Sensitivity and Uncertainty Analyses of Flux-based Ecosystem Model towards Improvement of Forest GPP Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Flux-based Ecosystem Model and Parameters
2.1.1. Leaf-level photosynthesis
2.1.2. Stomatal conductance
2.1.3. Canopy-level Photosynthesis
2.1.4. Ecosystem Respiration
2.2. Data
2.3. Extended Fourier Amplitude Sensitivity Test (EFAST)
2.4. Numerical Experiments for Sensitivity Analysis
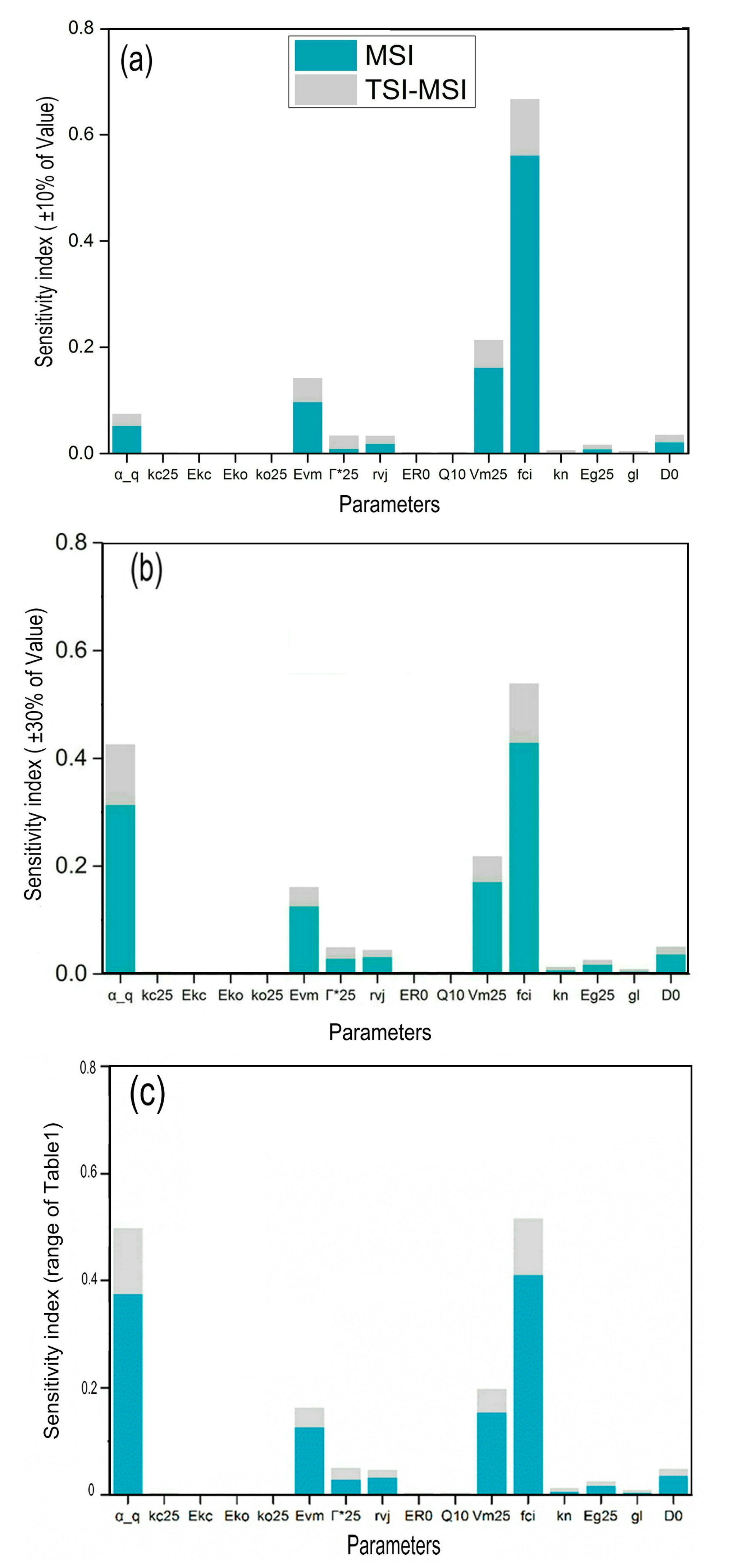
2.4.1. Parameters’ SI Variation with Parameter Range
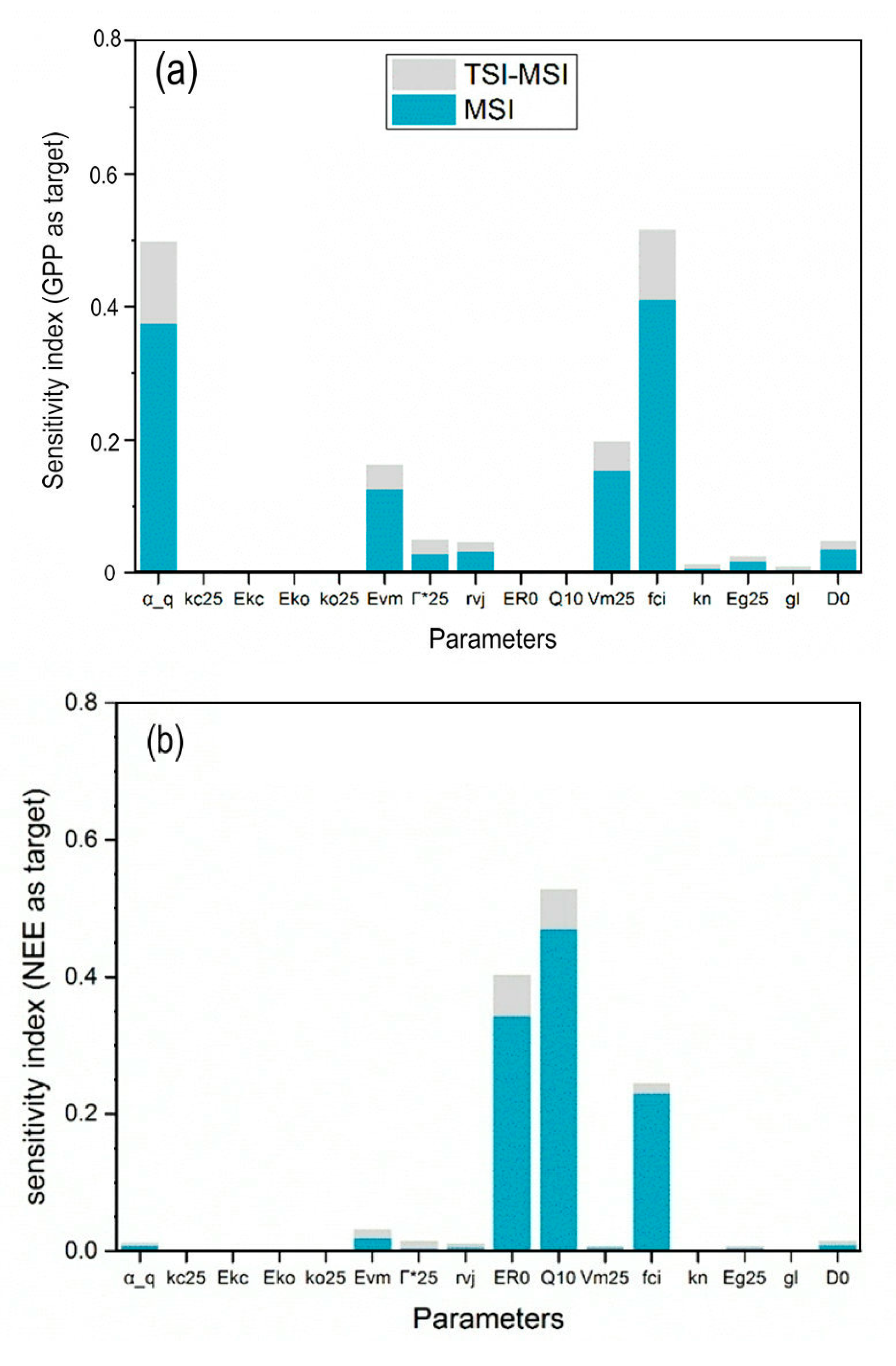
2.4.2. Comparison of Parameter SIs for GPP and NEE
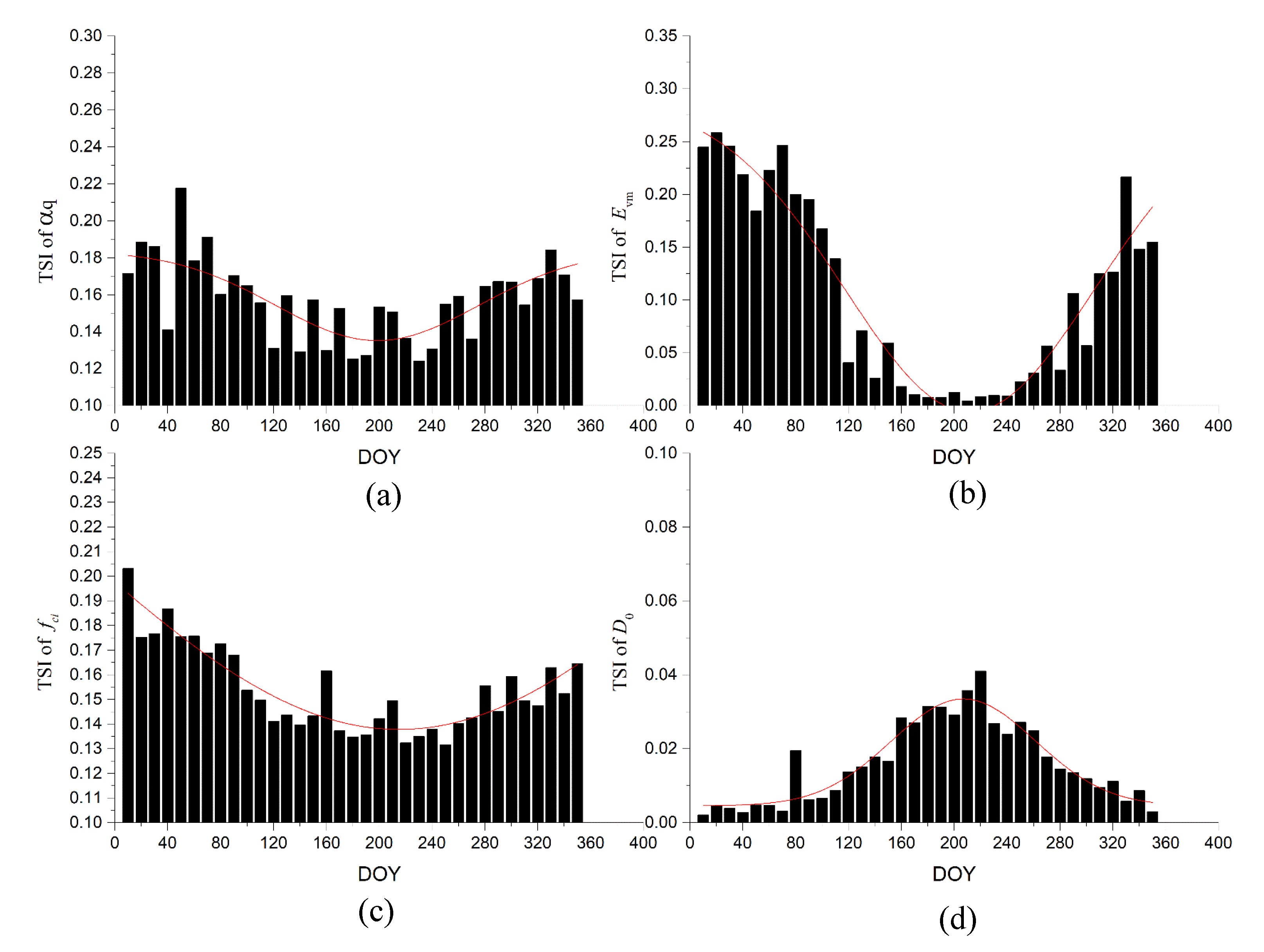
2.4.3. Temporal Characteristics of Parameter Sensitivity for GPP
2.4.4. Results of Uncertainty Analysis based on Parameter Sensitivity Analysis and Parameter Estimation
3. Results
3.1. Parameters’ SI variation with Parameter Range
3.2. Parameter Sensitivity for GPP and NEE
3.3. Temporal Characteristics of Parameter SA
3.4. Parameter Uncertainty Analysis based on Sensitivity Analysis
4. Discussion
4.1. Comparing to Previous Studies of Sensitive Parameters
4.2. Analysis of Variation of Parameters SIs
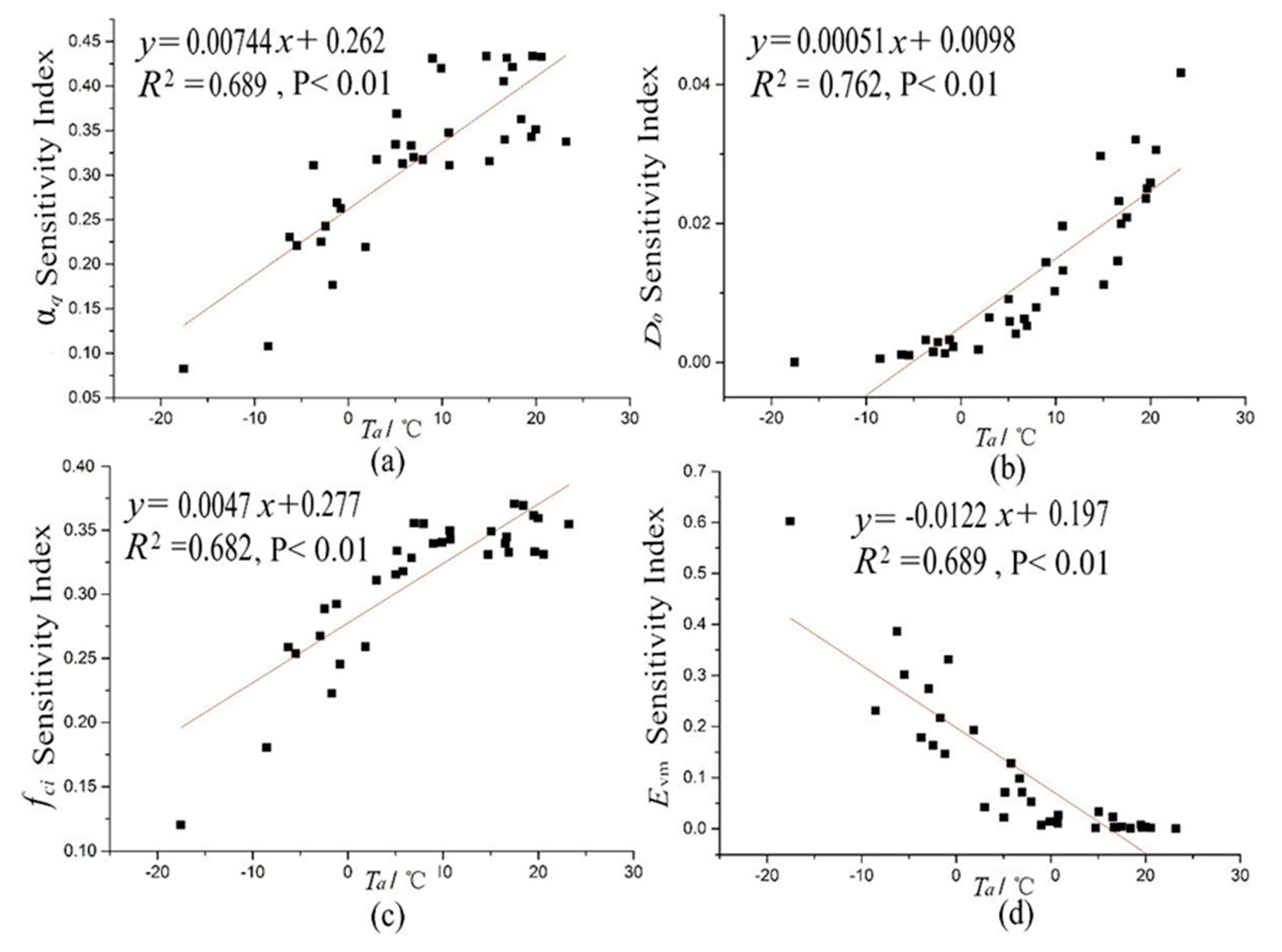
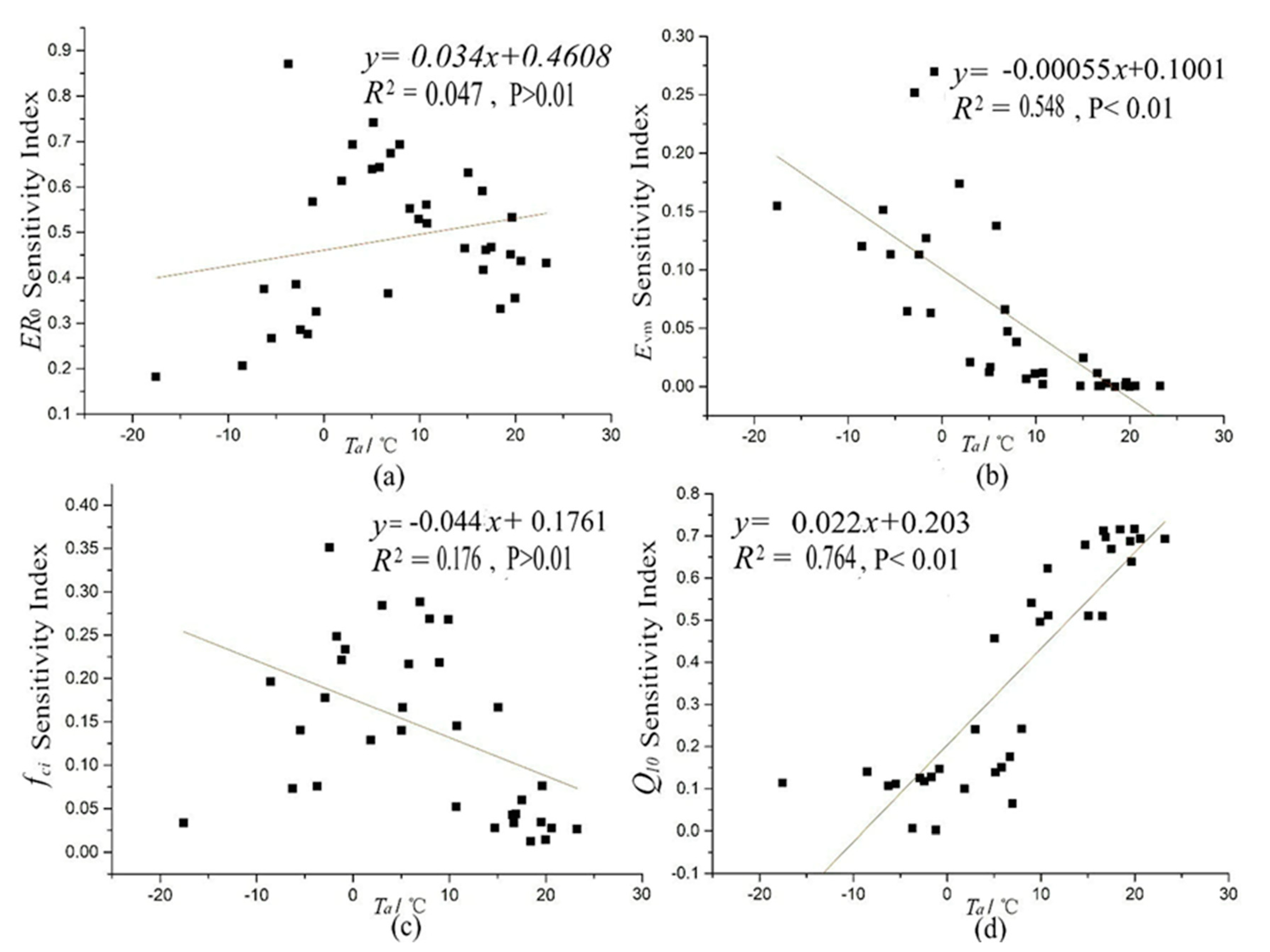
4.3. Environmental Factors Analyses of Temporal Variance of Parameter SIs
4.4. SA Improves the Effect of Parameter Optimization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Wu, C.; Liu, Y.; Wang, X.; Fang, B.; Yuan, W.; Ge, Q. Spring green-up date derived from GIMMS3g and SPOT-VGT NDVI of winter wheat cropland in the North China Plain. ISPRS J. Photogramm. Remote Sens. 2017, 130, 81–91. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, Y.; Huang, M.; Tao, B.; Liu, Z.; Hao, M.; Guo, R. Climate-driven uncertainties in modeling terrestrial ecosystem net primary productivity in China. Agric. For. Meteorol. 2017, 246, 123–132. [Google Scholar] [CrossRef]
- Sellers, P.J.; Randall, D.A.; Collatz, G.J.; Berry, J.A.; Field, C.B.; Dazlich, D.A.; Zhang, C.; Collelo, G.D.; Bounoua, L. A revised land surface parameterization (SiB2) for atmospheric GCMs .1. Model formulation. J. Clim. 1996, 9, 676–705. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, L.; Dickinson, R.E.; Norby, R.J.; Pallardy, S.G.; Hoffman, F.M. Impact of mesophyll diffusion on estimated global land CO2 fertilization. Proc. Natl. Acad. Sci. USA 2014, 111, 15774–15779. [Google Scholar] [CrossRef]
