Evaluation of the Importance of Some East Asian Tree Species for Refinement of Air Quality by Estimating Air Pollution Tolerance Index, Anticipated Performance Index, and Air Pollutant Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Site and Plant Materials
2.2. Experimental Design and Growth Conditions
2.3. Monitoring Physiological Parameters
2.4. Stomatal Ozone Uptake
2.5. Stomatal Sulfur Dioxide Uptake
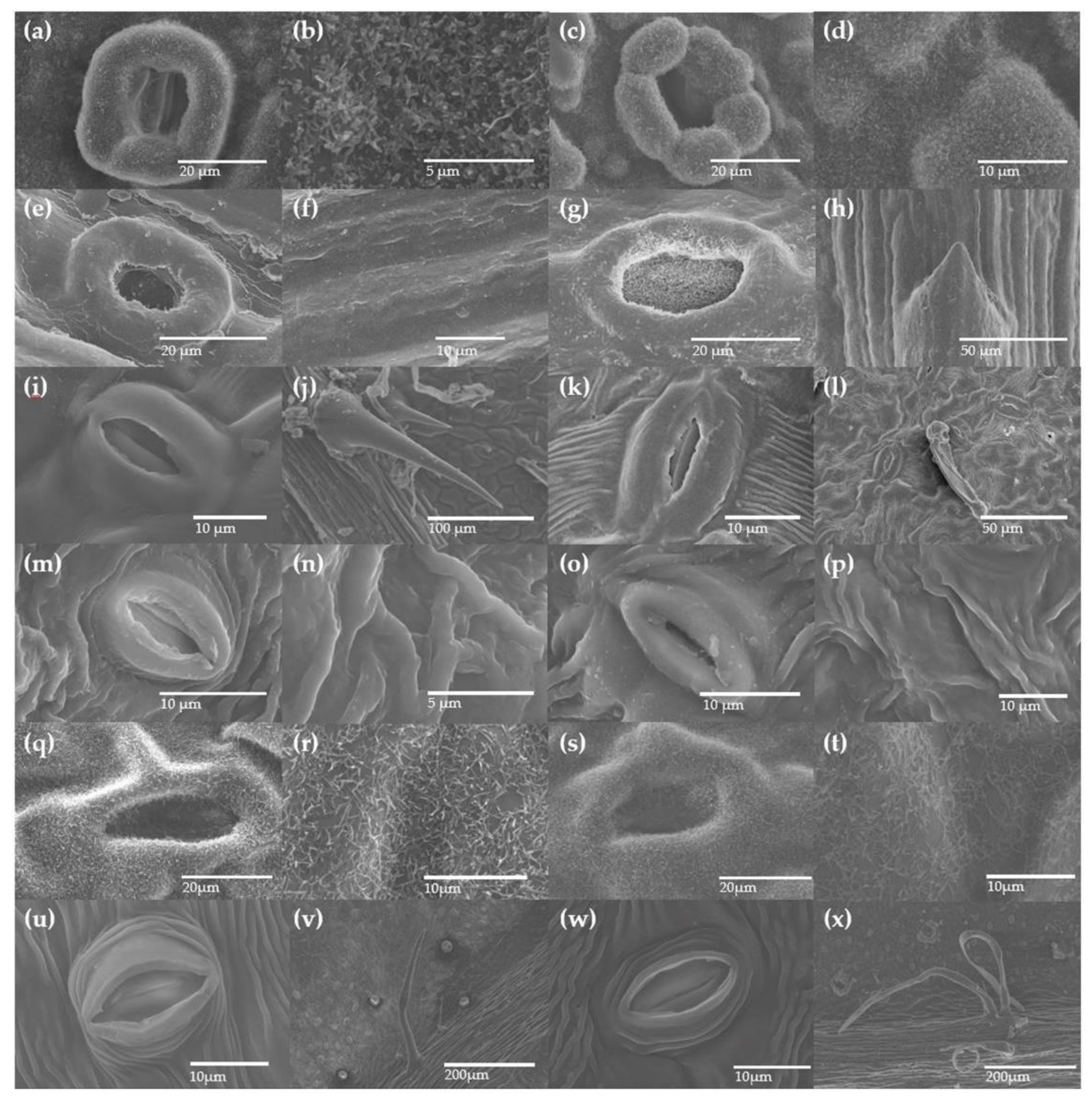
2.6. Determination of Leaf Microstructural Changes to Air Pollutants
2.7. Development of Air Pollution Tolerance Index (APTI) and Anticipated Performance Index (API)
2.8. Statistical Analysis
3. Results
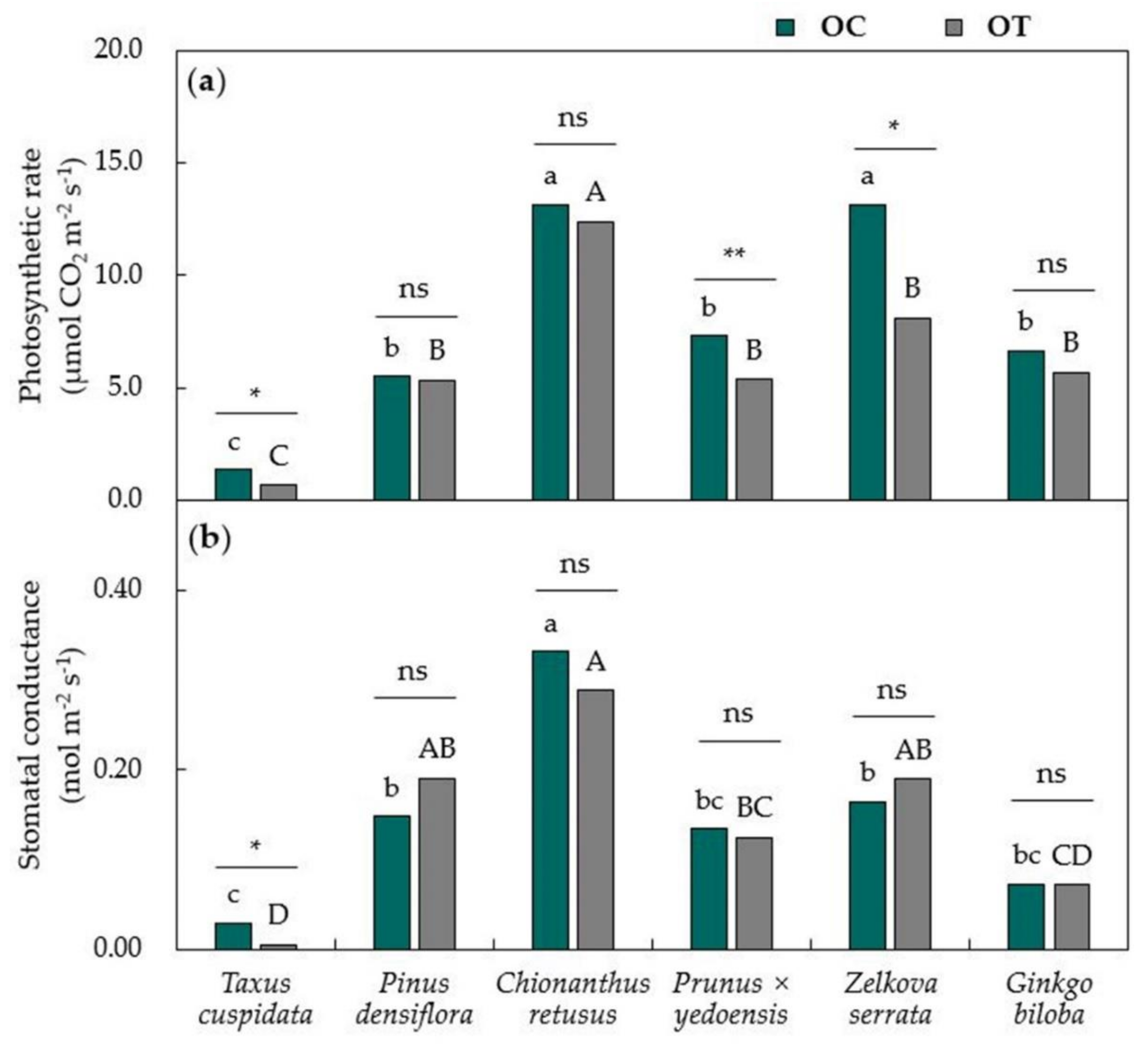
3.1. Physiological Responses of Urban Trees Towards Air Pollutants
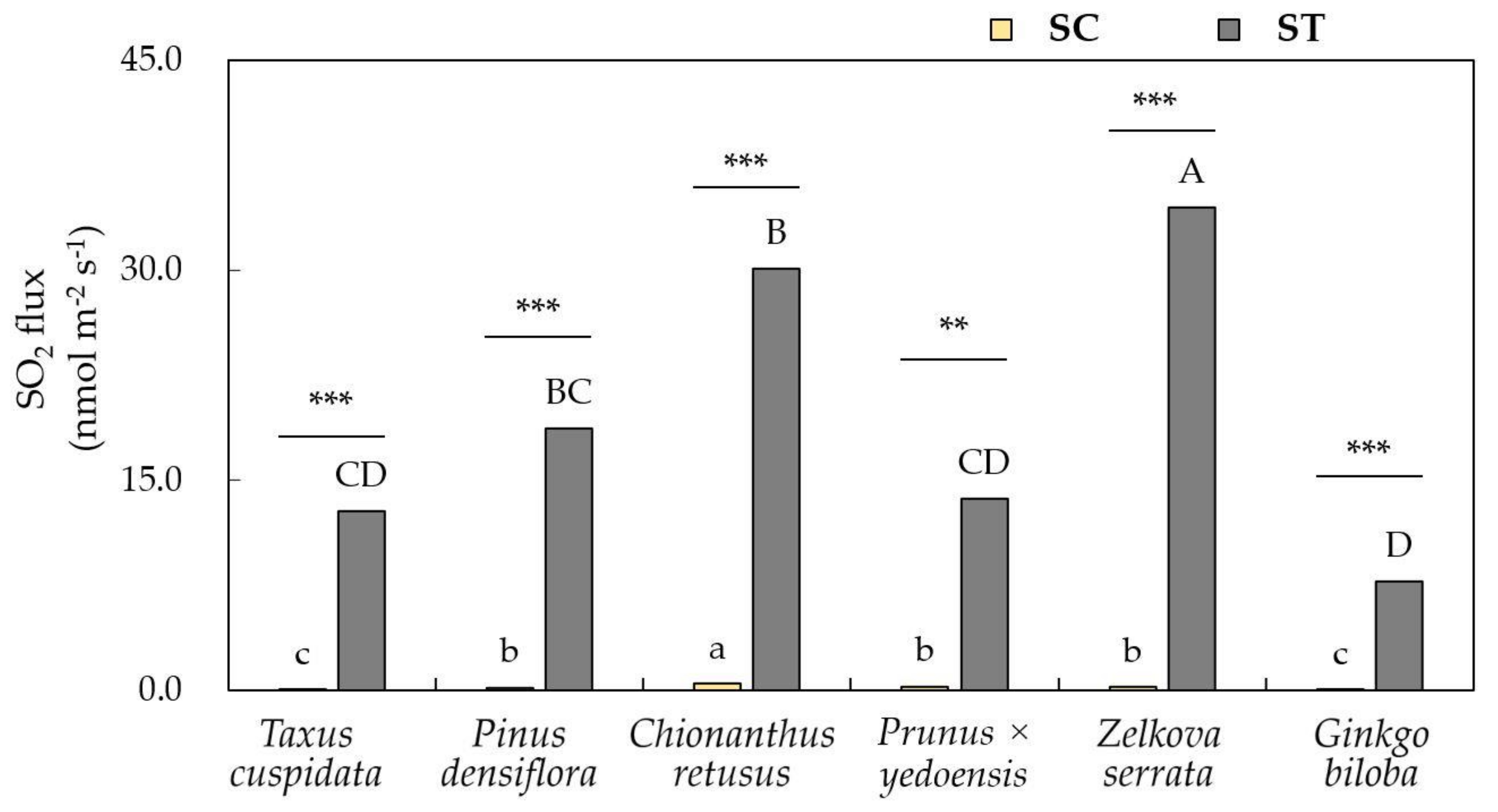
3.2. Leaf Response Towards Removal Efficiency of Air Pollutants
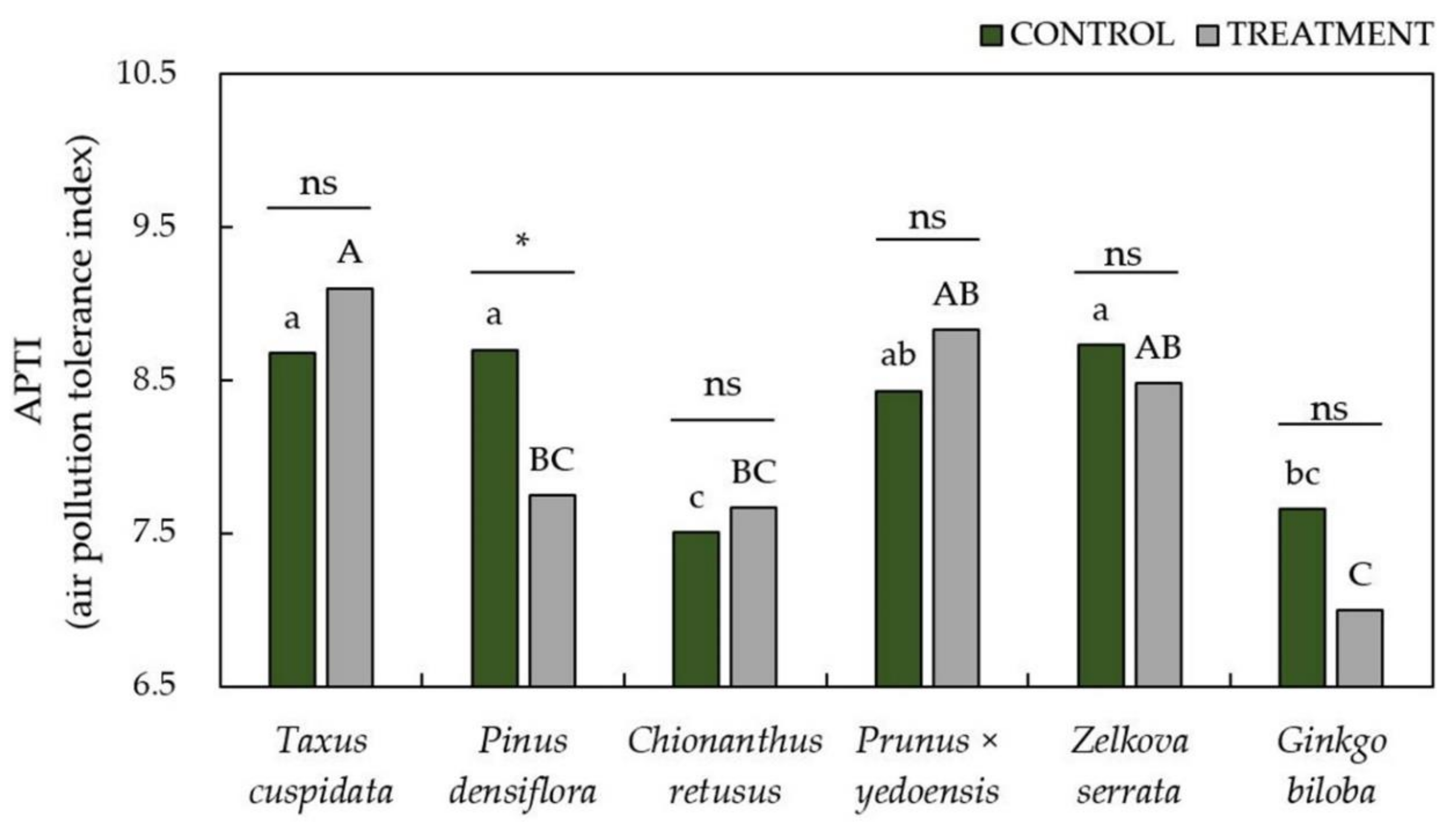
3.3. Evaluation of Air Pollution Tolerance Index (APTI) and Anticipated Performance Index (API) to Air Pollutants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalnay, E.; Cai, M. Impact of urbanization and land-use change on climate. Nature 2003, 423, 528–531. [Google Scholar] [CrossRef] [PubMed]
- UN DESA. World Urbanization Prospects: The 2018 Revision, Key Facts; United Nations Department of Economics and Social Affairs, Population Division: New York, NY, USA, 2018. [Google Scholar]
- Gerten, C.; Fina, S.; Rusche, K. The sprawling planet: Simplifying the measurement of global urbanization trends. Front. Environ. Sci. 2019, 7, 140. [Google Scholar] [CrossRef]
