1. Introduction
Phosphorus is an essential nutrient in water ecosystems and is considered a key limiting factor in water bodies’ eutrophication [
1,
2]. Population growth, increased industrialization, agricultural activities, and urbanization have led to the pollution of the water of many rivers, lakes, and reservoirs, seriously affecting water resources [
3,
4,
5]. Some phosphorus entering the river can be removed by self-purification, while some can be adsorbed by the particulate matter and sediment in the water and accumulated in the riverbed through natural sedimentation, and sediment acts as a phosphorus ‘sink’. Once the external environment changes, for example, the physical and chemical properties of the sediment change, the phosphorus concentration in the overlying water decreases, dissolved oxygen in the water decreases, and the sediment releases phosphorus into the overlying water and becomes an endogenous source of phosphorus in the river [
6,
7].
The determination of phosphorus fractions in sediments can help to understand the mechanism of phosphorus adsorption and sediment-overlying water phosphorus exchange, which is of great significance for water environment management and improvement [
8,
9,
10,
11]. Phosphorus in sediments is mainly inorganic and organic phosphorus. Different forms of phosphorus have different bioavailability and release capacities and contribute differently to eutrophication in water bodies. For most rivers, lakes, and reservoirs, sediments contain a relatively large proportion of inorganic phosphorus [
12,
13,
14,
15]. Based on the activity or potential bioavailability of phosphorus, Kaiserli et al. [
16] classified phosphorus forms in sediments as loosely adsorbed phosphorus (labile-P), iron-bound phosphorus (Fe-P), metal oxide-bound phosphorus (Al-P), organic phosphorus (OP), calcium-bound (Ca-P), and residual phosphorus (Res-P). Labile-P, Fe-P, and OP are considered relatively active forms in sediments and are easy transformed by transport at the sediment-water interface [
17,
18]. Labile-P is considered a significant indicator of sediment phosphorus bioavailability because it occurs as orthophosphate in pore water and can be taken up directly by algae, contributing to the water column eutrophication [
19]. It has been shown in some research that the amount of Fe/Al-P in sediments is influenced by human activities, generally from domestic sewage and industrial effluent discharges [
20,
21]. Based on the stability of OP, Ivanof et al. [
22,
23] classified organophosphorus as labile organic phosphorus (LOP), moderately labile organic phosphorus (MLOP) and non-labile organic phosphorus (NLOP). The amount and composition of organic phosphorus in the sediment affects the conversion of organic phosphorus to dissolved inorganic phosphorus during sediment resuspension. Additionally, OP can promote phytoplankton and algal growth through degradation and conversion to soluble reactive phosphorus (SRP) [
23,
24,
25]. Ca-P is usually closely related to endogenous element accumulation in carbonates from rock debris and soils in watersheds. Ca-P and Res-P are considered to be the most stable forms in sediments [
21].
Changes in external environmental factors significantly influence sediment phosphorus release; sediment phosphorus release is influenced by more than one factor and generally depends on a combination of influencing factors. Jiang et al. [
26] investigated the effects of living activity, light, temperature, and dissolved oxygen on sediment phosphorus release in Taihu Lake and found that: microorganism metabolism can change the microenvironmental state of sediment; and light, temperature, and dissolved oxygen have a more significant effect on phosphorus release. Li et al. [
27] studied the effect of substrate oxidation on phosphorus transformation in three shallow eutrophic lakes (Taihu Lake, Chaohu Lake, and Dianchi Lake) in China, and the results showed that substrate oxidation will promote the conversion between different forms of phosphorus in sediments, providing a scientific basis for eutrophic lake management. Wang et al. [
28] studied the effect of different disturbance conditions on sediment phosphorus release. The results showed that the release intensity of dissolved total phosphorus (DTP), soluble reactive phosphorus (SRP), and dissolved organic phosphorus (DOP) increased with the increase in disturbance intensity. The equilibrium time of DTP in the overlying water was about 16 h after the release of phosphorus from heavily polluted sediments and 8 h for lightly polluted sediments. After the cessation of disturbance, DTP, SRP, and DOP in the overlying water all showed a rapid decrease and gradually reached equilibrium, indicating the buffering capacity of the sediment in the disturbance conditions. These studies show that environmental factors on phosphorus release help understand the phosphorus exchange mechanism at the sediment-water interface and guide the water environment’s management.
