Soil Biodiversity Integrates Solutions for a Sustainable Future
Abstract
1. Introduction
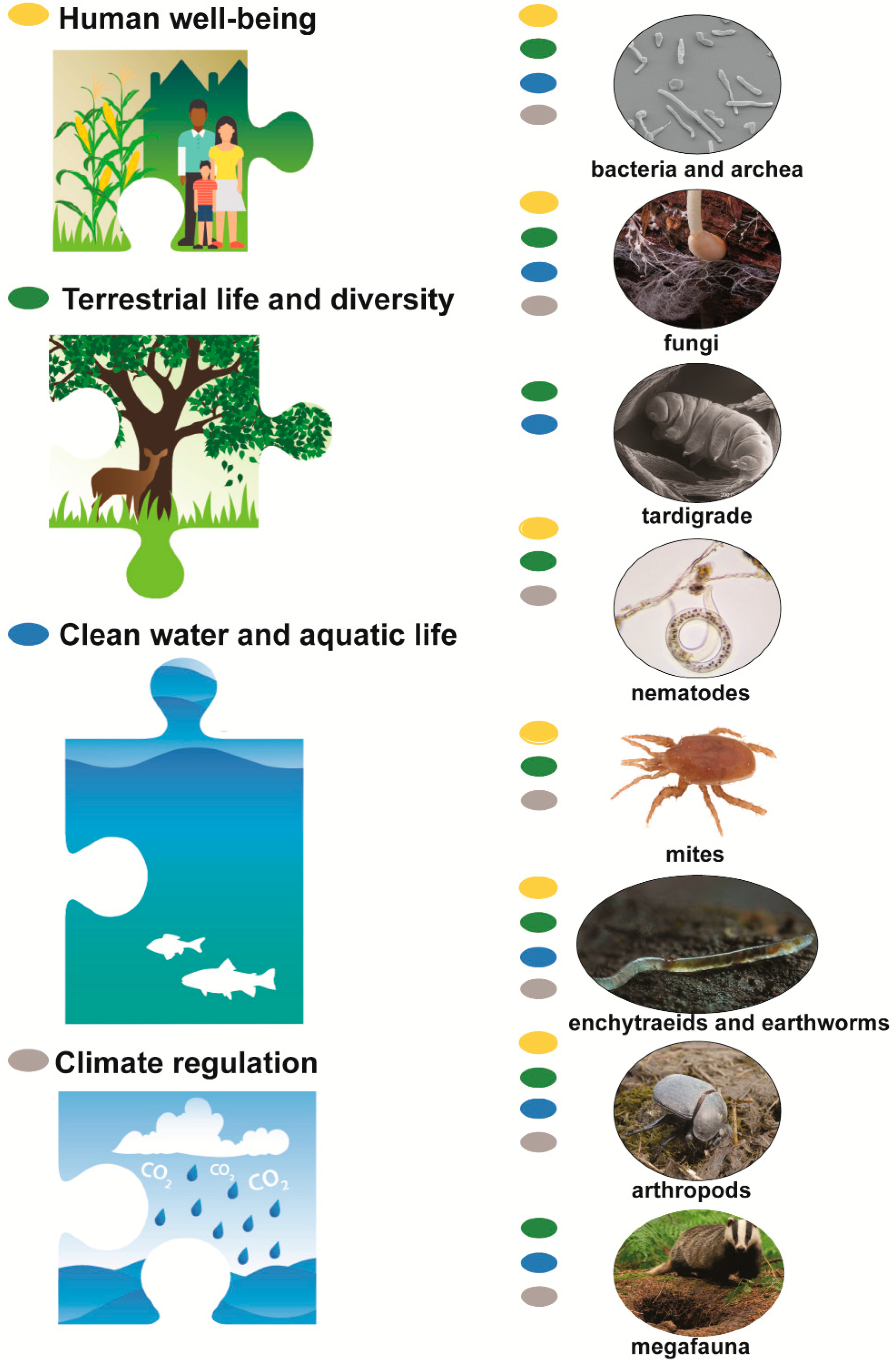
2. Soil Biodiversity Contributes to Sustainability
2.1. Soil Biodiversity Supports Human Well-Being (Sustainable Development Goals (SDGs) 1,2,3,8; Aichi Targets 13,18,19)
2.2. Soil Biodiversity Supports Terrestrial Life and Diversity (SDG 15; Entire Aichi Agenda)
2.3. Soil Biodiversity in Hydrological Processes (SDG 6,14; Aichi Targets 6,8,11)
2.4. Soil Biodiversity Regulates Climate (SDG 13; Aichi Target 15)
3. Threats to Soil Biodiversity
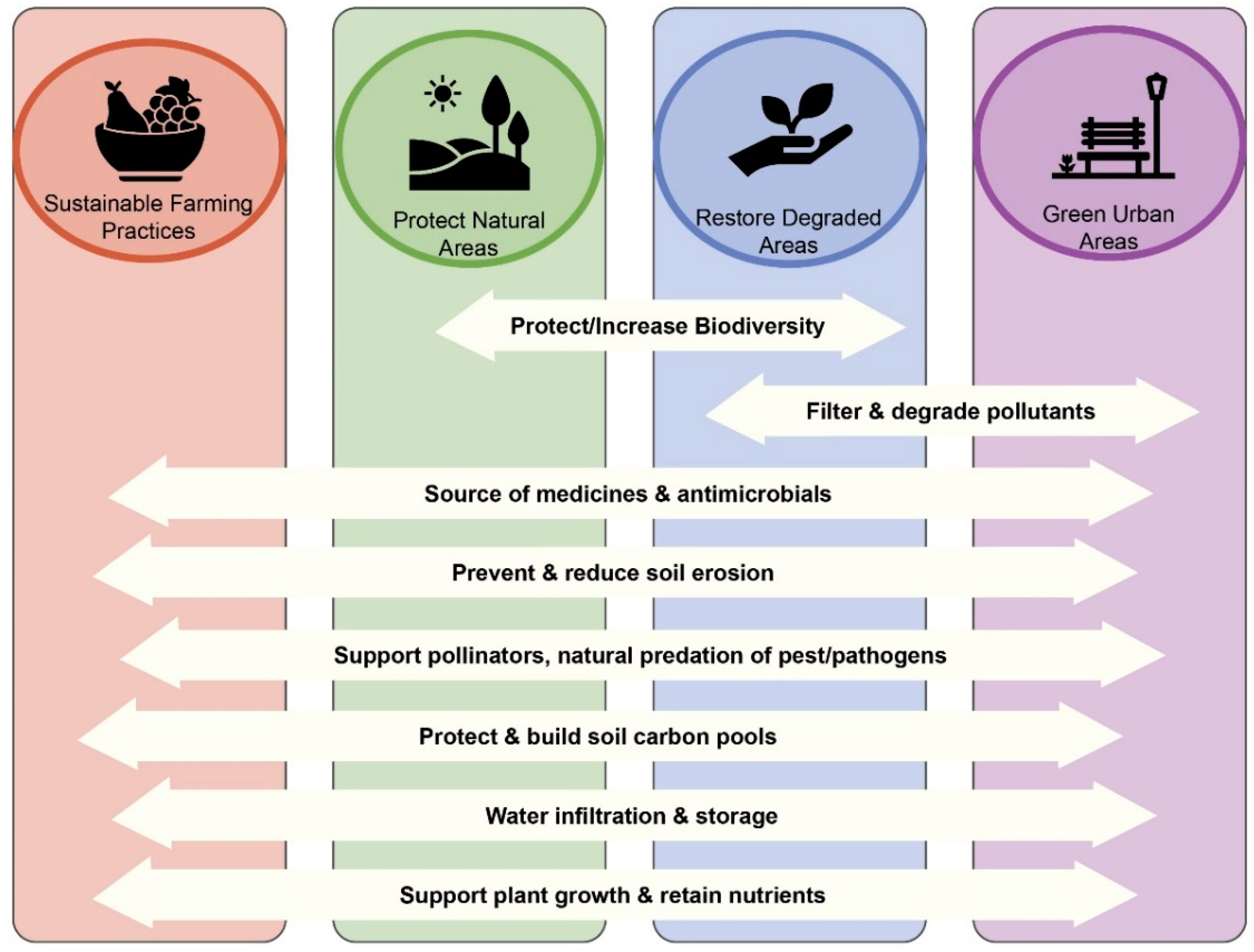
4. Protecting Soil Biodiversity
4.1. Protect Natural Areas
4.2. Restore Degraded Ecosystems
4.3. Employ Sustainable Agriculture Practices
4.4. Adapt Urban Areas for Biodiversity and People
5. Knowledge Gaps
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibbs, H.K.; Salmon, J.M. Mapping the world’s degraded lands. Appl. Geogr. 2015, 57, 12–21. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Scholes, R., Montanarella, L., Eds.; IPBES Secretariat: Bonn, Germany, 2018. [Google Scholar]
- Nilsson, M.; Griggs, D.; Visbeck, M. Policy: Map the interactions between Sustainable Development Goals. Nature 2016, 534, 320–322. [Google Scholar] [CrossRef]
- Soliveres, S.; van der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Decaëns, T.; Jiménez, J.J.; Gioia, C.; Measey, G.J.; Lavelle, P. The values of soil animals for conservation biology. Eur. J. Soil Biol. 2006, 42, S23–S38. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Bloem, J.; de Vries, F.T.; Kalbitz, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 2012, 4, 523–528. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Montanarella, L.; Pennock, D.J.; McKenzie, N.J.; Badraoui, M.; Chude, V.; Baptista, I.; Mamo, T.; Yemefack, M.; Singh Aulakh, M.; Yagi, K.; et al. World’s soils are under threat. SOIL Discuss. 2015, 2, 1263–1272. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Status of the World’s Soil Resources: Technical Summary; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Jenny, H. Factors of Soil Formation: A System of Quantitative Pedology; McGraw-Hill: New York, NY, USA; London, UK, 1941. [Google Scholar]
- UN Convention on Biological Diversity. CBD/COP/DEC/14/30 Cooperation with Other Conventions, International Organizations and Initiatives. 2018. Available online: https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-30-en.pdf (accessed on 24 September 2019).
