Carbon Mineralization under Different Saline—Alkali Stress Conditions in Paddy Fields of Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Design
2.2. Soil Analysis
2.2.1. Measurement of Soil Alkalinity
2.2.2. Measurements of Other Indicators
2.3. Experimental Design
2.4. Modeling Methods
2.5. Calculations and Statistical Analysis
3. Results
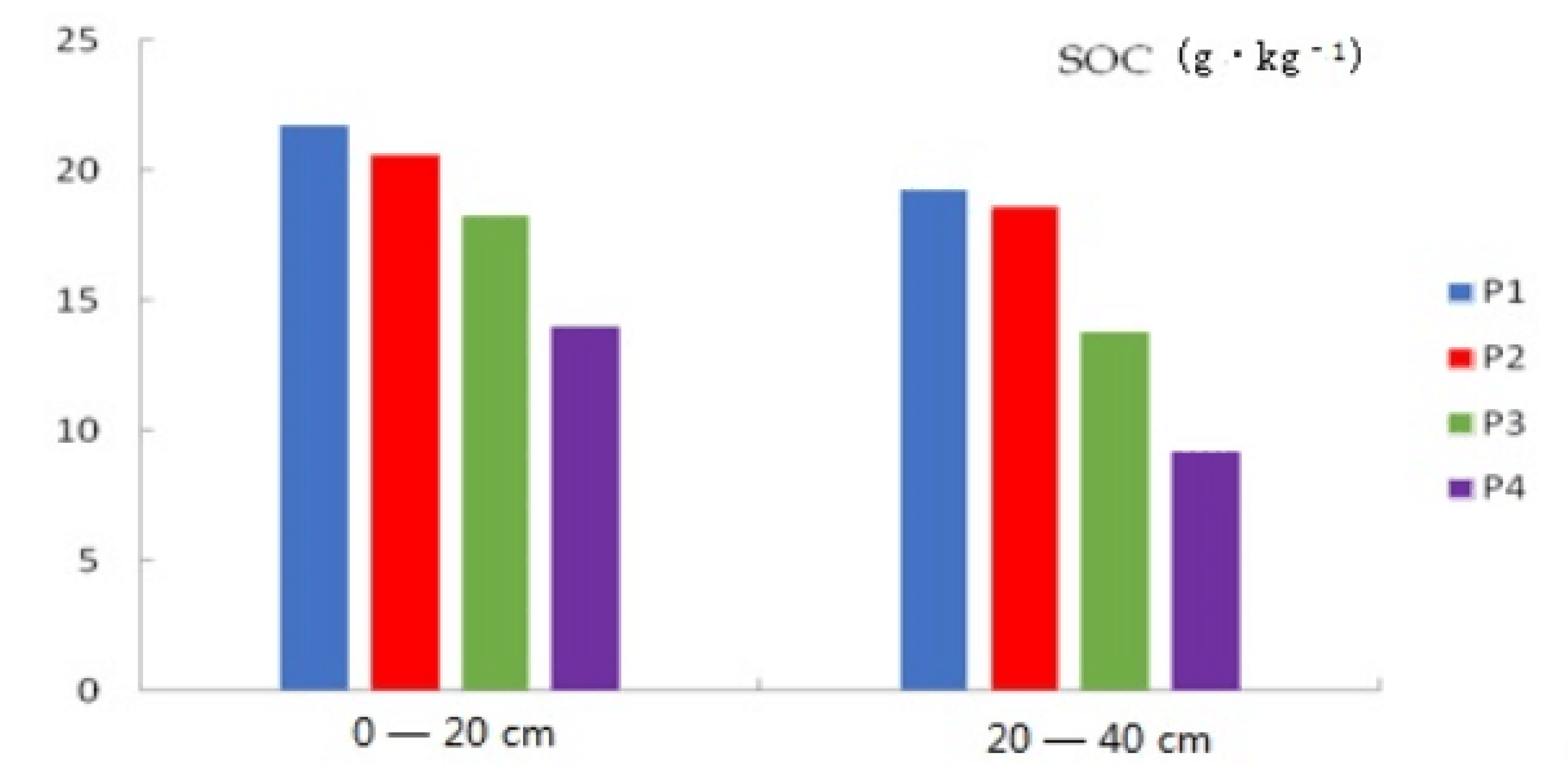
3.1. Characteristics of the SOC and Soil Alkalinity
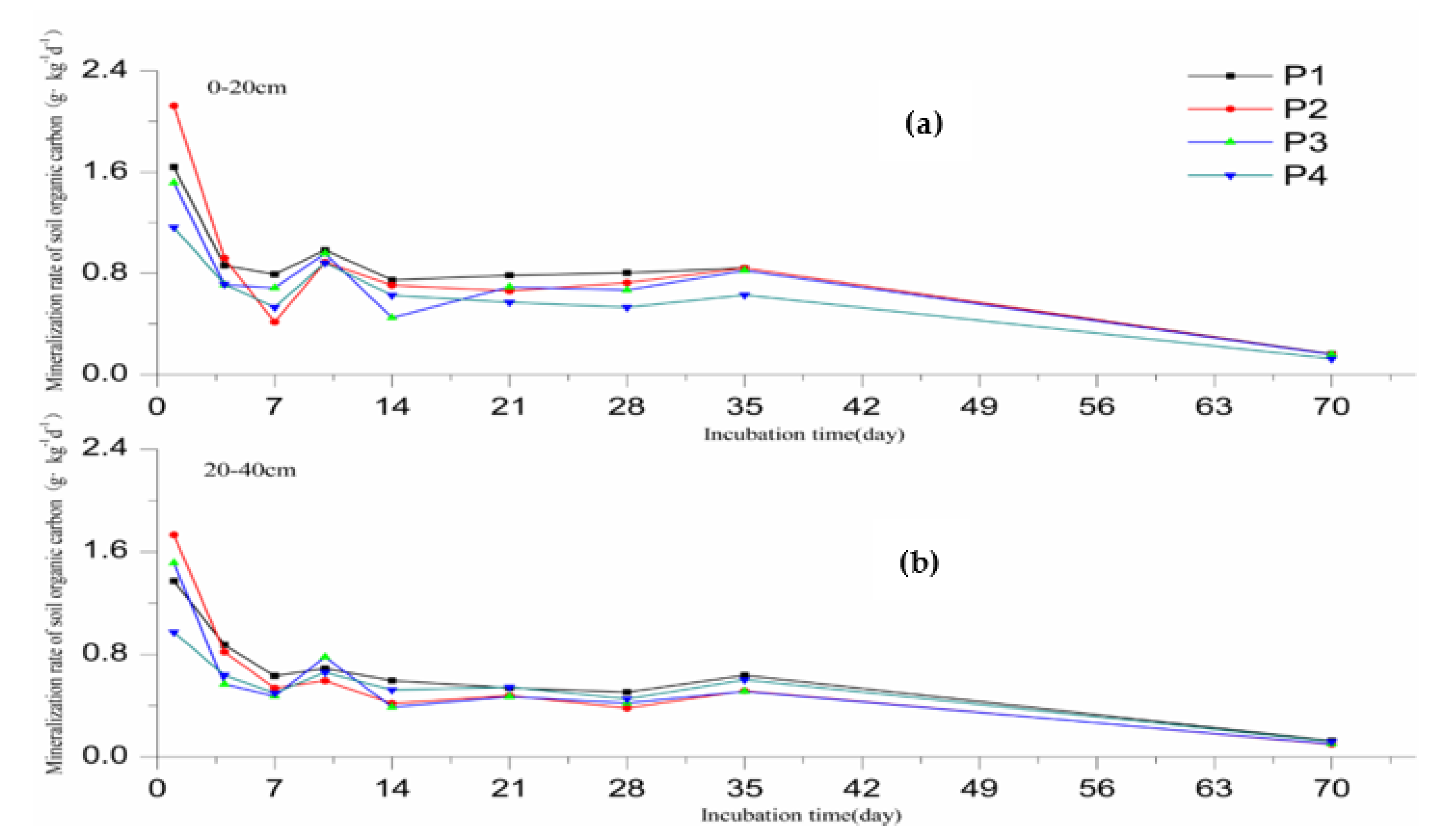
3.2. SOC Mineralization Rate
3.3. SOC Mineralization and Kinetic Equation Simulation
3.4. Accumulated SOC Mineralization Amount
3.5. Mineralization Potential of SOC
3.6. Soil Enzyme Activities
4. Discussion
4.1. Soil Enzyme Activities
4.2. Effects of Soil Salinization on SOC Mineralization Characteristics
4.3. Evaluation of First-Order Dynamics of the SOC Mineralization Process
4.4. Effects of Soil Enzyme Activities on the SOC Content and Mineralization
5. Conclusions
- 1.
