Habitat Quality and Social Behavioral Association Network in a Wintering Waterbirds Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Assessment of Habitat Availability
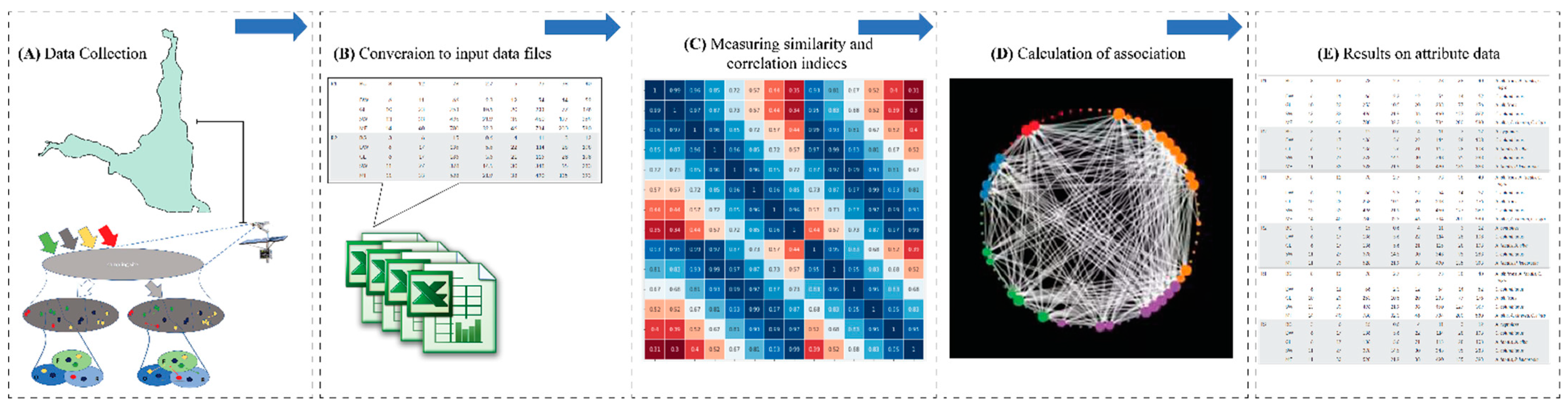
2.3. Linking Habitat Quality with Species Behavior and Network Analysis
2.4. Selection of Study Species
2.5. Data Collection on Activity-Based Habitat Selection Events
2.6. Social Behavioral Association Network Analysis
2.6.1. Aims and Assumptions
- We specifically hypothesized that:
- Species are free to be or not to be part of an MSG at any time so that they can freely select or leave a pre-occupied habitat.
- Community activity persistence solely depends upon the degree of sociality for activity synchronization within species {(i) or (j)} and between species {(i,j)}.
- Activity synchronization in species is directly proportional to the activity (foraging and roosting) of keystone species within the community.
2.6.2. SBAN Attributes
Density of the Network
Degree of the Network
2.7. Statistical Analysis
3. Results
3.1. Habitat Selection, Species Richness and Abundance Estimates
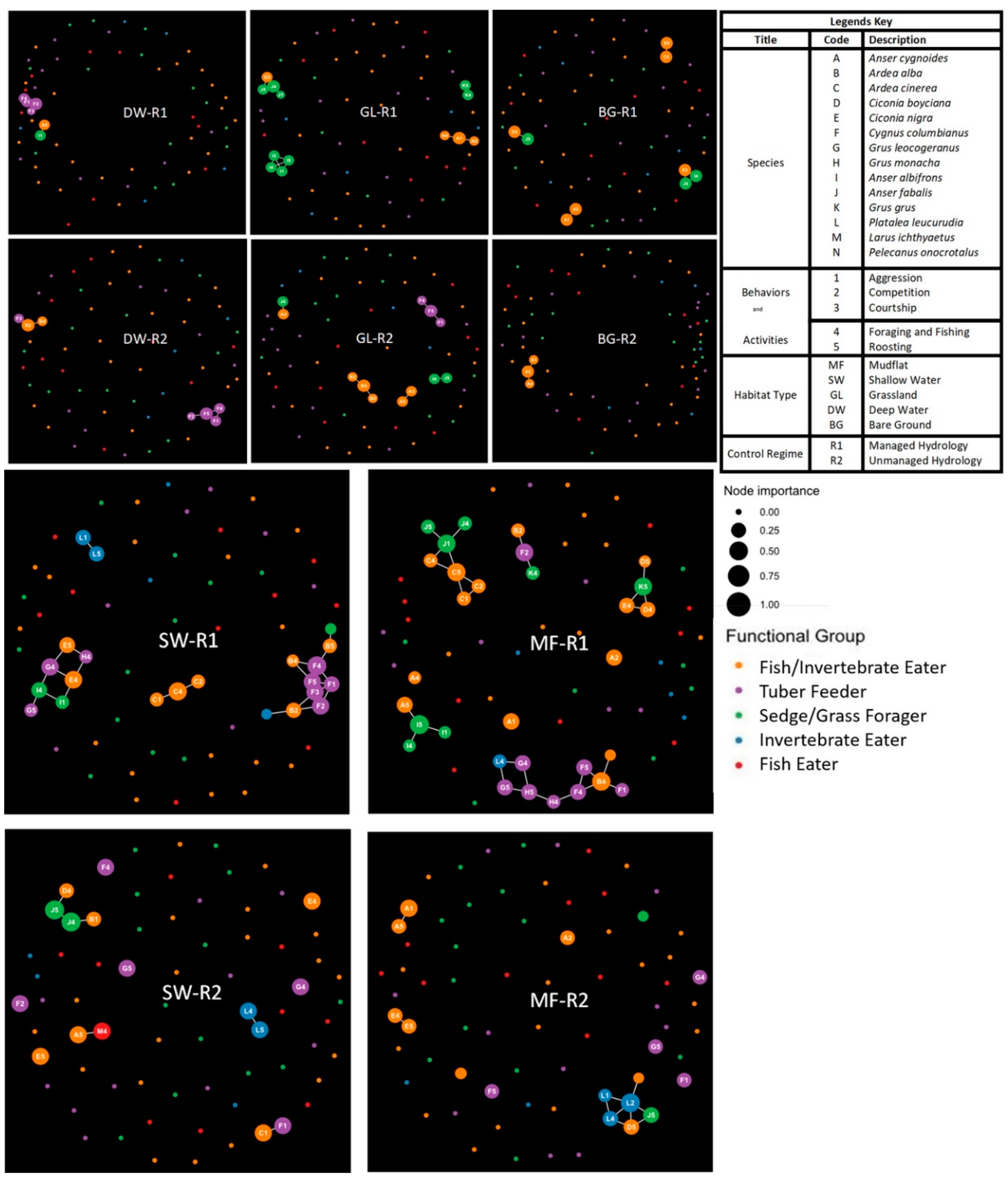
3.2. Species Keystoneness and Activity Synchrony Through Species Interaction Preference (SIP) and Behavior Interaction Preference (BIP)
3.3. Habitat Structure over Time
4. Discussion
4.1. Habitat Quality Alters Species Behavior and Network Structure
4.2. Social Interactions Alter Network Structure
4.3. Hydrology as Key Element for Structuring and Development of Wintering Habitats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Shallow Water | Deep Water | Mud Flat | Grass Land | Barren Land | Total |
|---|---|---|---|---|---|---|
| Anser albifrons | 340 | 88 | 305 | 1569 | 127 | 2429 |
| Anser cygnoides | 43 | 39 | 190 | 515 | 74 | 861 |
| Anser fabalis | 16 | N/A | 207 | 414 | 5 | 642 |
| Ardea alba | 583 | 24 | 305 | 128 | 19 | 1059 |
| Ardea cinerea | 766 | 71 | 798 | 37 | 17 | 1689 |
| Ciconia boyciana | 1 | N/A | 19 | N/A | N/A | 20 |
| Ciconia nigra | 25 | N/A | 88 | 2 | 6 | 121 |
| Cygnus columbianus | 2348 | 2045 | 1180 | 403 | 96 | 6072 |
| Grus grus | 3 | N/A | 5 | 42 | N/A | 50 |
| Grus leucogeranus | 20 | N/A | 114 | 12 | N/A | 146 |
| Grus monacha | 6 | N/A | 72 | 3 | N/A | 81 |
| Larus ichthyaetus ichthyaetus | 26 | 9 | 16 | N/A | 2 | 53 |
| Pelecanus onocrotalus | N/A | N/A | 5 | N/A | N/A | 5 |
| Platalea leucorodia | 1580 | N/A | 1409 | N/A | N/A | 2989 |
| Species | Shallow Water | Deep Water | Mud Flat | Grass Land | Barren Land | Total |
|---|---|---|---|---|---|---|
| Anser albifrons | 323 | 277 | 166 | 49 | N/A | 815 |
| Anser cygnoides | 30 | N/A | 120 | 198 | 75 | 423 |
| Anser fabalis | 24 | N/A | 83 | 138 | N/A | 245 |
| Ardea alba | 206 | 151 | 208 | 46 | N/A | 611 |
| Ardea cinerea | 342 | 101 | 426 | 5 | N/A | 874 |
| Ciconia boyciana | 4 | N/A | 6 | N/A | N/A | 10 |
| Ciconia nigra | 38 | N/A | 104 | N/A | N/A | 142 |
| Cygnus columbianus | 1366 | 2395 | 648 | 342 | 333 | 5084 |
| Grus leucogeranus | 9 | N/A | 60 | N/A | N/A | 69 |
| Larus ichthyaetus ichthyaetus | 10 | 1 | 14 | N/A | N/A | 25 |
| Platalea leucorodia | 207 | 2 | 345 | N/A | N/A | 554 |
References
- Morse, D.H. Ecological aspects of some mixed-species foraging flocks of birds. Ecol. Monogr. 1970, 40, 119–168. [Google Scholar] [CrossRef]