- Schulte, R.P.O.; Bampa, F.; Bardy, M.; Coyle, C.; Creamer, R.E.; Fealy, R.; Gardi, C.; Ghaley, B.B.; Jordan, P.; Laudon, H.; et al. Making the most of our land: Managing soil functions from local to continental scale. Front. Environ. Sci. 2015, 3, 81. [Google Scholar] [CrossRef]
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerdà, A.; Montanarella, L.; Quinton, J.N.; Pachepsky, Y.; van der Putten, W.H.; et al. The significance of soils and soil science towards realization of the United Nations Sustainable Development Goals. Soil 2016, 2, 111–128. [Google Scholar] [CrossRef]
- de Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Bracht Jørgensen, H.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; van der Heijden, M.G.A. Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Wall, D.H.; Bradford, M.A.; St. John, M.G.; Trofymow, J.A.; Bhean-Pelletier, V.; Bignell, D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voight, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar]
- Liu, Y.; Duan, M.; Yu, Z. Agricultural landscapes and biodiversity in China. Agric. Ecosyst. Environ. 2013, 166, 46–54. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Ferris, H. Suppressive service of the soil food web: Effects of environmental management. Agric. Ecosyst. Environ. 2007, 119, 75–87. [Google Scholar] [CrossRef]
- Crowder, D.W.; Jabbour, R. Relationships between biodiversity and biological control in agroecosystems: Current status and future challenges. Biol. Control 2014, 75, 8–17. [Google Scholar] [CrossRef]
- Hugh-Jones, M.; Blackburn, J. The ecology of Bacillus anthracis. Mol. Asp. Med. 2009, 30, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Weisberger, M. Worst anthrax outbreak in decades strikes farms in France. 2018. Available online: https://www.livescience.com/63381-anthrax-outbreak-farms-france.html (accessed on 26 August 2019).
- Guarino, B. Anthrax sickens 13 in western Siberia, and a thawed-out reindeer corpse may be to blame. Wash. Post 2016, 28, 2016. [Google Scholar]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Wall, D.H.; Six, J. Soil Biodiversity and the Environment. Annu. Rev. Environ. Resour. 2015, 40, 63–90. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hochard, J.P. Does land degradation increase poverty in developing countries? PLoS ONE 2016, 11, 13–15. [Google Scholar] [CrossRef]
- Pauli, N.; Abbott, L.K.; Negrete-Yankelevich, S.; Andrés, P. Farmer’s knowledge and use of soil fauna in agriculture: A worldwide review. Ecol. Soc. 2016, 21, 19. [Google Scholar] [CrossRef]
- Cunha, L.; Brown, G.G.; Stanton, D.W.G.; Da Silva, E.; Hansel, F.A.; Jorge, G.; McKey, D.; Vidal-Torrado, P.; Macedo, R.S.; Velasquez, E.; et al. Soil animals and pedogenesis. Soil Sci. 2016, 181, 110–125. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Phillips, H.R.P.; Cameron, E.K.; Ferlian, O.; Türke, M.; Winter, M.; Eisenhauer, N. Red list of a black box. Nat. Ecol. Evol. 2017, 1, 0103. [Google Scholar] [CrossRef]
- Boll, P.K.; Leal-Zanchet, A.M. Predation on invasive land gastropods by a Neotropical land planarian. J. Nat. Hist. 2015, 49, 983–994. [Google Scholar] [CrossRef]
- Wardle, D. Communities and Ecosystems: Linking the Aboveground and Belowground Components; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 0-691-07487-9. [Google Scholar]
- Andelt, W.F.; Kie, J.G.; Knowlton, F.F.; Cardwell, K. Variation in coyote diets associated with season and successional changes in vegetation. J. Wildl. Manag. 1987, 51, 273. [Google Scholar] [CrossRef]
