Abstract
As natural ecosystems in most parts of the world come under increasing human influence, fragmentation is becoming the major driving factor of the global biodiversity crisis. Therefore, connectivity between habitat patches is becoming even more important. China began building national parks with the primary purpose of protecting nationally representative natural ecosystems and maintaining the integrity of their structure, processes and functions. Research is necessary to improve the internal connectivity of national parks and to propose suggestions for existing functional zoning and biological corridors. In this study, Qianjiangyuan National Park was selected as an example park, and landscape fragmentation was evaluated exponentially and simulated visually. The habitat characteristics of protected species in the region, morphological spatial pattern analysis and the delta of the probability of connectivity were used together to identify key habitat patches and their importance levels in the study area. Potential habitat corridors in the region were then obtained using least-cost path analysis and gravity modeling methods based on the distribution of key habitat and the migration costs of target species. The results of this study show that the disturbed landscape of the study area is dominated by tea plantations and drylands, with central roads being an important factor affecting the overall landscape connectivity. In terms of the distribution of key habitat patches, the mountains have a high value. In terms of area, their size is not directly proportional to their importance for maintaining landscape connectivity in the region, but large area patches are generally of higher importance. In terms of distance, key habitats that are closer to each other have a stronger correlation and a greater possibility for species migration. Combined with the functional zoning of Qianjiangyuan National Park, the setting of strictly protected areas and recreational areas is reasonable, and traditional use areas and ecological conservation areas could be appropriately adjusted according to the distribution of key habitats. The important corridor in the middle of the ecological conservation area is crucial for the overall connectivity of the national park, and the connectivity between strict protected areas will depend on successful protection of the ecological conservation area.
1. Introduction
National parks are one of the most important types of protected area in China. China has been building a national park system since 2015 with the main aim of protecting the integrity and authenticity of the natural ecosystem. The first batch of parks, including 10 pilot national parks, was completed in 2020. Qianjiangyuan National Park, which focuses on the forest ecosystem, is one of the 10 existing pilots of the national park system in China and is in Zhejiang Province, one of China’s most economically developed provinces. With rapid urban and rural development, rates of habitat loss and fragmentation have increased [1,2]. In this context, connectivity between habitat patches is becoming even more important, as it may be the only way to provide adequate habitat for wildlife populations [3].
Maintaining or restoring landscape connectivity is a widely recommended strategy for adapting to climate change, as species are changing their distributions to adapt to emerging conditions [4]. Landscape connectivity refers to the degree to which the landscape facilitates or impedes movement among source patches [5] and is one of the most critical components of animal dispersal, population persistence, and maintenance of ecological function [6]. Landscape connectivity can be considered in terms of structural connectivity and functional connectivity, with the former referring to the apparent continuity that the landscape exhibits in space, which can be determined based on various types of maps, including satellite image maps, and the latter defining landscape continuity in terms of the characteristics of the ecological object or process under study [7].
Based on existing species-based studies of landscape connectivity, it is generally accepted that landscape connectivity plays an important role in biodiversity [8], which describes the role of the landscape in facilitating or hindering the dispersal of species between habitats [9]. Species characterized by low dispersal distances are more sensitive to landscape connectivity, and the barrier effect of specific land cover is species-specific [10]. Graph theory based approaches are widely used in different areas of landscape connectivity research, such as forests, protected areas, wildlife corridor design and ecological restoration [11].
Landscape connectivity studies in national parks and protected areas have mainly focused on the connectivity of watersheds and marine protected areas in analyses of hydrological connectivity status [12,13,14,15]. Terrestrial protected area connectivity studies have assessed landscape structures, connectivity and corridors of specific species within limited areas [16,17]. However, most studies have focused on regional protected area networks or connectivity between adjacent protected areas [18,19,20,21,22,23]. China’s national parks are integrated from different types of protected areas and can be regarded as networks of protected areas on a smaller scale. Currently, the most commonly used methods for quantitative analysis of the connectivity of protected areas are the Least-cost model [24], Circuit theory [25], Graph theory [26], the Resistant kernel model [27], Reserve design [28] and the Individual-based model [29].
In this study, we identified core habitats and corridors based on the habitat and dispersal distances of particular species and assigned migration cost levels to the preferences of target species for different landscape types. We selected two species under national protection in China at the first level as habitat representatives of the species in the region, and the final core habitat and potential corridors were obtained by overlaying the two species. The aims of this study were to analyze the status of landscape connectivity in Qianjiangyuan National Park, to identify priority sites for ecological restoration and corridor construction to improve landscape connectivity in the study area, and to provide useful information for future national park planning and conservation management.
2. Methods
2.1. Study Area
Qianjiangyuan National Park is one of the first 10 pilot national parks in China and is located on the western border of Zhejiang Province, one of the most economically developed provinces in China (longitude 118°01′–118°37′ East, latitude 28°54′–29°30′ North), at the junction of the three provinces of Zhejiang, Anhui, and Jiangxi (Figure 1). The national park covers an area of approximately 252 km2, consisting of the former Qianjiangyuan National Nature Reserve, Kaihua County Forestry Farm and the connecting area between these regions, which includes 4 townships, 19 administrative villages and 72 natural villages, with a total population of 9744.
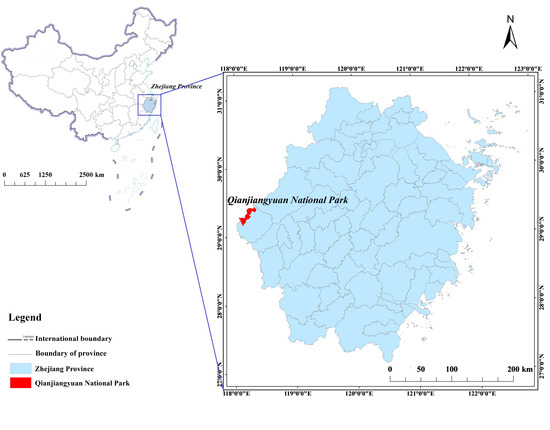
Figure 1.
Location of Qianjiangyuan National Park.
Qianjiangyuan National Park aims to protect the forest ecosystem that belongs to the low-elevation broad-leaved evergreen forest vegetation belt in the middle subtropical region, with five forest vegetation types distributed along the elevation gradient in the middle: subtropical evergreen broad-leaved forest, evergreen deciduous broad-leaved mixed forest, coniferous broad-leaved mixed forest, coniferous forest, and subalpine wetlands. The composition of flora is dominated by the typical East China flora, with transitional zone characteristics. The low elevation mid-subtropical broad-leaved evergreen forest in the national park is well preserved, representative, and typical; this type of ecosystem is rare in China and around the world, giving it global conservation value.