- Fisher, R.A.; Koven, C.D.; Anderegg, W.R.L.; Christoffersen, B.O.; Dietze, M.C.; Farrior, C.E.; Holm, J.A.; Hurtt, G.C.; Knox, R.G.; Lawrence, P.J.; et al. Vegetation demographics in Earth System Models: A review of progress and priorities. Glob. Chang. Biol. 2018, 24, 35–54. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z. Modelling for multi-scale ecosystems in the context of global climate change. Ecol. Model. 2013, 252, 1–2. [Google Scholar] [CrossRef]
- Bonan, G.B.; Doney, S.C. Climate, ecosystems, and planetary futures: The challenge to predict life in Earth system models. Science 2018, 359, 533. [Google Scholar] [CrossRef]
- Randerson, J.T.; Thompson, M.V.; Conway, T.J.; Fung, I.Y.; Field, C.B. The contribution of terrestrial sources and sinks to trends in the seasonal cycle of atmospheric carbon dioxide. Glob. Biogeochem. Cycles 1997, 11, 535–560. [Google Scholar] [CrossRef]
- Canadell, J.G.; Kirschbaum, M.U.F.; Kurz, W.A.; Sanz, M.-J.; Schlamadinger, B.; Yamagata, Y. Factoring out natural and indirect human effects on terrestrial carbon sources and sinks. Environ. Sci. Policy 2007, 10, 370–384. [Google Scholar] [CrossRef]
- Keenan, T.F.; Baker, I.; Barr, A.; Ciais, P.; Davis, K.; Dietze, M.; Dragon, D.; Gough, C.M.; Grant, R.; Hollinger, D.; et al. Terrestrial biosphere model performance for inter-annual variability of land-atmosphere CO2 exchange. Glob. Chang. Biol. 2012, 18, 1971–1987. [Google Scholar] [CrossRef]
- Saltelli, A.; Tarantola, S.; Chan, K.P.S. A quantitative model-independent method for global sensitivity analysis of model output. Technometrics 1999, 41, 39–56. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Lu, L.; Fang, F. Parameter sensitivity analysis of crop growth models based on the extended Fourier Amplitude Sensitivity Test method. Environ. Model. Softw. 2013, 48, 171–182. [Google Scholar] [CrossRef]
- Lagerwall, G.; Kiker, G.; Munoz-Carpena, R.; Wang, N. Global uncertainty and sensitivity analysis of a spatially distributed ecological model. Ecol. Model. 2014, 275, 22–30. [Google Scholar] [CrossRef]