- Balaban-Feld, J.; Mitchell, W.A.; Kotler, B.P.; Vijayan, S.; Tov Elem, L.T.; Rosenzweig, M.L.; Abramsky, Z. Individual willingness to leave a safe refuge and the trade-off between food and safety: A test with social fish. Proc. R. Soc. B 2019, 286, 20190826. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Bascompte, J.; Adler, P.B.; Allesina, S. Beyond pairwise mechanisms of species coexistence in complex communities. Nature 2017, 546, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.T.; Bonter, D.N.; Eldermire, C.; Freeman, B.G.; Greig, E.I.; Harmon, L.J.; Lisle, C.; Hochachka, W.M.; Stephens, D. Fighting over food unites the birds of North America in a continental dominance hierarchy. Behav. Ecol. 2017, 28, 1454–1463. [Google Scholar] [CrossRef]
- Gil, M.A.; Hein, A.M.; Spiegel, O.; Baskett, M.L.; Sih, A. Social information links individual behavior to population and community dynamics. Trends Ecol. Evol. 2018, 33, 535–548. [Google Scholar] [CrossRef]
- Balasubramaniam, K.N.; Beisner, B.A.; Berman, C.M.; De Marco, A.; Duboscq, J.; Koirala, S.; Majolo, B.; MacIntosh, A.J.; McFarland, R.; Molesti, S. The influence of phylogeny, social style, and sociodemographic factors on macaque social network structure. Am. J. Primatol. 2018, 80, e22727. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, L.; Yu, C.; Cheng, L.; Xu, W.; Song, Y. Do Geese Facilitate or Compete with Wintering Hooded Cranes (Grus monacha) for Forage Resources? Diversity 2020, 12, 105. [Google Scholar] [CrossRef]
- Rasool, M.A.; Zhang, X.; Hassan, M.A.; Hussain, T.; Lu, C.; Zeng, Q.; Peng, B.; Wen, L.; Lei, G. Construct social-behavioral association network to study management impact on waterbirds community ecology using digital video recording cameras. Ecol. Evol. 2021, 11, 2321–2335. [Google Scholar] [CrossRef] [PubMed]
- Berdahl, A.; Torney, C.J.; Ioannou, C.C.; Faria, J.J.; Couzin, I.D. Emergent sensing of complex environments by mobile animal groups. Science 2013, 339, 574–576. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, O.; Crofoot, M.C. The feedback between where we go and what we know—Information shapes movement, but movement also impacts information acquisition. Curr. Opin. Behav. Sci. 2016, 12, 90–96. [Google Scholar] [CrossRef]
- Hein, A.M.; McKinley, S.A. Sensing and decision-making in random search. Proc. Natl. Acad. Sci. USA 2012, 109, 12070–12074. [Google Scholar] [CrossRef]
- Hagen, M.; Kissling, W.D.; Rasmussen, C.; De Aguiar, M.A.; Brown, L.E.; Carstensen, D.W.; Alves-Dos-Santos, I.; Dupont, Y.L.; Edwards, F.K.; Genini, J. Biodiversity, species interactions and ecological networks in a fragmented world. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 46, pp. 89–210. [Google Scholar]
- Pike, T.W.; Samanta, M.; Lindström, J.; Royle, N.J. Behavioural phenotype affects social interactions in an animal network. Proc. R. Soc. B Biol. Sci. 2008, 275, 2515–2520. [Google Scholar] [CrossRef]
- Aplin, L.M.; Farine, D.R.; Morand-Ferron, J.; Cole, E.F.; Cockburn, A.; Sheldon, B.C. Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecol. Lett. 2013, 16, 1365–1372. [Google Scholar] [CrossRef]
- Gotelli, N.J.; McCabe, D.J. Species co-occurrence: A meta-analysis of JM Diamond’s assembly rules model. Ecology 2002, 83, 2091–2096. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Creamer, R.; Hannula, S.; Van Leeuwen, J.; Stone, D.; Rutgers, M.; Schmelz, R.; De Ruiter, P.; Hendriksen, N.B.; Bolger, T.; Bouffaud, M.-L. Ecological network analysis reveals the inter-connection between soil biodiversity and ecosystem function as affected by land use across Europe. Appl. Soil Ecol. 2016, 97, 112–124. [Google Scholar] [CrossRef]