- The SOC content of the surface layer in the paddy fields in Qianguo irrigation area is higher than that of the bottom layer, showing the phenomenon of surface enrichment. The higher the degree of soil alkalinity, the lower the SOC content, and the greater the effect on the SOC content in the bottom layer.
- 2.
- In the 70-day mineralization incubation experiment, the rate of CO2 release from the soil in the irrigated area show a tendency of first high and then reduced. The results of the incubation simulations are consistent with the first-order kinetic equation (Ct = C0 (1 − e−kt)).
- 3.
- For enzyme activities, amylase, and invertase are significantly positively correlated with the SOC content, and the difference of polyphenol oxidase activities between different soil layers is not as significant as the other enzymes.
- 4.
- The soil salinization and alkalinity is unfavorable to the SOC mineralization, and the extent of influence is related to the effects of saline–alkali, which is an important factor affecting the turnover of organic carbon pool, and the influence on the carbon sink is greater than that on the carbon source process.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Qiao, W.; Gao, D.; Dai, Y.; Deng, J.; Yang, G.; Han, X.; Ren, G. Temperature sensitivity of soil carbon dioxide and nitrous oxide emissions in mountain forest and meadow ecosystems in China. Atmos. Environ. 2016, 142, 340–350. [Google Scholar] [CrossRef]
- Tate, K.R.; Ross, D.J.; Saggar, S.; Hedley, C.B.; Dando, J.; Singh, B.K.; Lambie, S.M. Methane uptake in soils from Pinus radiata plantations, a reverting shrubland and adjacent pastures: Effects of land-use change, and soil texture, water and mineral nitrogen. Soil. Biol. Biochem. 2007, 39, 1437–1449. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.Y.; McPherson, B.; Griqq, R.; et al. CO2 Accounting and Risk Analysis for CO2 Sequestration at Enhanced Oil Recovery Sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Wang, W.; Dai, Z.; Duan, L.; Zhao, Y.; Zhang, J. An ecology-oriented exploitation mode of groundwater resources in the northern Tianshan Mountains, China. J. Hydrol. 2016, 543, 386–394. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y. Land exploitation resulting in soil salinization in a desert–oasis ecotone. Catena 2013, 100, 50–56. [Google Scholar] [CrossRef]
- Lehner, B.; Döll, P.; Alcamo, J.; Henrichs, T.; Kaspar, F. Estimating the impact of global change on flood and drought risks in Europe: A continental, integrated analysis. Nat. Clim. Chang. 2006, 75, 273–299. [Google Scholar] [CrossRef]
- Lou, Y.; Pan, Y.; Gao, C.; Jiang, M.; Lu, X.; Xu, Y.J. Response of Plant Height, Species Richness and Aboveground Biomass to Flooding Gradient along Vegetation Zones in Floodplain Wetlands, Northeast China. PLoS ONE 2016, 11, e0153972. [Google Scholar] [CrossRef]
- Sim, L.L.; Davis, J.A.; Chambers, J.M. When is it too salty for saline wetlands? Conceptual models for the examination of ecological regime shifts in saline wetlands. In Proceedings of the 29th Congress of the International-Association-of-Theoretical-and-Applied-Limnology, Lahti, Finland, 8–14 August 2004. [Google Scholar]
- Zhang, J.; Li, Z.; Ning, T.; Gu, S. Methane uptake in salt-affected soils shows low sensitivity to salt addition. Soil. Biol. Biochem. 2011, 43, 1434–1439. [Google Scholar] [CrossRef]
