Utilization of By-Products and Wastes as Supplementary Cementitious Materials in Structural Mortar for Sustainable Construction
Abstract
:1. Introduction
2. Structural Mortar
- floor covering mortar,
- rendering mortar, and
- structural mortar.
- binding material in masonry work,
- concrete repair material,
- matrix of thin reinforced cementitious products or composite systems (e.g., ferrocement, fabric-reinforced cementitious matrix (FRCM), textile-reinforced mortar (TRM), etc.),
- concrete equivalent mortar in scientific studies, and
- ultra-high performance concrete.
3. Major SCMs
3.1. SCMs from Industrial Processes
3.1.1. Fly Ash
3.1.2. Bottom Ash
3.1.3. Silica Fume
3.1.4. Ground Granulated Blast-Furnace Slag
3.1.5. Limestone Powder
3.2. SCMs from Natural Sources
3.2.1. Metakaolin
3.2.2. Volcanic Ash
3.3. SCMs from Agricultural Wastes
3.3.1. Palm Oil Fuel Ash
3.3.2. Rice Husk Ash
3.4. SCMs from Solid Wastes
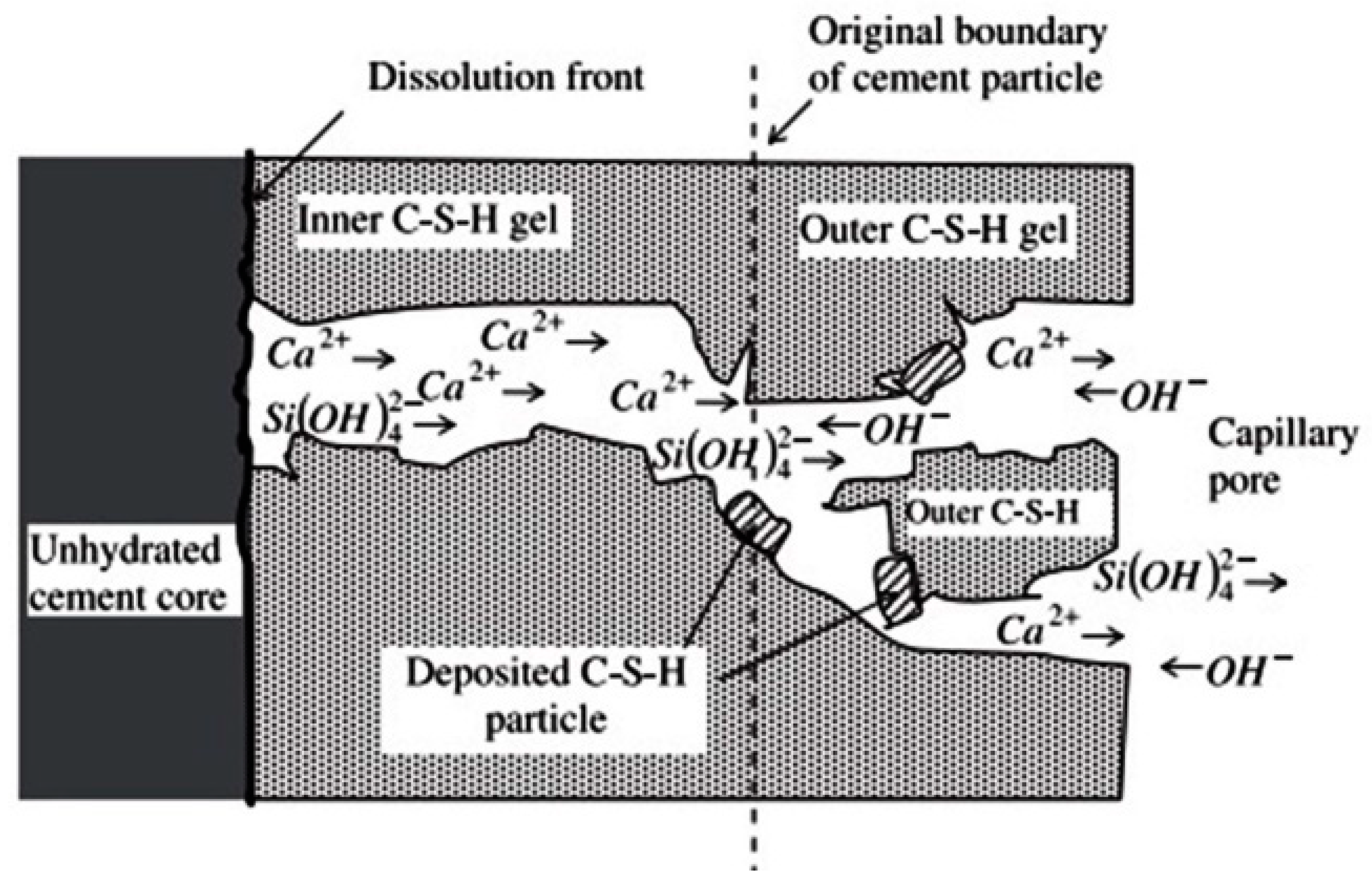
4. Principal Functioning Mechanisms of SCMs
- dispersing cement particles,
- modifying the kinetics of the hydration of cement,
- reacting with the secondary product of cement hydration, and
- physically filling the pore spaces in cement paste.
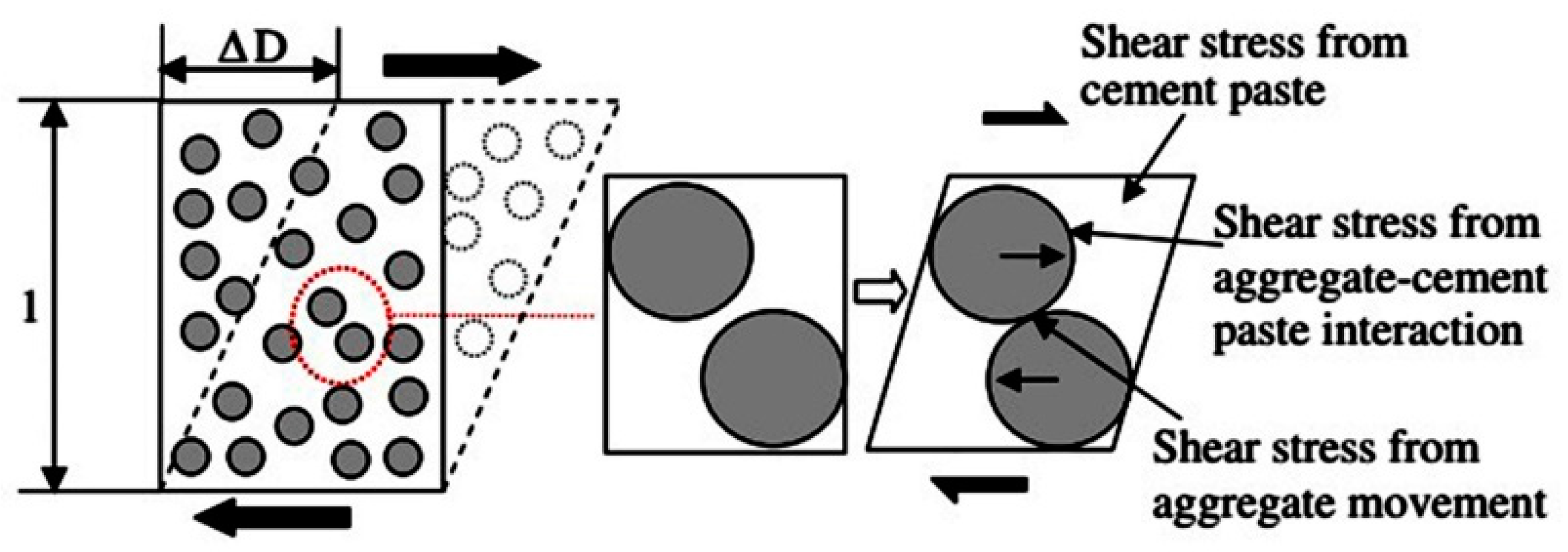
- the yield stress of binder paste,
- the viscous stress of binder paste,
- the interaction between the binder paste and aggregates, and
- the stress resulting from the movement of aggregates.