- Radomyski, A.; Giubilato, E.; Ciffroy, P.; Critto, A.; Brochot, C.; Marcomini, A. Modelling ecological and human exposure to POPs in Venice lagoon—Part II: Quantitative uncertainty and sensitivity analysis in coupled exposure models. Sci. Total Environ. 2016, 569, 1635–1649. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera, J.P.; van der Tol, C.; Magnani, F.; Mohammed, G.; Moreno, J. Global sensitivity analysis of the SCOPE model: What drives simulated canopy-leaving sun-induced fluorescence? Remote Sens. Environ. 2015, 166, 8–21. [Google Scholar] [CrossRef]
- Zhu, G.; Su, Y.; Li, X.; Zhang, K.; Li, C. Estimating actual evapotranspiration from an alpine grassland on Qinghai-Tibetan plateau using a two-source model and parameter uncertainty analysis by Bayesian approach. J. Hydrol. 2013, 476, 42–51. [Google Scholar] [CrossRef]
- Tian, S.; Youssef, M.A.; Amatya, D.M.; Vance, E.D. Global sensitivity analysis of DRAINMOD-FOREST, an integrated forest ecosystem model. Hydrol. Process. 2014, 28, 4389–4410. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Wang, S. A Global Sensitivity Analysis of Soil Parameters Associated With Backscattering Using the Advanced Integral Equation Model. IEEE Trans. Geosci. Remote Sens. 2015, 53, 5613–5623. [Google Scholar] [CrossRef]
- Tan, J.; Cui, Y.; Luo, Y. Assessment of uncertainty and sensitivity analyses for ORYZA model under different ranges of parameter variation. Eur. J. Agron. 2017, 91, 54–62. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Huang, Z.; Yan, W. Parameter optimization, sensitivity, and uncertainty analysis of an ecosystem model at a forest flux tower site in the United States. J. Adv. Model. Earth Syst. 2014, 6, 405–419. [Google Scholar] [CrossRef]
- Confalonieri, R.; Bellocchi, G.; Bregaglio, S.; Donatelli, M.; Acutis, M. Comparison of sensitivity analysis techniques: A case study with the rice model WARM. Ecol. Model. 2010, 221, 1897–1906. [Google Scholar] [CrossRef]
- Cariboni, J.; Gatelli, D.; Liska, R.; Saltelli, A. The role of sensitivity analysis in ecological modelling. Ecol. Model. 2007, 203, 167–182. [Google Scholar] [CrossRef]
- Saltelli, A.; Campolongo, F.; Cariboni, J. Screening important inputs in models with strong interaction properties. Reliab. Eng. Syst. Saf. 2009, 94, 1149–1155. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, X. Sensitivity analysis of modelled responses of vegetation dynamics on the Tibetan Plateau to doubled CO2 and associated climate change. Theor. Appl. Climatol. 2016, 124, 229–239. [Google Scholar] [CrossRef]
- Sobol’, I.M. Sensitivity estimates for nonlinear mathematical models. Math. Model. Comput. Exper. 1993, 14, 407–414. [Google Scholar]
