Grass–Legume Forage Systems Effect on Phosphorus Removal from a Grassland Historically Irrigated with Reclaimed Wastewater
Abstract
:1. Introduction
2. Materials and Methods
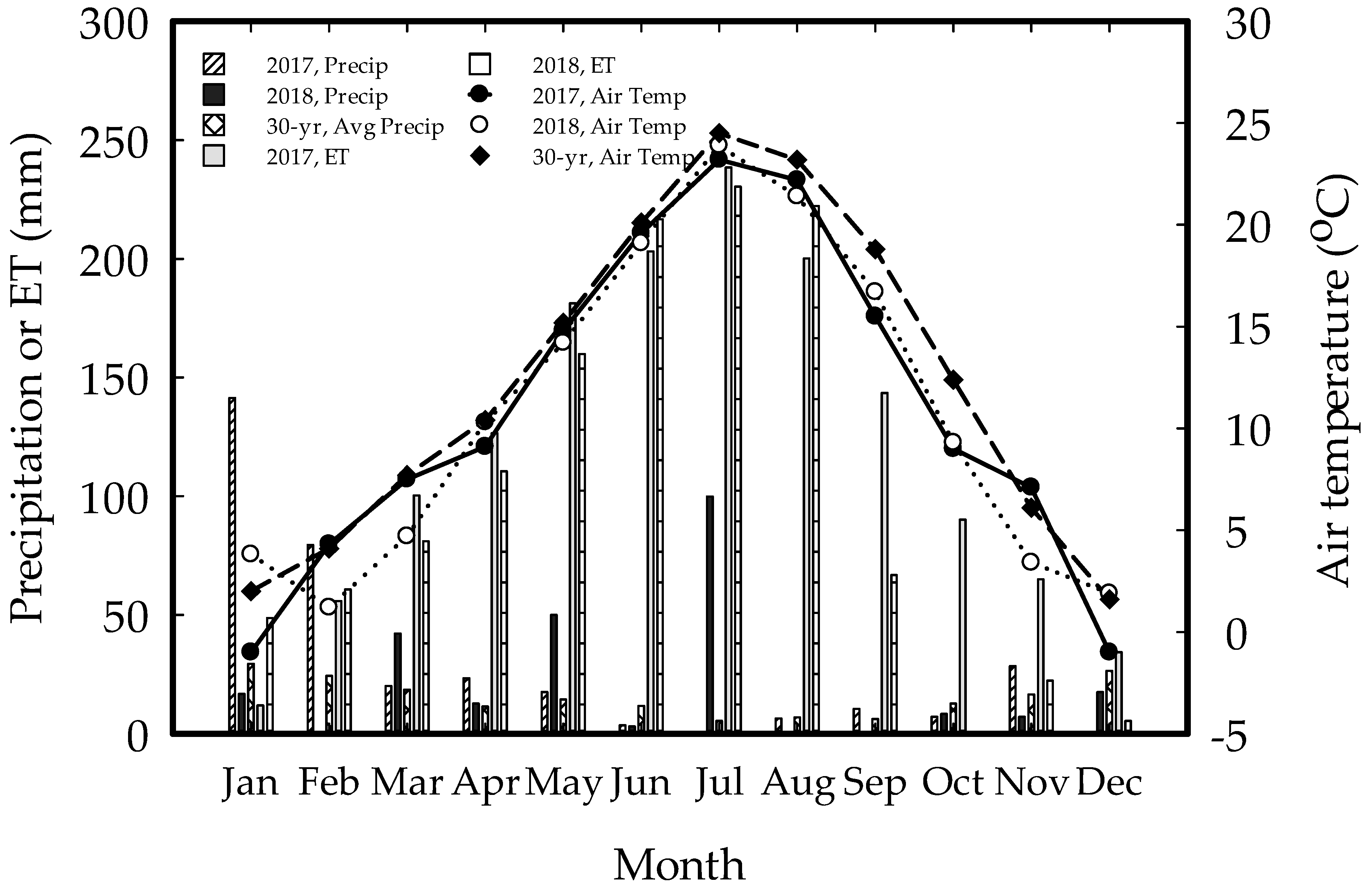
2.1. Description of the Study Area
2.2. Treatments and Experimental Design
2.3. Plot Establishment and Management
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Herbage Accumulation
3.2. Forage Tissue Phosphorus Concentration
3.3. Forage System Phosphorus Removal
4. Discussion
4.1. Herbage Accumulation
4.2. Forage Tissue Phosphorus Concentration
4.3. Phosphorus Removal
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maaß, O.; Grundmann, P. Added-value from linking the value chains of wastewater treatment, crop production and bioenergy production: A case study on reusing wastewater and sludge in crop production in Braunschweig (Germany). Resour. Conserv. Recycl. 2016, 107, 195–211. [Google Scholar] [CrossRef]
- Craig, C.A.; Feng, S.; Gilbertz, S. Water crisis, drought, and climate change in the southeast United States. Land Use Policy 2019, 88, 104110. [Google Scholar] [CrossRef]
- Khanpae, M.; Karami, E.; Maleksaeidi, H.; Keshavarz, M. Farmers’ attitude towards using treated wastewater for irrigation: The question of sustainability. J. Clean. Prod. 2020, 243, 118541. [Google Scholar] [CrossRef]
- Zvomuya, F.; Gupta, S.C.; Rosen, C.J. Phosphorus Leaching in Sandy Outwash Soils following Potato-Processing Wastewater Application. J. Environ. Qual. 2005, 34, 1277. [Google Scholar] [CrossRef] [PubMed]
- Keremane, G.B.; McKay, J. Successful wastewater reuse scheme and sustainable development: A case study in Adelaide. Water Environ. J. 2007, 21, 83–91. [Google Scholar] [CrossRef]
- Garcia, X.; Pargament, D. Reusing wastewater to cope with water scarcity: Economic, social and environmental considerations for decision-making. Resour. Conserv. Recycl. 2015, 101, 154–166. [Google Scholar] [CrossRef]
- Tran, Q.K.; Jassby, D.; Schwabe, K.A. The implications of drought and water conservation on the reuse of municipal wastewater: Recognizing impacts and identifying mitigation possibilities. Water Res. 2017, 124, 472–481. [Google Scholar] [CrossRef]
- Akhoundi, A.; Nazif, S. Sustainability assessment of wastewater reuse alternatives using the evidential reasoning approach. J. Clean. Prod. 2018, 195, 1350–1376. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Intriago, J.C.; López-Gálvez, F.; Allende, A.; Vivaldi, G.A.; Camposeo, S.; Nicolás Nicolás, E.; Alarcón, J.J.; Pedrero Salcedo, F. Agricultural reuse of municipal wastewater through an integral water reclamation management. J. Environ. Manag. 2018, 213, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Ray, I. Wastewater for agriculture: A reuse-oriented planning model and its application in peri-urban China. Water Res. 2010, 44, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, A.; Marecos Do Monte, M.H.; Bontoux, L.; Asano, T. The status of wastewater reuse practice in the Mediterranean basin: Need for guidelines. Water Res. 1999, 33, 2201–2217. [Google Scholar] [CrossRef]