- Naeem, S. Biodiversity and ecosystem functioning in restored ecosystems: Extracting principles for a synthetic perspective. In Foundations of Restoration Ecology; Island Press: Washington, DC, USA, 2006; pp. 210–237. [Google Scholar]
- Harvey, E.; Gounand, I.; Ward, C.L.; Altermatt, F. Bridging ecology and conservation: From ecological networks to ecosystem function. J. Appl. Ecol. 2017, 54, 371–379. [Google Scholar] [CrossRef]
- Borrett, S.R. Throughflow centrality is a global indicator of the functional importance of species in ecosystems. Ecol. Indic. 2013, 32, 182–196. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Seppänen, J.-T.; Forsman, J.T.; Mönkkönen, M.; Thomson, R.L. Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 2007, 88, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Allesina, S.; Levine, J.M. A competitive network theory of species diversity. Proc. Natl. Acad. Sci. USA 2011, 108, 5638–5642. [Google Scholar] [CrossRef]
- Carter, K.D.; Brand, R.; Carter, J.K.; Shorrocks, B.; Goldizen, A.W. Social networks, long-term associations and age-related sociability of wild giraffes. Anim. Behav. 2013, 86, 901–910. [Google Scholar] [CrossRef]
- Bock, C.E.; Jones, Z.F. Avian habitat evaluation: Should counting birds count? Front. Ecol. Environ. 2004, 2, 403–410. [Google Scholar] [CrossRef]
- Mammides, C.; Chen, J.; Goodale, U.M.; Kotagama, S.W.; Goodale, E. Measurement of species associations in mixed-species bird flocks across environmental and human disturbance gradients. Ecosphere 2018, 9, e02324. [Google Scholar] [CrossRef]
- Bastolla, U.; Fortuna, M.A.; Pascual-García, A.; Ferrera, A.; Luque, B.; Bascompte, J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 2009, 458, 1018. [Google Scholar] [CrossRef] [PubMed]
- Dakin, R.; Ryder, T.B. Reciprocity and behavioral heterogeneity govern the stability of social networks. Proc. Natl. Acad. Sci. USA 2020, 117, 2993–2999. [Google Scholar] [CrossRef]
- Pinter-Wollman, N.; Hobson, E.A.; Smith, J.E.; Edelman, A.J.; Shizuka, D.; De Silva, S.; Waters, J.S.; Prager, S.D.; Sasaki, T.; Wittemyer, G. The dynamics of animal social networks: Analytical, conceptual, and theoretical advances. Behav. Ecol. 2014, 25, 242–255. [Google Scholar] [CrossRef]
- Snijders, L.; Blumstein, D.T.; Stanley, C.R.; Franks, D.W. Animal social network theory can help wildlife conservation. Trends Ecol. Evol. 2017, 32, 567–577. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.; Blüthgen, N. Integrating network ecology with applied conservation: A synthesis and guide to implementation. In Aob Plants; Oxford University Press: Oxford, UK, 2015; p. 7. [Google Scholar] [CrossRef]
- Aharon-Rotman, Y.; McEvoy, J.; Zhaoju, Z.; Yu, H.; Wang, X.; Si, Y.; Xu, Z.; Yuan, Z.; Jeong, W.; Cao, L. Water level affects availability of optimal feeding habitats for threatened migratory waterbirds. Ecol. Evol. 2017, 7, 10440–10450. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.W. Toward an ecological synthesis: A case for habitat selection. Oecologia 2003, 136, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alahuhta, J.; Toivanen, M.; Hjort, J.; Ecke, F.; Johnson, L.B.; Sass, L.; Heino, J. A comparative analysis of the species richness and taxonomic distinctness of lake macrophytes in four regions: Similarities, differences and randomness along environmental gradients. In Proceedings of the Oikos Finland Conference for Ecologists and Evolutionary Biologists, Helsinki, Finland, 31 January–1 February 2017. [Google Scholar]