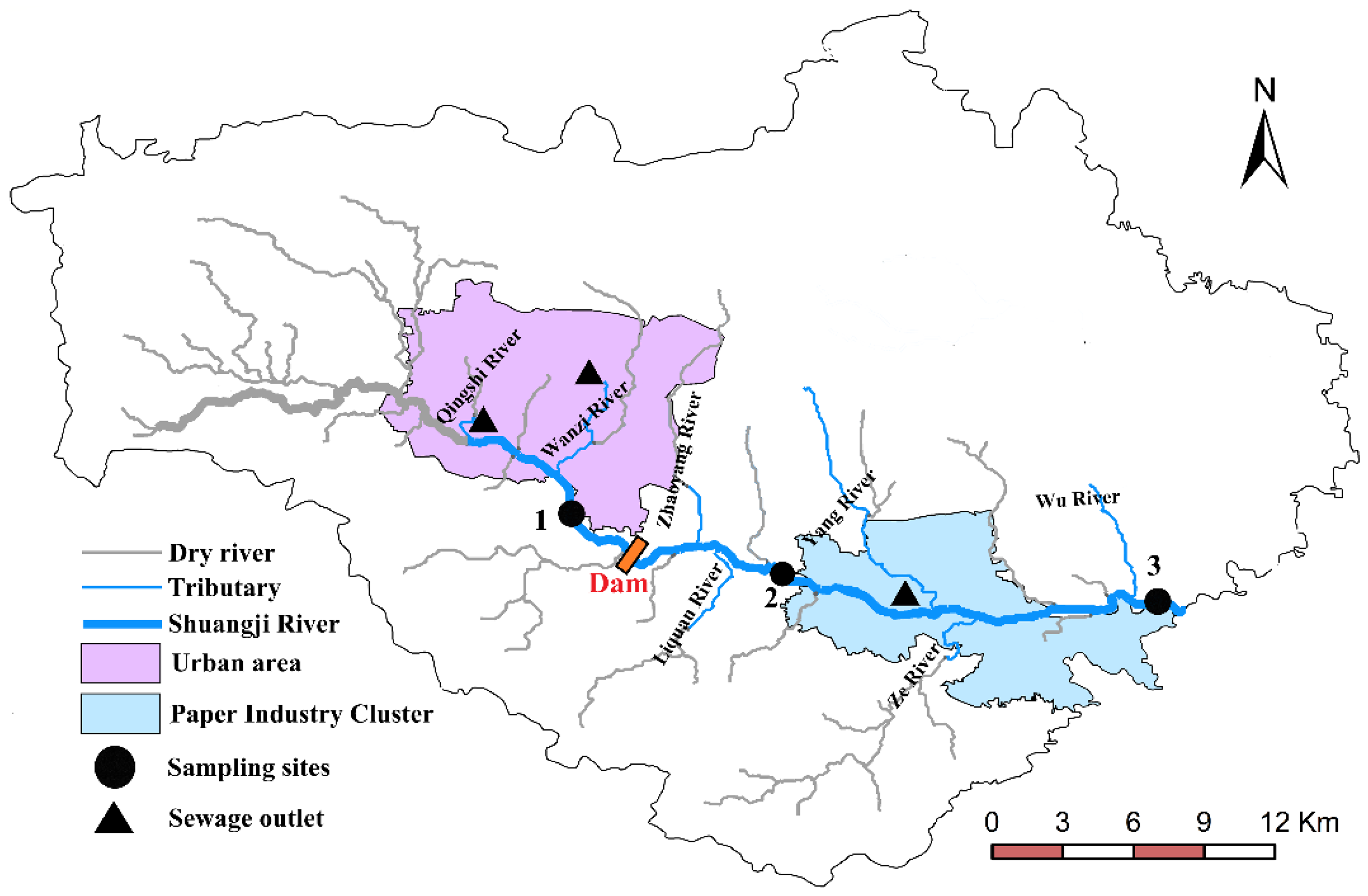
Shuangji River in Xinmi City is a tailwater-type river. The section above the river, where the Qingshi River joins the Shuangji River, was cut off. The natural runoff flow is small, and Shuangji River mainly consisting of the tailwater from the sewage treatment plant, the tailwater from the paper wastewater treatment plant, and the water from the coal mine, which is the main drainage and pollution channel in Xinmi city. With the implementation of point and non-point source pollution control measures in Xinmi city, the river’s phosphorus content has gradually decreased. However, the outflow section still often exceeds the surface water grade III standard, especially in summer and autumn. After site visits and indoor sediment phosphorus release experiments, it is suspected that high-temperature conditions have promoted the release of phosphorus, increasing the phosphorus exchange flux between the sediment-overlying water.
