Uptake Kinetics of \({\text{NH}_{4}^{+} }\), \({\text{NO}_{3}^{-} }\) and \({\text{H}_{2}\text{PO}_{4}^{-} }\) by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn
Abstract
1. Introduction
2. Materials and Methods
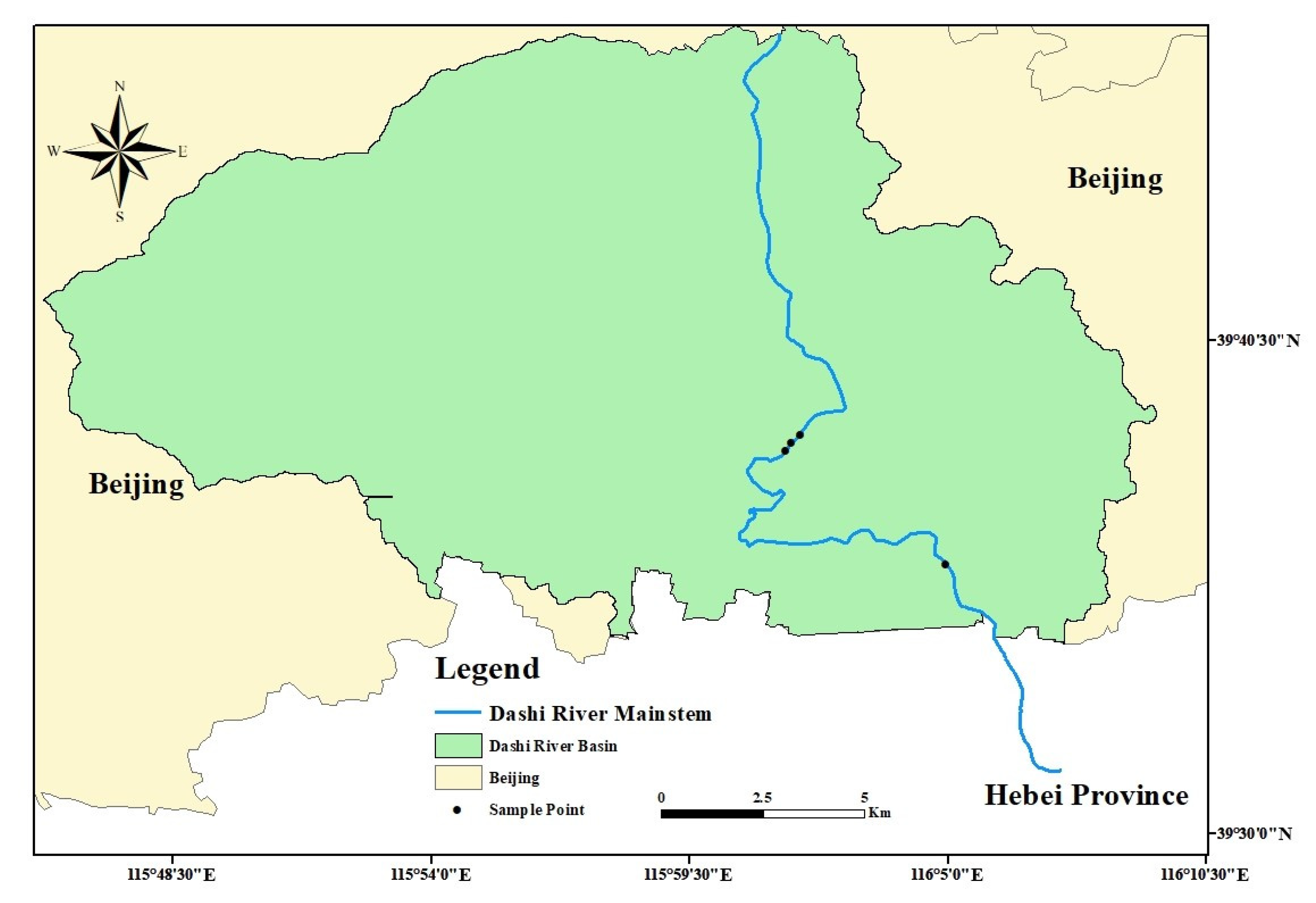
2.1. Experimental Design
2.2. Experimental Design
2.3. Determination Method for Nutrient
2.4. Calculation of Dynamic Parameters
2.5. Method of Data Analysis
3. Results
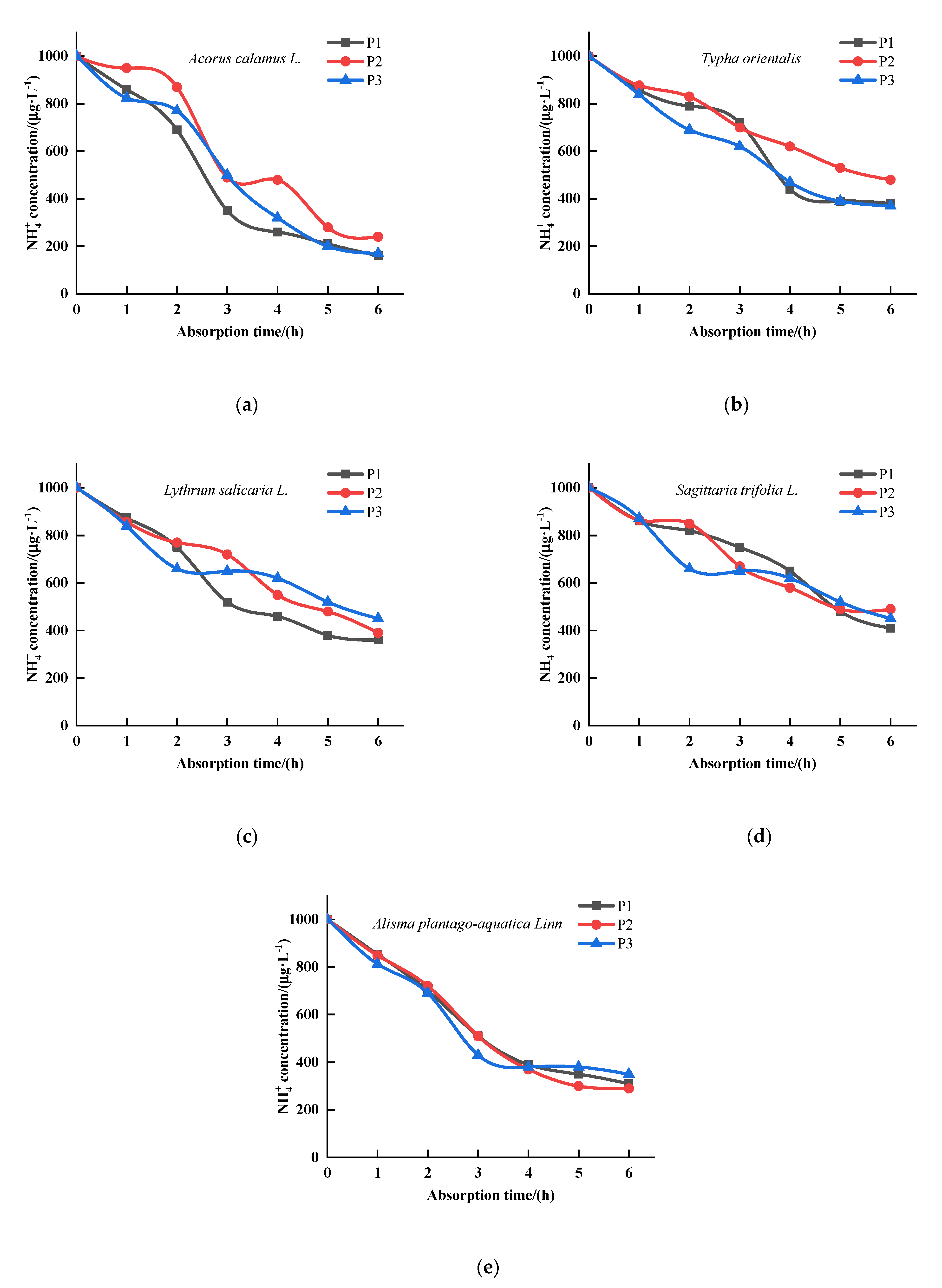
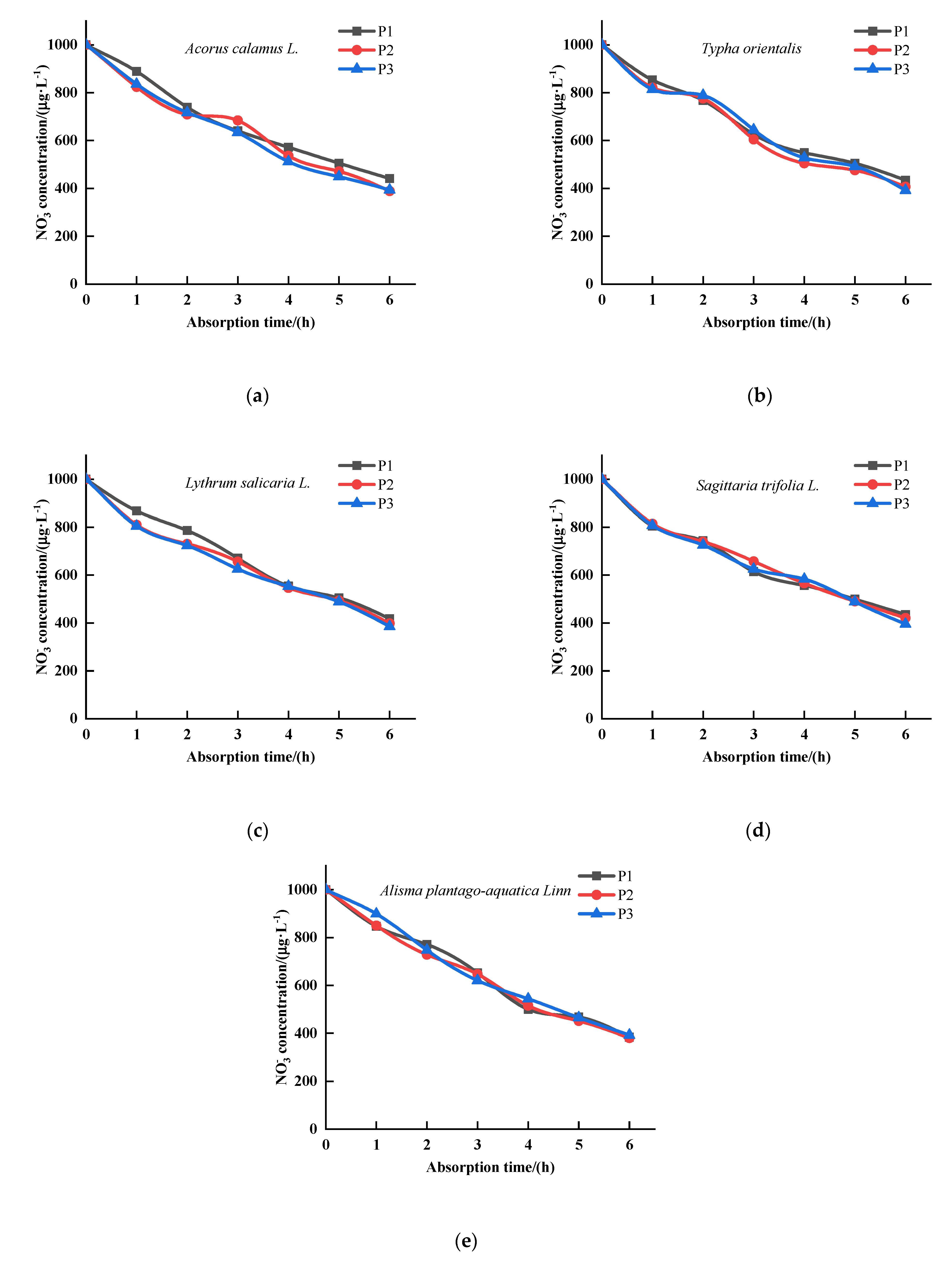
3.1. The Effects of P on the Kinetics of Absorption by Five Emergent Plants
3.2. The Effects of P on the Kinetics of Absorption by Five Emergent Plants
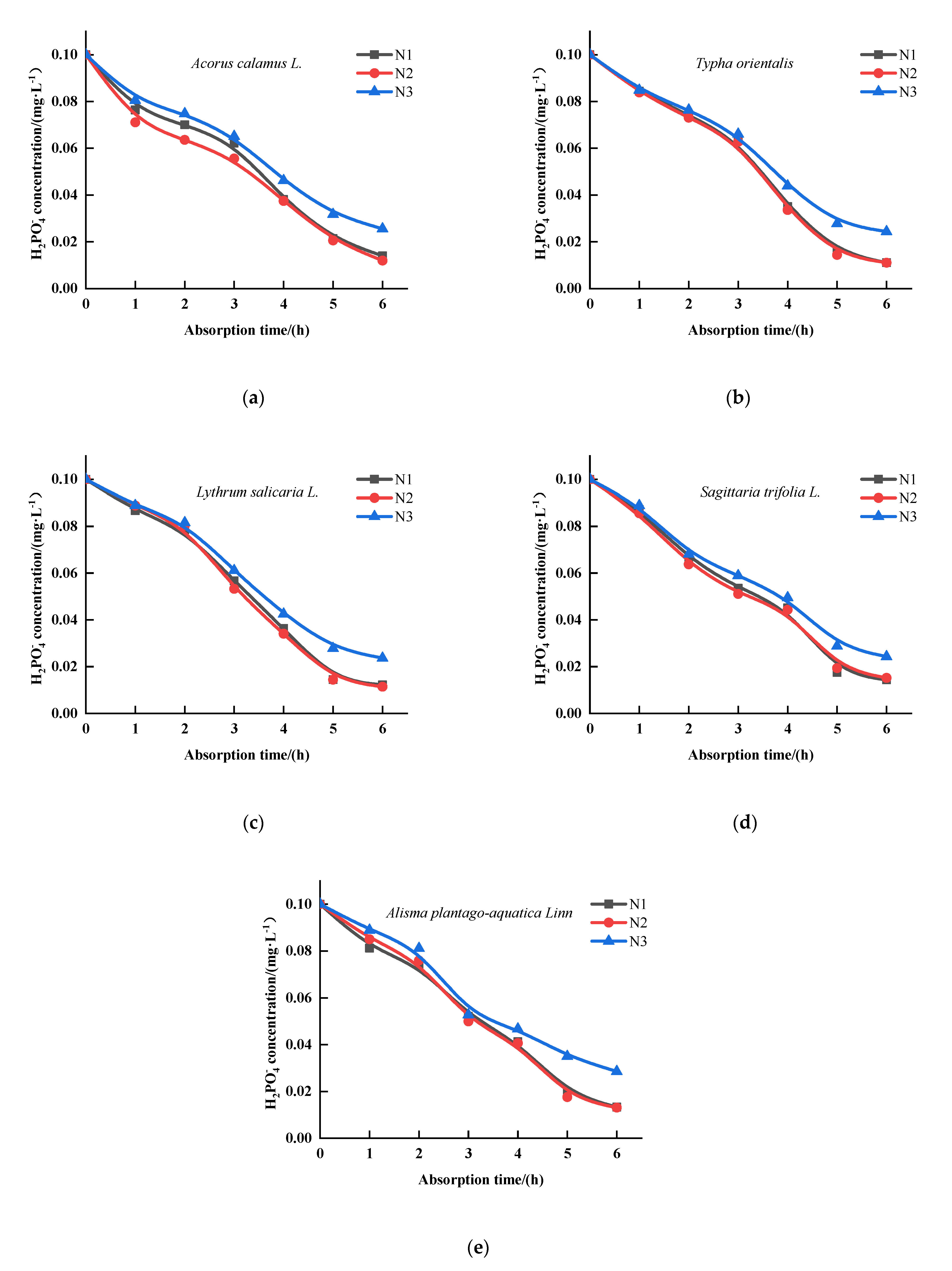
3.3. The Effect of N on the Kinetics of the Absorption by Five Emergent Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, T.; Ni, L.Y. The response of the antioxidant enzymein Ceratophyllum on inorganic nitrogen increased in water. Acta Hydroecologica Sin. 2004, 28, 299–302. [Google Scholar]