2.2. Data Sources
2.2.1. Landscape Distribution Map of Qianjiangyuan National Park
We used high-resolution remote sensing images taken by China’s Gaofen-1 (GF-1) satellite, which was successfully launched in 2013, as a data source. We selected high-resolution cloud-free remote sensing images (RS images) from March 2019 (resolution = 2 m, road area resolution = 0.8 m) based on the land cover characteristics of the study area. Based on ENVI 5.1 pre-processing of the images for geometric correction, full-color sharpening, cropping, and other pre-processing, followed by supervised classification and random sampling of the spots (381 sampling samples, sampling rate of 20%), the accuracy of the interpretation samples was tested by combining the Google Earth electronic map and field survey sample points. The accuracy of the interpretation layer and the image were checked for the degree of coincidence, missing judgment and misjudgment. The interpretation accuracy was >95%, and the minimum feature size of the map was 1000 m2, which met the accuracy requirements of this study (The remote sensing image interpretation and accuracy test of interpretation results in this study were commissioned by 21st Century Space Technology Co., LTD., Beijing, China). The landscape of Qianjiangyuan National Park is classified into 24 categories based on the habitat type and land cover characteristics of the target species: natural arboreal forest, shrubland, bamboo forest, other woodland, natural grassland, nursery land, tea plantation, pond, dry land, bare land, highway, road land, bridge, trail, urban residential land, rural residential land, detached house, house under construction, land for hydraulic construction, ditch, other construction land, reservoir, river and lake.
2.2.2. Vegetation Type Distribution Map of Qianjiangyuan National Park
The forest resources survey data for Qianjiangyuan National Park in 2017 were provided by the Zhejiang Forestry Resources Monitoring Center and used to determine the distribution of forest types, including broadleaf forest, coniferous forest, mixed coniferous forest, bamboo forest, shrubland, grassland, cultivated land, and non-forest land.
2.2.3. Habitat Identification and Dispersal Distance Threshold of Target Species
In this study, the analysis of landscape connectivity was based on the habitat characteristics of the target species in the study area. In landscape connectivity assessment, the selection and dispersal distance of the target species is crucial [30]. The landscape connectivity of protected species can guide the management of protected areas. Dispersal distance is a key process in determining distance thresholds and is species-specific. There are two species in Qianjiangyuan National Park that are important for protection at the national level in China, namely Elliot’s Pheasant (Syrmaticus ellioti) and the Black Muntjac (Muntiacus crinifrons), both of which are also CITES Appendix I listed species. In this study, a landscape connectivity analysis was conducted based on the dispersal distance threshold and habitat selections of these two species.
Elliot’s Pheasant is a typical ground-dwelling forest resident bird that can inhabit broad-leaved forests, coniferous forests, bamboo forests, and short-term shrublands close to forests, with evergreen or deciduous broad-leaved forests being the most suitable habitat [31]. Li [32] observed that the winter dispersal distance of Elliot’s Pheasant can span two or three hills with a diameter of 1.5–2 km, while Shi and Zheng [33] tracked the spring dispersal process over a long distance of more than 3 km using radio telemetry. Peng and Ding [34] used telemetry to determine the spring breeding dispersal linear distance to be 1.5–2.1 km. Zhang [35] used telescopes to observe and study the behavior of Elliot’s Pheasant during wilderness training using radio telemetry tracking equipment, GPS, compass, telescopes, and other equipment, and an analysis of the behavior of Elliot’s Pheasants released after wilderness training determined the dispersal distance to be 0.2–3.0 km.
The habitat of the Black Muntjac includes broadleaf forest, mixed coniferous forest, coniferous forest, scrub, and bamboo forest, but mixed coniferous forest and broadleaf forest are preferred [36]. The dispersal distance of the Black Muntjac is poorly studied, and its activity patterns are territorial, generally involving movement within the domain, with some individuals moving up to 2.5–5.0 km [37,38]. Based on the literature described above, we set a dispersal threshold of 3.0 km for Elliot’s Pheasant and 5.0 km for the Black Muntjac.
2.3. Data Processing
2.3.1. Landscape Fragmentation Analysis of Qianjiangyuan National Park
With reference to relevant studies [39], natural arboreal forests, shrublands, natural grasslands, bamboo forests, other woodlands, bare lands, rivers, lakes and reservoirs in Qianjiangyuan National Park are classified as protected landscapes, whereas tea plantations, nursery lands, dry lands, highways, road lands, trails, bridges, ponds, ditches, rural residential lands, urban residential lands, detached houses, houses under construction, lands for hydraulic construction and other construction lands are classified as non-protected landscapes. The area percentage (P), area weighted mean plaque fractal dimension (AWMPFD), fragmentation index (F) and relative agglomeration (Con of protected landscape patches were calculated to analyze the spatial characteristics of protected landscape patches at the landscape level and as a whole. The formulae for calculating each landscape index are shown in Table 1.

Table 1.
Landscape index calculation formulae.
Fragmentation reduces patch area and increases the number of patches, so the average patch area decreases [30]. Therefore, we used the average patch area to quantify fragmentation. The remote sensing images were cropped into grids (500 m × 500 m) using the Fishnet tool in ArcGIS. The area and number of patches in each grid were counted, and the average patch area was calculated to indicate the degree of fragmentation of the landscape.
2.3.2. Key Habitat and Connectivity Analysis of Qianjiangyuan National Park
This study refers to the method of combining morphological spatial pattern analysis (MSPA) and the delta of the probability of connectivity (dPC) adopted by Guo et al. [30] to identify the habitats in Qianjiangyuan National Park. First, according to the habitat types of Elliot’s Pheasant and the Black Muntjac, as well as the relevant literature, the areas where vegetation, elevation, and slope matched the habitat selection of the two species, respectively, were selected as potential habitat areas in Qianjiangyuan National Park, and the study area was reclassified into foreground (potential habitat) and background (all other areas) to obtain a binary map of potential and non-potential habitats. A MSPA was then conducted in the GuidosToolbox software (https://forest.jrc.ec.europa.eu/en/activities/lpa/gtb/) (accessed on 21 May 2021) to reclassify the landscape into seven categories based on morphological features: core, islet, loop, bridge, perforation, edge, and branch [40]. The edge width was set to five pixels, and “core” areas were extracted to identify habitats under the eight-neighbor rule. Unlike traditional methods that focus on the area or importance of individual patches without considering the overall landscape connectivity, this method provides four- or eight-neighbor rules because the connectivity analysis is performed on a raster grid, which allows for automatic classification based on pixel-level geometric concepts.