- Gotham, K.F.; King, A.J. Urbanization. In The Wiley Blackwell Companion to Sociology, 2nd ed.; Ritzer, G., Murphy, W.W., Eds.; Wiley–Blackwell: Hoboken, NJ, USA, 2019; pp. 267–282. [Google Scholar]
- Strohbach, M.W.; Döring, A.O.; Möck, M.; Sedrez, M.; Mumm, O.; Schneider, A.K.; Schröder, B. The “hidden urbanization”: Trends of impervious surface in lowdensity housing developments and resulting impacts on the water balance. Front. Environ. Sci. 2019, 7, 29. [Google Scholar] [CrossRef]
- Chen, X.; De Vries, S.; Assmuth, T.; Dick, J.; Hermans, T.; Hertel, O.; Jensen, A.; Jones, L.; Kabisch, S.; Lanki, T.; et al. Research challenges for cultural ecosystem services and public health in (peri-) urban environments. Sci. Total Environ. 2019, 651, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014, 193, 119–129. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hirabayashi, S.; Doyle, M.; McGovern, M.; Pasher, J. Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban For. Urban Green. 2018, 29, 40–48. [Google Scholar] [CrossRef]
- Weber, C. Ecosystem services provided by urban vegetation: A literature review. In Urban Environment; Rauch, S., Morrison, G., Norra, S., Schleicher, N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 119–131. [Google Scholar]
- Han, D.; Shen, H.; Duan, W.; Chen, L. A review on particulate matter removal capacity by urban forests at different scales. Urban For. Urban Green. 2019, 48, 126565. [Google Scholar] [CrossRef]
- Heo, S.; Bell, M.L. The influence of green space on the short–term effects of particulate matter on hospitalization in the US for 2000–2013. Environ. Res. 2019, 174, 61–68. [Google Scholar] [CrossRef]