- Kuwae, T.; Miyoshi, E.; Hosokawa, S.; Ichimi, K.; Hosoya, J.; Amano, T.; Moriya, T.; Kondoh, M.; Ydenberg, R.C.; Elner, R.W. Variable and complex food web structures revealed by exploring missing trophic links between birds and biofilm. Ecol. Lett. 2012, 15, 347–356. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Reich, P.B.; Isbell, F. Decomposer diversity and identity influence plant diversity effects on ecosystem functioning. Ecology 2012, 93, 2227–2240. [Google Scholar] [CrossRef]
- Wagg, C.; Jansa, J.; Schmid, B.; van der Heijden, M.G. a Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 2011, 14, 1001–1009. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- de Vries, F.T.; Caruso, T. Eating from the same plate? Revisiting the role of labile carbon inputs in the soil food web. Soil Biol. Biochem. 2016, 102, 4–9. [Google Scholar] [CrossRef]
- Allison, S.D. Brown ground: A soil carbon analogue for the green world hypothesis? Am. Nat. 2006, 167, 619–627. [Google Scholar] [CrossRef]
- Young, I.M.; Bengough, A.G. The search for the meaning of life in soil: An opinion. Eur. J. Soil Sci. 2018, 69, 31–38. [Google Scholar] [CrossRef]
- Havlicek, E.; Mitchell, E.A.D. Soils supporting biodiversity. In Choice Reviews Online; Dighton, J., Krumins, J.A., Eds.; Spring: Dordrecht, The Netherlands, 2015; Volume 52. [Google Scholar]
- Bardgett, R.D.; Anderson, J.M.; Behan-Pelletier, V.; Brussaard, L.; Coleman, D.C.; Ettema, C.; Moldenke, A.; Schimel, J.P.; Wall, D.H. The influence of soil biodiversity on hydrological pathways and the transfer of materials between terrestrial and aquatic ecosystems. Ecosystems 2001, 4, 421–429. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Pulleman, M.M.; Schmidt, O.; Faber, J.H.; Brussaard, L. Anecic earthworms (Lumbricus terrestris) alleviate negative effects of extreme rainfall events on soil and plants in field mesocosms. Plant Soil 2015, 397, 103–113. [Google Scholar] [CrossRef]
- Badorreck, A.; Gerke, H.H.; Hüttl, R.F. Effects of ground-dwelling beetle burrows on infiltration patterns and pore structure of initial soil surfaces. Vadose Zone J. 2012, 11, vzj2011.0109. [Google Scholar] [CrossRef]
- Kotliar, N.B.; Miller, B.J.; Reading, R.P.; Clark, T.W. The prairie dog as a keystone species. In Conservation of the Black-Tailed Prairie Dog: Saving North America; Hoogland, J.L., Ed.; Island Press: Washington, DC, USA, 2006; pp. 53–64. ISBN 1559634987. [Google Scholar]
- Wilson, M.C.; Smith, A.T. The pika and the watershed: The impact of small mammal poisoning on the ecohydrology of the Qinghai-Tibetan Plateau. Ambio 2014, 44, 16–22. [Google Scholar] [CrossRef]
- Kaiser, D.; Lepage, M.; Konaté, S.; Linsenmair, K.E. Ecosystem services of termites (Blattoidea: Termitoidae) in the traditional soil restoration and cropping system Zaï in northern Burkina Faso (West Africa). Agric. Ecosyst. Environ. 2017, 236, 198–211. [Google Scholar] [CrossRef]
- Bengough, A.G. Water dynamics of the root zone: Rhizosphere biophysics and its control on soil hydrology. Vadose Zone J. 2012, 11, vzj2011.0111. [Google Scholar] [CrossRef]
- Robinson, D.A.; Hopmans, J.W.; Filipovic, V.; van der Ploeg, M.; Lebron, I.; Jones, S.B.; Reinsch, S.; Jarvis, N.; Tuller, M. Global environmental changes impact soil hydraulic functions through biophysical feedbacks. Glob. Chang. Biol. 2019, 25, 1895–1904. [Google Scholar] [CrossRef]
- NOAA Gulf of Mexico ‘Dead Zone’ Is the Largest Ever Measured | National Oceanic and Atmospheric Administration. Available online: https://www.noaa.gov/media-release/gulf-of-mexico-dead-zone-is-largest-ever-measured (accessed on 30 July 2019).