The probability of connectivity (PC) is an area-based functional connectivity method that is well suited to identify key elements that maintain overall habitat connectivity to quantitatively describe landscape connectivity and identify patches with important connectivity [41]. The delta of PC (dPC) can be used to calculate the contribution of each patch to the overall connectivity of the ecological network. The formula for the calculation is as follows:
where and refer to the area of habitat i and j, respectively; the strength of the connection between any pair of patches is denoted by , which describes the ease of dispersion between patches i and j; and AL refers to area of the national park. Conefor Sensinode 2.6 software (http://www.conefor.org/) (accessed on 21 May 2021) was used to calculate dPC values. According to the different dispersal abilities of the target species, the dispersal distance thresholds were set at 3.0 km and 5.0 km for Elliot’s Pheasant and the Black Muntjac, respectively. If the distance between two patches was within the threshold, we set 0.5 as the probability of dispersion between patches [42]. Finally, the top 10 patches with the highest dPC values were selected as key habitats.
2.3.3. Potential Habitat Corridor Analysis of Qianjiangyuan National Park
Each pair of key habitats can be connected to each other by a least-cost path based on a least-cost model [6]. Least-cost paths are often used to optimize grid modules [30], and the grid’s resistance value describes its facilitating or hindering effect on species dispersal processes. Resistance values are attached to each land cover cell to calculate the connectivity between two habitats [43]. Therefore, using cost-path analysis in ArcGIS to calculate the path of least resistance for organisms moving along key habitat patches, potential corridors between key habitats can be obtained.
Resistance values for different landscape types were key factors that influenced the results. Based on the published literature related to the habitat selection of Elliot’s Pheasant and the Black Muntjac in the study area, the five resistance indicators of vegetation type, elevation, slope, distance from roads, and distance from settlements were selected to summarize the selection of different habitats by the target species in the literature and to assign habitat levels to them. The golden divide method [44] was used to assign resistance coefficients to each habitat level, construct cost surfaces for different resistance indicators based on the resistance coefficients, and superimpose the cost surfaces for each resistance indicator to construct a composite cost surface that was used in the least-cost path analysis to identify potential corridors for this study. We used the top 10 patches with the highest dPC values as key habitat areas and used the gravity model to identify general and important corridors in the potential corridors. The gravity model was used to quantitatively evaluate the interaction force between the source and the target [45], with a greater force indicating a more important corridor. Therefore, the relative importance of the corridor was evaluated as follows.
is the interaction force between core patches a and b, and are the weight values of the two patches, is the standardized value of the potential corridor resistance between patches a and b, is the resistance value of patch a, is the area of patch a, is the cumulative resistance value of the corridor between patches a and b, and is the maximum value of the cumulative resistance of all the corridors in the study area.
In this study, we constructed an interaction matrix between 10 key habitats based on the gravity model, and corridors with an interaction force greater than 0.1 were extracted according to the matrix evaluation results as important corridors, with the rest designated as general corridors. The general corridors, important corridors and key habitats were superimposed to construct a network of key habitats and corridors in Qianjiangyuan National Park.
3. Results
3.1. Analysis of Landscape Fragmentation
The landscape type distribution map of Qianjiangyuan National Park was obtained after interpreting the high-resolution remote sensing image to extract land cover information (Figure 2). Table 2 shows the landscape classification results, in which natural arboreal forest is the largest area, accounting for 78.37% of the total area of the national park, and other major landscape types include shrubs (accounting for 3.97%), bamboo forest (accounting for 2.85%), tea plantations (accounting for 7.41%), and dry lands (accounting for 4.39%).
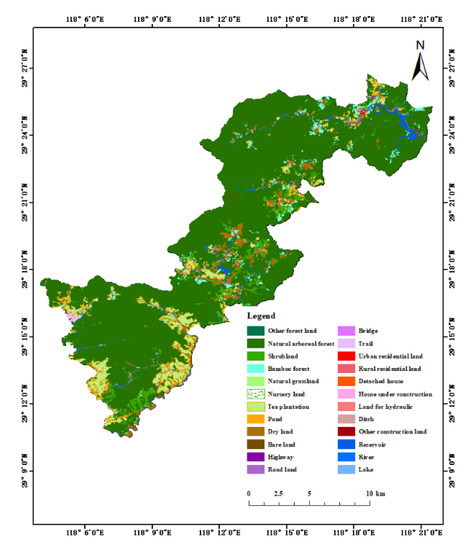
Figure 2.
Distribution of landscape in Qianjiangyuan National Park.

Table 2.
Area and proportion of different landscape categories of Qianjiangyuan National Park.
In Qianjiangyuan National Park, natural arboreal forests, shrublands, natural grasslands, bamboo forests, other woodlands, bare lands, rivers, lakes and reservoirs are classified as protected landscapes, whereas tea plantations, nursery lands, dry lands, highways, road lands, trails, bridges, ponds, ditches, rural residential lands, urban residential lands, detached houses, houses under construction, lands for hydraulic construction, and other construction lands are classified as non-protected landscapes. The distribution of protected and non-protected landscapes is shown in Figure 3. The average patch area of the protected landscapes was used to roughly simulate landscape fragmentation. As shown in Figure 4, the central and outer edge portions of the national park are more severely fragmented in comparison with other areas. The four landscape indices calculated for the proportion of protected landscape area (P), area weighted mean plaque fractal dimension (AWMPFD), fragmentation index (F), and relative aggregation (C′) are shown in Table 3.
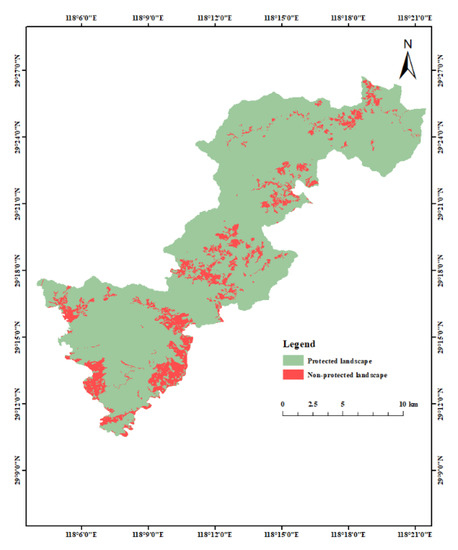
Figure 3.
Distribution of protected and unprotected landscapes in Qianjiangyuan National Park.
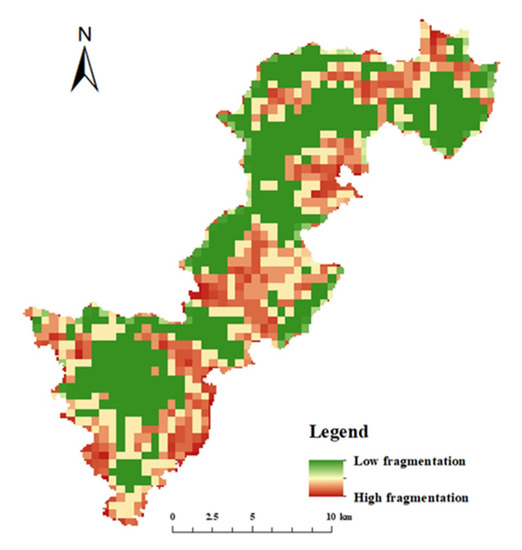
Figure 4.
