Abstract
Potentially toxic elements are persistent in the environment and plants have the ability to absorb and transfer them from soil in edible parts. The objectives of this study were to characterize the distribution of Cd and Pb in quinoa tissues and to investigate their accumulation and transfer from irrigated water in edible parts of quinoa. For the purpose of this study experiment and simulated pollution in the form of different metal concentration in water that was used for irrigation was designed. Distribution of metals in quinoa were determined and analyzed in seed formation and maturation stage. Bioaccumulation and translocation factors were calculated to characterize the efficiency of quinoa to absorb metals. The results of our study indicated that quinoa adopts potentially toxic metals from substrate but does not accumulate them. The potential of such a conclusion is useful for exploring the use of quinoa as lead and cadmium excluders.
1. Introduction
In numerous publications in recent decades, the term “heavy metals” is a group term for metals that are contaminants. The toxicity of a substance can only be defined if the relationship between dose and effect is known. This relationship is a characteristic of each substance and it is necessary to know this relationship when determining whether a substance is toxic or not. The term “Potentially Toxic Element(s)” (PTEs) can include uniform and comparative use, and meanings on the basis of more reliable characterization [1].
Potentially toxic elements (PTEs) are not susceptible to biodegradation, they are persistent in the environment and plants can absorb them from soil and accumulate them in edible parts. Consequently, PTEs can enter into the food chain, creating a risk to human health [2,3].
Cadmium (Cd) is extremely toxic at very low concentration for both plants and humans [4]. Toxic effects of cadmium in plants is reflected through its affinity for the thiol group as Cd can block functional groups of biomolecules, which results in the inhibition of enzyme activity of important plant metabolic processes, such as photosynthesis and respiration [5]. Since it belongs to the group of metals whose ions can rapidly be absorbed by plant roots, Cd is very mobile in soil and available to plants, and can easily be implemented into the food chain [6].
The toxic effect of lead (Pb) is more expressed at higher concentration and duration of exposure. These levels of Pb concentration in the plant affect germination, growth, photosynthesis, water regime, mineral nutrition and enzyme activity [7,8]. Accumulation of Pb by plants has been reported in roots, shoots, leaves and seeds. However, the majority of absorbed Pb by plants resides in the root, having only small amounts translocated to the aerial parts of plants [8].
Despite their relatively low concentration in edible parts of plant, both Cd and Pb are reported as food contaminants and food agencies highlight their threat to human health [9]. The uptake, accumulation and translocation of toxic metals by plants can differ widely even across cultivars within the same species [10].
Quinoa (Chenopodium quinoa Will) is a pseudocereal originated in the Andean region of South America. This cereal has received an increased interest across Europe lately due to its high protein content and compositions of protein [11]. Because of its nutritional value (rich in protein, fat, dietary fiber and minerals) quinoa is applied in the production of bread, pasta and baby food [12]. The Food and Agriculture Organization declared 2013 to be the “International Year of Quinoa” [13].
In 1995, 61.0 tons of quinoa was exported from Peru; in 2014, global demand for quinoa was 600 times higher, so they exported 36,000 tons of quinoa [14].
Estimated high nutritional value in alternative cereals, including quinoa, is associated with their qualitative and quantitative composition of protein [15,16]. The content of protein in quinoa ranges in value from 12.1% to 14.5% [17,18], with the maximum content being up to 22.08% [19,20].
The bioaccumulation and translocation potentials of PTEs by plants grown on contaminated soil were studied on Plantago major L. [21], Triticum aestivum [22,23], 30 varieties of Brassica pekinensis L. [24], miscanthus [25,26] and 32 hybrid rice varieties [27]. Transfer of PTEs from water-to-plant were studied on 14 sunflower hybrids [28], two eucalypt hybrid clones [29], wheat and different vegetables [30].
In spite of some research characterizing accumulation and transfer of PTEs in crops, the literature search revealed that quinoa has not been a focus of such research, and so this was identified as a research gap by the authors of this paper. The objectives of this study were to characterize the distribution of Cd and Pb in quinoa tissues and to investigate accumulation and transfer of Cd and Pb from irrigated water in edible parts of quinoa.