- surface forces (or colloidal interactions),
- Brownian forces,
- hydrodynamic forces, and
- various contact forces between particles.
5. Effects of SCMs on the Properties of Structural Mortar
5.1. Fly Ash (FA)
5.1.1. Effects on Flowability
5.1.2. Effects on Strength
5.1.3. Effects on Durability and Other Properties
5.2. Bottom Ash (BA)
5.2.1. Effects on Flowability
5.2.2. Effects on Strength
5.2.3. Effects on Durability and Other Properties
5.3. Silica Fume (SF)
5.3.1. Effects on Flowability
5.3.2. Effects on Strength
5.3.3. Effects on Durability and Other Properties
5.4. Ground Granulated Blast-Furnace Slag (GGBS)
5.4.1. Effects on Flowability
5.4.2. Effects on Strength
5.4.3. Effects on Durability and Other Properties
5.5. Limestone Powder (LP)
5.5.1. Effects on Flowability
5.5.2. Effects on Strength
5.5.3. Effects on Durability and Other Properties
5.6. Metakaolin (MK)
5.6.1. Effects on Flowability
5.6.2. Effects on Strength
5.6.3. Effects on Durability and Other Properties
5.7. Volcanic Ash (VA)
5.7.1. Effects on Flowability
5.7.2. Effects on Strength
5.7.3. Effects on Durability and Other Properties
5.8. Palm Oil Fuel Ash (POFA)
5.8.1. Effects on Flowability
5.8.2. Effects on Strength
5.8.3. Effects on Durability and Other Properties
5.9. Rice Husk Ash (RHA)
5.9.1. Effects on Flowability
5.9.2. Effects on Strength
5.9.3. Effects on Durability and Other Properties
5.10. Waste Glass Powder (WGP)
5.10.1. Effects on Flowability
5.10.2. Effects on Strength
5.10.3. Effects on Durability and Other Properties
6. Contributions of SCMs and Structural Mortar to Sustainable Construction
7. Research Gaps and Recommendations
- Some treatments (e.g., controlled burning and/or grinding) substantially improve SCM properties. The effects of such treatment processes on most SCMs were not studied thoroughly. The optimum treatment process needs to be determined for each SCM, especially for those obtained from agricultural sources.
- Different researchers experimented in different conditions for very specific purposes. Comparing SCMs for use in construction is difficult based on these situation-dependent data. The detailed characterization of each widely available SCM should be accomplished.
- Studies on how the behavior of SCM changes with its source and application conditions are still inadequate. The behavior of SCM incorporated mortars in every real-life situation (e.g., frost, high temperature, impact loading, exposure to seawater, etc.) should be determined.
- The effects of VA on the flowability of mortar mixture and its resistance against carbonation, acid attack, sulphate attack, and freezing and thawing are unknown. Therefore, broad research should be carried out to study these properties of VA mortar.
- The durability performance of BA mortar needs to be investigated thoroughly. Especially, the ASR resistance of BA mortar is very important, as some studies reported the signs of ASR due to the high concentration of K+ ions released from BA.
- RHA mortar should be investigated for its resistance against carbonation, ASR, sulphate attack, and freezing and thawing.
- The applications of POFA and GGBS as an SCM in mortar should be explored by examining their resistance against carbonation, acid attack, ASR, and freezing and thawing. Especially, the high content of K2O in POFA raises a concern about ASR.
- The behavior in acidic and freeze-thaw environments are still unexplored for the mortars containing LP and WGP. In addition, the influence of LP on ASR and chloride penetration needs broad investigation.
- The resistance of FA mortar against carbonation and acid attack needs to be examined systematically.
- Studies on the use of SCMs in structural mortar at different multilevel combinations, such as binary and ternary levels, were not performed meticulously.
- Dependable analytical models for predicting the effects of SCM on the mortar-performance are absent. Such models should be established considering all influencing parameters to predict the consequences of SCM inclusion.
- The carbon footprints of the mortars made with blended cement types, including various SCMs need to be determined.
- To ensure sustainable development, the total impact of the SCM incorporated structural mortar on the environment and economy should be quantified in a holistic manner, considering the treatment process, replacement level, and durability.
- To avoid contamination, the leaching of heavy metals from the cementitious products containing SCM should be quantified before any industrial application.
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halada, K. Progress of ecomaterials toward a sustainable society. Curr. Opin. Solid State Mater. Sci. 2003, 7, 209–216. [Google Scholar] [CrossRef]
- CEMBUREAU (The European Cement Association). Activity Report 2015; The European Cement Association: Brussels, Belgium, 2016. [Google Scholar]
- Tamanna, K.; Raman, S.N.; Jamil, M.; Hamid, R. Utilization of wood waste ash in construction technology: A review. Constr. Build. Mater. 2020, 237, 117654. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Jamil, M.; Karim, M.R.; Zain, M.F.M.; Kaish, A.B.M.A. Filler effect of pozzolanic materials on the strength and microstructure development of mortar. KSCE J. Civ. Eng. 2017, 21, 274–284. [Google Scholar] [CrossRef]
- Celik, K.; Jackson, M.D.; Mancio, M.; Meral, C.; Emwas, A.H.; Mehta, P.K.; Monteiro, P.J.M. High-volume natural volcanic pozzolan and limestone powder as partial replacements for portland cement in self-compacting and sustainable concrete. Cem. Concr. Compos. 2014, 45, 136–147. [Google Scholar] [CrossRef] [Green Version]
- CEMBUREAU. Cement 101: Key Facts & Figures. Available online: https://cembureau.eu/cement-101/key-facts-figures/ (accessed on 29 July 2017).
- Shubbar, A.A.; Jafer, H.; Abdulredha, M.; Al-khafaji, Z.S.; Nasr, M.S.; Al Masoodi, Z.; Sadique, M. Properties of cement mortar incorporated high volume fraction of GGBFS and CKD from 1 day to 550 days. J. Build. Eng. 2020, 30, 101327. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Mateus, R.; Camões, A.; Branco, F.G. Quality and durability properties and life-cycle assessment of high volume biomass fly ash mortar. Constr. Build. Mater. 2019, 197, 195–207. [Google Scholar] [CrossRef] [Green Version]
- WBCSD-CSI (World Business Council for Sustainable Development–Cement Sustainability Initiative. CO2 and Climate Protection. Available online: http://www.wbcsdcement.org/index.php/key-issues/climate-protection (accessed on 31 March 2017).
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Comans, R.N.J. Technical and environmental performance of lower carbon footprint cement mortars containing biomass fly ash as a secondary cementitious material. Resour. Conserv. Recycl. 2018, 134, 25–33. [Google Scholar] [CrossRef]
- UNFCCC (United Nations Framework Convention on Climate Change). Adoption of the Paris Agreement, FCCC/CP/2015/L.9/Rev.1. In Proceedings of the Conference Parties on Its Twenty First Session, Paris, France, 30 November–11 December 2015. [Google Scholar]
- UNFCCC (United Nations Framework Convention on Climate Change). Paris Agreement—Status of Ratification. Available online: http://unfccc.int/paris_agreement/items/9444.php (accessed on 5 July 2017).