Landscape fragmentation analysis of Qianjiangyuan National Park.

Table 3.
Landscape indexes in Qianjiangyuan National Park.
3.2. Morphological Spatial Pattern Analysis
Areas where land cover/vegetation type, elevation and slope conditions matched the habitat conditions were extracted based on the habitat characteristics of Elliot’s Pheasant and the Black Muntjac (Table 4). The suitable (including most suitable, sub-preferred and generally suitable) vegetation types for Elliot’s Pheasant are broad-leaved forest, coniferous forest, mixed forest, bamboo forest, shrub forest and farmland at an elevation of 200m, slope ≤50°, >700 m distance from roads and >700 m distance from settlements. Suitable vegetation types for Black Muntjac are broad-leaved forest, mixed-coniferous forest, coniferous forest and shrub forest, at altitudes 600 m, with slopes of ≤45°, ≥50 m distance from roads and ≥200 m distance from settlements. The habitat characteristics are shown in Figure 5.

Table 4.
Potential habitat range of Elliot’s Pheasant and Black Muntjac.
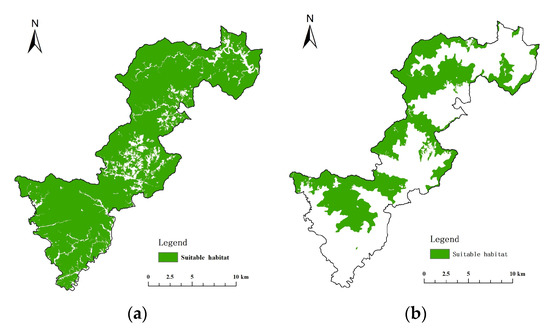
Figure 5.
Suitable habitat distribution for Elliot’s Pheasant and Black Muntjac. (a) Suitable habitat for Elliot’s Pheasant (b) Suitable habitat for Black Muntjac.
GuidosToolbox (https://forest.jrc.ec.europa.eu/en/activities/lpa/gtb/) (accessed on 21 May 2021) was applied to analyze the morphological features of the habitat map to reclassify the suitable habitats of Elliot’s Pheasant and Black-fronted Muntjac into seven categories: core, edge, perforation, bridge, loop, branch, and islet (Figure 6). In comparison with Elliot’s Pheasant, the Black Muntjac had fewer patches and fewer areas due to its habitat requirements, but both species were found to be relatively evenly distributed.
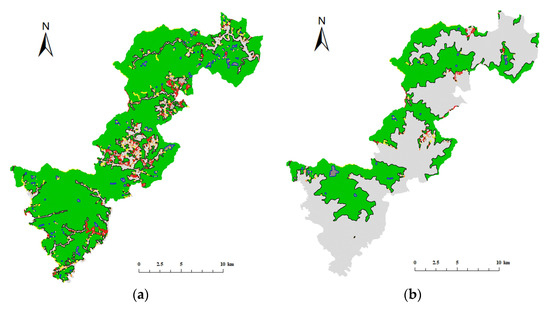
Figure 6.
Morphological spatial pattern analysis of Qianjiangyuan National Park. (a) MSPA results for Elliot’s Pheasant (b) MSPA results for Black-fronted Muntjac.
3.3. The Delta of the Probability of Connectivity Analysis
The core area from the morphological spatial pattern analysis was extracted, and the top 30 patches were selected in descending order according to the size of the area to generate the node files and link files required for the connectivity probability calculation, with different dispersal distances considered in the calculation (Elliot’s Pheasant = 3 km, Black Muntjac = 5 km). The delta of the probability of connectivity (dPC) for the 30 core patches was calculated using Conefor Sensinode2.6 software (http://www.conefor.org/) (accessed on 21 May 2021) (Table 5). The top 10 patches with dPC values were selected as key habitats to obtain a map of key habitats importance ranking (Figure 7).

Table 5.
Top 10 core habitat dPC vs. area.
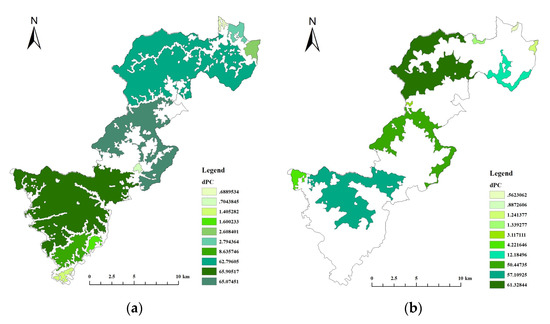
Figure 7.
Map of the top 10 core habitat importance levels. (a) Elliot’s Pheasant (b) Black Muntjac.
As shown in Table 5, the habitat patches that contributed most to maintaining landscape connectivity were not the largest area patches, and the size of the key habitat area for each target species was not proportional to its role in maintaining landscape connectivity in the national park, but the importance of large area patches was higher for both species, so the size of the key habitat was important for maintaining landscape connectivity in the region. The strict protected areas of Qianjiangyuan National Park are all located within large areas of habitat. Although there are strong interactions between a few small patches in the south with a high potential for species migration, these patches are small and are not key habitat areas that support the entire national park.
3.4. Analysis of Potential Habitat Corridors
The key habitat patches were transformed into particles, the minimum cost distance and cost back link between each particle were calculated, and then cost path analysis was performed. The cost raster data were crucial for this step, and the cost surface for each resistance indicator was constructed by determining the cost values of five resistance indicators: land cover/vegetation, elevation, slope, distance from road, and distance from residence (Figure 8 and Figure 9). Table 6 and Table 7 show the resistance and costs for each resistance indicator set at different levels for Elliot’s Pheasant and Black Muntjac, respectively, after reference to the relevant literature values. The final overlay yielded the respective cost raster data for Elliot’s Pheasant and Black Muntjac (Figure 10). Potential corridors between key habitats were obtained by cost-path analysis of 10 key habitats (Figure 11).