- Saltelli, A.; Annoni, P.; Azzini, I.; Campolongo, F.; Ratto, M.; Tarantola, S. Variance based sensitivity analysis of model output. Design and estimator for the total sensitivity index. Comput. Phys. Commun. 2010, 181, 259–270. [Google Scholar] [CrossRef]
- Li, W.; Peng, C.; Zhou, X.; Sun, J.; Zhu, Q.; Wu, H.; St-Onge, B. Application of the ecosystem model and Markov Chain Monte Carlo for parameter estimation and productivity prediction. Ecosphere 2015, 6. [Google Scholar] [CrossRef]
- Yuan, W.; Liang, S.; Liu, S.; Weng, E.; Luo, Y.; Hollinger, D.; Zhang, H. Improving model parameter estimation using coupling relationships between vegetation production and ecosystem respiration. Ecol. Model. 2012, 240, 29–40. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, Q. Parameterization and sensitivity analysis of a process-based terrestrial ecosystem model using adjoint method. J. Adv. Model. Earth Syst. 2014, 6, 315–331. [Google Scholar] [CrossRef]
- Pappas, C.; Fatichi, S.; Leuzinger, S.; Wolf, A.; Burlando, P. Sensitivity analysis of a process-based ecosystem model: Pinpointing parameterization and structural issues. J. Geophys. Res. Biogeosci. 2013, 118, 505–528. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Heng, L.; Steduto, P.; Rojas-Lara, B.; Raes, D.; Fereres, E. AquaCrop-The FAO Crop Model to Simulate Yield Response to Water: III. Parameterization and Testing for Maize. Agron. J. 2009, 101, 448–459. [Google Scholar] [CrossRef]
- Ferrari, A.; Gutierrez, S.; Sin, G. Modeling a production scale milk drying process: Parameter estimation, uncertainty and sensitivity analysis. Chem. Eng. Sci. 2016, 152, 301–310. [Google Scholar] [CrossRef]
- Varella, H.; Guerif, M.; Buis, S. Global sensitivity analysis measures the quality of parameter estimation: The case of soil parameters and a crop model. Environ. Model. Softw. 2010, 25, 310–319. [Google Scholar] [CrossRef]
- Tang, J.; Zhuang, Q. A global sensitivity analysis and Bayesian inference framework for improving the parameter estimation and prediction of a process-based Terrestrial Ecosystem Model. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Wang, Y.P.; Leuning, R.; Cleugh, H.A.; Coppin, P.A. Parameter estimation in surface exchange models using nonlinear inversion: How many parameters can we estimate and which measurements are most useful? Glob. Chang. Biol. 2001, 7, 495–510. [Google Scholar] [CrossRef]