- ASTM C219-14a. Standard Terminology Relating to Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Papayianni, I.; Karaveziroglou, M.; Athanassiou, F.; Georgisoudi, K.; Revithiadou, K. Mortars used for intervention in archaeological site of Ancient Olynthos. Trans. Built Environ. 1995, 15, 223–230. [Google Scholar]
- ASTM C270-14a. Standard Specification for Mortar for Unit Masonry; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- EN 1504-3. Products and systems for the protection and repair of concrete structures- Definitions, requirements, quality control and evaluation of conformity. In Part 3: Structural and Non-Structural Repair; CEN (European Committee for Standardization): Brussels, Belgium, 2006. [Google Scholar]
- FDOT (Florida Department of Transportation). Materials for concrete repair. In Standard Specifications for Road and Bridge Construction; FDOT: Tallahassee, FL, USA, 2017; Section 930; pp. 1069–1075. [Google Scholar]
- ACI 549.1R-93. Guide for the Design, Construction and Repair of Ferrocement; American Concrete Institute (ACI): Farmington Hills, MI, USA, 1993. [Google Scholar]
- Rao, S.; Silva, P.; de Brito, J. Experimental study of the mechanical properties and durability of self-compacting mortars with nano materials (SiO2 and TiO2). Constr. Build. Mater. 2015, 96, 508–517. [Google Scholar] [CrossRef]
- Parghi, A.; Alam, M.S. Physical and mechanical properties of cementitious composites containing recycled glass powder (RGP) and styrene butadiene rubber (SBR). Constr. Build. Mater. 2016, 104, 34–43. [Google Scholar] [CrossRef]
- Sigvardsen, N.M.; Ottosen, L.M. Characterization of coal bio ash from wood pellets and low-alkali coal fly ash and use as partial cement replacement in mortar. Cem. Concr. Compos. 2019, 95, 25–32. [Google Scholar] [CrossRef]
- Hsu, S.; Chi, M.; Huang, R. Effect of fineness and replacement ratio of ground fly ash on properties of blended cement mortar. Constr. Build. Mater. 2018, 176, 250–258. [Google Scholar] [CrossRef]
- Wongkeo, W.; Thongsanitgarn, P.; Ngamjarurojana, A.; Chaipanich, A. Compressive strength and chloride resistance of self-compacting concrete containing high level fly ash and silica fume. Mater. Des. 2014, 64, 261–269. [Google Scholar] [CrossRef]
- Supit, S.W.M.; Shaikh, F.U.A.; Sarker, P.K. Effect of ultrafine fly ash on mechanical properties of high volume fly ash mortar. Constr. Build. Mater. 2014, 51, 278–286. [Google Scholar] [CrossRef]
- Antoni; Chandra, L.; Hardjito, D. The impact of using fly ash, silica fume and calcium carbonate on the workability and compressive strength of mortar. Procedia Eng. 2015, 125, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Turk, K. Viscosity and hardened properties of self-compacting mortars with binary and ternary cementitious blends of fly ash and silica fume. Constr. Build. Mater. 2012, 37, 326–334. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Soriano, L.; Payá, J.; Monzó, J.; Borrachero, M.V.; Tashima, M.M. High strength mortars using ordinary Portland cement–fly ash–fluid catalytic cracking catalyst residue ternary system (OPC/FA/FCC). Constr. Build. Mater. 2016, 106, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Piedrahita, J.C.; Montes-García, P.; Mendoza-Rangel, J.M.; López Calvo, H.Z.; Valdez-Tamez, P.L.; Martínez-Reyes, J. Mechanical and durability properties of mortars prepared with untreated sugarcane bagasse ash and untreated fly ash. Constr. Build. Mater. 2016, 105, 69–81. [Google Scholar] [CrossRef]
- Kondraivendhan, B.; Bhattacharjee, B. Flow behavior and strength for fly ash blended cement paste and mortar. Int. J. Sustain. Built Environ. 2015, 4, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A. Effect of incinerator bottom ash properties on mechanical and pore size of blended cement mortars. Mater. Des. 2012, 36, 859–864. [Google Scholar] [CrossRef]
- Oruji, S.; Brake, N.A.; Nalluri, L.; Guduru, R.K. Strength activity and microstructure of blended ultra-fine coal bottom ash-cement mortar. Constr. Build. Mater. 2017, 153, 317–326. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Beltrán, M.G.; López, M.; Agrela, F. Effects of treatments on biomass bottom ash applied to the manufacture of cement mortars. J. Clean. Prod. 2017, 154, 424–435. [Google Scholar] [CrossRef]
- Menéndez, E.; Álvaro, A.M.; Hernández, M.T.; Parra, J.L. New methodology for assessing the environmental burden of cement mortars with partial replacement of coal bottom ash and fly ash. J. Environ. Manag. 2014, 133, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Maschio, S.; Tonello, G.; Piani, L.; Furlani, E. Fly and bottom ashes from biomass combustion as cement replacing components in mortars production: Rheological behaviour of the pastes and materials compression strength. Chemosphere 2011, 85, 666–671. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, H.J.; Kim, H.K.; Lee, H.K. Resistance of coal bottom ash mortar against the coupled deterioration of carbonation and chloride penetration. Mater. Des. 2016, 93, 160–167. [Google Scholar] [CrossRef]
- Kim, H.K. Utilization of sieved and ground coal bottom ash powders as a coarse binder in high-strength mortar to improve workability. Constr. Build. Mater. 2015, 91, 57–64. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H. Microstructural and strength characteristics of high-strength mortar using nontraditional supplementary cementitious materials. J. Mater. Civ. Eng. 2019, 31, 04019017. [Google Scholar] [CrossRef]
- Hot, J.; Cyr, M.; Augeard, E.; Eekhout, M. An investigation of CaSi silica fume characteristics and its possible utilization in cement-based and alkali-activated materials. Constr. Build. Mater. 2015, 101, 456–465. [Google Scholar] [CrossRef]
- Dawood, E.T.; Ramli, M. Development of high strength flowable mortar with hybrid fiber. Constr. Build. Mater. 2010, 24, 1043–1050. [Google Scholar] [CrossRef]
- Senhadji, Y.; Escadeillas, G.; Mouli, M.; Khelafi, H. Benosman Influence of natural pozzolan, silica fume and limestone fine on strength, acid resistance and microstructure of mortar. Powder Technol. 2014, 254, 314–323. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; İnan Sezer, G.; Ramyar, K. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures viewpoints. Constr. Build. Mater. 2014, 70, 17–25. [Google Scholar] [CrossRef]
- Rossen, J.E.; Lothenbach, B.; Scrivener, K.L. Composition of C-S-H in pastes with increasing levels of silica fume addition. Cem. Concr. Res. 2015, 75, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Y.; Ouyang, P.; Yang, Y. Hydration of the silica fume-Portland cement binary system at lower temperature. Constr. Build. Mater. 2015, 93, 919–925. [Google Scholar] [CrossRef]
- İnan Sezer, G. Compressive strength and sulfate resistance of limestone and/or silica fume mortars. Constr. Build. Mater. 2012, 26, 613–618. [Google Scholar] [CrossRef]
- Koksal, F.; Gencel, O.; Kaya, M. Combined effect of silica fume and expanded vermiculite on properties of lightweight mortars at ambient and elevated temperatures. Constr. Build. Mater. 2015, 88, 175–187. [Google Scholar] [CrossRef]