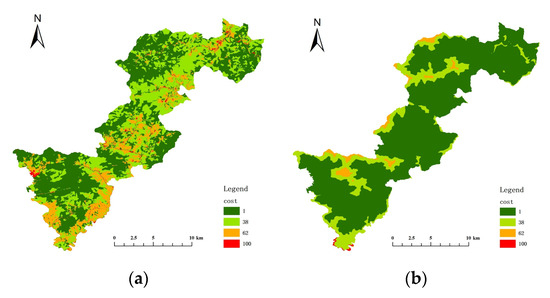
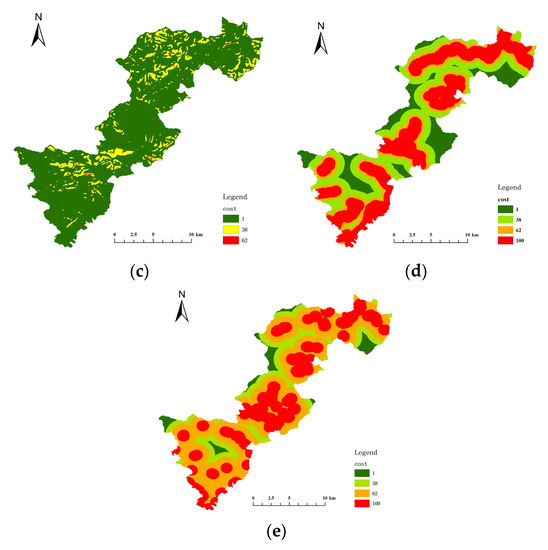
Figure 8.
Cost surface of each resistance indicator for Elliot’s Pheasant. (a) Cost of vegetation (b) Cost of elevation (c) Cost of slope (d) Cost of distance from road (e) Cost of distance from residential areas.
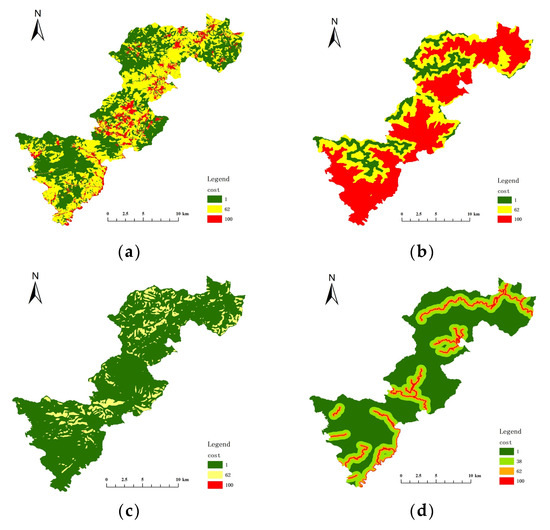
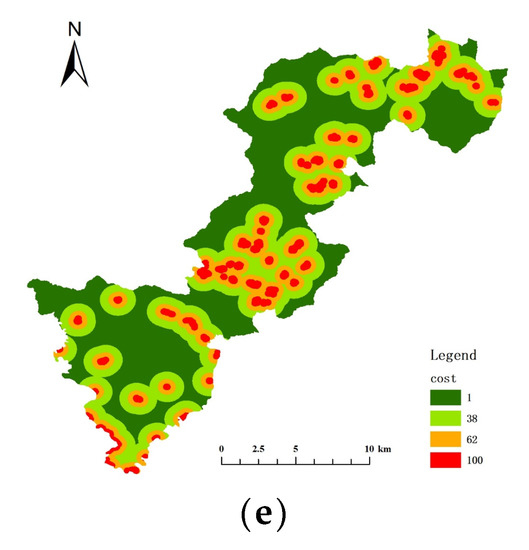
Figure 9.
Cost surface of each resistance indicator for the Black Muntjac. (a) Cost of vegetation (b) Cost of elevation (c) Cost of slop (d) Cost of distance from road (e) Cost of distance from residential areas.

Table 6.
Resistance and cost values of each resistance indicator for Elliot’s Pheasant.

Table 7.
Resistance and cost values of each resistance indicator for the Black Muntjac.
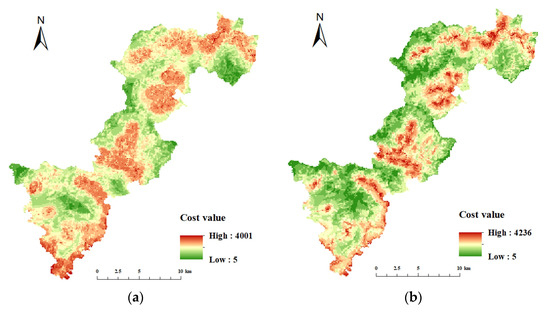
Figure 10.
Cost raster maps of Elliot’s Pheasant and the Black Muntjac in Qianjiangyuan National Park. (a) Cost raster of Elliot’s Pheasant (b) Cost raster of the Black Muntjac.
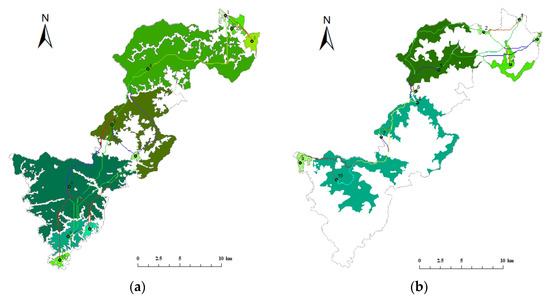
Figure 11.
Distribution of key habitats and potential corridors in Qianjiangyuan National Park. (a) Elliot’s Pheasant (b) Black Muntjac.
3.5. Key Habitat and Important Corridor Network Analysis
The interaction matrix between the 10 key habitats was constructed using a gravity model (Table 8 and Table 9). According to the matrix evaluation results, corridors with an interaction force greater than 0.1 for Elliot’s Pheasant were considered to be important corridors, and the rest were designated as general corridors (Figure 12 and Figure 13). The key habitats and corridors of the two species were overlaid to construct a network of key habitats and corridors in Qianjiangyuan National Park (Figure 14).

Table 8.
Gravity model-based interaction matrix of key habitat interactions for Elliot’s Pheasant.

Table 9.
Gravity model-based interaction matrix of key habitat interactions for the Black Muntjac.
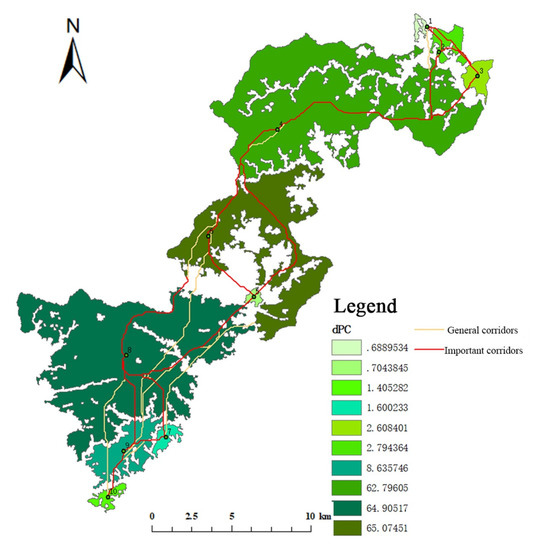
Figure 12.