- Vos, P.E.; Maiheu, B.; Vankerkom, J.; Janssen, S. Improving local air quality in cities: To tree or not to tree? Environ. Pollut. 2013, 183, 113–122. [Google Scholar] [CrossRef]
- Wu, J. Urban ecology and sustainability: The state-of-the-science and future directions. Landsc. Urban Plan. 2014, 125, 209–221. [Google Scholar] [CrossRef]
- Selmi, W.; Weber, C.; Rivière, E.; Blond, N.; Mehdi, L.; Nowak, D. Air pollution removal by trees in public green spaces in Strasbourg city, France. Urban For. Urban Green. 2016, 17, 192–201. [Google Scholar] [CrossRef]
- Paoletti, E.; Bardelli, T.; Giovannini, G.; Pecchioli, L. Air quality impact of an urban park over time. Procedia Environ. Sci. 2011, 4, 10–16. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Q.; Gong, P. Quantifying air pollution removal by green roofs in Chicago. Atmos. Environ. 2008, 42, 7266–7273. [Google Scholar] [CrossRef]
- Morani, A.; Nowak, D.J.; Hirabayashi, S.; Calfapietra, C. How to select the best tree planting locations to enhance air pollution removal in the MillionTreesNYC initiative. Environ. Pollut. 2011, 159, 1040–1047. [Google Scholar] [CrossRef]
- Smith, W.H. Forests as Sinks for Air Contaminants: Vegetative Compartment. In Air Pollution and Forests, 2nd ed.; Smith, W.H., Ed.; Springer-Verlag: New York, NY, USA, 1990; pp. 147–180. [Google Scholar]
