Abstract
The effects of indigenous microbial consortium on removal of As from As-contaminated soil using an Fe(III)-reducing bacterium Shewanella putrefaciens were investigated under circumneutral pH condition. Sequential extraction of As revealed that more than 30% of As was associated with Fe(III)-(oxy)hydroxides in the soil. Bioleaching experiments were conducted anaerobically with a supply of lactate as a carbon source. The highest As removal efficiency (57.5%) was obtained when S. putrefaciens and indigenous bacterial consortium coexisted in the soil. S. putrefaciens and indigenous bacteria solely removed 30.1% and 16.4% of As from the soil, respectively. The combination of S. putrefaciens and indigenous bacteria led to a higher amount of labile As after microbial dissolution of Fe(III)-(oxy)hydroxides. After microbial treatment, soil quality represented by pH and organic content appeared to be preserved. The results indicated that the ecological and physiological understanding of the indigenous microbiome might be important for the efficient application of bioleaching technology to remove As from contaminated soils.
1. Introduction
Arsenic occurs in nature with four main redox states: As(‒3), As(0), As(+3) and As(+5), with predominant forms of arsenate (As(+5)) as H2AsO4– and HAsO42– under aerobic and arsenite (As(+3)) as H3AsO30 and H2AsO3– under anaerobic conditions [1]. Though As accounts for only 0.0001% of crustal abundance, exposure with As compounds resulted in serious diseases for humans such as cancer of the skin, lungs, liver, and bladder and other organ systems [2,3,4,5]. Arsenic can be released to the environment from anthropogenic and natural sources [6,7].
In South Korea, significant As contamination has been mainly reported in the areas around abandoned metal mines, the number of which exceeded 1000. Up to 2500 mg/kg of As was identified from soils and waste materials in the vicinity of Dalsung Cu-W mine [8]. Agricultural soils, groundwater and vegetable samples around Songchun, Dongjung, Myoungbong, Dongil, and Duckum metal mines showed high contamination levels of As and heavy metals such as Cu, Pb and Zn [9]. It was also reported that the maximum of 167 µg/L of As existed in groundwater around the Gubong mine [10].
Bioleaching techniques using microorganisms are simple, efficient, cost-effective, and environmental-friendly methods without the generation of toxic byproducts when compared to chemical leaching [11,12,13]. Microbial bioleaching has been applied to remove a variety of toxic elements, including As from contaminated soils [14,15], sediments [16,17], and mine tailings [18,19].
To date, most bioleaching techniques employed chemolithotrophic bacteria such as Acidithiobacillus ferrooxidans, A. thiooxidans, and Leptospirillum ferrooxidans. These bacteria showed high tolerance against elevated concentrations of As [20] and converted insoluble metal sulfides into soluble sulfate [21,22]. However, in spite of the high efficiency of metal extraction from solids, the bacteria have a disadvantage to be applied for the remediation of, especially, agricultural soils. The reason lies in the fact that these acidophilic bacteria grow in low pH and produce sulfuric acid after sulfur oxidation during their metabolism [11]. An extremely acidic condition which is produced by the bacteria may destroy microbiota and reduce nutrients in the soil, resulting in the degradation of soil quality and productivity [14,23]. Therefore, it is necessary to develop a bioleaching technique, through which toxic elements such as As can be effectively extracted from agricultural soils under circumneutral pH condition without soil acidification.
Since As is mostly adsorbed onto or coprecipitated in Fe(III)-(hydr)oxides in soil, the reductive dissolution of Fe(III)-(hydr)oxides by iron-reducing bacteria (IRB) can induce As release into solution [24,25,26]. Among many IRB, Shewanella spp., dissimilatory IRB, which can gain energy for their growth and maintenance through reduction of Fe(III) to Fe(II) [27], have such a metabolic versatility that they can grow under not only anaerobic, but also aerobic condition. Under aerobic environments, Shewanella spp. used oxygen, nitrate, fumarate, ferric iron, Mn(IV) oxide, thiosulfate, sulfur, dimethyl sulfoxide, dimethyl sulfoxide, and anthraquinone-2,6-disulphonate as electron acceptors [28,29,30].
To date, many studies reported that Shewanella spp. could enhance As mobility by the dissimilatory reduction of Fe(III) to Fe(II) [31,32]. However, most studies have focused on the specific, Fe(III)-reducing and subsequent As-releasing function of only Shewanella in soil and sediment without the deep consideration of co-existing indigenous bacterial consortium until now. Recently many reports on human microbiome indicated that microorganisms in human gut played a large role in health and disease through ecological and physiological competition or cooperation between microbial members [33,34]. Thus, the role of indigenous bacteria which seemed not to be directly associated with geochemical mediation of Fe(III) and As in geological media to date should be investigated in the same vein.