- Xu, T.; White, L.; Hui, D.F.; Luo, Y.Q. Probabilistic inversion of a terrestrial ecosystem model: Analysis of uncertainty in parameter estimation and model prediction. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Xiao, X.M.; Zhang, Q.Y.; Hollinger, D.; Aber, J.; Moore, B. Modeling gross primary production of an evergreen needleleaf forest using modis and climate data. Ecol. Appl. 2005, 15, 954–969. [Google Scholar] [CrossRef]
- Li, Q.; Xia, J.; Shi, Z.; Huang, K.; Du, Z.; Lin, G.; Luo, Y. Variation of parameters in a Flux-Based Ecosystem Model across 12 sites of terrestrial ecosystems in the conterminous USA. Ecol. Model. 2016, 336, 57–69. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, W.; Xia, J.; Chen, J.; Liu, S.; Dong, W.; Merbold, L.; Law, B.; Arain, A.; Beringer, J.; et al. Global comparison of light use efficiency models for simulating terrestrial vegetation gross primary production based on the La Thuile database. Agric. For. Meteorol. 2014, 192, 108–120. [Google Scholar] [CrossRef]
- Knorr, W.; Kattge, J. Inversion of terrestrial ecosystem model parameter values against eddy covariance measurements by Monte Carlo sampling. Glob. Chang. Biol. 2005, 11, 1333–1351. [Google Scholar] [CrossRef]
- Van Wijk, M.T.; Clemmensen, K.E.; Shaver, G.R.; Williams, M.; Callaghan, T.V.; Chapin, F.S.; Cornelissen, J.H.C.; Gough, L.; Hobbie, S.E.; Jonasson, S.; et al. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: Generalizations and differences in ecosystem and plant type responses to global change. Glob. Chang. Biol. 2004, 10, 105–123. [Google Scholar] [CrossRef]
- Van Wijk, M.T.; Dekker, S.C.; Bouten, W.; Bosveld, F.C.; Kohsiek, W.; Kramer, K.; Mohren, G.M.J. Modeling daily gas exchange of a Douglas-fir forest: Comparison of three stomatal conductance models with and without a soil water stress function. Tree Physiol. 2000, 20, 115–122. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Caemmerer, S.V.; Berry, J.A. A biochemical-model of photosynthetic CO2 assimilation in leaves of C-3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Leuning, R. A critical-appraisal of a combined stomatal-photosynthesis model for C-3 plants. Plant Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Liu, Y.; Parolari, A.J.; Kumar, M.; Huang, C.-W.; Katul, G.G.; Porporato, A. Increasing atmospheric humidity and CO2 concentration alleviate forest mortality risk. Proc. Natl. Acad. Sci. USA 2017, 114, 9918–9923. [Google Scholar] [CrossRef] [PubMed]