- Skrynetska, I.; Karcz, J.; Barczyk, G.; Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A. Using Plantago major and Plantago lanceolata in environmental pollution research in an urban area of Southern Poland. Environ. Sci. Pollut. Res. 2019, 26, 23359–23371. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- McAdam, E.L.; Brodribb, T.J.; McAdam, S.A. Does ozone increase ABA levels by non-enzymatic synthesis causing stomata to close? Plant Cell Environ. 2017, 40, 741–747. [Google Scholar] [CrossRef]
- Choquette, N.E.; Ogut, F.; Wertin, T.M.; Montes, C.M.; Sorgini, C.A.; Morse, A.M.; Brown, P.J.; Leakey, A.D.B.; Ainsworth, E.A. Uncovering hidden genetic variation in photosynthesis of field-grown maize under ozone pollution. Glob. Chang. Biol. 2019, 25, 4327–4338. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, S.B.; Agrawal, M. Responses of tropical tree species to urban air pollutants: ROS/RNS formation and scavenging. Sci. Total Environ. 2020, 710, 136363. [Google Scholar] [CrossRef]
- Rai, R.; Rajput, M.; Agrawal, M.; Agrawal, S.B. Gaseous air pollutants: A review on current and future trends of emissions and impact on agriculture. J. Sci. Res. 2011, 55, 77–102. [Google Scholar]
- Ogunkunle, C.O.; Suleiman, L.B.; Oyedeji, S.; Awotoye, O.O.; Fatoba, P.O. Assessing the air pollution tolerance index and anticipated performance index of some tree species for biomonitoring environmental health. Agroforest. Syst. 2015, 89, 447–454. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pandey, M.; Mishra, A.; Tiwary, S.M.; Tripathi, B.D. Air pollution tolerance index and anticipated performance index of some plant species for development of urban forest. Urban For. Urban Green. 2015, 14, 866–871. [Google Scholar] [CrossRef]