The goal of this study was to investigate the effects of indigenous bacterial consortium living in As-contaminated agricultural soil on anaerobic As bioleaching efficiency by Shewanella putrefaciens, which was artificially inoculated. The extraction efficiency of Fe and As from the soil was compared through bioleaching experiments using only S. putrefaciens, only indigenous bacterial consortium, and a combination of both. Preservation of soil quality after bioleaching treatments was also examined by a measurement of pH and organic matter content. The results of the present study may provide insight into interaction between microorganisms in soil microbiota and information for the effective and practical application of bioleaching technique in the field.
2. Materials and Methods
2.1. Soil Characterization
A composite agricultural soil sample was collected from around 15 cm in depth in the vicinity of an abandoned Ag-Au-Cu-Zn Euirim mine in Gangwon province, South Korea. After air-drying, soil pH in water extracts, loss-on-ignition (LOI) [35], cation exchange capacity (CEC) [36], and soil texture [37] were measured.
Arsenic, Fe, Cd, Pb, Sb, Zn, Cu, Ni, and Mo in the soil sample were determined using inductively coupled plasma—optical emission spectroscopy (ICP-OES; Spectro Analytical Instruments GmbH, Germany) after aqua regia (HNO3: HCl = 3:1 (v/v)) digestion for 2 h at 70 °C. Chemical compositions of the soil including major elements were also determined by a X-ray fluorescence spectrometer (XRF; Supermini 200 benchtops, Germany). Mineralogical compositions were examined by X-ray diffractometer (XRD, MiniFlex 600, Germany).
Sequential extraction of the soil was carried out to identify As amounts in five fractions including ionically bound As, strongly adsorbed As, As associated with amorphous and poorly crystalline hydrous oxides of Fe-Mn, As associated with crystalline Fe oxyhydroxides, and residual As phase. For extraction of each fraction, 1 M MgCl2 (pH 8.0, 2 h), 1 M NaH2PO4 (pH 5.0, 12 h), 0.2 M (NH4)2C2O4 (pH 3.0, 2 h), mixture of 0.5 M Na-citrate and 1 M NaHCO3 (1 h) and then Na2S2O4 (30 min), and aqua regia (2 h) were employed, respectively [38].
2.2. Shewanella Putrefaciens
Shewanella putrefaciens was purchased from Korean Collection for Type Cultures (KCTC) and subcultured in 40 mL marine broth 2216 in 50 mL SPL conical tubes under condition of pH 6.8 ± 0.1 and 100 rpm agitation at 30 ± 1 °C. After several subculture, the bacterium was transferred to anaerobic marine broth 2216 and subcultured again. The marine broth 2216 consisted of 5 g/L peptone, 1 g/L yeast extract, 0.1 g/L Fe(III) citrate, 19.5 g/L NaCl, 5.9 g/L MgCl2, 3.24 g/L MgSO4, 1.8 g/L CaCl2, 0.55 g/L KCl, 0.16 g/L NaHCO3, 0.08 g/L KBr, 34 mg/L SrCl2, 22 mg/L H3BO3, 2.4 mg/L NaF, 1.6 mg/L NH4NO3, 8 mg/L Na2HPO4, and 4 mg/L Na2SiO3. The culture was supplemented with 5 mM sodium lactate as an energy source for bacterial growth. Prior to inoculation to the soil, S. putrefaciens was quantified by counting colony-forming units (CFU), developed on marine broth agar using a digital colony counter (KT 0074A, Korea). In addition, the anaerobic growth of S. putrefaciens was monitored by measuring optical density at 600 nm wavelength (OD600) with UV-vis spectrophotometer (UVmini-1240, Japan).
All the media and apparatus were sterilized by autoclave at 121 °C and 15 psi for 15 min, and sodium lactate was filtered through a 0.2 µm WhatmanTM syringe filter to remove irrelevant insoluble compounds and bacteria.
2.3. Bioleaching Experiments
Bioleaching experiments were conducted in 150 mL Wheaton narrow-mouthed round serum bottles without agitation under anaerobic condition at room temperature. Raw wet soil was used in the experiments for the activation of indigenous bacterial consortium. Sterile soil was prepared using autoclave at 121 °C and 15 psi for 15 min, to inactivate indigenous bacteria in the soil.
The soil was designed to be leached by only S. putrefaciens which was inoculated to sterilized soil, only indigenous bacterial consortium without S. putrefaciens inoculation, or the combination of both where S. putrefaciens was inoculated to non-sterilized soil. Abiotic control without any bacteria was included in the experimental set. The raw or autoclaved soil samples (3 g) were put in 100 mL of marine broth 2216 with 5 mM sodium lactate, supplied as an energy source. The inoculum of S. putrefaciens (7%, v/v) was obtained after 50 h of incubation (4.2 × 106 CFU/mL). Anaerobic condition was maintained in a glove box after purging the serum bottles with N2 gas. During incubation, an aliquot of solution in each bottle was periodically removed, filtered through a 0.2 µm WhatmanTM syringe filter and measured for pH, redox potential, and the concentrations of lactate, Fe2+, total dissolved Fe, and soluble As. The bioleaching experiments were conducted in duplicate. After bioleaching experiments, each residual soil was taken and the sequential extraction of As was carried out. In addition, quality of soils after bioleaching treatments was estimated. For this, the residual soil was removed after treatment and dried at 40 °C under anaerobic conditions for 7 days, and then soil pH and LOI were measured.