2. Materials and Methods
2.1. Experimental Design
For the purpose of this experiment, plant growth chamber (FITOCLIMA 20000HP MI234INR00—Ingles, 20–25 °C, 12–15 h photoperiod, RH 60%) was used. Composition of Floradur B (Floragard, Germany) was: 70% of German white peat + 30% German black peat + lime + macro and micro elements (Mg, B, Cu, Mn, Mo, Fe and Zn) + wetting agent. A Superfine structure was used with a pH of 5.6 and a salt content of 0.8 g/l.
Such an experiment simulated pollution in the form of different metal concentrations in water that was used for irrigation. The experiment had 12 treatments with 5 simultaneous repetitions, leading to a total of 60 variations. Solutions for plant watering were made by dissolving salts of lead (Pb (CH3COO)2 × 3H2O) and cadmium (3CdSO4 × 8H2O) in tap water.
During the entire duration of the experiment, soil moisture was maintained at level of 60% of the water retention capacity.
After 4 weeks, continuously to the end of the experiment, the following treatments were applied: I—tap water, II—5 mg/kg Pb, III—50 mg/kg Pb, IV—100 mg/kg Pb, V—5 mg/kg Cd, VI—50 mg/kg Cd, VII—100 mg/kg Cd, VIII—5 mg/kg Pb and Cd, IX—50 mg/kg Pb and Cd, X—100 mg/kg Pb and Cd, XI—100 mg/kg Pb and 5 mg/kg Cd, XII—5 mg/kg Pb and 100 mg/kg Cd (Table 1).

Table 1.
Experimental design, applied treatments.
Grain formation was observed after 100 days and two plants from each treatment were harvested. After 20 days, maturation of grain was completed and the rest of the plants were harvested.
2.2. Chemical Analysis
The first set of harvested plants were separated into root, shoot, leaf and seed; while the second set of harvested plants were separated into root, shoot and seed. The plant samples were first air-drayed at room temperature and then in a drying chamber at 105 °C to constant weights. After drying, the samples were powered with IKA A11 mill for plant material.
For analysis of total Cd and Pb concentration in substrate, 2.0000 g of sample was weighted and digested with 20 mL of concentrated HNO3 and slowly heated to boiling temperatures. The reaction mixture was heated until no brown vapors were generated. A mixture of 3 mL of concentrated H2O2 was added into the cooled reaction and the reaction mixture was heated slowly to boiling. After the termination of brown vapor generation, the reaction mixture was re-cooled and 3 mL of concentrated H2O2 was added. The reaction mixture was gradually heated to boiling and after no brown vapor was generated, the reaction mixture was cooled and quantitatively transferred to a volumetric flask of 100 mL [31]. A similar procedure was used for the plant samples digestion [32].
Preparation of the substrate samples for determination of the available forms of Pb and Cd was carried out by extraction with a DTPA (diethylenetriaminepentaacetic acid) buffer solution [33].
The concentration of cadmium and lead in the solution obtained by digestion were determined by the inductively coupled plasma-mass spectrometry, ICP-OES, Thermo ICAP 6300 duo [34].
2.3. Bioaccumulation Factor (BAF) and Translocation Factor (TF) Analysis
The bioaccumulation factor (BAF) was defined as the ratio of Cd or Pb concentration in the roots and those in the substrate used for plant growth [35], and it was defined using Equation (1). The BAF was calculated as:
where Cplant and Csoil represent Pb or Cd concentration in plants and substrate on dry weight basis, respectively.
BAF = Cplant/Csoil,
The translocation factor is defined as the ratio of Cd or Pb concentration in the seed to those in the root [21], as given in Equation (2):
where Cgrain and Croot represent Pb or Cd concentration in plants on dry weight basis.