This study investigates the content and distribution of various phosphorus fractions in sediments of the Shuangji River basin in Xinmi City and the influence of environmental factors on phosphorus release to provide practical information and control directions for the water environment managers.
2. Materials and Methods
2.1. Study Area
The source of the Shuangji River is located in the northwest mountainous area of Xinmi City, and the Shuangji River is part of the Shaying River system in the Huai River basin, with a total length of 204 km and a basin area of 1941 km2. The river flows from west to east, passes through Xinmi city, Xinzheng city, and finally merges into the Jialu River. The mainstream in Xinmi city is about 53 km long, with an average slope drop of about 3.3‰. Xinmi has a continental monsoon climate in the northern temperate zone, with an average annual temperature of 14.3 °C. The wind direction is mostly northwest and southeast, and the maximum wind speed is 22 m/s. The average annual precipitation is 650 mm. Due to the influence of the monsoon climate, the annual precipitation is uneven. Summer precipitation accounts for approximately 50% of the annual precipitation, spring precipitation approximately 21% of the annual precipitation, and autumn precipitation approximately 25% of the annual precipitation; winter precipitation only accounts for about 4% of the annual precipitation. The annual average evaporation is 1400 mm. The terrain of Xinmi City is complex, with an altitude above sea level of 114–1108.5 m. There are mountains in the west, north, and south, with hills and valleys in the middle and plains in the east. Mountainous area accounts for 21.2% of the total area; hilly area accounts for 57.3% of the total area.