- Been, A.; Ni, L. Effects of water column nutrient enrichment on the growth of Potamogeton maackianus. J. Aquat. Plant Manag. 2001, 39, 83–87. [Google Scholar]
- Arts, G.H.P. Deterioration of Atlantic soft water macrophyte communities by acidification, eutrophication and alkalinization. Aquat. Bot. 2002, 73, 373–393. [Google Scholar]
- Sagrario, M.A.G.; Jeppesen, E.; Goma, J.; Sondergaars, M.; Jensen, J.P.; Lauridsen, T.; Landkildehus, F. Does high nitrogen loading prevent clear-water conditions in shallow lakes at moderately high phosphorus concentrations? Freshw. Biol. 2005, 50, 27–41. [Google Scholar]
- Carr, G.M.; Duthine, H.C.; Taylor, W.D. Model of aquatic plant productivity: A review of that influence growth. Aquat. Bot. 1997, 59, 195–215. [Google Scholar]
- Xiong, H.; Tan, Q.; Hu, C. Structural and metabolic responses of Ceratophyllum demersum to eutrophic condition. Afr. J. Biotechnol. 2010, 9, 5722–5729. [Google Scholar]
- Farnsworth-Lee, L.A.; Baker, L.A. Conceptual model of aquatic plant decay and ammonia toxicity for shallow lakes. J. Environ. Eng. 2000, 126, 199–207. [Google Scholar]