- Garssen, A.G.; Pedersen, A.B.A.; Voesenek, L.A.C.J.; Verhoeven, J.A.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Li, Z.; Wang, S.; Qu, Y. Effects of irrigation regime and Nitrogen fertilizer management on CH4, N2O and CO2 emissions from saline–alkaline paddy fields in Northeast China. Sustainability 2018, 10, 475. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 12, 413–421. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Wang, X.; Wang, J.; Zhang, C.; Tian, C. Carbon dioxide fluxes and concentrations in a cotton field in Northwestern China: Effects of plastic mulching and drip irrigation. Pedosphere 2011, 21, 178–185. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Pan, G.; Smith, P.; Pan, W. The role of soil organic matter in maintaining the productivity and yield stability of cereals in China. Agric. Ecosyst. Environ. 2009, 129, 344–348. [Google Scholar] [CrossRef]
- Wei, X.; Ma, T.; Wang, Y.; W., Y.; Hao, M.; Shao, M.; Zhang, X. Long-term fertilization increases the temperature sensitivity of OC mineralization in soil aggregates of a highland agroecosystem. Geoderma 2016, 272, 1–9. [Google Scholar] [CrossRef]
- Kéraval, B.; Lehours, A.C.; Colombet, J.; Amblard, C.; Alvare, Z.; Fontaine, S. Soil carbon dioxide emissions controlled by an extracellular oxidative metabolism identifiable by its isotope signature. Biogeosciences 2016, 13, 6353–6362. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Becerra, C.A.; Schaeffer, S.M.; Schimel, J. Separating cellular metabolism from exoenzyme activity in soil organic matter decomposition. Soil Biol. Biochem. 2014, 71, 68–75. [Google Scholar] [CrossRef]
- Kéraval, B.; Fontaine, S.; Lallement, A.; Revaillot, S.; Billard, H.; Alvarez, G.; Maestre, F.; Amblard, C.; Lehours, A.C. Cellular and non-cellular mineralization of organic carbon in soils with contrasted physicochemical properties. Soil Biol. Biochem. 2018, 125, 286–289. [Google Scholar] [CrossRef]
- Ma, Y.F.; Gao, Y.H.; Feng, Q.L. Effects of pH and temperature on CaCO3 crystallization in aqueous solution with water soluble matrix of pearls. J. Cryst. Growth 2010, 312, 3165–3170. [Google Scholar] [CrossRef]
- Varadhrajan, C.; Tinnacher, R.M.; Pugh, J.D.; Trautz, R.C.; Zheng, L.; Spycher, N.F.; Birkholzer, J.T.; Castillo-Michel, H.; Esposito, R.A.; Nico, P.S. A laboratory study of the initial effects of dissolved carbon dioxide (CO2) on metal release from shallow sediments. Int. J. Greenh. Gas Control 2013, 19, 183–211. [Google Scholar] [CrossRef]
- Serrano-Ortiz, P.; Roland, M.; Sanchez-Moral, S.; Janssens, I.A.; Domingo, F.; Godderis, Y.; Kowalski, A.S. Hidden, abiotic CO2 flows and gaseous reservoirs in the terrestrial carbon cycle: Review and perspectives. Agric. For. Meteorol. 2010, 150, 321–329. [Google Scholar] [CrossRef]
- Yang, W.; Yang, M.; Wen, H.; Jiao, Y. Global Warming Potential of CH4, uptake and N2O emissions in saline–alkaline soils. Atmos. Environ. 2018, 191, 172–180. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Wang, J.; Stahr, K.; Kuzyakov, Y. CaCO3 recrystallization in saline and alkaline soils. Geoderma 2016, 282, 1–8. [Google Scholar] [CrossRef]