TF = Cgrain/Croot,
2.4. Statistical Analysis
Data were processed using one-way ANOVA and Tukey’s HSD post hoc test to distinguish statistical differences between the transfer of Cd and Pb from water—to—plant, bioaccumulation factors of Cd and Pb during formation and maturation of seed and transfer coefficients of Cd and Pb during formation and maturation of seed. An independent sample t-test was performed to analyze the bioaccumulation factor between Cd and Pb. In such a way, we analyzed the statistical difference between the two toxic metals. The level of statistical significance was set at 0.05. Statistical processing was performed using Microsoft Excel 2010 and SPSS Statistics 17.0. Two way ANOVA and Tukey’s post hoc test was also used to show interaction between treatments and PTEs.
3. Results
3.1. Transfer of PTEs from Water-to-Plant
Bioaccumulation and translocation of cadmium in quinoa during formation and maturation of seed within the 12 applied treatments is presented in Figure 1. Values in all parts of the plant for the treatments VII and IX and the difference between the content in the root and seed of the plant for the treatments IV, V, VI, VIII, X, XI and XII during formation of the seed were statistically significant (p < 0.05). At the stage of seed maturation, analysis showed a statistically significant difference (p < 0.05) between the root, stem and seed within treatments V, VI, VII, VIII, IX, X, XI and XII.
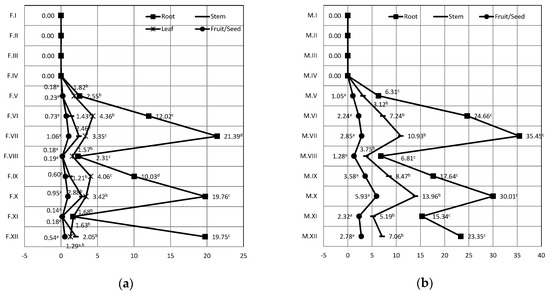
Figure 1.
The transfer of Cadmium from water—to—plant, Legend: (a) Seed formation, F.I–F.XII—treatment of plants during formation of seed; (b) Seed maturation, M.I–MXII—treatment of plants during maturation of seed. I—tap water; II—5 mg/kg of Pb; III—50 mg/kg Pb; IV—100 mg/kg Pb; V—5 mg/kg Cd; VI—50 mg/kg Cd; VII—100 pm Cd; VIII—5 mg/kg Pb and 5 mg/kg Cd; IX—50 mg/kg Pb and 50 mg/kg Cd; X—100 mg/kg Pb and 100 mg/kg Cd; XI—Pbmax Cdmin; XII—Pb min Cd max. Mean values within the same row with the different superscripts differ significantly (p < 0.05).
Figure 2 depicts the distribution of Pb in quinoa during the two observed stages of seed and the 12 treatments applied. Statistically significant differences were not found only within the treatments V, VI and VII (p < 0.05). For all other treatments, significant differences exist between the content in the roots and seeds.
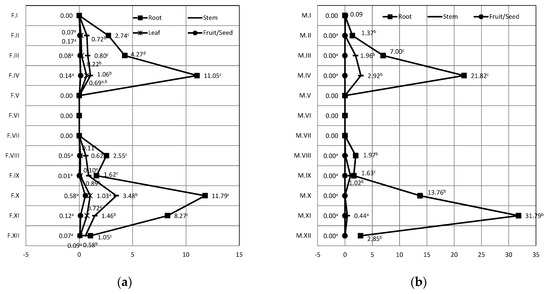
Figure 2.
The transfer of Lead from water—to—plant. Legend: (a) Seed formation, F.I–F.XII—treatments of plants during formation of seed; (b) Seed maturation, M.I–MXII—treatment of plants during maturation of seed. I—potable water; II—5 mg/kg of Pb; III—50 mg/kg Pb; IV—100 mg/kg Pb; V—5 mg/kg Cd; VI—50 mg/kg Cd; VII—100 pm Cd; VIII—5 mg/kg PB and 5 mg/kg Cd; IX—50 mg/kg Pb and 50 mg/kg Cd; X—100 mg/kg Pb and 100 mg/kg Cd; XI—Pbmax Cdmin; XII—Pb min Cd max. Mean values within the same row with the different superscripts differ significantly (p < 0.05).