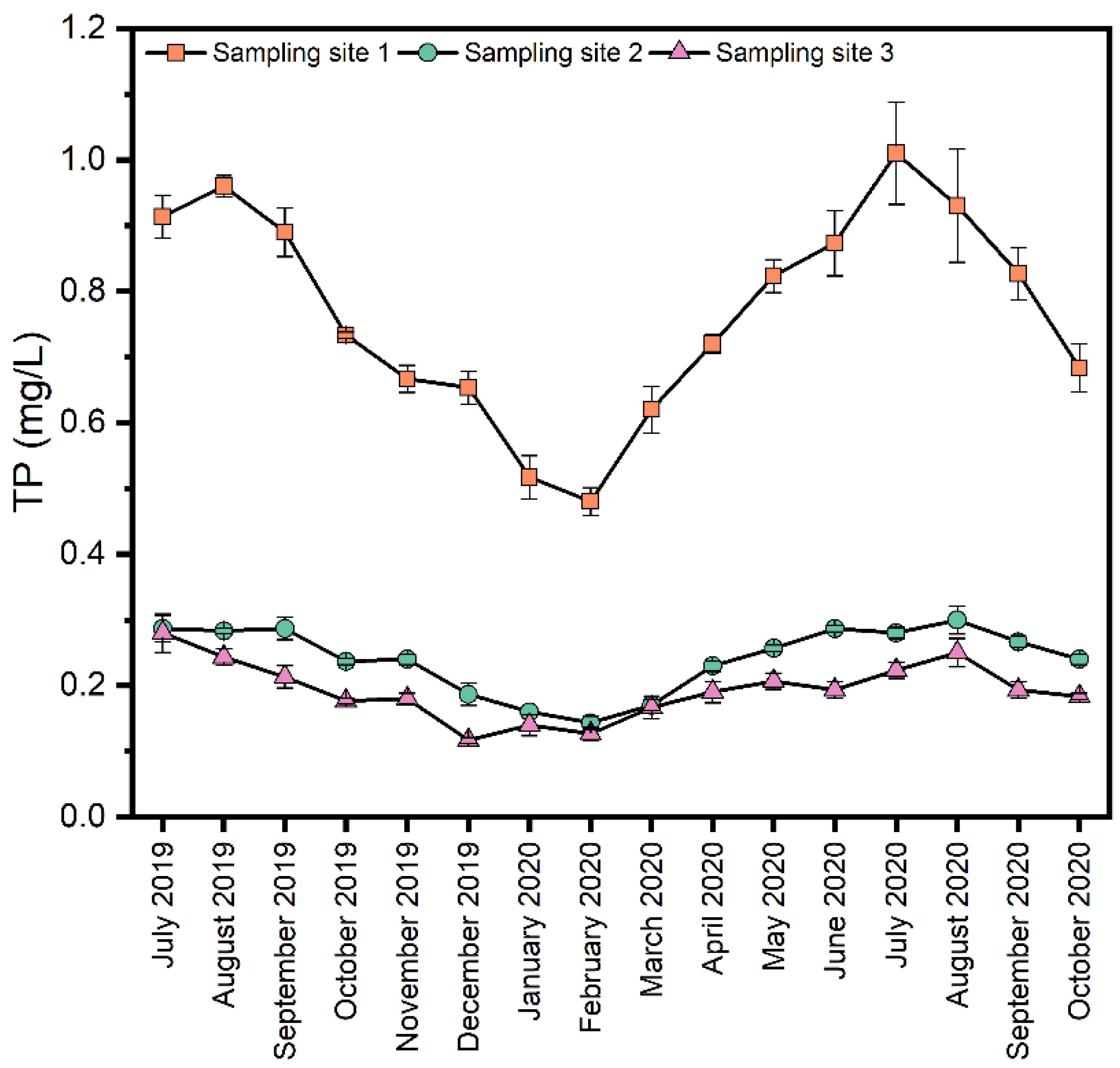
The Xinmi city section of the Shuangji River has very little natural runoff, and the upstream is dry. The main water volume comes from the domestic sewage plant’s tailwater, the papermaking wastewater, and the coal mine, which has become the main tailwater river in Xinmi city. The daily volume of outbound water is about 55,000 m3, the water quality is weakly alkaline, with an average pH of 7.94, and the hydrogeology is loose rock pores water. In 2017, the annual average values of chemical oxygen demand (COD), ammonia nitrogen (NH3-N), and total phosphorus (TP) of the Shuangji River provincial control monitoring section were 39.22, 1.74, and 0.26, respectively. It is urgent to control pollution sources in the Shuangji River basin to reach the Class III surface water goal.
The geographical location of the main river and tributaries in the study area is shown in
Figure 1. The flows are shown in
Table 1. The Shuangji River upper section was intercepted by a storage dam in Chaohua town. The storage dam is about 40 m long, 8 m wide and 2.5 m high, and the water surface area reaches 15,000 square meters. Most of the water stored in the storage dam flows to the Caomagou reservoir through the Dongfanghong irrigation canal. The Dongfanghong irrigation canal is the largest gravity irrigation canal in Xinmi City and the main canal is 30 km long and irrigates 43 villages in five townships (towns), with a total of about 2400 hectares of cultivated land. Part of the water transported to Caomagou reservoir is used as cooling water for nearby power plants, and a small part of the water (about 0.034 m
3/s,
Table 1) flows into the lower reaches of the Shuangji River through the Wu River.