- Chen, G.Y.; Li, G.X.; Tang, K. Kinetics of nitrogen and phosphorus uptake by root system of Iris pseudacorus L. and Typha angustifolia L. Chin. J. Environ. Eng. 2013, 7, 4638–4642. [Google Scholar]
- Zhang, X.L.; Wang, L.X.; Liu, H.M.; Qing, H.; Liu, D.W.; Wang, W.; Liang, C.Z.; Friedrich, R. Kinetics of nutrient uptake by three emergent plants, Phragmites australis, Typha orientalis and Scirpus triqueter. Acta Ecol. Sin. 2014, 34, 2238–2245. [Google Scholar]
- Xie, J.; Lv, X.W.; Li, H. Uptake dynamics of N and P in polluted water by 6 different wetland plants. Chin. J. Environ. Eng. 2016, 10, 4067–4072. [Google Scholar]
- Epstein, E.; Hagen, C.E. A kinetic study of the absorption of alkaline canons by barley roots. Plant Physiol. 1952, 27, 457–474. [Google Scholar] [PubMed]
- Nielsen, N.E.; Barber, S.A. Differences among genotypes of corn in the kinetics of P uptake. Agron. J. 1978, 70, 695–698. [Google Scholar]
- Classen, N.; Barber, S.A. A method for characterizing the relation between nutrient concentration and flux into roots of intact plants. Plant Physiol. 1974, 54, 564–568. [Google Scholar]