As for the seed maturation stage, the highest concentration of Pb was determined in the root for the treatment IV (21.82 mg/kg).
3.2. Interaction between Treatments and PTEs
A two-way ANOVA was conducted that examined the transfer of Cd and Pb from water to plants during formation and maturation of seed. Analysis of the transfer of Cd and Pb from water to the roots of the plant during formation of the seed showed that there was no statistically significant interaction between Pb and Cd (F = 1.67; p = 0.22). However, the transfer of Cd and Pb from water to the seed showed a statistically significant interaction between the two PTEs (F = 6.36; p = 0.028).
During maturation of the seed, analysis of the transfer of Cd and Pb from water to the roots of the plant showed no statistically significant interaction between Pb and Cd (F = 1.733; p = 0.21). The transfer of Cd and Pb from water to the seed showed a statistically significant interaction between two PTEs (F = 12.19; p = 0.005). Further analysis confirmed that Cd and Pb were not significantly affecting treatments during formation and maturation of seed (p = 0.22; p = 0.49, respectively).
3.3. Bioaccumulation Factor
Bioaccumulation factor (BAF), for values of total content of Pb and Cd in substrate, during seed formation are presented in Table 2. These values indicate that the absorption was of medium intensity. The highest value of BAF for Cd was within the treatment IV (1.27) and for Pb within the treatment X (0.35).

Table 2.
Bioaccumulation factor of Cd and Pb, total content in substrate, the seed formation.
The results show that statistical differences were observed for treatments II, VII, IX and X (p < 0.05). Medium intensity absorption was also observed, in the seed maturation stage (Table 3) with the highest value within the treatment XI for both Cd (0.78) and Pb (0.88). Results show statistical differences for treatments III, IV, V, VI, VII, VIII and IX (p < 0.05).

Table 3.
Bioaccumulation factor of Cd and Pb, total content in substrate, the seed maturation.
DTPA extractable BAF of Cd and Pb (Figure 3) indicate that bioaccumulation was generally medium to high intensity. For the seed formation the highest value of Cd was for the treatment VII (5.27), while treatment IV showed the highest values for Pb (2.58). The highest value of Cd for the seed maturation was for the treatment VI (4.75), and for Pb for the treatment XI (5.51).
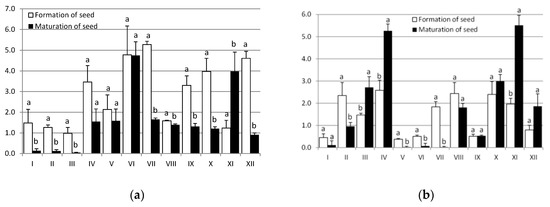
Figure 3.
DTPA (diethylenetriaminepentaacetic acid) extractable bioaccumulation factors of (a) Cadmium and (b) Lead during formation and maturation of seed. The diversities were expressed as different letters (p < 0.05).
The seed forming stage has stronger absorption of Cd, except for the treatment VI (Figure 3). In contrast for Pb, the stronger absorption is generally observed in the seed maturation stage.
Translocation factor (TF) of cadmium and lead during formation and maturation of seed is presented in the Figure 4. There is no significant difference between values of TF in these two stages for lead. However, for cadmium statistically significant difference exists for treatments VIII, IX, X and XII. TF’s values of metals in seed formation stage are lower than 1, except for control and treatment II for cadmium because of the low bioaccumulation in root for these two treatments.
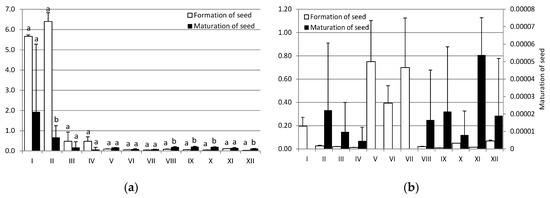
Figure 4.