Distribution of key habitats and corridors of Elliot’s Pheasant in Qianjiangyuan National Park.
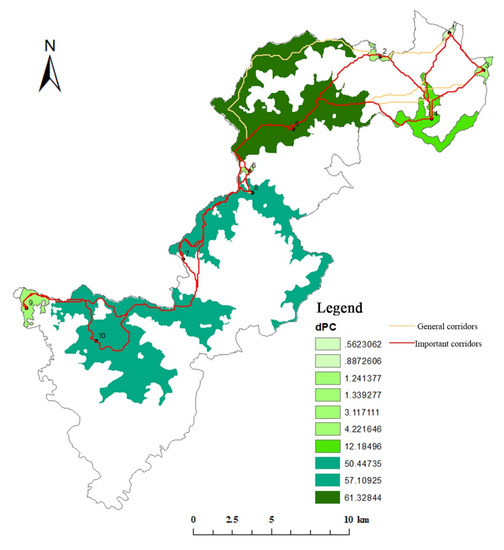
Figure 13.
Distribution of key habitat and corridors of the Black Muntjac in Qianjiangyuan National Park.
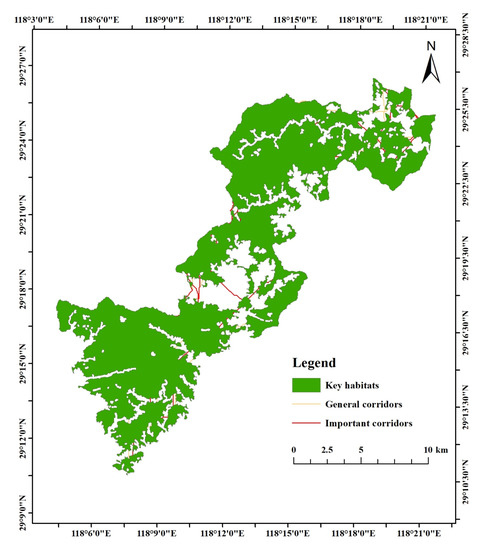
Figure 14.
Network map of key habitats and corridors in Qianjiangyuan National Park.
4. Discussion
Landscape fragmentation in Qianjiangyuan National Park occurs in the northern and southern edges where human disturbance is concentrated, and in the central part of the park. The disturbed landscapes in the southern and northern edges are dominated by tea plantations, while the central disturbed landscapes are dominated by drylands. Based on the distribution of key habitats, fragmentation and the distribution of corridors, the major roads in the central part of the national park are one of the main factors influencing the overall landscape connectivity in the region. Roads have also been highlighted in other relevant studies as major causes of landscape fragmentation and barriers to biological movement, resulting in reduced overall landscape connectivity for many native species [32].
The strictly protected areas are appropriately zoned settings for habitat protection, whereas the recreational areas are almost all within non-key habitats, which is reasonable. However, approximately half of the traditional use areas are located in key habitats, especially near the lower outer edges of the park, and excessive human intervention during recreational activities should be avoided in these areas. The ecological conservation area is the most widely distributed area within Qianjiangyuan National Park, and most potential corridors are located in this area, which is highly protected by strict Chinese laws and regulations, which favor the restoration of potential corridor areas. There are a number of distinct potential corridors in the ecological conservation area that are important for improving the overall connectivity of the national park, and due to their location close to traditional use areas, i.e., human disturbances such as settlements and roads, it is also particularly important to consider enhancing conservation management in this area to elevate its conservation status [46,47]. Qianjiangyuan National Park has three separate strictly protected areas located in the northern and southern regions of the park, so connectivity between these strictly protected areas is dependent on successful protection of the conservation area, highlighting the importance of protecting potential corridors in the central part of the park.
Functional zoning is a commonly accepted approach to national park planning and management. Previously, functional zoning schemes for national parks and protected areas have been based on the current status of natural resource characteristics and species distribution or developed by considering the compatibility of land use and landscape features or planning, or established by adjusting functional zones from the perspective of multiple stakeholders [48,49,50,51,52,53]. Habitat connectivity is severely affected by types of human activities and infrastructure that are rarely considered. Few habitat corridor studies have been conducted to support the zoning design of national parks. However, relevant studies have shown that interconnected habitat areas are critical for biodiversity conservation, especially in the face of climate change [54]. Therefore, it is important to consider habitat corridors in zoning design and as parts of functional zones. Noss and Harris proposed a conceptual model of core areas connected by corridors as a means of long-term conservation of protected area species, and their model can also be applied to zoning within protected areas [55]. Compared with the current functional zoning method of national parks which only considers the distribution of natural resources, this study provides support for the zoning design of national parks based on landscape connectivity and corridor design, which can improve the conservation efficiency of national parks.
5. Conclusions
This study analyzed landscape fragmentation in Qianjiangyuan National Park and identified key habitats and important corridors. We found that:
- (1)
- Roads, settlements, and cultivated land have a significant impact on the landscape connectivity of Qianjiangyuan National Park, with roads being one of the main reasons for the fragmentation of the overall landscape. We recommend that several potential corridors in the center of the park that connect key habitats on both sides of the road be protected to help link habitat patches, mitigate the impact of the road, and appropriate vegetation restoration and reforestation of tea plantations and drylands in the study area will increase the landscape connectivity of Qianjiangyuan National Park.
- (2)
- The area of each patch of key habitat is not proportional to its contribution to the landscape connectivity of Qianjiangyuan National Park, but the size of key habitats is important for maintaining landscape connectivity. At the landscape scale, large habitat patches of high importance should be prioritized for protection to promote habitat connectivity and species conservation in the study area. At the same time, groups of small patches with a high potential for species migration should also be protected as a whole to avoid further fragmentation or even area loss.
- (3)
- The locations and boundaries of strict protected areas and recreational areas of Qianjiangyuan National Park are relatively reasonable, and the scope of ecological conservation areas and traditional use areas could be adjusted to better match the distribution of key habitats. Special attention must be focused on protecting and managing ecological conservation areas because of the pressure of human disturbance around the area. However, at the same time, there are several important corridors in the area, and the connectivity between strictly protected areas depends on successful protection of ecological conservation areas.
Using high-resolution remote sensing image data, the landscape connectivity of different types of connected protected landscapes was analyzed as an integrated mosaic at the scale of an individual national park. In comparison with the more commonly used method of functional zoning of national parks, in which only the distribution of natural resources is considered, this study used detailed habitat characteristics to grade resistance and analyze landscape connectivity and corridors to support the zoning design of national parks. This method can improve the conservation effectiveness of national parks by ensuring that ecological connections are maintained and strengthened where they exist and restored where they are lost.