The values of pH and redox potential were measured by Orion 3 Star portable pH meter (Thermo Fisher, USA). Concentrations of Fe2+ were determined as following Stookey [39]. Briefly, 0.1 mL sample solution was added into 4.9 mL of ferrozine solution, including 0.1% (w/w) ferrozine (3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4’,4”-disulfonic acid sodium salt (C20H13N4NaO6S2)) in 0.5% ammonium acetate buffer of pH 7.0, and then the absorbance of color appeared was measured using 562 nm wavelength by UV-vis spectrophotometer (UVmini-1240, Japan). Concentrations of dissolved Fe3+ were calculated by subtracting Fe2+ from total dissolved Fe. The lactate concentration was measured with Metrosep Organic Acids column equipped in ion chromatography (Metrohm 830 IC+, Switzerland). Total dissolved Fe was determined by ICP-OES (Spectro Analytical Instruments GmbH, Germany), and dissolved As was analyzed by inductively coupled plasma mass spectrometer (ICP-MS; Agilent Technologies 7700 Series, USA). The removal efficiency of As (%) was calculated by Equation (1).
3. Results and Discussion
3.1. Physicochemical Properties of Soil
The results of the physicochemical analyses of the soil sample are listed in Table 1. The soil had a circumneutral pH of 8.2, with 8.1% of organic matter content and 13.3 meq/100 g of Na+ exchangeable capacity. Concentrations of metalloids and heavy metals in the soil indicated that As, Cd, Pb, and Sb exceeded the permissible criteria [40,41]. Especially, As concentration of 110 mg/kg in the soil was approximately four times higher than the Korean standard (25 mg/kg) [40], which indicated that the soil was significantly contaminated with As likely due to nearby mining activity. In addition, XRF analysis revealed that the predominant element of the soil was SiO2 with 58.22% followed by Al2O3, Fe2O3, K2O, CaO, and MgO, with the values of 20.82%, 8.70%, 3.74%, 2.82%, and 2.61%, respectively. Several trace elements were also detected, such as ZnO and As2O3. Sequential extraction results indicated that As mainly existed as residual fraction (48.4%) and crystalline or amorphous Fe oxyhydroxides fraction (33.9%). The soil texture was classified as sandy clay loam, with 65.1% sand, 11.1% silt, and 23.8% clay.

Table 1.
Physicochemical characteristics of the studied soil.
The XRD analysis showed that the mineralogical composition of the contaminated soil contained quartz (SiO2), chlorite ((Mg,Fe)3(Fe,Al)4O10(OH)2(Mg,Fe)3(OH)6), muscovite (KAl2(Si3AlO10)OH2), arsenopyrite (FeAsS), ankerite (Ca(Fe,Mg,Mn)(CO3)2) and berlinite (AlPO4) (Figure 1). The detection of arsenopyrite from the XRD analysis was consistent with the chemical analysis, in which As concentration was exceedingly high.
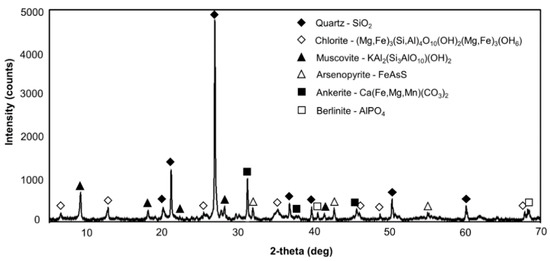
Figure 1.
X-ray diffraction (XRD) results of the contaminated soil.
3.2. Bioleaching of Soil
3.2.1. pH, Redox Potential, and Lactate
The growth curve of S. putrefaciens in marine broth under anaerobic condition is shown in Figure 2a. The gradual increase of OD600 in the first 10 days of incubation showed an exponential growth phase of the bacterial population. From 10 to 17 days, S. putrefaciens reached a stationary phase without any significant change of OD600 values.
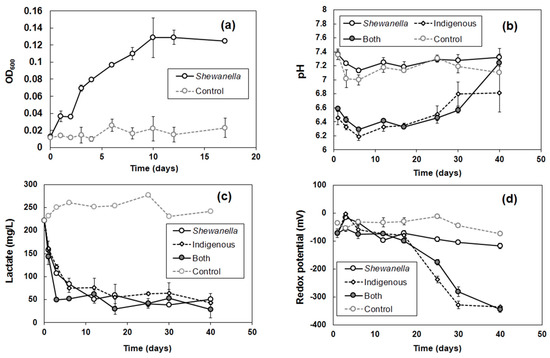
Figure 2.
(a) Growth curve of S. putrefaciens in marine broth under anaerobic condition at pH 7.0 ± 0.1. Variations in (b) pH, (c) lactate concentration, and (d) redox potential (Eh) during bioleaching experiments.