Transfer coefficients of (a) Cadmium and (b) Lead during formation and maturation of seed. The diversities were expressed as different letters (p < 0.05). Where no letters are presented, there is no significant difference.
4. Discussion
The dominant toxic forms, accessible fractions, of the PTEs included in this study are free ions. It has been proved that their bioavailability is decreasing when the soil pH and organic content is increasing [36].
Distribution of Cd in the seed formation stage shows that the highest content was in the root of quinoa, with the highest concentration Cd within the treatments VII, 100 mg/kg Cd, (21.39 mg/kg), X, 100 mg/kg Pb and Cd, (19.76 mg/kg) and XII, 5 mg/kg Pb and 100 mg/kg Cd, (19.75 mg/kg). These results are consistent with the fact that in the treatments VII, X and XII, the highest concentration of Cd were applied (100 mg/kg in water for irrigation). The lowest Cd content was determined in the seed. In the seed maturation stage, the highest content can be observed in root and the lowest content in the seed. The highest concentration of Cd in the root was again within the treatments VII (35.45 mg/kg). Similar studies have shown low levels of metals in the seed, as well as a low translocation factor [22]. Studies of Holm Oak (Quercus ilex L.) exposed to the urban environment showed a significant concentration of Pb and Cd in leaves [37].
The maximum content of Pb, observing the overall distribution in quinoa during the seed formation and maturation, was determined in the root and the lowest was found in the seed. Within the seed formation stage, the highest concentration of Pb in the roots was determined for the treatments IV at 100 mg/kg Pb (Cd: 11.05 mg/kg), and X at 100 mg/kg Pb, (Cd: 11.79 mg/kg). The highest concentration in the seed was determined for the treatment X (0.57 mg/kg) with the highest concentration of Pb applied. Investigations of lead content in 30 varieties of Chinese cabbage (Brassica pekinensis L.), in the experiment, have confirmed that the amount of lead in the soil had a significant effect on the lead concentration in plants [38].
As for seed maturation stage, the highest concentration of Pb was determined in the root for the treatment IV (21.82 mg/kg). Result of this study are consistent with the study of wheat (Triticum aestivum) that were grown on the contaminated soil in the vicinity of the mine, which contain high concentrations of Pb (17.23 mg/kg) and Cd (42 mg/kg), showing high accumulation of these elements in the root of the plants [23]. Similar distributions of both Cd and Pb, were observed also in the wheat with the highest content in the root [30].
According to some authors, the bioaccumulation factor is a compelling indicator of metal accumulation capacity [39]. Transfer of PTEs from soil into the plants is considered to be one of the main indicators of human exposure to contamination [24,40].
Plants’ ability to uptake and accumulate metals can vary greatly among different species or even within cultivars of the same species. An example of such a study is the research of 32 hybrid rice varieties grown on the contaminated soil. Results of this study indicated that there were significant differences in the accumulation and tolerance for lead and cadmium between all tested hybrids [27]. Also, 14 sunflower hybrids have shown significant differences in the accumulation of Cd and Zn [28]. The study of concentration levels of Cr, Cd and As in a soil-wheat system showed that bioaccumulation of Cd was much higher than other elements [41]. The experiment of the accumulation Pb by mandarin orange trees, irrigated with water containing different concentrations of Pb, showed that accumulation remained highest in plants that received the highest concentration of Pb via irrigation [42]. Investigation of bioaccumulation of PTEs by sorghum and sunflower on contaminated agricultural soil showed that the highest concentration of investigated elements (Cd, Cr, Cu, Ni, Pb) was in roots, while BF roots was the lowest [43].
Different plant species may accumulate lead with the highest concentration in the root [44] and, for this reason, an important step towards the control of health risks is obtaining an understanding of Pb accumulation mechanism, as well as its translocation to the aerial parts of plant.