- Sellers, P.J.; Berry, J.A.; Collatz, G.J.; Field, C.B.; Hall, F.G. Canopy reflectance, photosynthesis, and transpiration 3. A reanalysis using improved leaf models and a new canopy integration scheme. Remote Sens. Environ. 1992, 42, 187–216. [Google Scholar] [CrossRef]
- Hollinger, D.Y.; Aber, J.; Dail, B.; Davidson, E.A.; Goltz, S.M.; Hughes, H.; Leclerc, M.Y.; Lee, J.T.; Richardson, A.D.; Rodrigues, C.; et al. Spatial and temporal variability in forest-atmosphere CO2 exchange. Glob. Chang. Biol. 2004, 10, 1689–1706. [Google Scholar] [CrossRef]
- Hollinger, D.Y.; Goltz, S.M.; Davidson, E.A.; Lee, J.T.; Tu, K.; Valentine, H.T. Seasonal patterns and environmental control of carbon dioxide and water vapour exchange in an ecotonal boreal forest. Glob. Chang. Biol. 1999, 5, 891–902. [Google Scholar] [CrossRef]
- Xiao, X.M.; Hollinger, D.; Aber, J.; Goltz, M.; Davidson, E.A.; Zhang, Q.Y.; Moore, B. Satellite-based modeling of gross primary production in an evergreen needleleaf forest. Remote Sens. Environ. 2004, 89, 519–534. [Google Scholar] [CrossRef]
- Yan, M.; Tian, X.; Li, Z.Y.; Chen, E.X.; Wang, X.F.; Han, Z.T.; Sun, H. Simulation of Forest Carbon Fluxes Using Model Incorporation and Data Assimilation. Remote Sens. 2016, 8. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Z.; Sathyendranath, S.; Platt, T.; Jiang, C.; Sun, K. Canopy Reflectance Modeling of Aquatic Vegetation for Algorithm Development: Global Sensitivity Analysis. Remote Sens. 2018, 10, 837. [Google Scholar] [CrossRef]
- Prowse, T.A.A.; Bradshaw, C.J.A.; Delean, S.; Cassey, P.; Lacy, R.C.; Wells, K.; Aiello-Lammens, M.E.; Akcakaya, H.R.; Brook, B.W. An efficient protocol for the global sensitivity analysis of stochastic ecological models. Ecosphere 2016, 7. [Google Scholar] [CrossRef]
- Helton, J.C.; Davis, F.J.; Johnson, J.D. A comparison of uncertainty and sensitivity analysis results obtained with random and Latin hypercube sampling. Reliab. Eng. Syst. Saf. 2005, 89, 305–330. [Google Scholar] [CrossRef]