A slight difference of the initial pH was observed between autoclaved (pH 7.4 for S. putrefaciens and abiotic control) and non-autoclaved soils (pH 6.5–6.6 for indigenous bacterial consortium and combination of both) (Figure 2b). The result was likely due to a change in physicochemical properties of the soil during autoclave, though the reason could not be explained here. Increases of pH were observed over time in the samples of indigenous microbial consortium and combination of both. The result might be explained by the incomplete oxidation process of lactate to acetate, during which HCO3− was produced and alkalinity increased (Equation (2)) [43] in addition to the pH buffering capacity of soil [19].
Lactate‒ + 4 Fe(III) + 2 H2O → Acetate‒ + HCO3‒ + 4 Fe(II) + 5 H+
In contrast, pH did not change significantly in the samples of S. putrefaciens and abiotic control. It indicated that S. putrefaciens might completely oxidize lactate to carbon dioxide, as in Equation (3) [32]. A similar result was reported in the dissolution of heavy metals with various organic materials, including lactate by Shewanella HN 41 [44].
4 FeOOH + CH2O (organic matter) + 8 H+ → 4 Fe2+ + CO2 + 7 H2O
Lactate concentrations dramatically dropped in all biotic samples, while it was maintained in the abiotic control (Figure 2c). The result revealed that not only S. putrefaciens, but also some indigenous bacteria, used lactate for their metabolism and respiration process.
Redox potential (Eh) is also an important parameter to geochemical behavior of heavy metals. The influence of bacterial diversity on redox potential of the soil solution is presented in Figure 2d, where all the samples were under reducing condition. It was notable that the samples of indigenous microbial consortium and the combination of both showed a considerable decrease of redox potential to −340 ± 6 mV from the initial −73 mV, while the S. putrefaciens sample remained at −118 mV after 40 days of incubation. The difference of redox potential might result from metabolism of indigenous bacterial consortium in the soil.
3.2.2. Bioleaching of Fe
Figure 3 shows the concentrations of dissolved Fe(II), Fe(III) and total Fe extracted from the soil. Low concentrations of total Fe with less than 6 mg/L in the abiotic sample indicated that only a small amount of Fe could be chemically released from the soil (Figure 3d). Dissolved Fe in the abiotic control was likely due to ferric citrate in the supplied marine nutrient and electrochemical leaching under reducing environment. Microbiological reductive dissolution of Fe(III)-(hydr)oxides in the soil obviously appeared in the biotic samples (Figure 3a–c), where 5 mM of lactate was amended to stimulate bacteria. The elevated level of dissolved Fe implied the role of S. putrefaciens and indigenous bacterial consortium in Fe(III)-(hydr)oxides dissolution in the soil, as represented by the Equations (2) and (3). Increase in labile form of Fe might occur simultaneously with the oxidation of organic matter to HCO3‒ or CO2 [32,43].
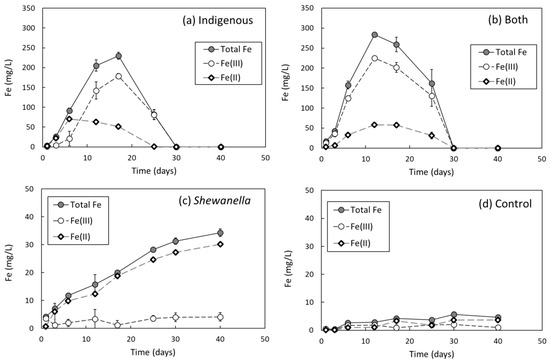
Figure 3.
Concentrations of Fe(II), Fe(III), and total dissolved Fe extracted from the soils during bioleaching experiments under anaerobic conditions. (a) bioleaching by indigenous bacterial consortium, (b) bioleaching by combination of S. putrefaciens and indigenous bacterial consortium, (c) bioleaching by only S. putrefaciens, and (d) abiotic control.
The extent and profile of microbial Fe(III)-(hydr)oxides leaching, however, were different between microorganisms. Total dissolved Fe reached maximum at 230 mg/L within 17 days in the sample of indigenous microbial consortium, while it was 284 mg/L in the sample of bacterial combination at 12 days of experimental running. Indigenous bacteria and S. putrefaciens in the combination sample together released more amount of total dissolved Fe, with a shorter time than only indigenous bacterial consortium. In addition, after maximum leaching, a decrease in total dissolved Fe appeared until no extracted Fe was observed after 30 days in the samples which indigenous bacteria were involved in. It was likely due to a formation of precipitates or adsorption back to the soil particles.
The amount of Fe lixiviated by only S. putrefaciens was much lower than by indigenous bacteria, with the highest values of 34 mg/L and 30 mg/L for total Fe and Fe(II), respectively, after 40 days; however, S. putrefaciens continuously increased the amount of total dissolved Fe and Fe(II) until the end of the experiment. Additionally, it was noticeable that S. putrefaciens obviously reduced almost all Fe(III) to Fe(II), which was likely due to dissimilatory reduction of Fe(III) by this well-known IRB. On the contrary, more amount of dissolved Fe existed as Fe(III) forms in the cases of indigenous bacteria involved for the experimental duration. Conceivably, dissolved Fe(III) might be present as complexes with inorganic or especially organic ligands, since Fe3+ is highly unstable under circumneutral pH conditions.