- Gorai, M.; Vadel, A.M.; Neffati, M.; Khemira, H. The effect of sodium chloride salinity on the growth, water status and ion content of Phragmites communis trin. Pak. J. Biol. Sci. 2007, 10, 2225–2230. [Google Scholar] [CrossRef][Green Version]
- Pagter, M.; Bragato, C.; Malagoli, M.; Brix, H. Osmotic and ionic effects of NaCl and Na2SO4 salinity on Phragmites australis. Aquat. Bot. 2009, 90, 43–51. [Google Scholar] [CrossRef]
- Zhou, C.; Shen, W.; Lu, C.; Wang, H.; Xiao, Y.; Zhao, Y.; An, S. Effects of Salinity on the Photosynthesis of Two Poaceous Halophytes. Clean Soil Air Water 2015, 43, 1660–1665. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, Y. Effect of various salt–alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Yang, C.; Chong, J.; Li, C.; Kim, C.M.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Sun, Z.; Chen, Y.; Yang, F. Resources and sustainable resource exploitation of salinized land in China. Agric. Res. Arid Areas 2005, 23, 154–158. (In Chinese) [Google Scholar]
- Zhang, H.; Tang, J.; Liang, S.; Li, Z.; Yang, P.; Wang, J.; Wang, S. The emissions of carbon dioxide, methane, and Nitrous Oxide during winter without cultivation in local saline-alkali rice and maize fields in northeast China. Sustainability 2017, 9, 1916. [Google Scholar] [CrossRef]
- Wang, Y. Effcets of Bensulfuron-Methyl and Its Degrader Brevibacterium sp. BH on Microbial Community and Bioremediation of Bensulfuron-Methyl in Paddy Soil. Master’s Thesis, Zhejiang University, Zhejiang, China, 2007. (In Chinese). [Google Scholar]
- Veen, J.; Ladd, J.; Amato, M. Turnover of carbon and nitrogen through the microbial biomass in a sandy loam and a clay soil incubated with [14C(U)]glucose and [15N](NH4)2SO4 under different moisture regimes. Soil Biol. Biochem. 1985, 17, 747–756. [Google Scholar] [CrossRef]
- Heuck, C.; Spohn, M. Carbon, nitrogen and phosphorus net mineralization in organic horizons of temperate forests: Stoichiometry and relations to organic matter quality. Biogeochemistry 2016, 131, 229–242. [Google Scholar] [CrossRef]
- Marklein, A.R.; Winbourne, J.B.; Enders, S.K.; Gonzale, D.J.X.; van Huysen, T.L.; Izquierdo, J.E.; Light, D.R.; Liptzin, D.; Miller, K.E.; Morford, S.L.; et al. Mineralization ratios of nitrogen and phosphorus from decomposing litter in temperate versus tropical forests. Glob. Ecol. Biogeogr. 2016, 25, 335–346. [Google Scholar] [CrossRef]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Liang, S.; Li, Z.; Wang, J.; Wang, S. Early thawing after snow removal and no straw mulching accelerates organic carbon cycling in a paddy soil in Northeast China. J. Environ. Manag. 2018, 209, 336–345. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. A Publ. Ecol. Soc. Am. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Carney, K.M.; Hungate, B.A.; Drake, B.G.; Megonigal, J.P. Altered soil microbial community at elevated CO₂ leads to loss of soil carbon. Proc. Natl. Acad. Sci. USA 2007, 104, 4990–4995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tan, X.; Cheng, J.; Haddix, M.L.; Cotufo, M.F. Interactions between aged biochar, fresh low molecular weight carbon and soil organic carbon after 3.5 years soil-biochar incubations. Geoderma 2019, 333, 99–107. [Google Scholar] [CrossRef]