2.2. Sampling Sites and Sample Collection
In this study, three sampling sites representing different overlying water types were set up on the mainstream, and the sampling sites were shown in
Figure 1.
Water samples were collected three times a month between July 2019 and October 2020. The water samples were collected from each sampling site at triplicate, mixed well and filled into pre-cleaned 500 mL polyethene plastic bottles, stored in a 4 °C portable refrigerator, and sent to the laboratory immediately after sampling for COD, NH3-N, and total TP. pH and dissolved oxygen (DO) were determined in situ using portable testing instruments.
In this study, four sediment collection activities were conducted at three sampling sites in January, April, July, and October 2020, and a total of 12 samples were collected. In each sampling activity, three groups of surface sediment (about 10 cm) were collected using a grabber-type sediment collector at each sampling site and mixed evenly and placed in plastic bags as final samples, drained of air, marked, and stored in a 4 °C portable refrigerator, and the samples were sent back to the laboratory for pre-processing immediately after the sampling was completed.
The collected sediments were freeze dried and ground and sieved through a 100 mesh screen before phosphorus fraction analysis.
2.3. Sample Analysis
COD was analyzed using the dichromate method, NH3-N was determined by Nessler’s reagent spectrophotometry, and TP was determined by the ammonium molybdate spectrometric method.
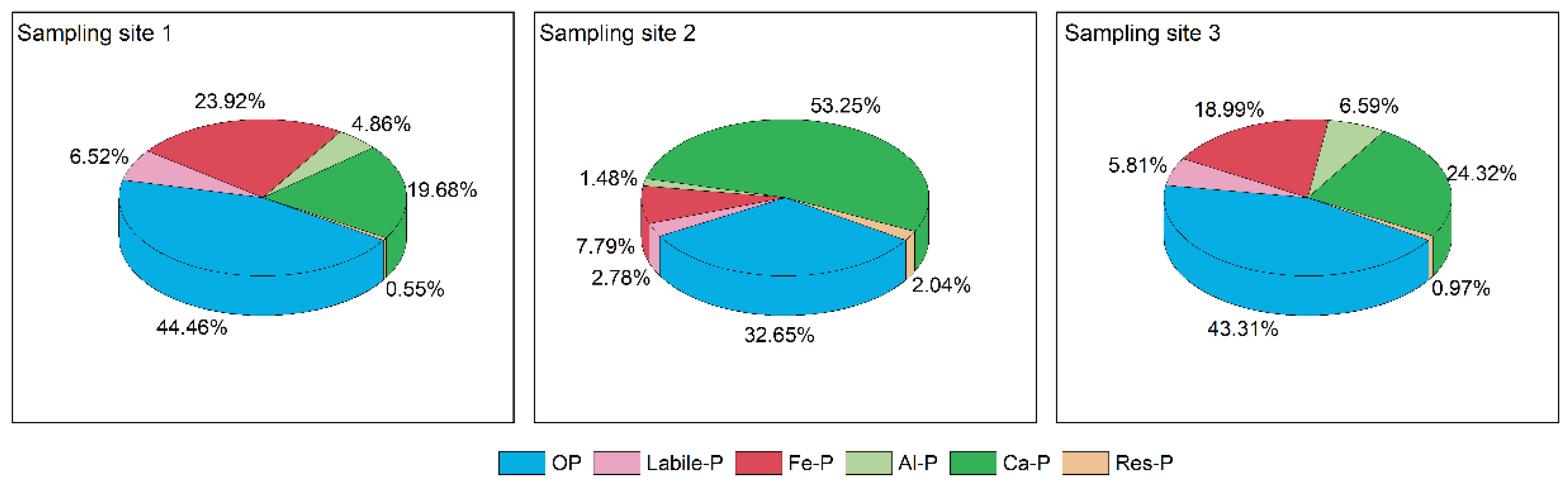
Phosphorus in sediments can be classified as inorganic phosphorus (IP) and organic phosphorus (OP). According to the sequential extraction method, inorganic phosphorus in sediments can be classified into labile-P, Fe-P, Al-P, Ca-P, and res-P [
4]. TP and IP were determined using the standards, measurements and testing program (SMT), and OP was the difference between TP and IP [
29].