TF’s values of metals in seed formation stage are lower than 1, except for control and treatment II, 5 mg/kg Pb, for cadmium because of the low bioaccumulation in root for these two treatments. These values, TF < 1, indicate a low level of translocation from the roots to the seed. Also, TF’s values of metals in seed maturation stage are lower than 1, showing a deficient transport of metals, from the root to the seed, especially for lead.
In this way, the edible parts of the plant are protected from potentially toxic elements contamination. These results indicate that quinoa only uptakes, but does not accumulate cadmium and lead, while the root is a barrier that prevents further translocation of metals to seed. Also, low metal accumulation indicates lower risk for primary consumers [45]. The high values of Cd and Pb in the root of pea plant with very low translocation to the edible parts of the plant were reported [45]. Similar studies of metal accumulation and translocation by miscanthus also reported limited translocation to aerial parts of plant [25,26]. Our results are not consistent with the study [39] that reported high TF values for Cd in wheat. The higher TF were found for Cd and Pb in Plantago major L. [21]. The highest concentration of Pb in beans was determined in shoot with very limited translocation to edible parts of the plant [46].
Metal excluders are defined as crops that can accumulate metals in small quantities in the edible parts, so their products are safe for consumption even when they are grown on the moderately contaminated soils [47]. This concept is based on the fact that several studies confirmed a significant difference in accumulation and distribution of metals between different varieties of the same culture.
On the other hand, the plants that are suitable for phytostabilization accumulate trace elements in the roots and translocation of these elements are limited, which prevents their transfer into the food chain [48].
5. Conclusions
The highest concentrations of metals were determined in the roots, for both studied growth stages of quinoa. BAF of Cd and Pb indicate that bioaccumulation was generally medium to high intensity. In addition, TF values were lower than 1, showing very limited translocation from root to seed and making quinoa suitable for Cd and Pb phytostabilization.
The results of our study indicated that quinoa adopts potentially toxic metals from substrate with scarce translocation to the plant parts, in particular to the seed. This conclusion paves the way for quinoa as a potential lead and cadmium excluder.
The limitation of this study is that they speciation/fractionation of Cd and Pb was not fully determined.
Future research should analyzes the safety of quinoa grown in contaminated environments for human consumption. Also, it is suggested to compare the bioaccumulation efficacy of other PTEs from water to quinoa under irrigation with wastewater.
Author Contributions
The manuscript was read, discussed, and approved by all authors. B.Z. and V.R. designed and performed the experiments; I.D. and V.R. analyzed the data and contributed to the writing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was supported by the Project: “Investigation of the possibility to use contaminated water in growing alternative, healthy cereals” (31006) financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia for the period 2011–2017.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pourret, O.; Hursthouse, A. It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.; Wu, W.; Yu, W.; Zhang, J.; Xu, J.; Rengel, Z.; Chen, L.; Cui, X.; Chen, Q. Nitrate reductase-mediated NO production enhances Cd accumulation in Panax notoginseng roots by affecting root cell wall properties. J. Plant Physiol. 2016, 193, 64–70. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Fornazier, R.F.; Vitória, A.P.; Lea, P.J.; Azevedo, R.A. Changes in antioxidant enzyme activities in soybean under cadmium stress. J. Plant Nutr. 2002, 25, 327–342. [Google Scholar] [CrossRef]