- Doligez, B.; Boulinier, T.; Fath, D. Habitat selection and habitat suitability preferences. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 5, pp. 1810–1830. [Google Scholar]
- Croll, D.A.; Maron, J.L.; Estes, J.A.; Danner, E.M.; Byrd, G.V. Introduced predators transform subarctic islands from grassland to tundra. Science 2005, 307, 1959–1961. [Google Scholar] [CrossRef]
- Fath, B.D.; Asmus, H.; Asmus, R.; Baird, D.; Borrett, S.R.; de Jonge, V.N.; Ludovisi, A.; Niquil, N.; Scharler, U.M.; Schückel, U. Ecological network analysis metrics: The need for an entire ecosystem approach in management and policy. Ocean Coast. Manag. 2019, 174, 1–14. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Zhang, Y.; Zha, D.; Zhao, B.; Yang, S.; Zhang, B.; de Boer, W.F. Predicting hydrological impacts of the Yangtze-to-Huaihe Water Diversion Project on habitat availability for wintering waterbirds at Caizi Lake. J. Environ. Manag. 2019, 249, 109251. [Google Scholar] [CrossRef] [PubMed]
- Shankman, D.; Liang, Q. Landscape changes and increasing flood frequency in China’s Poyang Lake region. Prof. Geogr. 2003, 55, 434–445. [Google Scholar] [CrossRef]
- Laird, R.A.; Schamp, B.S. Does local competition increase the coexistence of species in intransitive networks. Ecology 2008, 89, 237–247. [Google Scholar] [CrossRef]
- Farine, D.R.; Aplin, L.M.; Sheldon, B.C.; Hoppitt, W. Interspecific social networks promote information transmission in wild songbirds. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142804. [Google Scholar] [CrossRef] [PubMed]
- Berger-Tal, O.; Polak, T.; Oron, A.; Lubin, Y.; Kotler, B.P.; Saltz, D. Integrating animal behavior and conservation biology: A conceptual framework. Behav. Ecol. 2011, 22, 236–239. [Google Scholar] [CrossRef]
- Pomara, L.Y.; Cooper, R.J.; Petit, L.J. Mixed-species flocking and foraging behavior of four Neotropical warblers in Panamanian shade coffee fields and forests. Auk 2003, 120, 1000–1012. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; Lindig-Cisneros, R. Feathering the scene: The effects of ecological restoration on birds and the role birds play in evaluating restoration outcomes. Ecol. Restor. 2012, 30, 116–127. [Google Scholar] [CrossRef]
- Guan, L.; Wen, L.; Feng, D.; Zhang, H.; Lei, G. Delayed flood recession in central Yangtze floodplains can cause significant food shortages for wintering geese: Results of inundation experiment. Environ. Manag. 2014, 54, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lu, C.; Xia, Y.; Shi, L.; Zuo, A.; Lei, J.; Zhang, H.; Lei, G.; Wen, L. Effects of hydrological regime on development of Carex wet meadows in East Dongting Lake, a Ramsar Wetland for wintering waterbirds. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jia, Y.; Jing, L.; Zeng, Q.; Lei, J.; Zhang, S.; Lei, G.; Wen, L. Shifts in river-floodplain relationship reveal the impacts of river regulation: A case study of Dongting Lake in China. J. Hydrol. 2018, 559, 932–941. [Google Scholar] [CrossRef]
- Lei, J.; Jia, Y.; Wang, Y.; Lei, G.; Lu, C.; Saintilan, N.; Wen, L. Behavioural plasticity and trophic niche shift: How wintering geese respond to habitat alteration. Freshw. Biol. 2019, 64, 1183–1195. [Google Scholar] [CrossRef]
- Dong, R.; Wang, Y.; Lu, C.; Lei, G.; Wen, L. The seasonality of macroinvertebrate β diversity along the gradient of hydrological connectivity in a dynamic river-floodplain system. Ecol. Indic. 2021, 121, 107112. [Google Scholar] [CrossRef]