- Ouyang, L.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil carbon mineralisation and sequestration. Soil Res. 2014, 52, 46–54. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, X.; Li, M.; Wu, H. Impacts of biochar addition on soil dissolved organic matter characteristics in a wheat-maize rotation system in Loess Plateau of China. Chemosphere 2017, 186, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, J.; Li, Z.; Yang, W.; Duan, Y. The influence of soil physico-Chemical properties and enzyme activities on soil quality of saline-alkali agroecosystems in western Jilin Province, China. Sustainability 2018, 10, 1529. [Google Scholar] [CrossRef]
- Moore, J.; Jacobson, A.D.; Holmden, C.; Craw, D. Tracking the relationship between mountain uplift, silicate weathering, and long-term CO2 consumption with Ca isotopes: Southern Alps, New Zealand. Chem. Geol. 2013, 341, 110–127. [Google Scholar] [CrossRef]
- Manning, D.S.A.; Renforth, P.; Lopez-Capel, E.; Robertson, S.; Ghazireh, N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenh. Gas Control 2013, 17, 309–317. [Google Scholar] [CrossRef]
- Cheng, W.; Johnson, D.W.; Fu, S.L. Rhizosphere effects on decomposition: Controls of plant species, phenology, and fertilization. Soil Sci. Soc. Am. J. 2003, 67, 1418–1427. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barre, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Aslam, D.N.; Vandergheynst, J.S.; Rumsey, T.R. Development of models for predicting carbon mineralization and associated phytotoxicity in compost-amended soil. Bioresour. Technol. 2008, 99, 8735–8741. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Fangueiro, D.; Alves, F.; Vasconcelos, E.; Coutinho, J.; Bol, R.; Cabral, F. Carbon-mineralization kinetics in an organically managed Cambic Arenosol amended with organic fertilizers. J. Plant Nutr. Soil Sci. 2010, 173, 39–45. [Google Scholar] [CrossRef]
- Quan, Q.; Wang, C.; He, N.; Zhang, Z.; Wen, X.; Su, H.; Wang, Q.; Xue, J. Forest type affects the coupled relationships of soil C and N mineralization in the temperate forests of northern China. Sci. Rep. 2014, 4, 6584. [Google Scholar] [CrossRef] [PubMed]
- Trinsoutrot, I.; Recous, S.; Bentz, B.; Lineres, M.; Cheneby, D.; Nicolardot, B. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- Theuerl, S.; Buscot, F. Laccases: Toward disentangling their diversity and functions in relation to soil organic matter cycling. Biol. Fertil. Soils 2010, 46, 215–225. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Yang, G.; Miao, Y.; Wang, J.; Guo, Y. Changes in Labile Organic Carbon Fractions and Soil Enzyme Activities after Marshland Reclamation and Restoration in the Sanjiang Plain in Northeast China. Environ. Manag. 2012, 50, 418–426. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Lauber, C.; Weintraub, M.; Ahmed, B.; Allison, S.; Crenshaw, C.; Contosta, A.; Cusack, D.; Frey, S.; Gallo, M.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzyme and Its Research Methods Beijing; Agriculture Press: Beijing, China, 1986. [Google Scholar]





| Paddy Fields | Longitude | Latitude | Sampling Texture | Clay (%) | Silt (%) | Sand (%) |
|---|---|---|---|---|---|---|
| P1 | 124°43′03″ E | 45°00′19″ N | Loam | 14.07 | 40.18 | 45.75 |
| P2 | 124°40′41″ E | 45°00′25″ N | Black soil | 13.96 | 39.92 | 46.12 |
| P3 | 124°41′40″ E | 45°00′23″ N | Silt sandy loam | 10.75 | 67.33 | 21.92 |