While this study used two detailed habitat selections in the habitat corridor analysis and graded the resistance produced by different species based on landscape categories, it did not consider the region’s plant migration characteristics, the connectivity of aquatic organisms in the forest ecosystem, or the migration characteristics of the region’s less studied and less data-accessible species, such as insects, amphibians, reptiles, etc. In addition, this study only analyzed and optimized connectivity recommendations within the study area, and further ecosystem integrity and connectivity analyses can be conducted in conjunction with surrounding potential habitat patches to make useful recommendations for range optimization in Qianjiangyuan National Park. The current study assumes that highways, railroads, and national roads have a segmentation effect on habitats for connectivity analysis, but the form of road construction varies in different regions of China, especially in mountainous areas, where bridges and tunnels are more common, and the extent of impact on habitats is not clear, so the calculations in this study may underestimate the connectivity in some areas. It would be useful to analyze the impacts of different road types on the park ecosystem in future research.
Author Contributions
G.C. planned the project. Y.P. undertook most of the literature review and analyzed the data. Y.P. and M.M. wrote the manuscript. R.W. and Z.H. contributed additional literature and contributed substantially to modifying the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Technologies Research and Development Program of China [grant number 2018YFC0507203] and Shenzhen One Planet Foundation Research Plan [grant number ORS000-OPF024FRT/2018-1.3.02.01].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to the Zhejiang Forestry Resources Monitoring Center and the Qianjiangyuan National Park Administration for providing some of the relevant data for this study, and to the World Wide Fund for Nature (Beijing Office) for providing partial financial support for this project.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Ancillotto, L.; Bosso, L.; Conti, P.; Russo, D. Resilient responses by bats to a severe wildfire: Conservation implications. Anim. Conserv. 2020. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, D.; Wu, H.; Wang, J.; Li, S. Assessing the role of high-speed rail in shaping the spatial patterns of urban and rural development: A case of the Middle Reaches of the Yangtze River, China. Sci. Total Environ. 2020, 704, 135399. [Google Scholar] [CrossRef] [PubMed]
- Jordán, F. Adding function to structure—comments on Palmarola landscape connectivity. Community Ecol. 2001, 2, 133–135. [Google Scholar] [CrossRef]
- Magness, D.; Sesser, A.; Hammond, T. Using topographic geodiversity to connect conservation lands in the Central Yukon, Alaska. Landsc. Ecol. 2018, 33, 547–556. [Google Scholar] [CrossRef]
- Taylor, P.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity Is a Vital Element of Landscape Structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef]
- Adriaensen, F.; Chardon, J.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulinck, H.; Matthysen, E. The application of ‘least-cost’ modelling as a functional landscape model. Landsc. Urban Plan. 2003, 64, 233–247. [Google Scholar] [CrossRef]
- Wu, J. Landscape Ecology: Pattern, Process, Scale and Hierarchy, 2nd ed.; Higher Education Press: Beijing, China, 2007; ISBN 7-04-020879-2. [Google Scholar]
- He, J.; Huang, J.; Liu, D.; Wang, H.; Li, C. Updating the habitat conservation institution by prioritizing important connectivity and resilience providers outside. Ecol. Indic. 2018, 88, 219–231. [Google Scholar] [CrossRef]
- Cook, E.A. Landscape structure indices for assessing urban ecological networks. Landsc. Urban Plan. 2002, 58, 269–280. [Google Scholar] [CrossRef]
- Minor, E.S.; Lookingbill, T.R. A multiscale network analysis of protected-area connectivity for mammals in the United States. Conserv. Biol. 2010, 24, 1549–1558. [Google Scholar] [CrossRef]
- Machado, R.; Godinho, S.; Guiomar, N.; Gil, A.; Pirnat, J. Using graph theory to analyse and assess changes in Mediterranean woodland connectivity. Landsc. Ecol. 2020, 35, 1291–1308. [Google Scholar] [CrossRef]
- O’Leary, B.C.; Roberts, C.M. Ecological connectivity across ocean depths: Implications for protected area design. Glob. Ecol. Conserv. 2018, 15, e00431. [Google Scholar] [CrossRef]
- Job, N.; Roux, D.J.; Bezuidenhout, H.; Cole, N.S. A Multi-Scale, Participatory Approach to Developing a Protected Area Wetland Inventory in South Africa. Front. Environ. Sci. 2020, 8, 49. [Google Scholar] [CrossRef]
- Roberts, K.; Cook, C.; Beher, J.; Treml, E. Assessing the current state of ecological connectivity in a large marine protected area system. Conserv. Biol. 2020, 35, 699–710. [Google Scholar] [CrossRef]
- Cruz-Vazquez, C.; Rioja-Nieto, R.; Enriquez, C. Spatial and temporal effects of management on the reef seascape of a marine protected area in the Mexican Caribbean. Ocean Coast. Manag. 2019, 169, 50–57. [Google Scholar] [CrossRef]
- Amaral, Y.; Santos, E.; Ribeiro, M.; Barreto, L. Landscape structural analysis of the Lençóis Maranhenses National Park: Implications for conservation. J. Nat. Conserv. 2019, 51, 125725. [Google Scholar] [CrossRef]
- Lima, F.G.; Diniz, M.F.; Mendes, P. Ranking habitat importance for small wildcats in the Brazilian savanna: Landscape connectivity as a conservation tool. Biologia 2021, 76, 1–11. [Google Scholar] [CrossRef]
- Freeman, B.; Roehrdanz, P.R.; Peterson, A.T. Modeling endangered mammal species distributions and forest connectivity across the humid Upper Guinea lowland rainforest of West Africa. Biodivers. Conserv. 2018, 28, 671–685. [Google Scholar] [CrossRef]
- Bargelt, L.; Fortin, M.J.; Murray, D.L. Assessing connectivity and the contribution of private lands to protected area networks in the United States. PLoS ONE 2020, 15, e0228946. [Google Scholar] [CrossRef]
- Stewart, F.; Darlington, S.; Volpe, J.P.; Mcadie, M.; Fisher, J.T. Corridors best facilitate functional connectivity across a protected area network. Sci. Rep. 2019, 9, 10852. [Google Scholar] [CrossRef]
- Saura, S.; Bertzky, B.; Bastin, L.; Battistella, L.; Dubois, G. Global trends in protected area connectivity from 2010 to 2018. Biol. Conserv. 2019, 238, 1–8. [Google Scholar] [CrossRef]
- Huang, C.; Li, X.; Khanal, L.; Jiang, X. Habitat suitability and connectivity inform a co-management policy of protected area network for Asian elephants in China. PeerJ 2019, 7, e6791. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Hua, C.; Yu, G.; Ma, J.; Yang, F.; Wang, J.; Jin, T.; Long, Y.; Guo, Y.; Zhao, H. Assessing protected area overlaps and performance to attain China’s new national park system. Biol. Conserv. 2020, 241, 108382. [Google Scholar] [CrossRef]
- McRae, B.H.; Shah, V.B. Circuitscape User Guide. The University of California, Santa Barbara. 2011. Available online: https://www.researchgate.net/publication/265494222 (accessed on 21 May 2021).