- Lai, X.; Jiang, J.; Huang, Q. Effects of the normal operation of the Three Gorges Reservoir on wetland inundation in Dongting Lake, China: A modelling study. Hydrol. Sci. J. 2013, 58, 1467–1477. [Google Scholar] [CrossRef]
- Wiegand, T.; Naves, J.; Garbulsky, M.F.; Fernández, N. Animal habitat quality and ecosystem functioning: Exploring seasonal patterns using NDVI. Ecol. Monogr. 2008, 78, 87–103. [Google Scholar] [CrossRef]
- Woodget, A.S.; Austrums, R.; Maddock, I.P.; Habit, E. Drones and digital photogrammetry: From classifications to continuums for monitoring river habitat and hydromorphology. Wiley Interdiscip. Rev. Water 2017, 4, e1222. [Google Scholar] [CrossRef]
- Gross, F.; Etter, K.; Held, P.; Schneider von Deimling, J. Shallow water UAV based habitat monitoring of seagrass meadows in the Baltic Sea. In Proceedings of the EGU General Assembly Conference Abstracts, Online, 4–8 May 2020; p. 7510. [Google Scholar]
- Clerici, N.; Weissteiner, C.J.; Gerard, F. Exploring the use of MODIS NDVI-based phenology indicators for classifying forest general habitat categories. Remote Sens. 2012, 4, 1781–1803. [Google Scholar] [CrossRef]
- Wen, L.; Saintilan, N.; Yang, X.; Hunter, S.; Mawer, D. MODIS NDVI based metrics improve habitat suitability modelling in fragmented patchy floodplains. Remote Sens. Appl. Soc. Environ. 2015, 1, 85–97. [Google Scholar] [CrossRef]
- Ventura, D.; Bonifazi, A.; Gravina, M.F.; Ardizzone, G.D. Unmanned aerial systems (UASs) for environmental monitoring: A review with applications in coastal habitats. In Aerial Robots-Aerodynamics, Control and Applications; IntechOpen: London, UK, 2017; pp. 165–184. [Google Scholar]
- Kristan, I.; William, B. The role of habitat selection behavior in population dynamics: Source–sink systems and ecological traps. Oikos 2003, 103, 457–468. [Google Scholar] [CrossRef]
- Lindell, C.A. The value of animal behavior in evaluations of restoration success. Restor. Ecol. 2008, 16, 197–203. [Google Scholar] [CrossRef]
- Wang, C.; Dong, B.; Zhu, M.; Huang, H.; Cui, Y.-H.; Gao, X.; Liu, L.-P. Habitat selection of wintering cranes (Gruidae) in typical lake wetland in the lower reaches of the Yangtze River, China. Environ. Sci. Pollut. Res. 2019, 26, 8266–8279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, K.; Lu, C.; Awais, R.M.; Jia, Y.; Zhong, L.; Liu, P.; Dong, R.; Liu, D.; Zeng, W. Effects of origin and water depth on morphology and reproductive modes of the submerged plant Vallisneria natans. Glob. Ecol. Conserv. 2020, 24, e01330. [Google Scholar] [CrossRef]
- Wang, M.; Gu, Q.; Liu, G.; Shen, J.; Tang, X. Hydrological condition constrains vegetation dynamics for wintering waterfowl in China’s East Dongting Lake wetland. Sustainability 2019, 11, 4936. [Google Scholar] [CrossRef]
- Neumann, C.; Duboscq, J.; Dubuc, C.; Ginting, A.; Irwan, A.M.; Agil, M.; Widdig, A.; Engelhardt, A. Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 2011, 82, 911–921. [Google Scholar] [CrossRef]
- Wright, E.; Galbany, J.; McFarlin, S.C.; Ndayishimiye, E.; Stoinski, T.S.; Robbins, M.M. Male body size, dominance rank and strategic use of aggression in a group-living mammal. Anim. Behav. 2019, 151, 87–102. [Google Scholar] [CrossRef]
- Woodward, G.; Ebenman, B.; Emmerson, M.; Montoya, J.M.; Olesen, J.M.; Valido, A.; Warren, P.H. Body size in ecological networks. Trends Ecol. Evol. 2005, 20, 402–409. [Google Scholar] [CrossRef]
- Myers, P.; Espinosa, R.; Parr, C.S.; Jones, T.; Hammond, G.S.; Dewey, T.A. The Animal Diversity Web (Online). 2021. Available online: https://animaldiversity.org (accessed on 4 February 2021).