- Queste, B.Y.; Vic, C.; Heywood, K.J.; Piontkovski, S.A. Physical controls on oxygen distribution and denitrification potential in the north west Arabian Sea. Geophys. Res. Lett. 2018, 45, 4143–4152. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Zhang, J.; Huo, Y.; Shi, X.; Su, R.; et al. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef]
- Travaglia, C.; Masciarelli, O.; Fortuna, J.; Marchetti, G.; Cardozo, P.; Lucero, M.; Zorza, E.; Luna, V.; Reinoso, H. Towards sustainable maize production: Glyphosate detoxification by Azospirillum sp. and Pseudomonas sp. Crop Prot. 2015, 77, 102–109. [Google Scholar] [CrossRef]
- Bell, T.H.; Yergeau, E.; Maynard, C.; Juck, D.; Whyte, L.G.; Greer, C.W. Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel and nutrient disturbance. ISME J. 2013, 7, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.W.; Hugelius, G.; Ahlström, A.; Blankinship, J.C.; Bond-Lamberty, B.; Lawrence, C.R.; Loisel, J.; Malhotra, A.; Jackson, R.B.; Ogle, S.; et al. Networking our science to characterize the state, vulnerabilities, and management opportunities of soil organic matter. Glob. Chang. Biol. 2018, 24, e705–e718. [Google Scholar] [CrossRef] [PubMed]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; van Kessel, C.; Hungate, B.A.; Oenema, O.; Powlson, D.S.; van Groenigen, K.J. Sequestering soil organic carbon: A nitrogen dilemma. Environ. Sci. Technol. 2017, 51, 4738–4739. [Google Scholar] [CrossRef]
- de Graaff, M.-A.; Adkins, J.; Kardol, P.; Throop, H.L. A meta-analysis of soil biodiversity impacts on the carbon cycle. Soil 2015, 1, 257–271. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Wieder, W.R.; Grandy, A.S.; Kallenbach, C.M.; Bonan, G.B. Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 2014, 11, 3899–3917. [Google Scholar] [CrossRef]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; De Suiter, P.C.; Dong, Q.; Hastings, A.; Johnson, N.C.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Frouz, J.; Roubíčková, A.; Heděnec, P.; Tajovský, K. Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. Eur. J. Soil Biol. 2015, 68, 18–24. [Google Scholar] [CrossRef]
- Zhang, W.; Hendrix, P.F.; Dame, L.E.; Burke, R.A.; Wu, J.; Neher, D.A.; Li, J.; Shao, Y.; Fu, S. Earthworms facilitate carbon sequestration through unequal amplification of carbon stabilization compared with mineralization. Nat. Commun. 2013, 4, 2576. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, I.M.; Jan van Groenigen, K.; Fonte, S.J.; Six, J.; Brussaard, L.; Willem van Groenigen, J. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Mueller, R.C.; Paula, F.S.; Mirza, B.S.; Rodrigues, J.L.M.; Nüsslein, K.; Bohannan, B.J.M. Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J. 2014, 8, 1548–1550. [Google Scholar] [CrossRef]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Da, E.; Jesus, C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Sobral, B.W.; Silva, A.L.C.; Wall, D.H. Amazonian deforestation and soil biodiversity. Conserv. Biol. 2019, 33, 590–600. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; De Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 2010, 91, 460–473. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thebault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Domínguez, A.; Bedano, J.C.; Becker, A.R. Negative effects of no-till on soil macrofauna and litter decomposition in Argentina as compared with natural grasslands. Soil Tillage Res. 2010, 110, 51–59. [Google Scholar] [CrossRef]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2015, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, M.; Pigino, G.; Caruso, T.; Fanciulli, P.P.; Leonzio, C.; Bernini, F. Soil communities (Acari Oribatida; Hexapoda Collembola) in a clay pigeon shooting range. Pedobiologia 2005, 49, 1–13. [Google Scholar] [CrossRef]
- Hong, C.; Si, Y.; Xing, Y.; Li, Y. Illumina MiSeq sequencing investigation on the contrasting soil bacterial community structures in different iron mining areas. Environ. Sci. Pollut. Res. 2015, 22, 10788–10799. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. Bioscience 2005, 55, 477. [Google Scholar] [CrossRef]
- Zhang, P.; Li, B.; Wu, J.; Hu, S. Invasive plants differentially affect soil biota through litter and rhizosphere pathways: A meta-analysis. Ecol. Lett. 2019, 22, 200–210. [Google Scholar] [CrossRef]
- Cameron, E.K.; Vilà, M.; Cabeza, M. Global meta-analysis of the impacts of terrestrial invertebrate invaders on species, communities and ecosystems. Glob. Ecol. Biogeogr. 2016, 25, 596–606. [Google Scholar] [CrossRef]
- Bohlen, P.J.; Scheu, S.; Hale, C.; McLean, M.A.; Migge, S.; Groffman, P.M.; Parkinson, D. Non-native invasive earthworms as agents of change in northern temperate forests. Front. Ecol. Environ. 2004, 2, 427–435. [Google Scholar] [CrossRef]