- Cai, S.M.; Liu, W.T.; Zhang, Z.; Bai, Y.C.; Qian, X.Q. Phosphorus uptake kinetics of different types of duckweed. J. Ecol. Rural Environ. 2011, 27, 48–52. [Google Scholar]
- Chen, S.Y.; Xu, C.; Yao, Y.; Huang, L.Z.; Zhang, Y.T. Uptake kinetics of ammonia, nitrate and phosphorus by submerged macrophytes Hydrilla veticillata and Vallisneria natans. Environ. Sci. Technol. 2010, 16, 407–412. [Google Scholar]
- Tang, Y.X.; Zheng, J.M.; Lou, L.P. Comparisons of , and uptake kinetics in three different macrophytes in waterlogged condition. Chin. J. Eco-Agric. 2011, 19, 614–618. [Google Scholar]
- Shen, G.X.; Yao, F.; Ni, W.Z. The kinetics of ammonium and nitrate uptake by duckweed plant. Chin. J. Soil Sci. 2006, 37, 505–508. [Google Scholar]
- Jampeetong, A.; Brix, H. Nitrogen nutrition of Salvinia natans: Effects of inorganic nitrogen from on growth, morphology, nitrate reductase activity and uptake kinetics of ammonium and nitrate. Aquat. Bot. 2009, 90, 67–73. [Google Scholar]