- Kırca, Ö.; Özgür Yaman, İ.; Tokyay, M. Compressive strength development of calcium aluminate cement–GGBFS blends. Cem. Concr. Compos. 2013, 35, 163–170. [Google Scholar] [CrossRef]
- Kuo, W.T.; Chen, S.H.; Wang, H.Y.; Lin, J.C. A study on the mechanical and electricity properties of cement mortar added with GGBFS and piezoelectric powder. Constr. Build. Mater. 2013, 49, 251–256. [Google Scholar] [CrossRef]
- Memon, N.A.; Sumadi, S.R.; Ramli, M. Performance of high workability slag-cement mortar for ferrocement. Build. Environ. 2007, 42, 2710–2717. [Google Scholar] [CrossRef]
- Sakir, S.; Kaish, A.B.M.A.; Raman, S.; Mutalib, A. Recent trends in development of self-flowing mortar incorporating supplementary cementitious materials. In Proceedings of the 2nd International Electronic Conference on Materials, 2–16 May 2016; MDPI: Basel, Switzerland, 2016; Volume 2, p. 001. [Google Scholar]
- Lomboy, G.; Sundararajan, S.; Wang, K.; Subramaniam, S. A test method for determining adhesion forces and Hamaker constants of cementitious materials using atomic force microscopy. Cem. Concr. Res. 2011, 41, 1157–1166. [Google Scholar] [CrossRef]
- Onn, C.C.; Mo, K.H.; Radwan, M.K.H.; Liew, W.H.; Ng, C.G.; Yusof, S. Strength, carbon footprint and cost considerations of mortar blends with high volume ground granulated blast furnace slag. Sustainability 2019, 11, 7194. [Google Scholar] [CrossRef] [Green Version]
- Shubbar, A.A.; Al-Shaer, A.; AlKizwini, R.S.; Hashim, K.; Hawesah, H.A.; Sadique, M. Investigating the influence of cement replacement by high volume of GGBS and PFA on the mechanical performance of cement mortar. In Proceedings of the International Conference on Civil and Environmental Engineering Technologies, Najaf, Iraq, 23–24 April 2019; IOP Publishing: Bristol, UK, 2019; Volume 584, p. 012022. [Google Scholar]
- Benabed, B.; Kadri, E.H.; Azzouz, L.; Kenai, S. Properties of self-compacting mortar made with various types of sand. Cem. Concr. Compos. 2012, 34, 1167–1173. [Google Scholar] [CrossRef]
- Silva, P.R.D.; de Brito, J. Fresh-state properties of self-compacting mortar and concrete with combined use of limestone filler and fly ash. Mater. Res. 2015, 18, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, S.A.; Bier, T.A. Blends of limestone powder and fly-ash enhance the response of self-compacting mortars. Constr. Build. Mater. 2012, 27, 398–403. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Makhloufi, Z.; Chettih, M.; Bederina, M.; Kadri, E.L.H.; Bouhicha, M. Effect of quaternary cementitious systems containing limestone, blast furnace slag and natural pozzolan on mechanical behavior of limestone mortars. Constr. Build. Mater. 2015, 95, 647–657. [Google Scholar] [CrossRef]
- Ezziane, K.; Kadri, E.-H.H.; Bougara, A.; Bennacer, R. Analysis of mortar long-term strength with supplementary cementitious materials cured at different temperatures. ACI Mater. J. 2010, 107, 323–331. [Google Scholar]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Plé, O.; Alengaram, U.J. Combination effect of limestone filler and slag on delayed ettringite formation in heat-cured mortar. J. Mater. Civ. Eng. 2020, 32, 04019365. [Google Scholar] [CrossRef]
- Celik, K.; Hay, R.; Hargis, C.W.; Moon, J. Effect of volcanic ash pozzolan or limestone replacement on hydration of Portland cement. Constr. Build. Mater. 2019, 197, 803–812. [Google Scholar] [CrossRef]
- Vejmelková, E.; Pavlíková, M.; Keppert, M.; Keršner, Z.; Rovnaníková, P.; Ondráček, M.; Sedlmajer, M.; Černý, R. High performance concrete with Czech metakaolin: Experimental analysis of strength, toughness and durability characteristics. Constr. Build. Mater. 2010, 24, 1404–1411. [Google Scholar] [CrossRef]
- Mehdipour, I.; Vahdani, M.; Amini, K.; Shekarchi, M. Linking stability characteristics to material performance of self-consolidating concrete-equivalent-mortar incorporating fly ash and metakaolin. Constr. Build. Mater. 2016, 105, 206–217. [Google Scholar] [CrossRef]
- Cassagnabère, F.; Diederich, P.; Mouret, M.; Escadeillas, G.; Lachemi, M. Impact of metakaolin characteristics on the rheological properties of mortar in the fresh state. Cem. Concr. Compos. 2013, 37, 95–107. [Google Scholar] [CrossRef]
- Jiang, G.; Rong, Z.; Sun, W. Effects of metakaolin on mechanical properties, pore structure and hydration heat of mortars at 0.17 w/b ratio. Constr. Build. Mater. 2015, 93, 564–572. [Google Scholar] [CrossRef]
- Nadeem, A.; Memon, S.A.; Lo, T.Y. Mechanical performance, durability, qualitative and quantitative analysis of microstructure of fly ash and Metakaolin mortar at elevated temperatures. Constr. Build. Mater. 2013, 38, 338–347. [Google Scholar] [CrossRef]
- Al-Fadala, S.; Chakkamalayath, J.; Al-Bahar, S.; Al-Aibani, A.; Ahmed, S. Significance of performance based specifications in the qualification and characterization of blended cement using volcanic ash. Constr. Build. Mater. 2017, 144, 532–540. [Google Scholar] [CrossRef]
- Diaz-Loya, I.; Juenger, M.; Seraj, S.; Minkara, R. Extending supplementary cementitious material resources: Reclaimed and remediated fly ash and natural pozzolans. Cem. Concr. Compos. 2017, 101, 44–51. [Google Scholar] [CrossRef]
- Hossain, K.M.A.; Lachemi, M. Corrosion resistance and chloride diffusivity of volcanic ash blended cement mortar. Cem. Concr. Res. 2004, 34, 695–702. [Google Scholar] [CrossRef]
- Hossain, K.M.A. Blended cement using volcanic ash and pumice. Cem. Concr. Res. 2003, 33, 1601–1605. [Google Scholar] [CrossRef]
- Hassan, I.O.; Ismail, M.; Forouzani, P.; Majid, Z.A.; Mirza, J. Flow characteristics of ternary blended self-consolidating cement mortars incorporating palm oil fuel ash and pulverised burnt clay. Constr. Build. Mater. 2014, 64, 253–260. [Google Scholar] [CrossRef]
- Bamaga, S.O.; Ismail, M.A.; Majid, Z.A.; Ismail, M.; Hussin, M.W. Evaluation of sulfate resistance of mortar containing palm oil fuel ash from different sources. Arab. J. Sci. Eng. 2013, 38, 2293–2301. [Google Scholar] [CrossRef]
- Lim, N.H.A.S.; Ismail, M.A.; Lee, H.S.; Hussin, M.W.; Sam, A.R.M.; Samadi, M. The effects of high volume nano palm oil fuel ash on microstructure properties and hydration temperature of mortar. Constr. Build. Mater. 2015, 93, 29–34. [Google Scholar] [CrossRef]
- Chandara, C.; Mohd Azizli, K.A.; Ahmad, Z.A.; Saiyid Hashim, S.F.; Sakai, E. Heat of hydration of blended cement containing treated ground palm oil fuel ash. Constr. Build. Mater. 2012, 27, 78–81. [Google Scholar] [CrossRef]
- Farzadnia, N.; Noorvand, H.; Yasin, A.M.; Aziz, F.N. The effect of nano silica on short term drying shrinkage of POFA cement mortars. Constr. Build. Mater. 2015, 95, 636–646. [Google Scholar] [CrossRef]
- Jaturapitakkul, C.; Tangpagasit, J.; Songmue, S.; Kiattikomol, K. Filler effect and pozzolanic reaction of ground palm oil fuel ash. Constr. Build. Mater. 2011, 25, 4287–4293. [Google Scholar] [CrossRef]
- Ganesan, K.; Rajagopal, K.; Thangavel, K. Rice husk ash blended cement: Assessment of optimal level of replacement for strength and permeability properties of concrete. Constr. Build. Mater. 2008, 22, 1675–1683. [Google Scholar] [CrossRef]