- Craven, D.; Thakur, M.P.; Cameron, E.K.; Frelich, L.E.; Beauséjour, R.; Blair, R.B.; Blossey, B.; Burtis, J.; Choi, A.; Dávalos, A.; et al. The unseen invaders: Introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Glob. Chang. Biol. 2017, 23, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- García-Palacios, P.; Vandegehuchte, M.L.; Shaw, E.A.; Dam, M.; Post, K.H.; Ramirez, K.S.; Sylvain, Z.A.; de Tomasel, C.M.; Wall, D.H. Are there links between responses of soil microbes and ecosystem functioning to elevated CO2, N deposition and warming? A global perspective. Glob. Chang. Biol. 2015, 21, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Torode, M.; Barnett, K.L.; Facey, S.L.; Nielsen, U.; Power, S.; Johnson, S.N.J. Altered precipitation impacts on above- and belowground grassland invertebrates: Summer drought leads to outbreaks in spring. Front. Plant Sci. 2016, 7, 1468. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Shaw, E.A.; Bach, E.M.; Sala, O.E.; Wall, D.H. Drought suppresses soil predators and promotes root herbivores in mesic, but not in xeric grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 12883–12888. [Google Scholar] [CrossRef] [PubMed]
- Janion-Scheepers, C.; Phillips, L.; Sgrò, C.M.; Duffy, G.A.; Hallas, R.; Chown, S.L. Basal resistance enhances warming tolerance of alien over indigenous species across latitude. Proc. Natl. Acad. Sci. USA 2018, 115, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fronseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef]
- Fargione, J.E.; Bassett, S.; Boucher, T.; Bridgham, S.D.; Conant, R.T.; Cook-Patton, S.C.; Ellis, P.W.; Falcucci, A.; Fourqurean, J.W.; Gopalakrishna, T.; et al. Natural climate solutions for the United States. Sci. Adv. 2018, 4, eaat1869. [Google Scholar] [CrossRef]
- Kindermann, G.; Obersteiner, M.; Sohngen, B.; Sathaye, J.; Andrasko, K.; Rametsteiner, E.; Schlamadinger, B.; Wunder, S.; Beach, R. Global cost estimates of reducing carbon emissions through avoided deforestation. Proc. Natl. Acad. Sci. USA 2008, 105, 10302–10307. [Google Scholar] [CrossRef]
- Ahlering, M.; Fargione, J.; Parton, W. Potential carbon dioxide emission reductions from avoided grassland conversion in the northern Great Plains. Ecosphere 2016, 7, e01625. [Google Scholar] [CrossRef]
- Kral, K.C.; Limb, R.F.; Harmon, J.P.; Hovick, T.J. Arthropods and fire: Previous research shaping future conservation. Rangel. Ecol. Manag. 2017, 70, 589–598. [Google Scholar] [CrossRef]
- United Nations Convention to Combat Desertification. Global outlook working paper: Threats to soils. 2017. Available online: https://knowledge.unccd.int/publication/threats-soils-global-trends-and-perspectives-contribution-intergovernmental-technical (accessed on 1 December 2018).
- Wodika, B.R.; Klopf, R.P.; Baer, S.G. Colonization and recovery of invertebrate ecosystem engineers during prairie restoration. Restor. Ecol. 2014, 22, 456–464. [Google Scholar] [CrossRef]
- Wodika, B.R.; Baer, S.G. If we build it, will they colonize? A test of the field of dreams paradigm with soil macroinvertebrate communities. Appl. Soil Ecol. 2015, 91, 80–89. [Google Scholar] [CrossRef]
- Barber, N.A.; Lamagdeleine-Dent, K.A.; Willand, J.E.; Jones, H.P.; McCravy, K.W. Species and functional trait re-assembly of ground beetle communities in restored grasslands. Biodivers. Conserv. 2017, 26, 3481–3498. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Zahawi, R.A.; Wickey, P.; Townsend, A.R. Leaf litter arthropod responses to tropical forest restoration. Ecol. Evol. 2016, 6, 5158–5168. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.H.; Frère, C.; Banks, P.B. A review of fauna in mine rehabilitation in Australia: Current state and future directions. Biol. Conserv. 2012, 149, 60–72. [Google Scholar] [CrossRef]
- McCary, M.A.; Martínez, J.-C.; Umek, L.; Heneghan, L.; Wise, D.H. Effects of woodland restoration and management on the community of surface-active arthropods in the metropolitan Chicago region. Biol. Conserv. 2015, 190, 154–166. [Google Scholar] [CrossRef]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Baer, S.G.; Bach, E.M.; Meyer, C.K.; Du Preez, C.C.; Six, J. Belowground Ecosystem Recovery During Grassland Restoration: South African Highveld Compared to US Tallgrass Prairie. Ecosystems 2015, 18, 390–403. [Google Scholar] [CrossRef]
- Peralta, A.L.; Matthews, J.W.; Kent, A.D. Microbial community structure and denitrification in a wetland mitigation bank. Appl. Environ. Microbiol. 2010, 76, 4207–4215. [Google Scholar] [CrossRef]