When compared to the continuous dissolution of Fe(III)-(hydr)oxides by S. putrefaciens, the complete removal of dissolved Fe by co-existing indigenous bacteria after 30 days indicated that some metabolic or lytic products of indigenous microorganisms belonging to different linages from S. putrefaciens obviously influenced the geochemical behavior of dissolved Fe in the solution, though a biochemical or physiological investigation on such mechanisms lies beyond the scope of this study at present.
The result suggested that not only the ability of a single ‘special’ bacterial species, but also the effects of other co-existing ‘seemingly irrelevant’ microorganisms, should be considered for understanding geochemical fate of elements in field. Though identification of the indigenous bacteria was not conducted in the present study, some bacteria existing in the soil, for example, Geobacter or Enterobacter sp., might mediate the Fe(III) reduction process [44,45,46,47]. As well, there was a possibility that some bacteria, which were not known to be directly associated with Fe(III) reduction, might unintendedly enhance the activity of co-existing IRBs through certain pathways.
3.2.3. Bioleaching of As
The extraction of As from the soil during 40 days was presented in Figure 4. The concentrations of dissolved As from the soil were in the order of bacterial combination > S. putrefaciens > indigenous bacterial consortium > abiotic control with the maximum of 1447 µg/L, 726 µg/L, 413 µg/L, and 36 µg/L, respectively. While As was rarely released into solution in the abiotic control, bacteria enhanced As lixiviation from the soil during the bioleaching process. The results implied that microbial leaching could be effectively utilized in practical treatment of As-contaminated soil under neutral pH condition. Cummings et al. [31] also reported that S. alga BrY promoted As dissolution from sediment and a similar result was found in the study of Jiang et al. [48], as well.
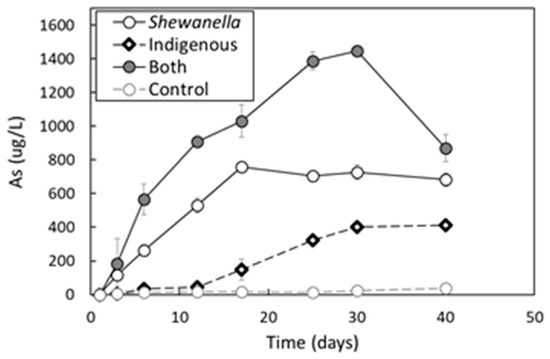
Figure 4.
The extracted As concentrations (µg/L) from the soil over time.
Correlation coefficients (R2) between dissolved As and Fe(II) were 0.909 in bioleaching by S. putrefaciens, which demonstrated that a strongly positive relationship existed between soluble As extracted from the soil and a reduction of Fe(III)-(hydr)oxides bearing As in the soil. When Fe extraction dramatically decreased from 17 days in the samples of indigenous bacteria and a combination of both, the extent of As extraction was still maintained, with high values. The formation of the significant reducing condition (‒340 mV of Eh) might contribute to the result, which controlled the chemical fate of released As [49].
Though the extent of Fe(III)-(hydr)oxides dissolution was much higher by the indigenous microbial consortium (Figure 3), S. putrefaciens showed a higher amount of dissolved As in the solution (Figure 4). The indigenous bacterial consortium has less potential to extract As from the soil than only S. putrefaciens under anaerobic condition. The result implied that S. putrefaciens was capable of maintaining As as a more labile form in the solution, and it might partly result from As respiratory system encoded by arr genes of S. putrefaciens, through which As(V) was converted to more mobile form, As(III). Though many bacteria have the As detoxifying system encoded by the ars gene, capable of As(V) reduction, the dissimilatory reduction of As(V) by S. putrefaciens might played an important role for the enhancement of soluble As [50].
Microbial activities led to the reductive dissolution of Fe(III)-(hydr)oxides and increased As dissolution from the soil. The maximum As removal efficiency in the experiment of bacterial combination was 57.5%, while the values of S. putrefaciens and indigenous microbial consortium were 30.1% and 16.4%, respectively. This observation showed a similar tendency of Fe extraction, since the values of total dissolved Fe and Fe(II) in the sample of bacterial combination were highest, as presented in Figure 3.