- Antiohos, S.K.; Papadakis, V.G.; Tsimas, S. Rice husk ash (RHA) effectiveness in cement and concrete as a function of reactive silica and fineness. Cem. Concr. Res. 2014, 61–62, 20–27. [Google Scholar] [CrossRef]
- Zunino, F.; Lopez, M. Decoupling the physical and chemical effects of supplementary cementitious materials on strength and permeability: A multi-level approach. Cem. Concr. Compos. 2016, 65, 19–28. [Google Scholar] [CrossRef]
- Chatveera, B.; Lertwattanaruk, P. Evaluation of nitric and acetic acid resistance of cement mortars containing high-volume black rice husk ash. J. Environ. Manag. 2014, 133, 365–373. [Google Scholar] [CrossRef]
- Lim, J.L.G.; Raman, S.N.; Lai, F.-C.; Zain, M.F.M.; Hamid, R. Synthesis of nano cementitious additives from agricultural wastes for the production of sustainable concrete. J. Clean. Prod. 2018, 171, 1150–1160. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Gupta, S.; Kua, H.W. Application of rice husk biochar and thermally treated low silica rice husk ash to improve physical properties of cement mortar. Theor. Appl. Fract. Mech. 2019, 104, 102376. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Durability of mortar using waste glass powder as cement replacement. Constr. Build. Mater. 2012, 36, 205–215. [Google Scholar] [CrossRef]
- Calmon, J.L.; Sauer, A.S.; Vieira, G.L.; Teixeira, J.E.S.L. Effects of windshield waste glass on the properties of structural repair mortars. Cem. Concr. Compos. 2014, 53, 88–96. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of curing temperature and glass type on the pozzolanic reactivity of glass powder. Cem. Concr. Res. 2014, 58, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Zanwar, A.B.; Patil, Y.D. Enhancement of Sustainable Mortar by Using Fine Glass Powder. In Lecture Notes in Civil Engineering; Shukla, S.K., Barai, S., Mehta, A., Eds.; Springer: Singapore, 2020; Volume 35, pp. 65–72. ISBN 9789811374807. [Google Scholar]
- Patel, D.; Tiwari, R.P.; Shrivastava, R.; Yadav, R.K. Effective utilization of waste glass powder as the substitution of cement in making paste and mortar. Constr. Build. Mater. 2019, 199, 406–415. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Mohseni, E.; Yazdi, M.A.; Sarker, P.; Ranjbar, M.M. Effect of nano-CuO and fly ash on the properties of self-compacting mortar. Constr. Build. Mater. 2015, 94, 758–766. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rukzon, S. Strength and chloride resistance of the blended Portland cement mortar containing rice husk ash and ground river sand. Mater. Struct. 2015, 48, 3771–3777. [Google Scholar] [CrossRef]
- Scheetz, B.E.; Earle, R. Utilization of fly ash. Curr. Opin. Solid State Mater. Sci. 1998, 3, 510–520. [Google Scholar] [CrossRef]
- Jow, J.; Dong, Y.; Zhao, Y.; Ding, S.; Li, Q. Fly Ash-based Technologies and Value-added Products Based on Materials Science. In Proceedings of the 2015 World of Coal Ash (WOCA) Conference, Nashville, TN, USA, 5–7 May 2015. [Google Scholar]
- NETL (National Energy Technology Laboratory). American Coal Ash Association 2012 Coal Combustion Product Report. Available online: https://www.netl.doe.gov/research/Coal/energy-systems/gasification/gasifipedia/solid-waste-bg (accessed on 22 February 2020).
- Lima, A.T.; Ottosen, L.M.; Ribeiro, A.B. Assessing fly ash treatment: Remediation and stabilization of heavy metals. J. Environ. Manag. 2012, 95, 110–115. [Google Scholar] [CrossRef]
- Yin, K.; Ahamed, A.; Lisak, G. Environmental perspectives of recycling various combustion ashes in cement production—A review. Waste Manag. 2018, 78, 401–416. [Google Scholar] [CrossRef]
- Siriruang, C.; Toochinda, P.; Julnipitawong, P.; Tangtermsirikul, S. CO2 capture using fly ash from coal fired power plant and applications of CO2-captured fly ash as a mineral admixture for concrete. J. Environ. Manag. 2016, 170, 70–78. [Google Scholar] [CrossRef]
- Beltrán, M.G.; Barbudo, A.; Agrela, F.; Jiménez, J.R.; de Brito, J. Mechanical performance of bedding mortars made with olive biomass bottom ash. Constr. Build. Mater. 2016, 112, 699–707. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.-P.; Dou, X.; Lisak, G.; Chang, V.W.-C. Co-complexation effects during incineration bottom ash leaching via comparison of measurements and geochemical modeling. J. Clean. Prod. 2018, 189, 155–168. [Google Scholar] [CrossRef]
- Ban, C.C.; Ramli, M. The implementation of wood waste ash as a partial cement replacement material in the production of structural grade concrete and mortar: An overview. Resour. Conserv. Recycl. 2011, 55, 669–685. [Google Scholar]
- Paris, J.M.; Roessler, J.G.; Ferraro, C.C.; DeFord, H.D.; Townsend, T.G. A review of waste products utilized as supplements to Portland cement in concrete. J. Clean. Prod. 2016, 121, 1–18. [Google Scholar] [CrossRef]
- Rao, G.A. Investigations on the performance of silica fume-incorporated cement pastes and mortars. Cem. Concr. Res. 2003, 33, 1765–1770. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Wei, J.; Li, J.; Zhang, P. Preparation of high performance blended cements and reclamation of iron concentrate from basic oxygen furnace steel slag. Resour. Conserv. Recycl. 2011, 56, 48–55. [Google Scholar] [CrossRef]
- Van Oss, H.G. Iron and Steel Slag. In Mineral Commodity Summaries; U.S. Geological Survey: Reston, VI, USA, 2015; pp. 82–83. [Google Scholar]
- Zhang, T.; Gao, P.; Gao, P.; Wei, J.; Yu, Q. Effectiveness of novel and traditional methods to incorporate industrial wastes in cementitious materials—An overview. Resour. Conserv. Recycl. 2013, 74, 134–143. [Google Scholar] [CrossRef]
- Rashad, A.M. An overview on rheology, mechanical properties and durability of high- volume slag used as a cement replacement in paste, mortar and concrete. Constr. Build. Mater. 2018, 187, 89–117. [Google Scholar] [CrossRef]
- Willett, J.C.; Neely, P.R. Stone, Crushed [Advance Release]. In 2014 Minerals Yearbook; U.S. Geological Survey: Leiston, VA, USA, 2016; pp. 71.1–71.24. [Google Scholar]
- Elgalhud, A.A.; Dhir, R.K.; Ghataora, G. Limestone addition effects on concrete porosity. Cem. Concr. Compos. 2016, 72, 222–234. [Google Scholar] [CrossRef]
- Siddique, R. Effect of volcanic ash on the properties of cement paste and mortar. Resour. Conserv. Recycl. 2011, 56, 66–70. [Google Scholar] [CrossRef]
- Masmoudi, R.; Kupwade-Patil, K.; Bumajdad, A.; Büyüköztürk, O. In situ Raman studies on cement paste prepared with natural pozzolanic volcanic ash and Ordinary Portland Cement. Constr. Build. Mater. 2017, 148, 444–454. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Megat Johari, M.A.; Tayeh, B.A.; Yusuf, M.O. Pozzolanic reactivity of ultrafine palm oil fuel ash waste on strength and durability performances of high strength concrete. J. Clean. Prod. 2017, 144, 511–522. [Google Scholar] [CrossRef]
- Noorvand, H.; Ali, A.A.A.; Demirboga, R.; Noorvand, H.; Farzadnia, N. Physical and chemical characteristics of unground palm oil fuel ash cement mortars with nanosilica. Constr. Build. Mater. 2013, 48, 1104–1113. [Google Scholar] [CrossRef]
- USDA (United States Department of Agriculture). Rice Yearbook; USDA Economic Research Service: Washington, DC, USA, 2017.