- Son, J.-K.; Sung, H.-C.; Kang, B.-H. The Study on the Selection of Suitable site for Palustrine Wetland Creation at Habitat Restoration Areas for Oriental stork (Ciconia boyciana). J. Wetl. Res. 2011, 13, 95–104. [Google Scholar]
- Zhou, L.; Xue, W.; Zhu, S.; Shan, K.; Chen, J. Foraging habitat use of oriental white stork (Ciconia boyciana) recently breeding in China. Zool. Sci. 2013, 30, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, N.; Liu, X. Habitat utilization of common crane (Grus grus) during winter in Lashi Lake, Yunnan Province. Sichuan J. Zool. 2008, 27, 356–362. [Google Scholar]
- Tortosa, F.S.; Villafuerte, R. Habitat selection by flocking wintering common cranes (Grus grus) at los Pedroches valley, Spain. Etologia 2000, 8, 21–24. [Google Scholar]
- Sullender, B.K.; Barzen, J.; Silbernagel, J. Foraging success and habitat selection of the Eurasian Spoonbill (Platalea leucorodia) at Poyang Lake, China. Waterbirds 2016, 39, 356–364. [Google Scholar] [CrossRef]
- Guo-Gang, Z.; Dong-Ping, L.; Yun-Qiu, H.; Hong-Xing, J.; Ming, D.; Fa-Wen, Q.; Jun, L.; Tian, M.; Li-Xia, C.; Zhi, X. Migration routes and stopover sites of Pallas’s Gulls Larus ichthyaetus breeding at Qinghai Lake, China, determined by satellite tracking. Forktail 2014, 30, 104–108. [Google Scholar]
- Bridges, A.S.; Noss, A.J. Behavior and activity patterns. In Camera Traps in Animal Ecology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 57–69. [Google Scholar]
- Caravaggi, A.; Banks, P.B.; Burton, A.C.; Finlay, C.M.; Haswell, P.M.; Hayward, M.W.; Rowcliffe, M.J.; Wood, M.D. A review of camera trapping for conservation behaviour research. Remote Sens. Ecol. Conserv. 2017, 3, 109–122. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Hansen, D.L.; Shneiderman, B.; Smith, M.A.; Himelboim, I. Social network analysis: Measuring, mapping, and modeling collections of connections. In Analyzing Social Media Networks with NodeXL; Elsevier: Amsterdam, The Netherlands, 2011; pp. 31–51. [Google Scholar]
- Laporta, L.; Afonso, J.; Mesquita, I. The need for weighting indirect connections between game variables: Social Network Analysis and eigenvector centrality applied to high-level men’s volleyball. Int. J. Perform. Anal. Sport 2018, 18, 1067–1077. [Google Scholar] [CrossRef]
- Laporta, L.; Afonso, J.; Mesquita, I. Interaction network analysis of the six game complexes in high-level volleyball through the use of Eigenvector Centrality. PLoS ONE 2018, 13, e0203348. [Google Scholar] [CrossRef] [PubMed]
- Cysouw, M.; Cysouw, M.M.; Matrix, D.; Suggests, M. Package ‘qlcMatrix’ 2018. Available online: https://cran.r-project.org/web/packages/qlcMatrix/qlcMatrix.pdf (accessed on 4 February 2021).
- Croft, D.P.; James, R.; Krause, J. Exploring Animal Social Networks; Princeton University Press: Woodstock, UK, 2008. [Google Scholar]
- Croft, D.P.; Krause, J.; Darden, S.K.; Ramnarine, I.W.; Faria, J.J.; James, R. Behavioural trait assortment in a social network: Patterns and implications. Behav. Ecol. Sociobiol. 2009, 63, 1495–1503. [Google Scholar] [CrossRef]
- Briatte, F. Ggnetwork: Geometries to Plot Networks with’ggplot2′; R Package Version 0.5; CCSD: Las Vegas, NV, USA, 2016; p. 1. Available online: https://cran.r-project.org/web/packages/ggnetwork/index.html (accessed on 4 February 2021).