- Moura, D.R.; Silveira, M.L.; O’Connor, G.A.; Wise, W.R. Long-term reclaimed water application effects on phosphorus leaching potential in rapid infiltration basins. J. Environ. Monit. 2011, 13, 2457. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol. Adv. 2016, 34, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Jalali, M. Assessment risk of phosphorus leaching from calcareous soils using soil test phosphorus. Chemosphere 2017, 171, 106–117. [Google Scholar] [CrossRef]
- Liu, X.-P.; Bi, Q.-F.; Qiu, L.-L.; Li, K.-J.; Yang, X.-R.; Lin, X.-Y. Increased risk of phosphorus and metal leaching from paddy soils after excessive manure application: Insights from a mesocosm study. Sci. Total Environ. 2019, 666, 778–785. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [Green Version]
- Sharpley, A. Managing agricultural phosphorus to minimize water quality impacts. Sci. Agric. 2016, 73, 1–8. [Google Scholar] [CrossRef]
- Motew, M.; Chen, X.; Booth, E.G.; Carpenter, S.R.; Pinkas, P.; Zipper, S.C.; Loheide, S.P.; Donner, S.D.; Tsuruta, K.; Vadas, P.A.; et al. The Influence of Legacy P on Lake Water Quality in a Midwestern Agricultural Watershed. Ecosystems 2017, 20, 1468–1482. [Google Scholar] [CrossRef]
- Jaiswal, D.; Elliott, H.A. Long-Term Phosphorus Fertility in Wastewater-Irrigated Cropland. J. Environ. Qual. 2011, 40, 214. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.K.; Adjei, M.B.; Scholberg, J.M.S.; Chambliss, C.G.; Rechcigl, J.E. Forage production and phosphorus phytoremediation in manure-impacted soils. Agron. J. 2004, 96, 1780–1786. [Google Scholar] [CrossRef] [Green Version]
- Read, J.J.; Sistani, K.R.; Oldham, J.L.; Brink, G.E. Double-cropping annual ryegrass and bermudagrass to reduce phosphorus levels in soil with history of poultry litter application. Nutr. Cycl. Agroecosystems 2009, 84, 93–104. [Google Scholar] [CrossRef]
- Abe, K.; Ozaki, Y. Comparison of useful terrestrial and aquatic plant species for removal of nitrogen and phosphorus from domestic wastewater. Soil Sci. Plant Nutr. 1998, 44, 599–607. [Google Scholar] [CrossRef]
- Vervoort, R.W.; Radcliffe, D.E.; Cabrera, M.L.; Latimore, M. Field-Scale Nitrogen and Phosphorus Losses from Hayfields Receiving Fresh and Composted Broiler Litter. J. Environ. Qual. 1998, 27, 1246. [Google Scholar] [CrossRef]
- Brink, G.E.; Rowe, D.E.; Sistani, K.R. Broiler Litter Application Effects on Yield and Nutrient Uptake of ‘Alicia’ Bermudagrass. Agron. J. 2002, 94, 911. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.D. Winter Cereal–Corn Double Crop Forage Production and Phosphorus Removal. Soil Sci. Soc. Am. J. 2006, 70, 1951. [Google Scholar] [CrossRef] [Green Version]