- Liu, F.; Li, H.L. A preliminary study under on uptake kinetics of Potamogeton different nutrient. J. Wuhan Bot. Res. 2009, 27, 98–101. [Google Scholar]
- Chang, H.Q.; Li, N.; Xu, X.F. and uptake kinetics of three aquatic macrophytes. Ecol. Environ. 2008, 17, 511–514. [Google Scholar]
- Chen, S.Y.; Xu, C.; Yao, Y. Uptake Kinetics of Ammonia Nitrate and Phosphorus by Submerged Macrophytes Hydrilla verticillate and Vallisneria natas. Environ. Sci. Technol. 2012, 35, 34–36. [Google Scholar]
- Zhang, A.W.; Cao, T.; Zhang, M. Uptake of ammonium by Vallisneria natans and dark regimes spicatum under light. J. Lake Sci. 2013, 25, 289–294. [Google Scholar]
- Cao, T.; Ni, L.Y. The role of toxicity in the decline of the submersed macrophyte Vallisneria natans in lakes of the Yangtze River basin, China. Mar. Freshw. Res. 2007, 58, 581–587. [Google Scholar] [CrossRef]
- Runcie, J.W.; Ritchie, R.J.; Larkum, A.W.D. Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquat. Bot. 2003, 76, 155–174. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. Environmental Quality Standards for Surface Water; China Environmental Science Press: Beijing, China, 2002; Available online: https://wenku.baidu.com/view/911e9f3c195f312b3169a5e8.html (accessed on 15 November 2020).
- Ministry of Environmental Protection of the People’s Republic of China. Water Quality-Determination of Ammonia Nitrogen-Nessler’s Reagent Spectrophotometry; China Environmental Science Press: Beijing, China, 2009; Available online: https://www.yunzhan365.com/50616095.html (accessed on 15 November 2020).
- Ministry of Environmental Protection of the People’s Republic of China. Water Quality-Determination of Nitrate Nitrogen-Ultraviolet Spectrophotometry; China Environmental Science Press: Beijing, China, 2007; Available online: https://www.yunzhan365.com/87559483.html (accessed on 15 November 2020).
- Ministry of Environmental Protection of the People’s Republic of China. Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method; China Environmental Science Press: Beijing, China, 1989; Available online: https://www.yunzhan365.com/30053506.html (accessed on 15 November 2020).