- Hollinger, D.Y.; Richardson, A.D. Uncertainty in eddy covariance measurements and its application to physiological models. Tree Physiol. 2005, 25, 873–885. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Weng, E.; White, L.; Ma, Y.; Zhou, X. Conditional inversion to estimate parameters from eddy-flux observations. J. Plant Ecol. 2009, 2, 55–68. [Google Scholar] [CrossRef]
- Mosegaard, K.; Sambridge, M. Monte Carlo analysis of inverse problems. Inverse Probl. 2002, 18, R29–R54. [Google Scholar] [CrossRef]
- Bonan, G.B.; Lawrence, P.J.; Oleson, K.W.; Levis, S.; Jung, M.; Reichstein, M.; Lawrence, D.M.; Swenson, S.C. Improving canopy processes in the Community Land Model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Rogers, A. The use and misuse of Vc,max in Earth System Models. Photosynth. Res. 2014, 119, 15–29. [Google Scholar] [CrossRef]
- Walker, A.P.; Quaife, T.; van Bodegom, P.M.; De Kauwe, M.G.; Keenan, T.F.; Joiner, J.; Lomas, M.R.; MacBean, N.; Xu, C.; Yang, X.; et al. The impact of alternative trait-scaling hypotheses for the maximum photosynthetic carboxylation rate (V-cmax) on global gross primary production. New Phytol. 2017, 215, 1370–1386. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; de Angelis, P.; Freeman, M.; Wingate, L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Chang. Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef]
- Tan, Z.-H.; Wu, Z.-X.; Hughes, A.C.; Schaefer, D.; Zeng, J.; Lan, G.-Y.; Yang, C.; Tao, Z.-L.; Chen, B.-Q.; Tian, Y.-H.; et al. On the ratio of intercellular to ambient CO2 (c(i)/c(a)) derived from ecosystem flux. Int. J. Biometeorol. 2017, 61, 2059–2071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shao, Q.; Tao, J.; Chi, W. Intra-annual variability of satellite observed surface albedo associated with typical land cover types in China. J. Geogr. Sci. 2015, 25, 35–44. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, P.; Hui, D.; Luo, Y. Global pattern of temperature sensitivity of soil heterotrophic respiration (Q(10)) and its implications for carbon-climate feedback. J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Spatial and seasonal variations of Q(10) determined by soil respiration measurements at a Sierra Nevadan forest. Glob. Biogeochem. Cycles 2001, 15, 687–696. [Google Scholar] [CrossRef]
- Yuan, W.; Xu, W.; Ma, M.; Chen, S.; Liu, W.; Cui, L. Improved snow cover model in terrestrial ecosystem models over the Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2016, 218, 161–170. [Google Scholar] [CrossRef]


| Parameter | Definition | Unit | Value | Range | Reference | |