- Sleugh, B.B.; Gilfillen, R.A.; Willian, W.T.; Henderson, H.D. Nutritive Value and Nutrient Uptake of Sorghum–Sudangrass under Different Broiler Litter Fertility Programs. Agron. J. 2006, 98, 1594. [Google Scholar] [CrossRef] [Green Version]
- Newman, Y.C.; Agyin-Birikorang, S.; Adjei, M.B.; Scholberg, J.M.; Silveira, M.L.; Vendramini, J.M.B.; Rechcigl, J.E.; Sollenberger, L.E.; Chrysostome, M. Enhancing Phosphorus Phytoremedation Potential of Two Warm-Season Perennial Grasses with Nitrogen Fertilization. Agron. J. 2009, 101, 1345. [Google Scholar] [CrossRef]
- Silveira, M.L.; Vendramini, J.M.B.; Sui, X.; Sollenberger, L.E.; O’Connor, G.A. Use of Warm-Season Grasses Managed as Bioenergy Crops for Phytoremediation of Excess Soil Phosphorus. Agron. J. 2013, 105, 95–100. [Google Scholar] [CrossRef]
- Gotcher, M.J.; Zhang, H.; Schroder, J.L.; Payton, M.E. Phytoremediation of soil phosphorus with crabgrass. Agron. J. 2014, 106, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Read, J.J.; Lang, D.J.; Adeli, A.; Jenkins, J.N. Harvest Management Effects on ‘Tifton 44’ Bermudagrass Phosphorus Removal and Nutritive Value. Agron. J. 2018, 110, 879. [Google Scholar] [CrossRef]
- Brink, G.E.; Pederson, G.A.; Sistani, K.R.; Fairbrother, T.E. Uptake of Selected Nutrients by Temperate Grasses and Legumes. Agron. J. 2001, 93, 887–890. [Google Scholar] [CrossRef]
- Evers, G.W. Ryegrass-bermudagrass production and nutrient uptake when combining nitrogen fertilizer with broiler litter. Agron. J. 2002, 94, 905–910. [Google Scholar] [CrossRef]
- Pederson, G.A.; Brink, G.E.; Fairbrother, T.E. Nutrient Uptake in Plant Parts of Sixteen Forages Fertilized with Poultry Litter. Agron. J. 2002, 94, 895. [Google Scholar]
- Timmermans, B.G.H.; van Eekeren, N. Phytoextraction of soil phosphorus by potassium-fertilized grass-clover swards. J. Environ. Qual. 2016, 45, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Aponte, A.; Samarappuli, D.; Berti, M.T. Alfalfa–Grass Mixtures in Comparison to Grass and Alfalfa Monocultures. Agron. J. 2019, 111, 628. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [Green Version]
- Bélanger, G.; Castonguay, Y.; Lajeunesse, J. Benefits of mixing timothy with alfalfa for forage yield, nutritive value, and weed suppression in northern environments. Can. J. Plant Sci. 2014, 94, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Foster, A.; Biligetu, B.; Darambazar, E. Forage Accumulation, Nutritive Value, and Botanical Composition of Grass–Cicer Milkvetch Mixtures under Two Harvest Managements. Crop Sci. 2019, 59, 2876–2885. [Google Scholar] [CrossRef]