- Gowda, M.R.; Narasimhan, M.C.; Karisiddappa. Development and study of the strength of self-compacting mortar mixes using local materials. J. Mater. Civ. Eng. 2011, 23, 526–532. [Google Scholar] [CrossRef]
- Jani, Y.; Hogland, W. Waste glass in the production of cement and concrete—A review. J. Environ. Chem. Eng. 2014, 2, 1767–1775. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of combined glass particles on hydration in cementitious systems. J. Mater. Civ. Eng. 2015, 27, 1–13. [Google Scholar] [CrossRef]
- Kim, S.; Hanif, A.; Jang, I. Incorporating liquid crystal display (LCD) glass waste as supplementary cementing material (SCM) in cement mortars—Rationale based on hydration, durability, and pore characteristics. Materials 2018, 11, 2538. [Google Scholar] [CrossRef] [Green Version]
- ASTM C618-15. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Taha, B.; Nounu, G. Using lithium nitrate and pozzolanic glass powder in concrete as ASR suppressors. Cem. Concr. Compos. 2008, 30, 497–505. [Google Scholar] [CrossRef]
- Dodson, V.H. Concrete Admixtures; Springer Science+Business Media: New York City, NY, USA, 1990; ISBN 978-1-4757-4845-1. [Google Scholar]
- Roussel, N.; Lemaître, A.; Flatt, R.J.; Coussot, P. Steady state flow of cement suspensions: A micromechanical state of the art. Cem. Concr. Res. 2010, 40, 77–84. [Google Scholar] [CrossRef]
- Lu, G.; Wang, K.; Rudolphi, T.J. Modeling rheological behavior of highly flowable mortar using concepts of particle and fluid mechanics. Cem. Concr. Compos. 2008, 30, 1–12. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Safiuddin, M.; West, J.S.; Soudki, K.A. Self-Consolidating High Performance Concrete with Rice Husk Ash: Components, Properties and Mixture Design; VDM Publishing House Ltd.: Saarbrücken, Germany, 2009; ISBN 978-3-639-14506-9. [Google Scholar]
- Shen, L.; Struble, L.; Lange, D. Modeling dynamic segregation of self-consolidating concrete. ACI Mater. J. 2009, 106, 375–380. [Google Scholar]
- Flatt, R.J. Dispersion forces in cement suspensions. Cem. Concr. Res. 2004, 34, 399–408. [Google Scholar] [CrossRef]
- Flatt, R.J.; Bowen, P. Electrostatic repulsion between particles in cement suspensions: Domain of validity of linearized Poisson–Boltzmann equation for nonideal electrolytes. Cem. Concr. Res. 2003, 33, 781–791. [Google Scholar] [CrossRef]
- Maheswaran, S.; Iyer, N.R.; Palani, G.S.; Pandi, R.A.; Dikar, D.D.; Kalaiselvam, S. Effect of high temperature on the properties of ternary blended cement pastes and mortars. J. Therm. Anal. Calorim. 2015, 122, 775–786. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lee, H.S. Simulation of a temperature rise in concrete incorporating fly ash or slag. Mater. Struct. 2010, 43, 737–754. [Google Scholar] [CrossRef]
- Narmluk, M.; Nawa, T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem. Concr. Res. 2011, 41, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, P.; Cyr, M.; Ringot, E. Mineral admixtures in mortars: Effect of inert materials on short-term hydration. Cem. Concr. Res. 2003, 33, 1939–1947. [Google Scholar] [CrossRef]
- ACI 222R-01. Protection of Metals in Concrete against Corrosion (Reapproved 2010); American Concrete Institute (ACI): Farmington Hills, MI, USA, 2011. [Google Scholar]
- Young, J.F. Cement-based materials. Curr. Opin. Solid State Mater. Sci. 1998, 3, 505–509. [Google Scholar] [CrossRef]
- Kwan, A.K.H.; Li, Y. Effects of fly ash microsphere on rheology, adhesiveness and strength of mortar. Constr. Build. Mater. 2013, 42, 137–145. [Google Scholar] [CrossRef]
- Tudjono, S.; Purwanto Apsari, K.T. Study the effect of adding nano fly ash and nano lime to compressive strength of mortar. Procedia Eng. 2014, 95, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kwan, A.K.H. Ternary blending of cement with fly ash microsphere and condensed silica fume to improve the performance of mortar. Cem. Concr. Compos. 2014, 49, 26–35. [Google Scholar] [CrossRef]
- Shannag, M.J.; Mourad, S.M. Flowable high strength cementitious matrices for ferrocement applications. Constr. Build. Mater. 2012, 36, 933–939. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Argiz, C.; Gálvez, J.C.; Moragues, A. Effect of silica fume fineness on the improvement of Portland cement strength performance. Constr. Build. Mater. 2015, 96, 55–64. [Google Scholar] [CrossRef]
- Benabed, B.; Kenai, S.; Azzouz, L.; Kadri, E.H.; Belaidi, A.S.E. Effects of limestone quarry dust content on rheology and strength of self-compacting mortar. In Proceedings of the Twelfth International Conference on Recent Advances in Concrete Technology and Sustainability Issues, Prague, Czech Republic, 30 October–2 November 2012; pp. 377–388. [Google Scholar]
- Kwan, A.K.H.; McKinley, M. Effects of limestone fines on water film thickness, paste film thickness and performance of mortar. Powder Technol. 2014, 261, 33–41. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. A study on hydration, compressive strength, and porosity of Portland-limestone cement mixes containing SCMs. Cem. Concr. Compos. 2014, 51, 1–13. [Google Scholar] [CrossRef]
- Yerramala, A.; Ramachandurdu, C.; Bhaskar Desai, V. Flexural strength of metakaolin ferrocement. Compos. Part B Eng. 2013, 55, 176–183. [Google Scholar] [CrossRef]
- Yerramala, A.; Rama Chandurdu, C.; Bhaskar Desai, V. Impact strength of metakaolin ferrocement. Mater. Struct. 2014, 49, 5–15. [Google Scholar] [CrossRef]
- Kostuch, J.A.; Walter, G.V.; Jones, T.R. High performance concretes containing Metakaolin—A review. In Proceedings of the Concrete 2000: Economic and Durable Construction through Excellence, Dundee, Scotland, 7–9 September 1993; pp. 1799–1811. [Google Scholar]
- Taylor-Lange, S.C.; Riding, K.A.; Juenger, M.C.G. Increasing the reactivity of metakaolin-cement blends using zinc oxide. Cem. Concr. Compos. 2012, 34, 835–847. [Google Scholar] [CrossRef]