3.2.4. Sequential Extraction of As in Soil
Before bioleaching, the proportions of ionically bound As, strongly adsorbed As, As associated with amorphous and poorly crystalline hydrous oxides of Fe-Mn, As associated with crystalline Fe oxyhydroxides, and residual As, were 0.8%, 17.0%, 7.5%, 26.4%, and 48.4%, respectively (Figure 5). After bioleaching, the abiotic control soil showed similar amounts of As with the untreated original soil in all five fractions. However, As associated with crystalline Fe oxyhydroxides decreased dramatically from 26.4% of the original soil to 1.5%, 3.7%, and 15.1% in the samples of bacterial combination, S. putrefaciens, and indigenous microbial consortium, respectively. Such decreasing tendency was also found in the fractions of strongly adsorbed As and amorphous and poorly crystalline hydrous oxides of Fe–Mn in the sample of bacterial combination, with only 5.6% and 0.5% remaining, respectively. The highest As removal in the sample indicated that the combined activities of S. putrefaciens and indigenous bacteria could efficiently extract As from the soil. Those fractions also decreased in both S. putrefaciens and indigenous microbial consortium samples. On the other hand, As percentages in the residual fraction of all samples were almost unchanged before and after treatment, and the result showed that the microorganisms could not influence the solubilization of As in this fraction. The residual fraction seemed to be hardly extracted and mainly unaltered by bioleaching. The result was similar with the previous studies, where bioleaching technique was applied for removal of As [12] or heavy metals such as Cu, Pb, and Zn [51]. However, when considering the practical application of the bioleaching technique to As-contaminated soil in field, the presence of As in the residual fraction might not be environmentally problematic, since any dissolution process occurring in nature could not readily extract this non-bioavailable fraction of As.
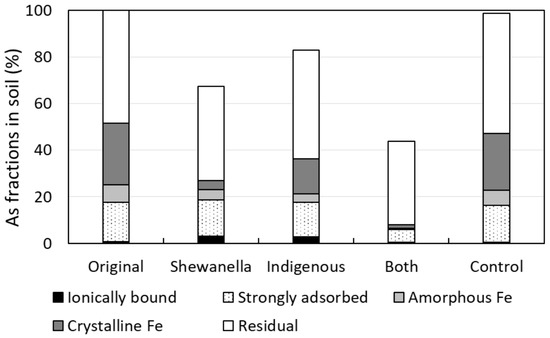
Figure 5.
Result of sequential extraction of As in soil. The sums of extracted As concentrations from each step were 108, 73, 89, 47, and 106 mg/kg from the leftmost (Original) bar.
3.3. Soil Quality Preservation
After bioleaching treatment, LOI and pH of the soil were measured to examine soil quality of the treated soil. The LOI of the abiotic control sample (7.6%) showed similar results to the original soil without treatment (7.9%). A slight increase of LOI was observed in the samples of indigenous microbial consortium (9.2%) and S. putrefaciens (9.0%), and the highest LOI value up to 11.2% took place in the sample of bacterial combination. The results were probably due to the effect of microbial growth under the condition of lactate supplement. Soil pH remained as a weakly alkaline condition after bioleaching treatment with values of 8.1, 7.7, 7.9, and 8.1 for indigenous bacterial consortium, S. putrefaciens, bacterial combination, and abiotic control, respectively. The microbiological leaching technique employed in this study seemed to completely preserve soil quality, when compared to conventional chemical leaching techniques [12]. In addition, this bioleaching technique performed under circumneutral pH could be chosen as a sustainable environmental remediation of agricultural soils, since effective extraction of heavy metals from soils by some acidophilic bacteria such as Acidithiobacillus [47] might reduce nutrients and possibly destroy the indigenous bacterial ecosystem in the soil by extremely low pH.
4. Conclusions
This study investigated the effects of S. putrefaciens, indigenous bacterial consortium, and the combination of both on the efficiency of As bioleaching from contaminated soil. When S. putrefaciens was inoculated to indigenous bacterial consortium in As-contaminated soil, it performed the highest As removal of 57.5%. Either S. putrefaciens or indigenous bacterial consortium solely showed lower As removal efficiency. According to the result of sequential extraction of As, the extraction of As was related to the microbial dissolution of Fe-associated fractions in the soil. After bioleaching using the combination of S. putrefaciens and indigenous bacterial consortium, 81.5% of remaining As in the soil existed as a residual fraction, which indicated that the treated soil retained a long-term stability against any kind of change in environmental conditions. Considering the preservation of soil quality after the bioleaching process, our results demonstrated that application of bioleaching under neutral pH condition using S. putrefaciens and indigenous bacteria could provide a sustainable and eco-friendly technique to reduce As from the contaminated agricultural soils.
To date, the study on microorganisms to release As from contaminated soil had been focused on a few specific functions of some bacteria, such as redox transformation by Shewanella or Geobacter without consideration of other bacterial ecosystem in the natural soil. However, during the practical application of bioleaching using such bacteria, it would be impossible to exclude the potential effects of other unidentified co-existing bacteria. They might enhance or interrupt the leaching process or accelerate or retard its kinetics. For designing more effective treatment processes, therefore, soil microbiome and interactions among its microbiological members, geochemical and mineralogical properties, and environments surrounding them should be identified. Further works of this study would include the identification of indigenous bacterial species existing in the soil and the biochemical, physiological and ecological investigation on the combined function of indigenous bacteria and S. putrefaciens. In addition, it would be necessary to investigate bioleaching of other heavy metals, such as Cd, Pb, and Zn from soils significantly contaminated with these metals, for practical operation of the bioleaching technique.