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadottir, A.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-year continental-scale field experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Zhang, L.; Li, W.; van der Werf, W.; Sun, J.; Spiertz, H.; Li, L. Yield advantage and water saving in maize/pea intercrop. F. Crop. Res. 2012, 138, 11–20. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere Processes and Management for Improving Nutrient Use Efficiency and Crop Productivity. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; pp. 1–32. [Google Scholar]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Institute The SAS system for Windows 9.4; SAS Institute Inc.: Cary, NC, USA, 2015.
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. Analysis of repeated measures data. In SAS Systems for Mixed Models; SAS Institute Inc.: Cary, NC, USA, 1996. [Google Scholar]
- Obour, A.K.; Vendramini, J.M.B.; Silveira, M.L.; Sollenberger, L.E.; O’Connor, G.A.; Jawitz, J.W. Phosphorus Fertilization Responses on Bahiagrass Pastures: Forage Production and Water Quality. Agron. J. 2011, 103, 324–330. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hasan, M.M.; Teixeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell. Mol. Biol. Lett. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Bork, E.W.; Gabruck, D.T.; McLeod, E.M.; Hall, L.M. Five-Year Forage Dynamics Arising from Four Legume-Grass Seed Mixes. Agron. J. 2017, 109, 2789–2799. [Google Scholar] [CrossRef]
- Solomon, J.K.Q.; Macoon, B.; Lang, D.J.; Parish, J.A.; Vann, R.C. A novel approach to grass–legume management. Crop Sci. 2011, 51, 1865–1876. [Google Scholar] [CrossRef]
- Cox, S.; Peel, M.D.; Creech, J.E.; Waldron, B.L.; Eun, J.S.; Zobell, D.R.; Miller, R.L.; Snyder, D.L. Forage production of grass–legume binary mixtures on intermountain western USA irrigated pastures. Crop Sci. 2017, 57, 1742–1753. [Google Scholar] [CrossRef]
- Andrews, J.S.; Sanders, Z.P.; Cabrera, M.L.; Hill, N.S.; Radcliffe, D.E. Simulated nitrate leaching in annually cover cropped and perennial living mulch corn production systems. J. Soil Water Conserv. 2020, 75, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Sleugh, B.; Moore, K.J.; George, J.R.; Brummer, E.C. Binary Legume-Grass Mixtures Improve Forage Yield, Quality, and Seasonal Distribution. Agron. J. 2000, 92, 24–29. [Google Scholar] [CrossRef]
- Leep, R.; Jeranyama, P.; Min, D.-H.; Dietz, T.; Bughrara, S.; Isleib, J. Grazing Effects on Herbage Mass and Composition in Grass-Birdsfoot Trefoil Mixtures. Agron. J. 2002, 94, 1257–1262. [Google Scholar] [CrossRef]
- Bélanger, G.; Tremblay, G.F.; Seguin, P.; Lajeunesse, J.; Bittman, S.; Hunt, D. Cutting management of alfalfa-based mixtures in contrasting agroclimatic regions. Agron. J. 2020. [Google Scholar] [CrossRef]