- Lavado, R.S.; Rodríguez, M.; Alvarez, R.; Taboada, M.A.; Zubillaga, M.S. Transfer of potentially toxic elements from biosolid-treated soils to maize and wheat crops. Agric. Ecosyst. Environ. 2007, 118, 312–318. [Google Scholar] [CrossRef]
- He, J.; Ji, Z.X.; Wang, Q.Z.; Liu, C.F.; Zhou, Y.B. Effect of Cu and Pb pollution on the growth and antionxidant enzyme activity of Suaeda heteroptera. Ecol. Eng. 2016, 87, 102–109. [Google Scholar] [CrossRef]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Fernández, J.M.; Puschenreiter, M.; Williams, P.N.; Plaza, C. Availability and transfer to grain of As, Cd, Cu, Ni, Pb and Zn in a barley agri-system: Impact of biochar, organic and mineral fertilizers. Agric. Ecosyst. Environ. 2016, 219, 171–178. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, T.; Wang, X.; Zhou, F.; Yang, Y.; Yin, Y. Effects of soil type and genotype on lead concentration in rootstalk vegetables and the selection of cultivars for food safety. J. Environ. Manag. 2013, 122, 8–14. [Google Scholar] [CrossRef]
- Lambert, N.; Yarwood, J.N. Engineering legume seed storage proteins. In Plant Protein Engineering; Cambridge University Press: Cambridge, UK, 1992; pp. 167–187. [Google Scholar]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Bazile, D.; Bertero, D.; Nieto, C. (Eds.) State of the Art Report of Quinoa in the World in 2013; FAO & CIRAD: Rome, Italy, 2015. [Google Scholar]
- Bedoya-Perales, N.S.; Pumi, G.; Mujica, A.; Talamini, E.; Padula, A.D. Quinoa expansion in peru and its implications for land use management. Sustainability 2018, 10, 532. [Google Scholar] [CrossRef]
- Gorinstein, S.; Pawelzik, E.; Delgado-Licon, E.; Haruenkit, R.; Weisz, M.; Trakhtenberg, S. Characterisation of pseudocereal and cereal proteins by protein and amino acid analyses. J. Sci. Food Agric. 2002, 82, 886–891. [Google Scholar] [CrossRef]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa—An Indian perspective. Ind. Crops Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Varisi, V.A.; Camargos, L.S.; Aguiar, L.F.; Christofoleti, R.M.; Medici, L.O.; Azevedo, R.A. Lysine biosynthesis and nitrogen metabolism in quinoa (Chenopodium quinoa): Study of enzymes and nitrogen-containing compounds. Plant Physiol. Biochem. 2008, 46, 11–18. [Google Scholar] [CrossRef]
- Galal, T.M.; Shehata, H.S. Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol. Indic. 2015, 48, 244–251. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.K. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere 2008, 70, 1264–1272. [Google Scholar] [CrossRef]
- Boussen, S.; Soubrand, M.; Bril, H.; Ouerfelli, K.; Abdeljaouad, S. Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 2013, 192, 227–236. [Google Scholar] [CrossRef]
- Liu, X.; Song, Q.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil–vegetable system: A multi-medium analysis. Sci. Total Environ. 2013, 463, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services. A review. J. Environ. Manag. 2014, 143, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a productive biofuel crop for phytoremediation. Crit. Rev. Plant Sci. 2014, 33, 1–19. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, M.; Zhou, X.; Liao, B.H.; Peng, P.Q.; Hu, M.; Zhu, W.; Wu, Y.J.; Zou, Z.J. Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant Soil 2015, 386, 317–329. [Google Scholar] [CrossRef]
- Laporte, M.A.; Sterckeman, T.; Dauguet, S.; Denaix, L.; Nguyen, C. Variability in cadmium and zinc shoot concentration in 14 cultivars of sunflower (Helianthus annuus L.) as related to metal uptake and partitioning. Environ. Exp. Bot. 2015, 109, 45–53. [Google Scholar] [CrossRef]
- Pietrini, F.; Iori, V.; Bianconi, D.; Mughini, G.; Massacci, A.; Zacchini, M. Assessment of physiological and biochemical responses, metal tolerance and accumulation in two eucalypt hybrid clones for phytoremediation of cadmium-contaminated waters. J. Environ. Manag. 2015, 162, 221–231. [Google Scholar] [CrossRef]