- Strickland, M.S.; Callaham, M.A.; Gardiner, E.S.; Stanturf, J.A.; Leff, J.W.; Fierer, N.; Bradford, M.A. Response of soil microbial community composition and function to a bottomland forest restoration intensity gradient. Appl. Soil Ecol. 2017, 119, 317–326. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Wall, D.H. Soil biological responses to, and feedbacks on, trophic rewilding. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170448. [Google Scholar] [CrossRef] [PubMed]
- Andriuzzi, W.S.; Wall, D.H. Responses of belowground communities to large aboveground herbivores: Meta-analysis reveals biome-dependent patterns and critical research gaps. Glob. Chang. Biol. 2017, 23, 3857–3868. [Google Scholar] [CrossRef] [PubMed]
- Wubs, E.R.J.; van der Putten, W.H.; Mortimer, S.R.; Korthals, G.W.; Duyts, H.; Wagenaar, R.; Bezemer, T.M. Single introductions of soil biota and plants generate long-term legacies in soil and plant community assembly. Ecol. Lett. 2019, 22, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Ribas, C.R.; Schmidt, F.A.; Solar, R.R.C.; Campos, R.B.F.; Valentim, C.L.; Schoereder, J.H. Ants as indicators of the success of rehabilitation efforts in deposits of gold mining tailings. Restor. Ecol. 2012, 20, 712–720. [Google Scholar] [CrossRef]
- Mergeay, M.; Monchy, S.; Vallaeys, T.; Auquier, V.; Benotmane, A.; Bertin, P.; Taghavi, S.; Dunn, J.; van der Lelie, D.; Wattiez, R. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: Towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 2003, 27, 385–410. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Klopf, R.P.; Baer, S.G.; Bach, E.M.; Six, J. Restoration and management for plant diversity enhances the rate of belowground ecosystem recovery. Ecol. Appl. 2017, 27, 355–362. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. AMF, phylogeny, and succession: Specificity of response to mycorrhizal fungi increases for late-successional plants. Ecosphere 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Maul, J.E.; Szlavecz, K. Managing soil biodiversity and ecosystem services. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 335–356. ISBN 978-0-19-957592-3. [Google Scholar]
- Briones, M.J.I.; Schmidt, O. Conventional tillage decreases the abundance and biomass of earthworms and alters their community structure in a global meta-analysis. Glob. Chang. Biol. 2017, 23, 4396–4419. [Google Scholar] [CrossRef]
- Brennan, A.; Fortune, T.; Bolger, T. Collembola abundances and assemblage structures in conventionally tilled and conservation tillage arable systems. Pedobiologia 2006, 50, 135–145. [Google Scholar] [CrossRef]
- Bedano, J.C.; Cantú, M.P.; Doucet, M.E. Influence of three different land management practices on soil mite (Arachnida: Acari) densities in relation to a natural soil. Appl. Soil Ecol. 2006, 32, 293–304. [Google Scholar] [CrossRef]
- Spurgeon, D.; Keith, A.; Schmidt, O.; Lammertsma, D.; Faber, J. Land-use and land-management change: Relationships with earthworm and fungi communities and soil structural properties. BMC Ecol. 2013, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. 2005, 29, 189–217. [Google Scholar] [CrossRef]
- Erktan, A.; McCormack, M.L.; Roumet, C. Frontiers in root ecology: Recent advances and future challenges. Plant Soil 2018, 424, 1–9. [Google Scholar] [CrossRef]
- Smith, C.M.; David, M.B.; Mitchell, C.A.; Masters, M.D.; Anderson-Teixeira, K.J.; Bernacchi, C.J.; DeLucia, E.H. Reduced nitrogen losses after conversion of row crop agriculture to perennial biofuel crops. J. Environ. Qual. 2013, 42, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Crews, T.E.; Blesh, J.; Culman, S.W.; Hayes, R.C.; Jensen, E.S.; Mack, M.C.; Peoples, M.B.; Schipanski, M.E. Going where no grains have gone before: From early to mid-succession. Agric. Ecosyst. Environ. 2016, 223, 223–238. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanksi, M.E.; Grandy, A.S. Managing agroecosystems for soil microbial carbon use efficiency: Ecological unknowns, potential outcomes, and a path forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global meta-analysis of the relationship between soil organic matter and crop yields. Soil Discuss. 2018, 5, 15–32. [Google Scholar] [CrossRef]
- Pauli, N.; Barrios, E.; Conacher, A.J.; Oberthür, T. Farmer knowledge of the relationships among soil macrofauna, soil quality and tree species in a smallholder agroforestry system of western Honduras. Geoderma 2012, 189, 186–198. [Google Scholar] [CrossRef]
- McKey, D.; Rostain, S.; Iriarte, J.; Glaser, B.; Birk, J.J.; Holst, I.; Renard, D. Pre-Columbian agricultural landscapes, ecosystem engineers, and self-organized patchiness in Amazonia. Proc. Natl. Acad. Sci. USA 2010, 107, 7823–7828. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs Population Division. World urbanization prospects 2018: Highlights. 2019. Available online: https://population.un.org/wup/Publications/Files/WUP2018-Highlights.pdf (accessed on 25 July 2019).