- Santos, L.A.; Santos, W.A.; Sperandio, M.V.L.; Bucher, C.A.; de Souza, S.R.; Fernandes, M.S. Nitrate uptake kinetics and metabolic parameters in two rice varieties grown in high and low nitrate. J. Plant Nutr. 2011, 34, 988–1002. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Y.P.; Zhang, T.T.; Zhao, Y.; Shen, Y.; Huang, L.; Gao, X.; Guo, J.S. The logistic growth of duckweed (Lemna minor) and kinetics of ammonium uptake. Environ. Technol. 2014, 35, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.H.; Zheng, S.J.; Shi, J.Q.; Hu, A.T.; Shi, R.H.; Xu, M. Several considerations in kinetic research on nutrients uptake by plants. Plant Nutr. Fertil. Sci. 1995, 1, 11–17. [Google Scholar]
- Zhang, Z.H.; Rengel, Z.; Meney, K. Kinetics of ammonium, nitrate and phosphorus uptake by Canna indica and Schoenoplectus validus. Aquat. Bot. 2009, 91, 71–74. [Google Scholar] [CrossRef]
- Fageria, N.K. Kinetics of phosphate absorption by intact rice plants. Aust. J. Agric. 1974, 25, 395–400. [Google Scholar] [CrossRef]
- Cacco, G.; Ferrari, G.; Saccomani, M. Pattern of sulfate uptake during root elongation in maize: Its correlation with productivity. Physiol. Plant. 2006, 48, 375–378. [Google Scholar] [CrossRef]
- Brix, H.; Dyhr-Jensen, K.; Lorenzen, B. Root-zone acidity and nitrogen source affects Typha latifolia L. growth and uptake kinetics of ammonium and nitrate. J. Exp. Bot. 2002, 53, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Y.; Babourina, O.; Rengel, Z.; Yang, X.E.; Pu, P.M. Ammonium and nitrate uptake by the floating plant Landoltia punctata. Ann. Bot. 2007, 99, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, Z.H.; He, Q.F.; Wang, X.Y.; Wang, C.R.; Chen, D.F.; Lai, Z.L. Root growth of wetland plants with different toot types. Acta Ecol. Sin. 2007, 27, 450–457. [Google Scholar] [CrossRef]
- Chen, S.P. Advances in biological fundamental studies on artificial wetland wastewater treatment system. J. Lake Sci. 1996, 8, 268–272. [Google Scholar]
| Total Phosphorus Level | Total Nitrogen Level | ||||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | N1 | N2 | N3 | ||
1 | 0.04 | 0.08 | 0.5 | 0.2 | 0.6 | 1.2 | |
1 | 0.04 | 0.08 | 0.5 | ||||
| Plant Species | P1 | P2 | P3 |
|---|---|---|---|
| Acorus calamus L. | |||
| Typha orientalis | |||
| Lythrum salicaria L. | |||
| Sagittaria trifolia L. | |||
| Alisma plantago-aquatica Linn |
| Plant Species | Vmax (mg−1·g−1·h−1) | Km (mg·L−1) | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | |
| Acorus calamus L. | 0.3571 ± | 0.2500 ± | 0.1887 ± | 0.2121 ± | 0.2242 ± | 0.2616 ± |
| Typha orientalis | 0.1851 ± | 0.1491 ± | 0.2174 ± | 0.3594 ± | 0.3949 ± | 0.3077 ± |
| Lythrum salicaria L. | 0.3333 ± | 0.2083 ± | 0.1843 ± | 0.3490 ± | 0.4406 ± | 0.4677 ± |
| Sagittaria trifolia L. | 0.2222 ± | 0.2128 ± | 0.2083 ± | 0.5308 ± | 0.5683 ± | 0.6078 ± |