- Meng, W.; Wang, Z.; Hu, B.; Wang, Z.; Li, H.; Goodman, R.C. Heavy metals in soil and plants after long-term sewage irrigation at Tianjin China: A case study assessment. Agric. Water Manag. 2016, 171, 153–161. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Epa Method 3050b—Acid Digestion of Sediments, Sludges, and Soils; Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Association of Official Analytical Chemists, Inc. Official Methods of Analysis of Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- International Organization for Standardization. ISO 14870:2005—Soil Quality—Extraction with a DTPA Buffer Solution; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- International Organization for Standardization. ISO 22036:2008—Soil Quality—Determination of Trace Elements in Extracts of Soil by Inductively Coupled Plasma—Atomic Emission Spectrometry (ICP—AES); International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- Boim, A.G.F.; Melo, L.C.A.; Moreno, F.N.; Alleoni, L.R.F. Bioconcentration factors and the risk concentrations of potentially toxic elements in garden soils. J. Environ. Manag. 2016, 170, 21–27. [Google Scholar] [CrossRef]
- Sydow, M.; Chrzanowski, Ł.; Cedergreen, N.; Owsianiak, M. Limitations of experiments performed in artificially made OECD standard soils for predicting cadmium, lead and zinc toxicity towards organisms living in natural soils. J. Environ. Manag. 2017, 198, 32–40. [Google Scholar] [CrossRef]
- Arena, C.; Santorufo, L.; Cataletto, P.R.; Memoli, V.; Scudiero, R.; Maisto, G. Eco-physiological and Antioxidant Responses of Holm Oak (Quercus ilex L.) Leaves to Cd and Pb. Water Air Soil Pollut. 2017, 228, 459. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Q.; Zhang, Y.; Wei, S. Lead accumulation in different Chinese cabbage cultivars and screening for pollution-safe cultivars. J. Environ. Manag. 2010, 91, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, X.; Li, T.; Hu, S.; Ji, J.; Wang, C. Characteristics of heavy metal transfer and their influencing factors in different soil–crop systems of the industrialization region, China. Ecotoxicol. Environ. Saf. 2016, 126, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Adamo, P.; Iavazzo, P.; Albanese, S.; Agrelli, D.; De Vivo, B.; Lima, A. Bioavailability and soil-to-plant transfer factors as indicators of potentially toxic element contamination in agricultural soils. Sci. Total Environ. 2014, 500, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Li, C.; Song, Z.; Gao, X.; Wu, M. Pollution and health risk assessment of carcinogenic elements As, Cd, and Cr in multiple media—A case of a sustainable farming area in China. Sustainability 2019, 11, 5208. [Google Scholar] [CrossRef]
- Zhang, A.; Cortes, V.; Phelps, B.; Ryswyk, H.V.; Srebotnjak, T. Experimental Analysis of soil and mandarin orange plants treated with heavy metals found in oilfield-produced wastewater. Sustainability 2018, 10, 1493. [Google Scholar] [CrossRef]
- Memoli, V.; Esposito, F.; Marco, A.D.; Arena, C.; Vitale, L.; Tedeshi, A.; Magliulo, V.; Maisto, G. Metal compartmentalization in different biomass portions of Helianthus annuus L. and Sorghum bicolor L. grown in an agricultural field inside an urban fabric. Appl. Soil Ecol. 2017, 121, 118–126. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Lu, C.; Peng, H.; Luo, M.; Li, G.; Shen, Y.; Ding, H.; Zhang, Z.; Pan, G.; et al. The development dynamics of the maize root transcriptome responsive to heavy metal Pb pollution. Biochem. Biophys. Res. Commun. 2015, 458, 287–293. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Boersch, J.; Frohne, T.; Du Laing, G.; Rinklebe, J. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J. Environ. Manag. 2017, 186, 192–200. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Wei, S.; Ren, W. Identification of cadmium-excluding welsh onion (Allium fistulosum L.) cultivars and their mechanisms of low cadmium accumulation. Environ. Sci. Pollut. Res. 2012, 19, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Buscaroli, A.; Zannoni, D.; Menichetti, M.; Dinelli, E. Assessment of metal accumulation capacity of Dittrichia viscosa (L.) Greuter in two different Italian mine areas for contaminated soils remediation. J. Geochem. Explor. 2016, 182, 123–131. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).