- Morsy, M.S.; Al-Salloum, Y.A.; Abbas, H.; Alsayed, S.H. Behavior of blended cement mortars containing nano-metakaolin at elevated temperatures. Constr. Build. Mater. 2012, 35, 900–905. [Google Scholar] [CrossRef]
- Argiz, C.; Menéndez, E.; Moragues, A. Advances in coal bottom ash use as a new common Portland cement constituent. In Service Life and Durability of Reinforced Concrete Structures: Selected Papers of the 8th International RILEM PhD Workshop held in Marne-la-Vallée, France, 26–27 September 2016; Andrade, C., Gulikers, J., Marie-Victoire, E., Eds.; Springer: Cham, Switzerland, 2019; Volume 17, pp. 43–53. ISBN 978-3-319-90236-4. [Google Scholar]
- Yao, Y.; Sun, H. Durability and leaching analysis of a cementitious material composed of high volume coal combustion byproducts. Constr. Build. Mater. 2012, 36, 97–103. [Google Scholar] [CrossRef]
- Hossack, A.M.; Thomas, M.D.A. Varying fly ash and slag contents in Portland limestone cement mortars exposed to external sulfates. Constr. Build. Mater. 2015, 78, 333–341. [Google Scholar] [CrossRef]
- Hossack, A.M.; Thomas, M.D.A. Evaluation of the effect of tricalcium aluminate content on the severity of sulfate attack in Portland cement and Portland limestone cement mortars. Cem. Concr. Compos. 2015, 56, 115–120. [Google Scholar] [CrossRef]
- Li, L.G.; Zhu, J.; Huang, Z.H.; Kwan, A.K.H.; Li, L.J. Combined effects of micro-silica and nano-silica on durability of mortar. Constr. Build. Mater. 2017, 157, 337–347. [Google Scholar] [CrossRef]
- Mlinarik, L.; Kopecskó, K. The influence of combined application of two SCMs on the corrosion and acid attack durability of mortars. Period. Polytech. Civ. Eng. 2016, 61, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Matos, A.M.; Sousa-Coutinho, J. ASR and sulphate performance of mortar containing industrial waste. Struct. Concr. 2016, 17, 84–95. [Google Scholar] [CrossRef]
- Siddique, R.; Bennacer, R. Use of iron and steel industry by-product (GGBS) in cement paste and mortar. Resour. Conserv. Recycl. 2012, 69, 29–34. [Google Scholar] [CrossRef]
- Hadj-sadok, A.; Kenai, S.; Courard, L.; Darimont, A. Microstructure and durability of mortars modified with medium active blast furnace slag. Constr. Build. Mater. 2011, 25, 1018–1025. [Google Scholar] [CrossRef]
- Torres, S.M.; Sharp, J.H.; Swamy, R.N.; Lynsdale, C.J.; Huntley, S.A. Long term durability of Portland-limestone cement mortars exposed to magnesium sulfate attack. Cem. Concr. Compos. 2003, 25, 947–954. [Google Scholar] [CrossRef]
- Yazıcı, Ş.; Arel, H.Ş.; Anuk, D. Influences of metakaolin on the durability and mechanical properties of mortars. Arab. J. Sci. Eng. 2014, 39, 8585–8592. [Google Scholar] [CrossRef]
- Bamaga, S.O.; Hussin, M.W.; Ismail, M.A. Palm Oil Fuel Ash: Promising supplementary cementing materials. KSCE J. Civ. Eng. 2013, 17, 1708–1713. [Google Scholar] [CrossRef]
- Chatveera, B.; Lertwattanaruk, P. Evaluation of sulfate resistance of cement mortars containing black rice husk ash. J. Environ. Manag. 2009, 90, 1435–1441. [Google Scholar] [CrossRef]
- ASTM C1437-15. Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Sakir, S.; Raman, S.N.; Kaish, A.B.M.A.; Mutalib, A.A. Self-flowing mortar for ferrocement in strengthening applications. Perspect. Sci. 2016, 8, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Phua, Z.; Giannis, A.; Dong, Z.L.; Lisak, G.; Ng, W.J. Characteristics of incineration ash for sustainable treatment and reutilization. Environ. Sci. Pollut. Res. 2019, 26, 16974–16997. [Google Scholar] [CrossRef]
- ASTM C33/C33M—16e1. Standard Specification for Concrete Aggregates; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Hunger, M.; Brouwers, H.J.H. Flow analysis of water-powder mixtures: Application to specific surface area and shape factor. Cem. Concr. Compos. 2009, 31, 39–59. [Google Scholar] [CrossRef]
- Kwan, A.K.H.; Fung, W.W.S.; Wong, H.H.C. Water film thickness, flowability and rheology of cement–sand mortar. Adv. Cem. Res. 2010, 22, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Aïtcin, P.C. Cements of yesterday and today – concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Holland, T.C. Silica Fume User’s Manual; Federal Highway Administration, U.S. Department of Transportation & Silica Fume Association: Washington, DC, USA, 2005. [Google Scholar]
- LNEC E-462. Resistance of Cements to Sulphate Attack; National Laboratory of Civil Engineering: Lisbon, Portugal, 2004. [Google Scholar]
- ASTM C1202-17. Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C1567-13. Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar-Bar Method); ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- CEB-FIP. Diagnosis and Assessment of Concrete Structures—State-of-Art Report; FIB—International Federation for Structural Concrete: Lausanne, Switzerland, 1989. [Google Scholar]
- Irassar, E.F. Sulfate attack on cementitious materials containing limestone filler—A review. Cem. Concr. Res. 2009, 39, 241–254. [Google Scholar] [CrossRef]
- Jamil, M.; Kaish, A.B.M.A.; Raman, S.N.; Zain, M.F.M. Pozzolanic contribution of rice husk ash in cementitious system. Constr. Build. Mater. 2013, 47, 588–593. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Leese, R.; Casey, D. Embodied CO2e of UK Cement, Additions and Cementitious Material; MPA Cement, Mineral Products Association (MPA): London, UK; Available online: https://cement.mineralproducts.org/documents/Factsheet_18.pdf (accessed on 24 March 2020).
- BS 8500-1:2006. Concrete—Complementary British Standard to BS EN 206-1—Part 1: Method of Specifying and Guidance for the Specifier; British Standards Institution: London, UK, 2006; p. 38. [Google Scholar]


| Purpose | Minimum Strength at 28 days (MPa) | Reference |
|---|---|---|
| Masonry work (ASTM Type M) | 17.2 | [15] |
| Structural repair (EN Class R3) | 25.0 | [16] |
| Concrete repair | 27.6 | [17] |
| Thin reinforced cementitious products | 35.0 | [18] |
| Structural repair (EN Class R4) | 45.0 | [16] |