- Pouyat, R.V.; Yesilonis, I.D.; Dombos, M.; Szlavecz, K.; Setälä, H.; Cilliers, S.; Hornung, E.; Kotze, D.J.; Yarwood, S. A global comparison of surface soil characteristics across five cities. Soil Sci. 2015, 180, 136–145. [Google Scholar] [CrossRef]
- Epp Schmidt, D.J.; Pouyat, R.; Szlavecz, K.; Setälä, H.; Kotze, D.J.; Yesilonis, I.; Cilliers, S.; Hornung, E.; Dombos, M.; Yarwood, S.A. Urbanization erodes ectomycorrhizal fungal diversity and may cause microbial communities to converge. Nat. Ecol. Evol. 2017, 1, 0123. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Insect rarity, extinction and conservation in urban Rome (Italy): A 120-year-long study of tenebrionid beetles. Insect Conserv. Divers. 2011, 4, 307–315. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Leff, J.W.; Barberan, A.; Bates, S.T.; Betley, J.; Crowther, T.W.; Kelly, E.F.; Oldfield, E.E.; Shaw, E.A.; Steenbock, C.; et al. Biogeographic patterns in below-ground diversity in New York City’s Central Park are similar to those observed globally. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141988. [Google Scholar] [CrossRef] [PubMed]
- Byrne, L.B.; Bruns, M.A.; Kim, K.C. Ecosystem properties of urban land covers at the aboveground-belowground interface. Ecosystems 2008, 11, 1065–1077. [Google Scholar] [CrossRef]
- Mehring, A.S.; Levin, L.A. Potential roles of soil fauna in improving the efficiency of rain gardens used as natural stormwater treatment systems. J. Appl. Ecol. 2015, 52, 1445–1454. [Google Scholar] [CrossRef]
- McGuire, K.L.; Payne, S.G.; Palmer, M.I.; Gillikin, C.M.; Keefe, D.; Kim, S.J.; Gedallovich, S.M.; Discenza, J.; Rangamannar, R.; Koshner, J.A.; et al. Digging the New York city skyline: Soil fungal communities in green roofs and city parks. PLoS ONE 2013, 8, e58020. [Google Scholar] [CrossRef]
- Kyrö, K.; Brenneisen, S.; Kotze, D.J.; Szallies, A.; Gerner, M.; Lehvävirta, S. Local habitat characteristics have a stronger effect than the surrounding urban landscape on beetle communities on green roofs. Urban For. Urban Green. 2018, 29, 122–130. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.K.; Martins, I.S.; Lavelle, P.; Mathieu, J.; Tedersoo, L.; Bahram, M.; Gottschall, F.; Guerra, C.A.; Hines, J.; Patoine, G.; et al. Global mismatches in aboveground and belowground biodiversity. Conserv. Biol. 2019, 33, 1187–1192. [Google Scholar] [CrossRef]

| Strategic Goal | Targets |
|---|---|
| Address the underlying causes of biodiversity loss by mainstreaming biodiversity across government and society. | 1, 2, 3, 4 |
| Reduce the direct pressures on biodiversity and promote sustainable use. | 5, 6, 7, 8, 9, 10 |
| Improve the status of biodiversity by safeguarding ecosystems, species, and genetic diversity. | 11, 12, 13 |
| Enhance the benefits to all from biodiversity and ecosystem services. | 14, 15, 16 |
| Enhance implementation through participatory planning, knowledge management, and capacity building. | 17, 18, 19, 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach, E.M.; Ramirez, K.S.; Fraser, T.D.; Wall, D.H. Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability 2020, 12, 2662. https://doi.org/10.3390/su12072662
Bach EM, Ramirez KS, Fraser TD, Wall DH. Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability. 2020; 12(7):2662. https://doi.org/10.3390/su12072662
Chicago/Turabian StyleBach, Elizabeth M., Kelly S. Ramirez, Tandra D. Fraser, and Diana H. Wall. 2020. "Soil Biodiversity Integrates Solutions for a Sustainable Future" Sustainability 12, no. 7: 2662. https://doi.org/10.3390/su12072662
APA StyleBach, E. M., Ramirez, K. S., Fraser, T. D., & Wall, D. H. (2020). Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability, 12(7), 2662. https://doi.org/10.3390/su12072662