- Kurvers, R.H.; Krause, J.; Croft, D.P.; Wilson, A.D.; Wolf, M. The evolutionary and ecological consequences of animal social networks: Emerging issues. Trends Ecol. Evol. 2014, 29, 326–335. [Google Scholar] [CrossRef]
- Sih, A.; Hanser, S.F.; McHugh, K.A. Social network theory: New insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 2009, 63, 975–988. [Google Scholar] [CrossRef]
- Shafii, B.; Price, W.J.; Minshall, G.W.; Holderman, C.; Anders, P.J.; Lester, G.; Barrett, P. Characterizing benthic macroinvertebrate community responses to nutrient addition using NMDS and BACI analyses. In Applied Statistics in Agriculture; Kansas State University: New York, NY, USA, 2013; pp. 64–79. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Valdes, M.A. Non-Metric Multidimensional Scaling (NMDS) as a Basis for a Plant Functional Group Classification and a Bayesian Belief Network Formulation for California Oak Woodlands; University of California: Davis, CA, USA, 2008. [Google Scholar]
- Cao, Y.; Hawkins, C.P. Simulating biological impairment to evaluate the accuracy of ecological indicators. J. Appl. Ecol. 2005, 42, 954–965. [Google Scholar] [CrossRef]
- Kenkel, N. On selecting an appropriate multivariate analysis. Can. J. Plant Sci. 2006, 86, 663–676. [Google Scholar] [CrossRef]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Harmon, J.P.; Barton, B.T. On their best behavior: How animal behavior can help determine the combined effects of species interactions and climate change. Ann. N. Y. Acad. Sci. 2013, 1297, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Boggie, M.A.; Collins, D.P.; Donnelly, J.P.; Carleton, S.A. Land Use, anthropogenic disturbance, and riverine features drive patterns of habitat selection by a wintering waterbird in a semi-arid environment. PLoS ONE 2018, 13, e0206222. [Google Scholar] [CrossRef] [PubMed]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals: Ecological Archives E095-178. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]
- Martin, A.E.; Fahrig, L. Habitat specialist birds disperse farther and are more migratory than habitat generalist birds. Ecology 2018, 99, 2058–2066. [Google Scholar] [CrossRef]
- Bennett, V.A.; Doerr, V.A.; Doerr, E.D.; Manning, A.D.; Lindenmayer, D.B.; Yoon, H.-J. Habitat selection and behaviour of a reintroduced passerine: Linking experimental restoration, behaviour and habitat ecology. PLoS ONE 2013, 8, e54539. [Google Scholar] [CrossRef]
- French, A.R.; Smith, T.B. Importance of body size in determining dominance hierarchies among diverse tropical frugivores 1. Biotropica J. Biol. Conserv. 2005, 37, 96–101. [Google Scholar]
- Barnagaud, J.Y.; Daniel Kissling, W.; Sandel, B.; Eiserhardt, W.L.; Şekercioğlu, Ç.H.; Enquist, B.J.; Tsirogiannis, C.; Svenning, J.C. Ecological traits influence the phylogenetic structure of bird species co-occurrences worldwide. Ecol. Lett. 2014, 17, 811–820. [Google Scholar] [CrossRef]
- Laird, R.A.; Schamp, B.S. Competitive intransitivity promotes species coexistence. Am. Nat. 2006, 168, 182–193. [Google Scholar] [CrossRef]
- Isope, P.; Dieudonne, S.; Barbour, B. Temporal organization of activity in the cerebellar cortex: A manifesto for synchrony. Ann. N. Y. Acad. Sci. 2002, 978, 164–174. [Google Scholar] [CrossRef] [PubMed]
- de Solages, C.; Szapiro, G.; Brunel, N.; Hakim, V.; Isope, P.; Buisseret, P.; Rousseau, C.; Barbour, B.; Léna, C. High-frequency organization and synchrony of activity in the Purkinje cell layer of the cerebellum. Neuron 2008, 58, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Kruse, K.L.; Collins, D.P.; Conring, C.M.; Grisham, B.A.; Conway, W.C.; Knetter, J.M. Summer habitat selection of the Lower Colorado River Valley Population of greater sandhill cranes. J. Fish Wildl. Manag. 2017, 8, 436–448. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, Y.; Guan, L.; Lu, C.; Lei, G.; Wen, L.; Liu, G. Optimising hydrological conditions to sustain wintering waterbird populations in Poyang Lake National Natural Reserve: Implications for dam operations. Freshw. Biol. 2013, 58, 2366–2379. [Google Scholar] [CrossRef]
- Xia, S.; Liu, Y.; Wang, Y.; Chen, B.; Jia, Y.; Liu, G.; Yu, X.; Wen, L. Wintering waterbirds in a large river floodplain: Hydrological connectivity is the key for reconciling development and conservation. Sci. Total Environ. 2016, 573, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, Y.; Zeng, G.; Liang, J.; Guo, S.; Huang, L.; Hua, S.; Wu, H.; Zhu, Y.; An, H. Influence of hydrological regime and climatic factor on waterbird abundance in Dongting Lake Wetland, China: Implications for biological conservation. Ecol. Eng. 2016, 90, 473–481. [Google Scholar] [CrossRef]