Author Contributions
Conceptualization, J.-U.L.; methodology, T.M.T. and J.-U.L.; validation, T.M.T. and J.-U.L.; formal analysis, T.M.T. and H.-J.H.; investigation, T.M.T. and H.-J.H.; resources, J.-I.K.; data curation, T.M.T., H.-J.H., J.-I.K. and J.-U.L.; writing—original draft preparation, T.M.T.; writing—review and editing, J.-U.L.; visualization, T.M.T. and J.-U.L.; supervision, J.-U.L.; project administration, J.-I.K. and J.-U.L.; funding acquisition, J.-U.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project of Mine Hazard Prevention Technology Development by Mine Reclamation Corporation of Korea in 2019.
Acknowledgments
The authors appreciate Ji-Won Kang for his technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oremland, R.S.; Stolz, J.F. The ecology of arsenic. Science 2003, 300, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Surdu, S. Non-melanoma skin cancer: Occupational risk from UV light and arsenic exposure. Rev. Environ. Health 2014, 29, 255–264. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, S.; Zhang, D. Association of inorganic arsenic exposure with liver cancer mortality: A meta-analysis. Environ. Res. 2014, 135, 120–125. [Google Scholar] [CrossRef]
- Sanchez, T.R.; Perzanowski, M.; Graziano, J.H. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environ. Res. 2016, 147, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Reimann, C.; Matschullat, J.; Birke, M.; Salminen, R. Arsenic distribution in the environment: The effects of scale. Appl. Geochem. 2009, 24, 1147–1167. [Google Scholar] [CrossRef]
- Jung, M.C.; Thornton, I.; Chon, H.-T. Arsenic, Sb and Bi contamination of soils, plants, waters and sediments in the vicinity of the Dalsung Cu-W mine in Korea. Sci. Total Environ. 2002, 295, 81–89. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, K.-W.; Ahn, J.S.; Ko, I.; Lee, C.-H. Investigation and risk assessment modeling of As and other heavy metals contamination around five abandoned metal mines in Korea. Environ. Geochem. Health 2005, 27, 193–203. [Google Scholar] [CrossRef]
- Woo, N.C.; Choi, M.C. Arsenic and metal contamination of water resources from mining wastes in Korea. Environ. Geol. 2001, 40, 305–311. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J.-U. A comparison of microbial leaching and chemical leaching of arsenic and heavy metals from mine tailings. Biotechnol. Bioprocess Eng. 2015, 20, 91–99. [Google Scholar] [CrossRef]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Does bioleaching represent a biotechnological strategy for remediation of contaminated sediments? Sci. Total Environ. 2016, 563–564, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.-S.; Park, H.-S.; Kim, K.-W.; Lee, J.-U. The role of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in arsenic bioleaching from soil. Environ. Geochem. Health 2013, 35, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Chai, L.; Wang, Y.; Liu, Y.; Xiao, R. Bioleaching remediation of heavy metal-contaminated soils using Burkholderia sp. Z-90. J. Hazard. Mater. 2016, 301, 145–152. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Tran, T.; Han, H.-J.; Lee, S.-H.; Lee, J.-U. Possibility of bacterial leaching of antimony, chromium, copper, manganese, nickel, and zinc from contaminated sediment. J. Geochem. Explor. 2015, 156, 153–161. [Google Scholar] [CrossRef][Green Version]
- Gan, M.; Jie, S.; Li, M.; Zhu, J.; Liu, X. Bioleaching of multiple metals from contaminated sediment by moderate thermophiles. Mar. Pollut. Bull. 2015, 97, 47–55. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Ha, M.-G.; Shin, S.; Seo, M.; Jang, J.; Jo, S.; Kim, D.; Lee, S.; Jung, Y.; Kang, P.; et al. Electrochemical effect on bioleaching of arsenic and manganese from tungsten mine wastes using Acidithiobacillus spp. J. Environ. Manag. 2018, 223, 852–859. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Ni, Y.; Yang, X.; Li, H. Bioleaching of arsenic from medicinal realgar by pure and mixed cultures. Process Biochem. 2007, 42, 1265–1271. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Lee, M.H.; Park, H.J.; Lee, J.-U. Bioleaching of arsenic and heavy metals from mine tailings by pure and mixed cultures of Acidithiobacillus spp. J. Ind. Eng. Chem. 2015, 21, 451–458. [Google Scholar] [CrossRef]
- Villar, L.D.; Garcia Jr, O. Assessment of anaerobic sewage sludge quality for agricultural application after metal bioleaching. Environ. Technol. 2003, 24, 1553–1559. [Google Scholar] [CrossRef]
- Nickson, R.T.; McArthur, J.M.; Ravenscroft, P.; Burgess, W.G.; Ahmed, K.M. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Postma, D.; Jakobsen, R. Release of arsenic associated with the reduction and transformation of iron oxides. Geochim. Cosmochim. Acta 2006, 70, 4116–4129. [Google Scholar] [CrossRef]