- Kashyap, R.; Sharma, R.; Uniyal, S.K. Bioindicator responses and performance of plant species along a vehicular pollution gradient in western Himalaya. Environ. Monit. Assess. 2018, 190, 302. [Google Scholar] [CrossRef]
- Jain, S.; Bhattacharya, T.; Chakraborty, S. Comparison of plant tolerance towards air pollution of rural, urban and mine sites of Jharkhand: A biochemical approach to identify air pollutant sink. In Advances in Waste Management; Kalamdhad, A., Singh, J., Dhamodharan, K., Eds.; Springer: Singapore, 2019; pp. 123–142. [Google Scholar]
- Kaur, M.; Nagpal, A.K. Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ. Sci. Pollut. Res. 2017, 24, 18881–18895. [Google Scholar] [CrossRef]
- Karmakar, D.; Padhy, P.K. Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 2019, 237, 124522. [Google Scholar] [CrossRef]
- Wei, X.; Lyu, S.; Yu, Y.; Wang, Z.; Liu, H.; Pan, D.; Chen, J. Phylloremediation of air pollutants: Exploiting the potential of plant leaves and leaf-associated microbes. Front. Plant Sci. 2017, 8, 1318. [Google Scholar] [CrossRef]
- Khalid, N.; Noman, A.; Sanaullah, T.; Akram, M.A.; Aqeel, M. Vehicle pollution toxicity induced changes in physiology, defence system and biochemical characteristics of Calotropis procera L. Chem. Ecol. 2018, 34, 565–581. [Google Scholar] [CrossRef]
- Khalid, N.; Masood, A.; Noman, A.; Aqeel, M.; Qasim, M. Study of the responses of two biomonitor plant species (Datura alba & Ricinus communis) to roadside air pollution. Chemosphere 2019, 235, 832–841. [Google Scholar] [CrossRef]
- Seoul Open Data Plaza. Available online: http://data.seoul.go.kr/dataList/367/S/2/datasetView.do (accessed on 10 April 2019).