| Alisma plantago-aquatica Linn | 0.1961 ± | 0.2174 ± | 0.3571 ± | 0.2498 ± | 0.2388 ± | 0.1692 ± |
| Plant Species | P1 | P2 | P3 |
|---|---|---|---|
| Acorus calamus L. | |||
| Typha orientalis | |||
| Lythrum salicaria L. | |||
| Sagittaria trifolia L. | |||
| Alisma plantago-aquatica Linn |
| Plant Species | Vmax (mg−1·g−1·h−1) | Km (mg·L−1) | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | |
| Acorus calamus L. | 0.3333 ± | 0.4167 ± | 0.3846 ± | 1.2592 ± | 1.1920 ± | 1.2175 ± |
| Typha orientalis | 0.400 ± | 0.8333 ± | 0.6667 ± | 1.5271 ± | 1.0808 ± | 1.382 ± |
| Lythrum salicaria L. | 0.1818 ± | 0.1923 ± | 0.2083 ± | 0.3872 ± | 0.3885 ± | 0.3716 ± |
| Sagittaria trifolia L. | 0.2703 ± | 0.2083 ± | 0.2381 ± | 0.5077 ± | 0.5537 ± | 0.5288 ± |
| Alisma plantago-aquatica Linn | 0.2272 ± | 0.2083 ± | 0.1852 ± | 0.3362 ± | 0.3541 ± | 0.3801 ± |
| Plant Species | N1 | N2 | N3 |
|---|---|---|---|
| Acorus calamus L. | |||
| Typha orientalis | |||
| Lythrum salicaria L. | |||
| Sagittaria trifolia L. | |||
| Alisma plantago-aquatica Linn |
| Plant Species | Vmax (mg−1·g−1·h−1) | Km (mg·L−1) | ||||
|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N1 | N2 | N3 | |
| Acorus calamus L. | 0.0189 ± | 0.0191 ± | 0.0171 ± | 0.0047 ± | 0.0037 ± | 0.0091 ± |
| Typha orientalis | 0.0174 ± | 0.0179 ± | 0.0164 ± | 0.0017 ± | 0.0014 ± | 0.0018 ± |
| Lythrum salicaria L. | 0.0166 ± | 0.0175 ± | 0.0170 ± | 0.0021 ± | 0.0017 ± | 0.0016 ± |
| Sagittaria trifolia L. | 0.0168 ± | 0.0186 ± | 0.0163 ± | 0.0063 ± | 0.0042 ± | 0.0062 ± |
| Alisma plantago-aquatica Linn | 0.0212 ± | 0.0206 ± | 0.0164 ± | 0.0060 ± | 0.0065 ± | 0.0077 ± |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Hu, Q.; Wei, Y.; Yin, H.; Sun, C.; Liu, G. Uptake Kinetics of \({\text{NH}_{4}^{+} }\), \({\text{NO}_{3}^{-} }\) and \({\text{H}_{2}\text{PO}_{4}^{-} }\) by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn. Sustainability 2021, 13, 434. https://doi.org/10.3390/su13010434
Wang K, Hu Q, Wei Y, Yin H, Sun C, Liu G. Uptake Kinetics of \({\text{NH}_{4}^{+} }\), \({\text{NO}_{3}^{-} }\) and \({\text{H}_{2}\text{PO}_{4}^{-} }\) by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn. Sustainability. 2021; 13(1):434. https://doi.org/10.3390/su13010434
Chicago/Turabian StyleWang, Kunlun, Qian Hu, Yumin Wei, Hui Yin, Changhong Sun, and Guizhong Liu. 2021. "Uptake Kinetics of \({\text{NH}_{4}^{+} }\), \({\text{NO}_{3}^{-} }\) and \({\text{H}_{2}\text{PO}_{4}^{-} }\) by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn" Sustainability 13, no. 1: 434. https://doi.org/10.3390/su13010434
APA StyleWang, K., Hu, Q., Wei, Y., Yin, H., Sun, C., & Liu, G. (2021). Uptake Kinetics of \({\text{NH}_{4}^{+} }\), \({\text{NO}_{3}^{-} }\) and \({\text{H}_{2}\text{PO}_{4}^{-} }\) by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn. Sustainability, 13(1), 434. https://doi.org/10.3390/su13010434