| Chemical Composition (wt.%) | FA 1 | BA 1 | SF 1 | GGBS 1 | LP 1 | MK 1 | VA 1 | POFA 1 | RHA 1 | WGP 1 | OPC 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 36–65 | 44–59 | 85–99 | 28–41 | 0–8 | 49–69 | 45–65 | 47–69 | 17–94 | 56–81 | 16–23 |
| CaO | 1–19 | 1–17 | 0–4 | 37–50 | 45–55 | 0–2 | 3–11 | 4–12 | 0–2 | 5–11 | 49–69 |
| Al2O3 | 17–29 | 5–32 | 0–6 | 5–14 | 0–3 | 25–44 | 11–18 | 1–9 | 0–3 | 0–6 | 4–7 |
| Fe2O3 | 4–31 | 2–9 | 0–3 | 0–1 | 0–2 | 0–3 | 1–13 | 1–10 | 0–2 | 0–1 | 2–7 |
| MgO | 0–7 | 1–3 | 0–5 | 4–10 | 0–7 | 0–3 | 1–9 | 2–6 | 0–1 | 0–4 | 0–5 |
| SO3 | 0–3 | 0–2 | 0–2 | 0–3 | 0–1 | 0–1 | 0–1 | 0–3 | 0–1 | 0–1 | 0–1 |
| Na2O | 0–2 | 0–1 | 0–2 | 0–3 | 0–1 | 0–1 | 3–4 | 0–1 | 0–1 | 7–16 | 0–1 |
| K2O | 0–3 | 1–8 | 0–2 | 0–2 | 0–1 | 0–2 | 1–6 | 5–11 | 0–5 | 0–1 | 0–1 |
| P2O5 | 0–2 | 0–1 | 0–1 | – | – | 0–1 | 0–1 | 3–5 | – | – | – |
| TiO2 | 0–2 | 0–3 | – | 0–1 | – | 0–1 | 0–3 | – | – | – | – |
| LOI | 0–5 | 1–13 | 0–6 | 1–2 | 36–45 | 0–4 | 1–6 | 1–21 | 0–6 | 0–12 | – |
| Sp. gravity | 2.26 | 2.64 | 2.24 | 2.88 | 2.72 | 2.51 | 2.66 | 2.42 | 2.16 | 2.50 | 3.15 |
| Ref. | [19,20,21,22,23,24,25,26,27,28,29,30] | [31,32,33,34,35,36,37,38] | [20,23,39,40,41,42,43,44,45,46,47] | [7,48,49,50,51,52,53,54] | [41,46,55,56,57,58,59,60,61,62] | [43,58,63,64,65,66,67] | [60,62,68,69,70,71] | [38,72,73,74,75,76,77] | [78,79,80,81,82,83] | [84,85,86,87,88] | [20,40,50,51,55,72,89,90] |
| Flowability | Strength | |||||
|---|---|---|---|---|---|---|
| SCM | Effect | Replacement Ratio [% w/w] | Ref. | Effect | Replacement Ratio [% w/w] | Ref. |
| FA | ▲ | 5–30 | [20,24,25,29,56,89] | ▲ | 0–40 | [22,24,25,43,133,134] |
| ▼ | 25–30 | [26] | ▼ | 10–70 | [22,24,26,28,29,30,43,89] | |
| SF | ▲ | 7.3 | [135] | ▲ | 5–25 | [26,39,46,135,136,137] |
| ▼ | 5–20 | [26,46,101] | ▼ | 5–20 | [39] | |
| GGBS | ▲ | 10–30 | [49] | ▲ | 20–60 | [49,50] |
| ▼ | 10–70 | [49,53] | ||||
| WGP | ▼ | 5–25 | [20] | ▲ | 5–25 | [20,87] |
| ▼ | 5–40 | [84,85,87,88] | ||||
| LP | ▲ | 5–70 | [25,46,56,138,139] | ▲ | 2.4–15 | [55,138,139,140] |
| ▼ | 20–30 | [138] | ▼ | 5–50 | [41,46,59,62] | |
| MK | ▼ | 12.5–25 | [64,65] | ▲ | 5–20 | [43,64,67,141,142,143,144] |
| ▼ | 5–15 | [145] | ||||
| POFA | ▲ | 5–50 | [72] | ▲ | 10–80 | [74,77] |
| ▼ | 10–30 | [76,111] | ▼ | 10–30 | [38,76,111] | |
| RHA | ▼ | 5–20 | [113] | ▲ | 5–25 | [78,83] |
| ▼ | 5–30 | [79,80,83,113] | ||||
| BA | ▲ | 9–41 | [32,37] | ▲ | 5–33 | [32,34,35,146] |
| ▼ | 6–41 | [32,33,34,35,37,38,97,146] | ||||
| VA | – | – | – | ▲ | 20–30 | [60] |
| ▼ | 20–50 | [60,62,68,69,70,71] | ||||
| Carbonation Resistance | Acid Resistance | ASR Resistance | Sulphate Resistance | Chloride Resistance | Freezing and Thawing Resistance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCM | Effect | Ref. | Effect | Ref. | Effect | Ref. | Effect | Ref. | Effect | Ref. | Effect | Ref. |
| FA | – | – | – | – | ▲ | [147] | ▲ | [43,148,149] | ▲ | [29] | ▲ | [43] |
| SF | ▲ | [150] | ▲ | [151] | ▲ | [152] | ▲ | [43,84,148,152] | ▲ | [43,84] | ▲ | [43] |
| ▼ | [84] | |||||||||||
| GGBS | – | – | – | – | – | – | ▲ | [148,153] | ▲ | [154] | – | – |
| WGP | ▼ | [84] | – | – | ▲ | [84,152] | ▲ | [84,152] | ▲ | [84] | – | – |
| LP | ▼ | [58] | – | – | – | – | No effect | [148,149] | – | – | – | – |
| ▼ | [155] | |||||||||||
| MK | ▼ | [58] | ▲ | [151] | ▲ | [156] | ▲ | [43,148,156] | ▲ | [43,151] | ▲ | [64] |
| ▼ | [67] | |||||||||||
| POFA | – | – | – | – | – | – | ▲ | [157] | – | – | – | – |
| RHA | – | – | ▲ | [81] | – | – | ▲ | [158] | ▲ | [79,80,90] | – | – |
| BA | ▼ | [146] | – | – | ▲ | [146] | ▲ | [146] | – | – | ▼ | [146] |
| ▼ | [35] | |||||||||||
| VA | – | – | – | – | ▲ | [69] | – | – | ▲ | [70] | – | – |
| Flow Spread | Compressive Strength (28 days) | |||||||
|---|---|---|---|---|---|---|---|---|
| SCM | Value [mm] | Replacement Ratio [% w/w] | w/b Ratio (% w/w) | Ref. | Value [MPa] | Replacement Ratio [% w/w] | w/b Ratio (% w/w) | Ref. |
| FA | 241 | 25.0 | 0.43 | [26] | 122 | 35.0 | 0.21 | [133] |
| SF | 369 | 7.3 | 0.46 | [135] | 118 | 7.3 | 0.20 | [135] |
| GGBS | 232 | 30.0 | 0.70 | [49] | 57 | 50.0 | – | [50] |
| WGP | 216 | 5.0 | 0.40 | [20] | 56 | 20.0 | 0.35 | [87] |
| LP | 445 | 30.0 | 0.34 | [139] | 69 | 15.0 | 0.35 | [25] |
| MK | – | – | – | – | 120 | 20.0 | 0.30 | [67] |
| POFA | 310 | 5.0 | 0.35 | [72] | 75 | 10.0 | 0.35 | [111] |
| RHA | 255 | 20.0 | 0.84 | [113] | 70 | 20.0 | 0.40 | [83] |
| BA | 230 | 21.0 | 0.38 | [37] | 82 | 5.0 | 0.52 | [35] |
| VA | – | – | – | – | 45 | 10.0 | 0.40 | [68] |
| Cement Designation [175] | SCM Content | Equivalent CO2 (kg/ton) |
|---|---|---|
| CEM I | 0% | 860 |
| CEM II/A-LL or L | 6%–20% LP | 842–721 |
| CEM II/A-V | 6%–20% FA | 825–686 |
| CEM II/B-V | 21%–35% FA | 694–555 |
| CEM II/B-S | 21%–35% GGBS | 712–585 |
| CEM III/A | 36%–65% GGBS | 594–350 |
| CEM III/B | 66%–80% GGBS | 359–232 |
| CEM IV/B-V | 36%–55% FA | 564–381 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakir, S.; Raman, S.N.; Safiuddin, M.; Kaish, A.B.M.A.; Mutalib, A.A. Utilization of By-Products and Wastes as Supplementary Cementitious Materials in Structural Mortar for Sustainable Construction. Sustainability 2020, 12, 3888. https://doi.org/10.3390/su12093888
Sakir S, Raman SN, Safiuddin M, Kaish ABMA, Mutalib AA. Utilization of By-Products and Wastes as Supplementary Cementitious Materials in Structural Mortar for Sustainable Construction. Sustainability. 2020; 12(9):3888. https://doi.org/10.3390/su12093888
Chicago/Turabian StyleSakir, Shamir, Sudharshan N. Raman, Md. Safiuddin, A. B. M. Amrul Kaish, and Azrul A. Mutalib. 2020. "Utilization of By-Products and Wastes as Supplementary Cementitious Materials in Structural Mortar for Sustainable Construction" Sustainability 12, no. 9: 3888. https://doi.org/10.3390/su12093888
APA StyleSakir, S., Raman, S. N., Safiuddin, M., Kaish, A. B. M. A., & Mutalib, A. A. (2020). Utilization of By-Products and Wastes as Supplementary Cementitious Materials in Structural Mortar for Sustainable Construction. Sustainability, 12(9), 3888. https://doi.org/10.3390/su12093888