- McRae, B. Isolation by resistance. Evol. Int. J. Org. Evol. 2006, 60, 1551–1561. [Google Scholar] [CrossRef]
- Theobald, D.M. A general model to quantify ecological integrity for landscape assessments and US application. Landsc. Ecol. 2013, 28, 1859–1874. [Google Scholar] [CrossRef]
- Compton, B.; McGarigal, K.; Cushman, S.; Gamble, L. A Resistant-Kernel Model of Connectivity for Amphibians that Breed in Vernal Pools. Conserv. Biol. J. Soc. Conserv. Biol. 2007, 21, 788–799. [Google Scholar] [CrossRef] [PubMed]
- White, J.W.; Scholz, A.J.; Rassweiler, A.; Steinback, C.; Botsford, L.W.; Kruse, S.; Costello, C.; Mitarai, S.; Siegel, D.A.; Drake, P.T. A comparison of approaches used for economic analysis in marine protected area network planning in California. Ocean Coast. Manag. 2013, 74, 77–89. [Google Scholar] [CrossRef]
- Allen, C.H.; Parrott, L.; Kyle, C. An individual-based modelling approach to estimate landscape connectivity for bighorn sheep (Ovis canadensis). PeerJ 2016, 4, e2001. [Google Scholar] [CrossRef]
- Guo, S.; Kaoru, S.; Yin, W.; Chang, S. Landscape Connectivity as a Tool in Green Space Evaluation and Optimization of the Haidan District, Beijing. Sustainability 2018, 10, 1979. [Google Scholar] [CrossRef]
- Ding, P.; Zhuge, Y. Elliot’s Pheasant. Chin. J. Zool 1989, 24, 39–42. [Google Scholar] [CrossRef]
- Li, B. The Elliot’s pheasant in Southern Anhui. Chin. Wildl. 1985, 5, 18–20. [Google Scholar] [CrossRef]
- Shi, J. The seasonal changes of habitats of Elliot’s Pheasant. Zool. Res. 1997, 18, 275–283. [Google Scholar]
- Yanbo, P.; Ping, D. Factors Affecting Movement of Spring Dispersal of Elliot’s Pheasants. Zool. Res. 2005, 26, 373–378. [Google Scholar]
- Zhang, G. The Natural Dispersion and Habitat Selection of Syrmaticus Ellioti; Guangxi Normal University: Guilin, China, 2015. [Google Scholar]
- Zheng, X.; Bao, Y.; Ge, B.; Zheng, R. Seasonal changes in habitat use of black muntjac (Muntiacus crinifr ons) in Zhejiang. Acta Theriol. Sin. 2006, 26, 201–205. [Google Scholar] [CrossRef]
- Wang, Q. Anhui Chronicles of the Animals; Anhui Science & Technology Publishing House: Hefei, China, 1990; ISBN 7-5337-0410-5. [Google Scholar]
- Sheng, H. Chinese Deer Species; East China Normal University Press: Shanghai, China, 1992; ISBN 7-5617-0795-9/Q.008. [Google Scholar]
- Bennett, A.F.; Saunders, D. Habitat Fragmentation and Landscape Change; Oxford University Press: Oxford, UK, 2010; ISBN 9780191720666. [Google Scholar]
- Soille, P.; Vogt, P. Morphological segmentation of binary patterns. Pattern Recognit. Lett. 2009, 30, 456–459. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Guo, G.; Wu, Z.; Xiao, R.; Chen, Y.; Liu, X.; Zhang, X. Impacts of urban biophysical composition on land surface temperature in urban heat island clusters. Landsc. Urban Plan. 2015, 135, 1–10. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Cui, G. The Efficacy of Landscape-Level Conservation in Changbai Mountain Biosphere Reserve, China. PLoS ONE 2014, 9, e95081. [Google Scholar] [CrossRef]
- Sklar, F.H.; Costanza, R. The development of dynamic spatial models for landscape ecology: A review and prognosis. Ecol. Stud. Anal. Synth. 1991, 82, 239–288. [Google Scholar] [CrossRef]
- Smeraldo, S.; Bosso, L.; Fraissinet, M.; Bordignon, L.; Brunelli, M.; Ancillotto, L.; Russo, D. Modelling risks posed by wind turbines and power lines to soaring birds: The black stork (Ciconia nigra) in Italy as a case study. Biodivers. Conserv. 2020, 29, 1959–1976. [Google Scholar] [CrossRef]
- Lhoest, S.; Fonteyn, D.; Danou, K.; Delbeke, L.; Fayolle, A. Conservation value of tropical forests: Distance to human settlements matters more than management in Central Africa. Biol. Conserv. 2020, 241, 108351. [Google Scholar] [CrossRef]
- Del Carmen Sabatini, M.; Verdiell, A.; Rodríguez Iglesias, R.M.; Vidal, M. A quantitative method for zoning of protected areas and its spatial ecological implications. J. Environ. Manage. 2007, 83, 198–206. [Google Scholar] [CrossRef]
- Fu, M. Identification of functional zones and methods of target management in Sanjiangyuan National Park. Biodivers. Sci. 2017, 25, 52–63. [Google Scholar] [CrossRef]
- Fu, M.; Tian, J.; Ren, Y.; Li, J.; Liu, W.; Zhu, Y. Functional zoning and space management of Three-River-Source National Park. J. Geogr. Sci. 2019, 29, 2069–2084. [Google Scholar] [CrossRef]
- Habtemariam, B.T.; Fang, Q. Zoning for a multiple-use marine protected area using spatial multi-criteria analysis: The case of the Sheik Seid Marine National Park in Eritrea. Mar. Policy 2016, 63, 135–143. [Google Scholar] [CrossRef]
- Ruiz-Labourdette, D.; Schmitz, M.F.; Montes, C.; Pineda, F.D. Zoning a Protected Area: Proposal Based on a Multi-thematic Approach and Final Decision. Env. Model Assess 2010, 15, 531–547. [Google Scholar] [CrossRef]
- Verdiell, A.; Sabatini, M.C.; Maciel, M.C.; Iglesias, R. A mathematical model for zoning of protected natural areas. Int. Trans. Oper. Res. 2005, 12, 203–213. [Google Scholar] [CrossRef]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.J.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Guidelines for Conserving Connectivity Through Ecological Networks and Corridors; IUCN: Gland, Switzerland, 2020; ISBN 9782831720524. [Google Scholar]
- Noss, R.F.; Harris, L.D. Nodes, networks, and MUMs: Preserving diversity at all scales. Environ. Manag. 1986, 10, 299–309. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).