- Northrup, J.M.; Rivers, J.W.; Yang, Z.; Betts, M.G. Synergistic effects of climate and land-use change influence broad-scale avian population declines. Glob. Chang. Biol. 2019, 25, 1561–1575. [Google Scholar] [CrossRef]


| Spp. Code | Color Code | Description | Potential Wintering Habitat | Species |
|---|---|---|---|---|
| A |  | Fish, clam and invertebrate eaters | Marshes and swaps along Yangzi [67] | Anser cygnoides |
| B | Mudflats and freshwater marshes [67] | Ardea alba | ||
| C | Shallow waters in lakes and streams [67] | Ardea cinerea | ||
| D | Wetlands, lakes, marshes [68,69] | Ciconia boyciana | ||
| E | Marshy wetlands and rivers [67] | Ciconia nigra | ||
| F |  | Tuber feeder | Lakes, grasslands and marshes [67] | Cygnus columbianus |
| G | Marshes and shallow fresh water [67] | Grus leucogeranus | ||
| H | Mudflats, marshes and shallow water [67] | Grus monacha | ||
| I |  | Sedge/grass forager | Shallow fresh water and marshes [67] | Anser albifrons |
| J | Lakes and marshes [67] | Anser fabalis | ||
| K | Cropland and praires [70,71] | Grus grus | ||
| L |  | Invertebrate eater | Shallow water lakes [72] | Platalea leucorodea |
| M |  | Fish eater | Lakes and rivers [73] | Larus spp. |
| N | Shallow waters at freshwater lakes [67] | Pelecanus onocrotalus |
| Control Regime | Habitat | n | No. of Nodes | No. of Edges | Eigen Value | Edge Density | SIP | BIP | Competition | Aggression | Keystone Species |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Managed lakes (R1) | Bare ground | 8 | 12 | 66 | 2.163 | 0.25 | −2.46 | 0.02 | 0 | 14 | A. albifrons, A. fabalis, C. nigra |
| Deep water | 6 | 11 | 55 | 1.918 | 0.33 | 0.25 | −2.64 | 55 | 98 | C. columbianus | |
| Grass land | 10 | 23 | 253 | 2.479 | 0.95 | −1.05 | −1.66 | 71 | 126 | A. albifrons | |
| Shallow water | 13 | 33 | 528 | 4.498 | 2.6 | −1.89 | −1.58 | 53 | 238 | C. columbianus | |
| Mud flat | 14 | 40 | 780 | 3.834 | 2.9 | −1.94 | −1.39 | 98 | 176 | A. alba, A. cinerea, C. nigra | |
| Unmanaged lakes (R2) | Bare ground | 3 | 6 | 15 | 0.700 | 0.06 | 1.36 | −3.51 | 0 | 12 | A. cygnoides |
| Deep water | 6 | 17 | 136 | 2.495 | 0.73 | −1.22 | −2.08 | 119 | 177 | C. columbianus | |
| Grass land | 6 | 17 | 136 | 1.969 | 0.6 | −0.39 | −2.41 | 8 | 52 | A. fabalis, A. alba | |
| Shallow water | 11 | 27 | 351 | 2.234 | 1.18 | −2.15 | −1.55 | 82 | 128 | C. columbianus | |
| Mud flat | 11 | 33 | 528 | 3.431 | 2.05 | −1.89 | −1.77 | 107 | 78 | A. fabalis, P. leucorodea |
| Source of Variation | DF | Sum of Squares | Mean Square | p Value | Significance Level |
|---|---|---|---|---|---|
| Interaction 1 | 11 | 25,753 | 2341.2 | p = 0.0249 | * |
| Network metrics 1 | 11 | 759,260 | 69,024 | p < 0.0001 | **** |
| Lakes 1 | 1 | 5530.9 | 5530.9 | p = 0.0237 | * |
| Habitats 1 | 48 | 575,435 | 11,988 | p < 0.0001 | **** |
| Interaction 2 | 7 | 13.58 | 1.939 | p = 0.0001 | *** |
| Habitat areas between treatments 2 | 7 | 59.74 | 8.534 | p < 0.0001 | **** |
| Wintering periods 2 | 1 | 0.6547 | 0.6547 | p = 0.0285 | * |
| Habitat Areas among wintering periods 2 | 8 | 0.3665 | 0.04581 | p = 0.8281 | Not significant |
| Habitat transition between treatments 3 | 16 | 33.65 | 2.103 | p = 0.0113 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasool, M.A.; Hassan, M.A.; Zhang, X.; Zeng, Q.; Jia, Y.; Wen, L.; Lei, G. Habitat Quality and Social Behavioral Association Network in a Wintering Waterbirds Community. Sustainability 2021, 13, 6044. https://doi.org/10.3390/su13116044
Rasool MA, Hassan MA, Zhang X, Zeng Q, Jia Y, Wen L, Lei G. Habitat Quality and Social Behavioral Association Network in a Wintering Waterbirds Community. Sustainability. 2021; 13(11):6044. https://doi.org/10.3390/su13116044
Chicago/Turabian StyleRasool, Muhammad Awais, Muhammad Azher Hassan, Xiaobo Zhang, Qing Zeng, Yifei Jia, Li Wen, and Guangchun Lei. 2021. "Habitat Quality and Social Behavioral Association Network in a Wintering Waterbirds Community" Sustainability 13, no. 11: 6044. https://doi.org/10.3390/su13116044
APA StyleRasool, M. A., Hassan, M. A., Zhang, X., Zeng, Q., Jia, Y., Wen, L., & Lei, G. (2021). Habitat Quality and Social Behavioral Association Network in a Wintering Waterbirds Community. Sustainability, 13(11), 6044. https://doi.org/10.3390/su13116044