| P4 | 124°42′27″ E | 45°00′05″ N | Sandy loam | 4.58 | 31.30 | 64.12 |
| Paddy Fields | Soil Depth | pH | EC (us·cm−1) | ESP (%) | TN (g·kg−1) | C/N | WHC (%) | SOC (g·kg−1) | CEC (cmol kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 0–20 cm | 7.74 | 392.67 | 7.16 | 0.54 | 40.22 | 43.20 | 21.76 | 16.36 |
| 20–40 cm | 8.26 | 219.33 | 8.00 | 0.48 | 39.81 | 39.10 | 19.31 | 17.42 | |
| P2 | 0–20 cm | 8.79 | 202.27 | 7.22 | 0.49 | 42.54 | 44.20 | 20.63 | 18.21 |
| 20–40 cm | 9.09 | 184.80 | 8.41 | 0.47 | 39.91 | 38.70 | 18.64 | 19.03 | |
| P3 | 0–20 cm | 8.84 | 231.00 | 7.45 | 0.46 | 40.04 | 43.50 | 18.30 | 16.02 |
| 20–40 cm | 9.16 | 190.90 | 10.02 | 0.42 | 32.93 | 39.70 | 13.83 | 16.68 | |
| P4 | 0–20 cm | 9.19 | 361.67 | 15.06 | 0.45 | 31.27 | 37.50 | 14.01 | 14.02 |
| 20–40 cm | 9.41 | 263.00 | 16.57 | 0.39 | 23.57 | 35.20 | 9.24 | 16.15 |
| Samples | Ct/g kg−1 | C0/g kg−1 | k/d−1 | T1/2 | R2 | Ct/SOC | Ct/C0 | |
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| P1 | 0–20 cm | 3.523 a | 4.211 a | 0.028 c | 4.254 b,c | 0.9844 | 0.194 c,d | 0.837 |
| 20–40 cm | 2.653 c | 3.030 c | 0.032 b | 4.139 d | 0.9911 | 0.191 f | 0.876 | |
| P2 | 0–20 cm | 3.281 a,b | 3.932 b | 0.028 c | 4.278 b | 0.9827 | 0.208 d | 0.835 |
| 20–40 cm | 2.23 d,e | 2.434 e | 0.037 a | 4.044 e | 0.9903 | 0.222 g | 0.917 | |
| P3 | 0–20 cm | 3.117 b | 3.812 b | 0.026 d | 4.335 a | 0.9839 | 0.157 c | 0.818 |
| 20–40 cm | 2.190 e | 2.448 e | 0.037 a | 4.309 a,b | 0.9846 | 0.295 e | 0.895 | |
| P4 | 0–20 cm | 2.657 c | 3.116 c | 0.030 b,c | 4.205 b,c | 0.9884 | 0.131 b | 0.853 |
| 20–40 cm | 2.446 d | 2.974 d | 0.027 c,d | 3.987 e | 0.9887 | 0.177 a | 0.822 | |
| Indicators | SOC | EC | pH | ESP | CO2-C | C0 | C0/soc | k |
|---|---|---|---|---|---|---|---|---|
| EC | 0.569 | |||||||
| pH | 0.786 | −0.788 | ||||||
| ESP | −0.945 | 0.470 | −0.118 | |||||
| CO2-C | 0.927 | −0.062 | −0.565 | −0.838 | ||||
| C0 | 0.952 * | −0.139 | −0.495 | −0.895 | 0.993 * | |||
| C0/SOC | −0.985 * | 0.544 | 0.076 | 0.975 * | −0.860 | −0.904 | ||
| k | −0.678 | 0.706 | −0.331 | 0.857 | −0.435 | −0.538 | 0.792 | |
| W | 0.696 | −0.049 | −0.386 | −0.850 | 0.724 | 0.781 | −0.721 | −0.725 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Tang, J.; Li, Z.; Liu, Y.; Zhou, Z.; Wang, J.; Qu, Y.; Dai, Z. Carbon Mineralization under Different Saline—Alkali Stress Conditions in Paddy Fields of Northeast China. Sustainability 2020, 12, 2921. https://doi.org/10.3390/su12072921
Wang S, Tang J, Li Z, Liu Y, Zhou Z, Wang J, Qu Y, Dai Z. Carbon Mineralization under Different Saline—Alkali Stress Conditions in Paddy Fields of Northeast China. Sustainability. 2020; 12(7):2921. https://doi.org/10.3390/su12072921
Chicago/Turabian StyleWang, Sining, Jie Tang, Zhaoyang Li, Yuqing Liu, Zihao Zhou, Jingjing Wang, Yunke Qu, and Zhenxue Dai. 2020. "Carbon Mineralization under Different Saline—Alkali Stress Conditions in Paddy Fields of Northeast China" Sustainability 12, no. 7: 2921. https://doi.org/10.3390/su12072921
APA StyleWang, S., Tang, J., Li, Z., Liu, Y., Zhou, Z., Wang, J., Qu, Y., & Dai, Z. (2020). Carbon Mineralization under Different Saline—Alkali Stress Conditions in Paddy Fields of Northeast China. Sustainability, 12(7), 2921. https://doi.org/10.3390/su12072921





