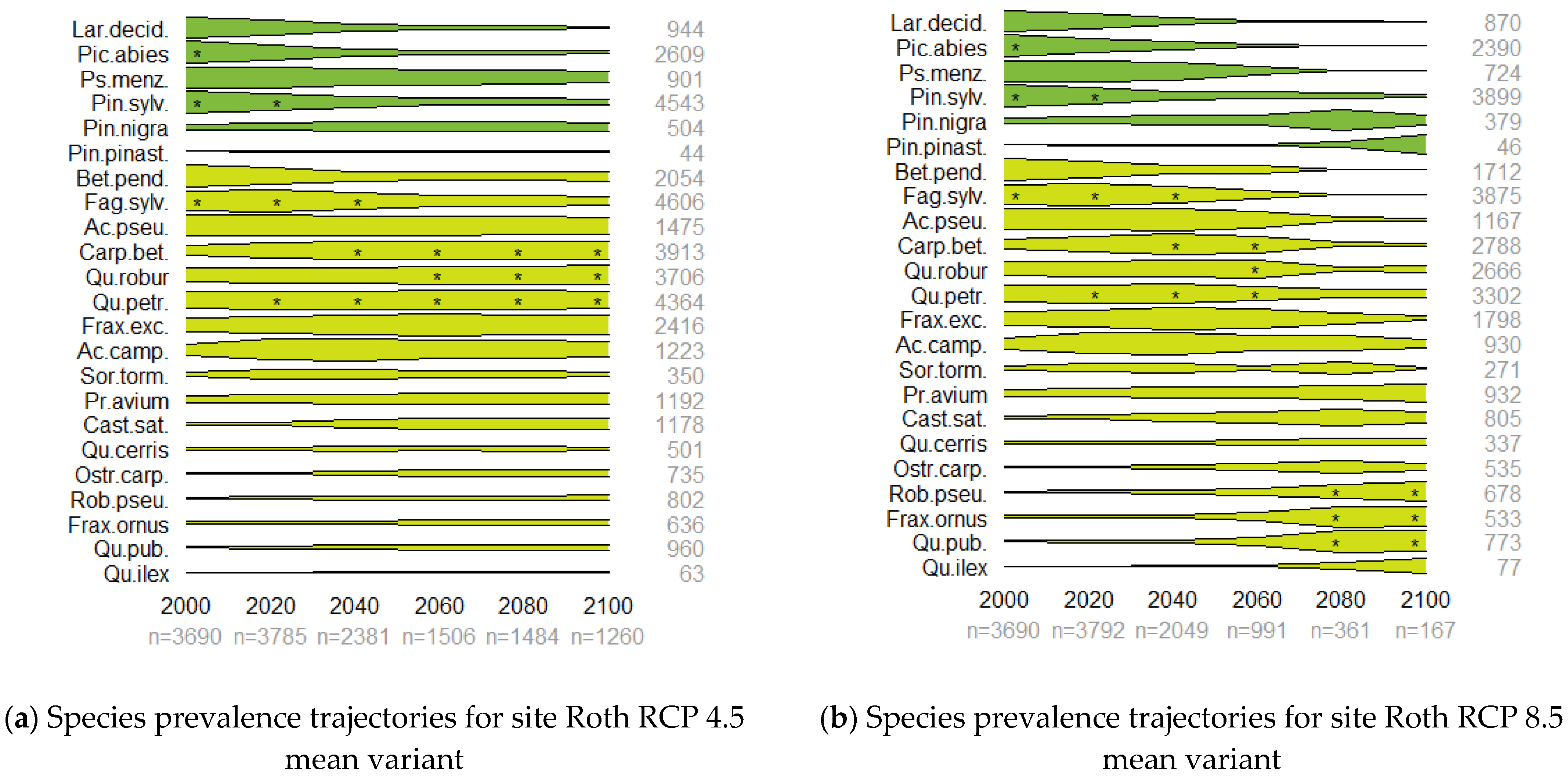
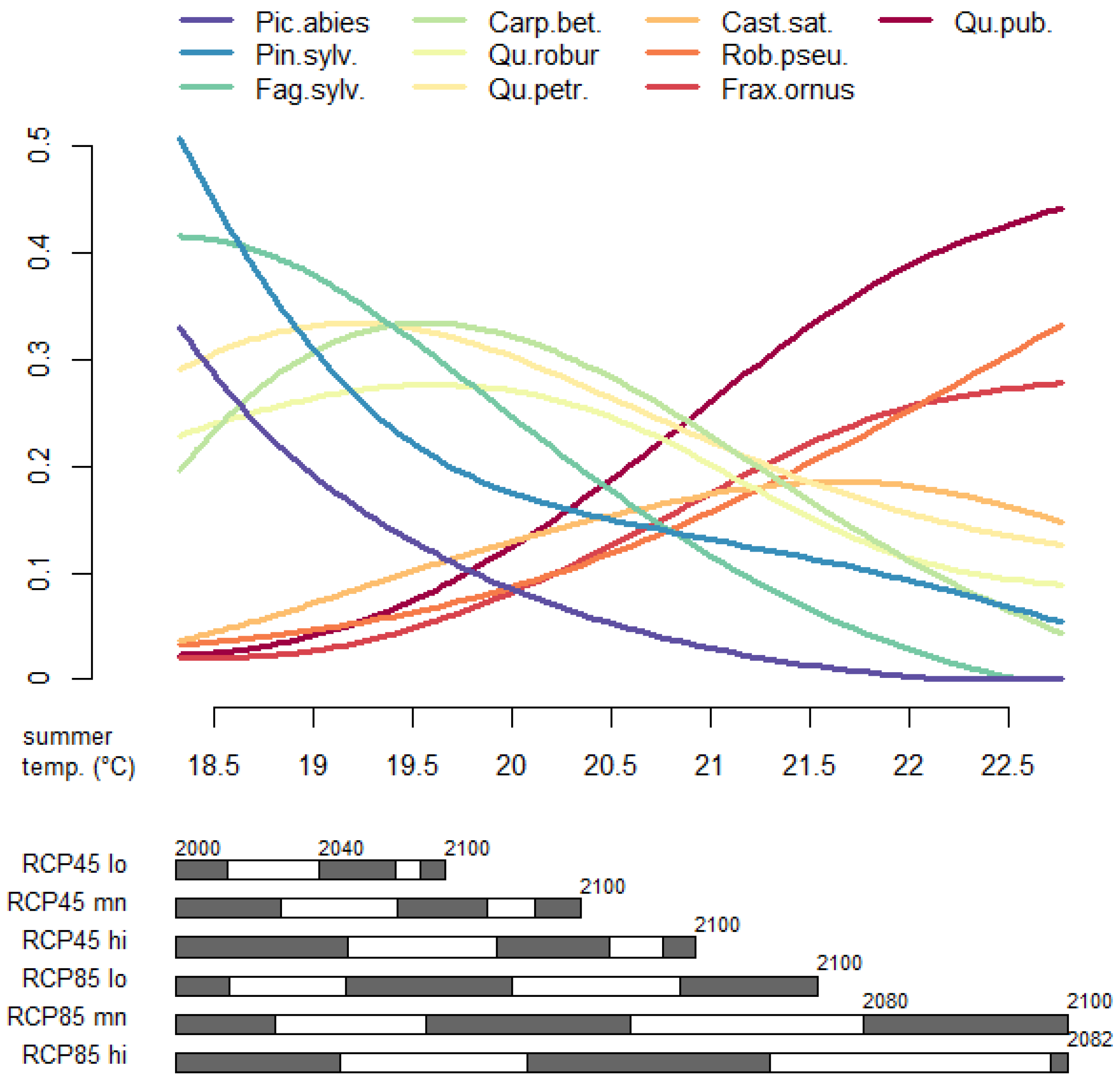
| RCP Variant | Temperature (°C) | Precipitation (mm) | |||||
|---|---|---|---|---|---|---|---|
| Year | Annual | Summer | Winter | Annual | Summer | Winter | |
| All | 2000 | 9.48 | 18.33 | 0.89 | 677 | 208 | 145 |
| RCP 4.5 low | 2100 | 11.20 | 19.67 | 3.32 | 789 | 216 | 176 |
| RCP 4.5 mean | 2100 | 11.55 | 20.35 | 3.25 | 750 | 213 | 172 |
| RCP 4.5 high | 2100 | 11.81 | 20.91 | 3.39 | 709 | 195 | 172 |
| RCP 8.5 low | 2100 | 13.08 | 21.49 | 5.6 | 836 | 209 | 223 |
| RCP 8.5 mean | 2100 | 13.85 | 22.85 | 5.85 | 797 | 190 | 214 |
| RCP 8.5 high | 2100 | 14.34 | 23.81 | 5.84 | 727 | 162 | 196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mette, T.; Brandl, S.; Kölling, C. Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions. Sustainability 2021, 13, 6522. https://doi.org/10.3390/su13126522
Mette T, Brandl S, Kölling C. Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions. Sustainability. 2021; 13(12):6522. https://doi.org/10.3390/su13126522
Chicago/Turabian StyleMette, Tobias, Susanne Brandl, and Christian Kölling. 2021. "Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions" Sustainability 13, no. 12: 6522. https://doi.org/10.3390/su13126522
APA StyleMette, T., Brandl, S., & Kölling, C. (2021). Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions. Sustainability, 13(12), 6522. https://doi.org/10.3390/su13126522