- Fendorf, S.; Michael, H.A.; van Green, A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef]
- DiChristina, T.J. Effects of nitrate and nitrite on dissimilatory iron reduction by Shewanella putrefaciens 200. J. Bacteriol. 1992, 174, 1891–1896. [Google Scholar] [CrossRef]
- Beliaev, A.S.; Klingeman, D.M.; Klappenbach, J.A.; Wu, L.; Romine, M.F.; Tiedje, J.M.; Nealson, K.H.; Fredrickson, J.K.; Zhou, J. Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. J. Bacteriol. 2005, 187, 7138–7145. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Romine, M.F.; Beliaev, A.S.; Auchtung, J.M.; Driscoll, M.E.; Gardner, T.S.; Nealson, K.H.; Osterman, A.L.; Pinchuk, G.; Reed, J.L.; et al. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 2008, 6, 592–603. [Google Scholar] [CrossRef]
- Yoon, S.; Sanford, R.A.; Löffler, F.E. Shewanella spp. use acetate as an electron donor for denitrification but not ferric iron or fumarate reduction. Appl. Environ. Microbiol. 2013, 79, 2818–2822. [Google Scholar] [CrossRef]
- Cummings, D.E.; Caccavo, F.; Fendorf, S.; Rosenzweig, R.F. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 1999, 33, 723–729. [Google Scholar] [CrossRef]
- Lee, S.-W. Enhancement of arsenic mobility by Fe(III)-reducing bacteria from iron oxide minerals. J. Mater. Cycles Waste Manag. 2013, 15, 362–369. [Google Scholar] [CrossRef]
- Parfrey, L.W.; Walters, W.A.; Knight, R. Microbial eukaryotes in the human microbiome: Ecology, evolution, and future directions. Front. Microbiol. 2011, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- McNally, L.; Brown, S.P. Microbiome: Ecology of stable gut communities. Nat. Microbiol. 2016, 1, 15016. [Google Scholar] [CrossRef] [PubMed]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Busenberg, E.; Clemency, C.V. Determination of the cation exchange capacity of clays and soils using an ammonia electrode. Clays Clay Miner. 1973, 21, 213–217. [Google Scholar] [CrossRef]
- Kroetsch, D.; Wang, C. Particle size distribution. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 713–726. [Google Scholar]
- Ahn, J.S.; Park, Y.S.; Kim, J.Y.; Kim, K.W. Mineralogical and geochemical characterization of arsenic in an abandoned mine tailings of Korea. Environ. Geochem. Health 2005, 27, 147–157. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Korea Ministry of Environment. Soil Contamination Prevention and Restoration. Available online: http://eng.me.go.kr/eng/web/index.do?menuId=313 (accessed on 25 March 2020).
- US EPA. Regional Screening Levels (RSLs). 2009. Available online: http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/index.htm (accessed on 13 August 2019).
- Moen, J.E.T.; Cornet, J.P.; Evers, C.W.A. Soil protection and remedial actions: Criteria for decision making and standardization of requirements. In Contaminated Soil; Springer: Dordrecht, The Netherlands, 1986; pp. 441–448. [Google Scholar]
- Lovely, D.R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 1991, 52, 259–287. [Google Scholar] [CrossRef]
- Ayyasamy, P.M.; Chun, S.; Lee, S. Desorption and dissolution of heavy metals from contaminated soil using Shewanella sp. (HN-41) amended with various carbon sources and synthetic soil organic matters. J. Hazard. Mater. 2009, 161, 1095–1102. [Google Scholar] [CrossRef]
- Call, D.F.; Logan, B.E. Lactate oxidation coupled to iron or electrode reduction by Geobacter sulfurreducens PCA. Appl. Environ. Microbiol. 2011, 77, 8791–8794. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Phillips, E.J.; Lonergan, D.J.; Jenter, H.; Lovley, D.R. Isolation of Geobacter species from diverse sedimentary environments. Appl. Environ. Microbiol. 1996, 62, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H. Characterization of Fe(III)-reducing enrichment culture and isolation of Fe(III)-reducing bacterium Enterobacter sp. L6 from marine sediment. J. Biosci. Bioeng. 2016, 122, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lee, J.-H.; Kim, D.; Kanaly, R.A.; Kim, M.-G.; Hur, H.-G. Differential arsenic mobilization from As-bearing ferrihydrite by iron-respiring Shewanella strains with different arsenic-reducing activities. Environ. Sci. Technol. 2013, 47, 8616–8623. [Google Scholar] [CrossRef]
- Lim, M.-S.; Yeo, I.W.; Roh, Y.; Lee, K.-K.; Jung, M.C. Arsenic reduction and precipitation by shewanella sp.: Batch and column tests. Geosci. J. 2008, 12, 151–157. [Google Scholar] [CrossRef]
- Saltikov, C.W.; Wildman, R.A.; Newman, D.K. Expression dynamics of arsenic respiration and detoxification in Shewanella sp. strain ANA-3. J. Bacteriol. 2005, 187, 7390–7396. [Google Scholar] [CrossRef]
- Liu, Y.-G.; Zhou, M.; Zeng, G.-M.; Wang, X.; Li, X.; Fan, T.; Xu, W.-H. Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: Effects of substrate concentration. Bioresour. Technol. 2008, 99, 4124–4129. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).