2.4. Sediment Phosphorus Release Experiment
10 g of the original sediment was evenly laid flat in a 1000 mL beaker and 500 mL of distilled water was slowly injected along the beaker wall; 20 mL of the overlying water was collected with a syringe every 12 h, filtered through a 0.45 μm fiber filter membrane, and the DTP content in the water was determined by the ammonium molybdate spectrometric method after sampling, immediately supplemented with 20 mL of distilled water.
2.4.1. Effect of Temperature on Phosphorus Release
To simulate the differences in phosphorus release from sediments caused by temperature changes in the four seasons in the Shuangji River basin, three sets of beakers were placed in 5, 15, and 25 °C for phosphorus release experiments.
2.4.2. Effect of pH on Phosphorus Release
NaHCO3 or dilute sulfuric acid was dissolved in 500 mL of distilled water, and the pH was adjusted to 4, 6, 7, 8, and 10, and then slowly injected along the beaker walls to perform phosphorus release experiments in the same room temperature (about 15 °C).
2.4.3. The Effect of Dissolved Oxygen on Phosphorus Release
Aerobic experiments were conducted by micro-aeration of the overlying water through a micro-inflatable pump, and dissolved oxygen in the water was measured at intervals to maintain it at DO > 5 mg/L. The anaerobic experiment replaced the beaker with a 1000 mL pumping bottle with a thin tube inserted on the cap and a clamp controlling the tube’s mouth. Nitrogen was passed through the micro-inflatable pump once a day for about 30 min each time. Immediately after the end of the aeration, the exhaust pipe was tightly sealed. The two groups of experiments were conducted at room temperature (about 15 °C).
2.4.4. Effect of Hydraulic Disturbance on Phosphorus Release
This experiment simulated four groups of experiments with different flow velocities by using different rotational speeds of the thermostatic oscillator, with flow velocities of 0, 0.2, and 0.4 m/s. The purpose was to investigate the effect of flow velocity on phosphorus release from the sediment, and the empirical equation between oscillator speed and flow velocity was adopted from the research results of Wang et al. [
30]; the equation is shown in (1), and the set rotational speeds were 0, 110 and 160 r/min:
where
v refers to the flow velocity (m/s) and
ω refers to the rotational speed (r/min).
2.4.5. Sediment Phosphorus Release Fluxes and Release Rates
As time increases, phosphorus is gradually released from the sediment into the overlying water, and the release rate and amount released can be calculated using Equations (2) and (3).
In the equations: r represents the release rate in mg/(m2·h); V is the volume of overlying water in L; , and are, respectively, the nth, initial concentration and j − 1th collection phosphorus concentration in the overlying water at the time of the water sample in mg/L; represents the volume of the water sample collected for the j − 1th time in L; is the concentration of the supplementary overlying water in mg/L; A is the sediment and the contact area of the overlying water in m2; t is the time in hours; and W is the amount of phosphorus released from the sediment during tin mg.
2.5. Statistical Analysis
The water samples and sediment samples measured in the experiment were expressed by the mean (±SD). The difference of pollutant concentration and sediment phosphorus content among different sampling sites and the influence of different environmental factors on phosphorus release in sediments were analyzed by nonparametric tests based on the K independent sample test (Kruskal-Wallis test). Pearson’s correlation coefficients calculated by Equation (4) were used to test the correlation between variables. We use OriginPro 2019b (OriginLab, Northampton, MA, USA) to generate graphics, using IBM SPSS Statistics 26.0 for Windows for data statistics and performing analyses.
and represent the values of two variables; and represent the mean value of two variables; n represents the number of samples; and and represent the sample standard deviation of the two variables.