- Lee, J.K.; Woo, S.Y.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Park, J.H.; Lee, K.A. Effects of Elevated Temperature and Ozone in Brassica juncea L.: Growth, Physiology, and ROS Accumulation. Forests 2020, 11, 68. [Google Scholar] [CrossRef]
- Laisk, A.; Kull, O.; Moldau, H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol. 1989, 90, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Winner, W.E.; Mooney, H.A. Ecology of SO2 resistance: I. Effects of fumigations on gas exchange of deciduous and evergreen shrubs. Oecologia 1979, 44, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Lee, S.H.; Khaine, I.; Je, S.M.; Lee, T.Y.; You, H.N.; Lee, H.K.; Jang, J.H.; Kim, I.; Woo, S.Y. Stomatal movements depend on interactions between external night light cue and internal signals activated by rhythmic starch turnover and abscisic acid (ABA) levels at dawn and dusk. Acta Physiol. Plant. 2017, 39, 162. [Google Scholar] [CrossRef]
- Thambavani, S.D.; Sabitha, M.A. Variation in air pollution tolerance index and anticipated performance index of plants near a sugar factory: Implications for landscape-plant species selection for industrial areas. J. Res. Biol. 2011, 7, 494–502. [Google Scholar]
- Prajapati, S.K.; Tripathi, B.D. Anticipated Performance Index of some tree species considered for green belt development in and around an urban area: A case study of Varanasi city, India. J. Environ. Manag. 2008, 88, 1343–1349. [Google Scholar] [CrossRef]
- Hoshika, Y.; Katata, G.; Deushi, M.; Watanabe, M.; Koike, T.; Paoletti, E. Ozone-induced stomatal sluggishness changes carbon and water balance of temperate deciduous forests. Sci. Rep. 2015, 5, 9871. [Google Scholar] [CrossRef]
- Martin, M.J.; Host, G.E.; Lenz, K.E.; Isebrands, J.G. Simulating the growth response of aspen to elevated ozone: A mechanistic approach to scaling a leaf-level model of ozone effects on photosynthesis to a complex canopy architecture. Environ. Pollut. 2001, 115, 425–436. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Käffer, M.I.; Domingos, M.; Lieske, I.; Vargas, V.M. Predicting ozone levels from climatic parameters and leaf traits of Bel-W3 tobacco variety. Environ. Pollut. 2019, 248, 471–477. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Kangasjärvi, J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef]
- Kollist, H.; Nuhkat, M.; Roelfsema, M.R.G. Closing gaps: Linking elements that control stomatal movement. New Phytol. 2014, 203, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, F.; Jia, H.; Hu, J.; Feng, Z. Molecular response of poplar to single and combined ozone and drought. Sci. Total Environ. 2019, 655, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Bytnerowicz, A.; Karlsson, P.E.; Paoletti, E.; Sanz, M.; Schaub, M.; Wieser, G. Promoting the O3 flux concept for European forest trees. Environ. Pollut. 2007, 146, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2020, 22, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.N.; Lytle, C.M. Air pollutants. In Plant Ecophysiology; Prasad, M.N.V., Ed.; John Wiley and Sons: New York, NY, USA, 1997; pp. 375–392. [Google Scholar]
- Tausz, M. Sulfur in forest ecosystems. In Sulfur in Plants an Ecological Perspective. Plant Ecophysiology; Hawkesford, M.J., De Kok, L.J., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 6, pp. 59–75. [Google Scholar]
- Elliott-Kingston, C.; Haworth, M.; McElwain, J.C. Damage structures in leaf epidermis and cuticle as an indicator of elevated atmospheric sulphur dioxide in early Mesozoic floras. Rev. Palaeobot. Palynol. 2014, 208, 25–42. [Google Scholar] [CrossRef]