|---|---|---|---|---|---|---|
| Minimum | Maximum | |||||
| Canopy quantum efficiency of photon conversion | mol mol−1 photon | 0.28 | 0 | 0.5 | [40] | |
| Michaelis–Menten constant for carboxylation | mol mol−1 | 460 | 50 | 600 | [40] | |
| Activation energy of | Jmol−1 | 59,356 | 30,000 | 150,000 | [40] | |
| Activation energy of | Jmol−1 | 35,948 | 10,000 | 60,000 | [40] | |
| Michaelis–Menten constant for oxygenation | mol mol−1 | 0.33 | 0.2 | 0.5 | [40] | |
| Activation energy of | Jmol−1 | 58,520 | 10,000 | 100,000 | [40] | |
| CO2 compensation point without dark respiration | μmol mol−1 | 42.5 | 10 | 200 | [40] | |
| Ratio of to at 25 ℃ | - | 1.79 | 1 | 5 | [40] | |
| Whole ecosystem respiration at 0 °C | μmol CO2 m−2 s−1 | 2.5 | 1 | 5 | [41] | |
| Temperature dependency of ecosystem respiration | - | 2 | 1 | 3 | [41] | |
| Maximum carboxylation rate at 25 °C | μmol CO2 m−2 s−1 | 29 | 10 | 300 | [40] | |
| Ratio of internal CO2 to air CO2 | - | 0.87 | 0.5 | 0.9 | [40] | |
| Canopy extinction coefficient for light | - | 0.8 | 0.7 | 0.9 | [40] | |
| Activation energy of CO2 compensation point at 25 °C | J mol−1 | 60,000 | 30,000 | 100,000 | [40] | |
| Empirical coefficient in Leuning model | - | 1657 | 100 | 2000 | [42] | |
| Empirical coefficient in Leuning model | kPa | 2.74 | 1 | 10 | [42] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Ma, C.; Li, X.; Yuan, W.; Liu, Z.; Zhu, G. Sensitivity and Uncertainty Analyses of Flux-based Ecosystem Model towards Improvement of Forest GPP Simulation. Sustainability 2020, 12, 2584. https://doi.org/10.3390/su12072584
Ma H, Ma C, Li X, Yuan W, Liu Z, Zhu G. Sensitivity and Uncertainty Analyses of Flux-based Ecosystem Model towards Improvement of Forest GPP Simulation. Sustainability. 2020; 12(7):2584. https://doi.org/10.3390/su12072584
Chicago/Turabian StyleMa, Hanqing, Chunfeng Ma, Xin Li, Wenping Yuan, Zhengjia Liu, and Gaofeng Zhu. 2020. "Sensitivity and Uncertainty Analyses of Flux-based Ecosystem Model towards Improvement of Forest GPP Simulation" Sustainability 12, no. 7: 2584. https://doi.org/10.3390/su12072584
APA StyleMa, H., Ma, C., Li, X., Yuan, W., Liu, Z., & Zhu, G. (2020). Sensitivity and Uncertainty Analyses of Flux-based Ecosystem Model towards Improvement of Forest GPP Simulation. Sustainability, 12(7), 2584. https://doi.org/10.3390/su12072584