- Ooi, L.; Matsuura, T.; Munemasa, S.; Murata, Y.; Katsuhara, M.; Hirayama, T.; Mori, I.C. The mechanism of SO2-induced stomatal closure differs from O3 and CO2 responses and is mediated by nonapoptotic cell death in guard cells. Plant Cell Environ. 2018, 42, 437–447. [Google Scholar] [CrossRef]
- Reich, P.B. Quantifying plant response to ozone: A unifying theory. Tree Physiol. 1987, 3, 63–91. [Google Scholar] [CrossRef]
- Hoshika, Y.; Watanabe, M.; Carrari, E.; Paoletti, E.; Koike, T. Ozone-induced stomatal sluggishness changes stomatal parameters of Jarvis-type model in white birch and deciduous oak. Plant Biol. 2018, 20, 20–28. [Google Scholar] [CrossRef]
- Manes, F.; Vitale, M.; Fabi, A.M.; De Santis, F.; Zona, D. Estimates of potential ozone stomatal uptake in mature trees of Quercus ilex in a Mediterranean climate. Environ. Exp. Bot. 2007, 59, 235–241. [Google Scholar] [CrossRef]
- Fares, S.; Conte, A.; Chabbi, A. Ozone flux in plant ecosystems: New opportunities for long-term monitoring networks to deliver ozone-risk assessments. Environ. Sci. Pollut. Res. 2018, 25, 8240–8248. [Google Scholar] [CrossRef]
- Pfanz, H.; Martinoia, E.; Lange, O.L.; Heber, U. Flux of SO2 into leaf cells and cellular acidification by SO2. Plant Physiol. 1987, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, P.; Ottiger, M.; Günthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
- Haworth, M.; Elliott-Kingston, C.; Gallagher, A.; Fitzgerald, A.; McElwain, J.C. Sulphur dioxide fumigation effects on stomatal density and index of non-resistant plants: Implications for the stomatal palaeo-[CO2] proxy method. Rev. Palaeobot. Palynol. 2012, 182, 44–54. [Google Scholar] [CrossRef]
- Bartiromo, A.; Guignard, G.; Lumaga, M.R.B.; Barattolo, F.; Chiodini, G.; Avino, R.; Guerriero, G.; Barale, G. The cuticle micromorphology of in situ Erica arborea L. exposed to long-term volcanic gases. Environ. Exp. Bot. 2013, 87, 197–206. [Google Scholar] [CrossRef]
- Steinthorsdottir, M.; Elliott-Kingston, C.; Bacon, K.L. Cuticle surfaces of fossil plants as a potential proxy for volcanic SO2 emissions: Observations from the Triassic–Jurassic transition of East Greenland. Paleobiodivers. Paleoenviron. 2018, 98, 49–69. [Google Scholar] [CrossRef]
- Granieri, D.; Vita, F.; Inguaggiato, S. Volcanogenic SO2, a natural pollutant: Measurements, modeling and hazard assessment at Vulcano Island (Aeolian Archipelago, Italy). Environ. Pollut. 2017, 231, 219–228. [Google Scholar] [CrossRef]
- Rai, P.K.; Panda, L.L. Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: An Indo-Burma hot spot region. Air Qual. Atmos. Health 2014, 7, 93–101. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, A.; Katnoria, J.; Nagpal, A. Air Pollution Tolerance Index (APTI): An Important Determinant for the Development of Green Space in and Around Industrial/Urban Area. In Bioremediation: Current Research and Applications; Rathoure, A.K., Ed.; IK International Publishing House Pvt. Ltd.: New Delhi, India, 2017; pp. 422–449. [Google Scholar]


| Grading Character | Pattern of Assessment | Grading Allotted 1 | |
|---|---|---|---|
| (a) Tolerance | APTI | 2.0–6.0 | + |
| 6.1–10.0 | ++ | ||
| 10.1–14.0 | +++ | ||
| 14.1–18.0 | ++++ | ||
| 18.1–22.0 | +++++ | ||
| (b) Biological | Plant habit | Small | − |
| parameters | Medium | + | |
| Large | ++ | ||
| Crown structure | Sparse/irregular/globular | − | |
| Spreading crown/open/semi-dense | + | ||
| Spreading dense | ++ | ||
| Plant type | Deciduous | − | |
| Evergreen | + | ||
| (c) Morphological | Size | Small | − |
| laminar structure | Medium | + | |
| Large | ++ | ||
| Texture | Smooth | − | |
| Coriaceous | + | ||
| Hardiness | Not hardy | − | |
| Hardy | + | ||
| (d) Socio-economic | Frequency | <3 uses | − |
| importance | 3–4 uses | + | |
| >4 uses | ++ | ||
| Score (%) | API Value | Assessment of Category |
|---|---|---|
| Up to 30.0 | 0 | Not recommended |
| 31.0–40.0 | 1 | Very poor |
| 41.0–50.0 | 2 | Poor |
| 51.0–60.0 | 3 | Moderate |
| 61.0–70.0 | 4 | Good |
| 71.0–80.0 | 5 | Very Good |
| 81.0–90.0 | 6 | Excellent |
| 91.0–100.0 | 7 | Best |
| Tree Species | AsA (mg/g FW) | pH | TChl (mg/g FW) | RWC (%) | |
|---|---|---|---|---|---|
| Taxus cuspidata | Con. | 1.07 ± 0.30 a | 5.56 ± 0.10 b | 2.69 ± 0.51 c,d | 78 ± 6 b,c |
| Tre. | 1.02 ± 0.25 a | 5.54 ± 0.02 b | 2.46 ± 0.23 b | 83 ± 6 a | |
| Pinus densiflora | Con. | 1.12 ± 0.34 a | 3.85 ± 0.08 d | 1.60 ± 0.25 d | 81 ± 3 a |
| Tre. | 1.12 ± 0.34 a | 3.79 ± 0.10 d | 1.48 ± 0.22 b | 72 ± 8 a,b,c ** | |
| Chionanthus retusus | Con. | 0.55 ± 0.12 b | 5.36 ± 0.19 c | 11.7 ± 4.44 a | 66 ± 6 d |
| Tre. | 0.47 ± 0.01 b | 5.49 ± 0.02 b | 11.0 ± 4.82 a | 69 ± 5 b,c | |
| Prunus × yedoensis | Con. | 1.08 ± 0.32 a | 5.66 ± 0.13 b | 3.32 ± 0.11 c,d | 75 ± 3 b,c |
| Tre. | 1.11 ± 0.37 a | 5.57 ± 0.07 b | 2.22 ± 0.51 b *** | 80 ± 5 a,b * | |
| Zelkova serrata | Con. | 1.14 ± 0.37 a | 6.03 ± 0.04 a | 7.37 ± 0.83 b | 72 ± 5 c,d |
| Tre. | 1.16 ± 0.43 a | 6.01 ± 0.02 a | 3.93 ± 0.96 b *** | 74 ± 8 a,b,c | |
| Ginkgo biloba | Con. | 0.51 ± 0.17 b | 3.94 ± 0.03 d | 5.07 ± 3.28 b,c | 73 ± 4 c,d |
| Tre. | 0.50 ± 0.17 b | 3.99 ± 0.18 c | 4.15 ± 2.23 b | 66 ± 10 c | |
| Species | APTI | PH | CS | PT | LS 1 | EV | Grade Allotted | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | T | H | Score (%) | API Value | Category | ||||||
| Taxus cuspidata | ++ | ++ | − | + | − | − | + | − | 37.5 | 1 | Very poor |
| Pinus densiflora | ++ | ++ | ++ | + | ++ | − | + | − | 62.5 | 4 | Good |
| Chionanthus retusus | ++ | ++ | + | − | ++ | − | + | − | 50.0 | 2 | Poor |
| Prunus × yedoensis | ++ | ++ | ++ | − | ++ | + | − | + | 62.5 | 4 | Good |
| Zelkova serrata | ++ | ++ | ++ | − | + | + | + | − | 56.3 | 3 | Moderate |
| Ginkgo biloba | ++ | ++ | ++ | − | ++ | − | + | + | 56.3 | 3 | Moderate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, M.J.; Lee, J.K.; Park, S.; Lim, Y.J.; Kim, H.; Kim, K.N.; Je, S.M.; Park, C.R.; Woo, S.Y. Evaluation of the Importance of Some East Asian Tree Species for Refinement of Air Quality by Estimating Air Pollution Tolerance Index, Anticipated Performance Index, and Air Pollutant Uptake. Sustainability 2020, 12, 3067. https://doi.org/10.3390/su12073067
Kwak MJ, Lee JK, Park S, Lim YJ, Kim H, Kim KN, Je SM, Park CR, Woo SY. Evaluation of the Importance of Some East Asian Tree Species for Refinement of Air Quality by Estimating Air Pollution Tolerance Index, Anticipated Performance Index, and Air Pollutant Uptake. Sustainability. 2020; 12(7):3067. https://doi.org/10.3390/su12073067
Chicago/Turabian StyleKwak, Myeong Ja, Jong Kyu Lee, Sanghee Park, Yea Ji Lim, Handong Kim, Kyeong Nam Kim, Sun Mi Je, Chan Ryul Park, and Su Young Woo. 2020. "Evaluation of the Importance of Some East Asian Tree Species for Refinement of Air Quality by Estimating Air Pollution Tolerance Index, Anticipated Performance Index, and Air Pollutant Uptake" Sustainability 12, no. 7: 3067. https://doi.org/10.3390/su12073067
APA StyleKwak, M. J., Lee, J. K., Park, S., Lim, Y. J., Kim, H., Kim, K. N., Je, S. M., Park, C. R., & Woo, S. Y. (2020). Evaluation of the Importance of Some East Asian Tree Species for Refinement of Air Quality by Estimating Air Pollution Tolerance Index, Anticipated Performance Index, and Air Pollutant Uptake. Sustainability, 12(7), 3067. https://doi.org/10.3390/su12073067