- Adjesiwor, A.T.; Islam, M.A.; Zheljazkov, V.D.; Ritten, J.P.; Garcia y Garcia, A. Grass–legume Seed Mass Ratios and Nitrogen Rates Affect Forage Accumulation, Nutritive Value, and Profitability. Crop Sci. 2017, 57, 2852–2864. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Brink, G.; Stout, R.; Ruth, L. Grass–Legume Proportions in Forage Seed Mixtures and Effects on Herbage Yield and Weed Abundance. Agron. J. 2013, 105, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Tracy, B.F.; Sanderson, M.A. Productivity and Stability Relationships in Mowed Pasture Communities of Varying Species Composition. Crop Sci. 2004, 44, 2180–2186. [Google Scholar] [CrossRef]
- Orloff, S.B.; Brummer, E.C.; Shrestha, A.; Putnam, D.H. Cool-Season Perennial Grasses Differ in Tolerance to Partial-Season Irrigation Deficits. Agron. J. 2016, 108, 692–700. [Google Scholar] [CrossRef]
- Davison, J.; Solomon, J.K.; Lawry, T. Alfalfa Variety Trial in Western Nevada, Initial Results; University of University of Nevada, Reno, Extension: Reno, NV, USA, 2016. [Google Scholar]
- White, J.; Lemus, R.; Saunders, J.R.; Rivera, D.; Brett, R. Mississippi Perennial Cool-Season Forage Crop Variety Trials, 2016; MAFES Information Bulletin 522: Mississippi, MA, USA, 2017. [Google Scholar]
- Springer, T.L.; Aiken, G.E. Harvest frequency effects on white clover forage biomass, quality, and theoretical ethanol yield. Biomass Bioenergy 2015, 78, 1–5. [Google Scholar] [CrossRef]
- Tracy, B.F.; Albrecht, K.; Flores, J.; Hall, M.; Islam, A.; Jones, G.; Lamp, W.; MacAdam, J.W.; Skinner, H.; Teutsch, C. Evaluation of Alfalfa-Tall Fescue Mixtures across Multiple Environments. Crop Sci. 2016, 56, 2026–2034. [Google Scholar] [CrossRef]
- Minneé, E.M.K.; McCready, T.B.; Woodward, S.L. Herbage production, botanical composition and survival of perennial ryegrass- and tall fescue-based swards in simple and diverse species mixtures in a dryland environment. Anim. Prod. Sci. 2017, 57, 1405. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Karn, J.F.; Hendrickson, J.R. Dry Matter Yields of Cool-Season Grass Monocultures and Grass-Alfalfa Binary Mixtures. Agron. J. 2001, 93, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Bélanger, G.; Brégard, A.; Michaud, R. Phosphorous Uptake and Concentration of Timothy Genotypes under Varying N Applications. Crop Sci. 2002, 42, 2044–2048. [Google Scholar] [CrossRef]
- Foster, A.; Vera, C.L.; Malhi, S.S.; Clarke, F.R. Forage yield of simple and complex grass–legume mixtures under two management strategies. Can. J. Plant Sci. 2014, 94, 41–50. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Godwin, G.; Kilcer, T.F.; Barney, P.; Hunter, M.; Cherney, J.H.; Beer, S. Nitrogen, phosphorus, potassium, magnesium and calcium removal by brown midrib sorghum sudangrass in the northeastern USA. J. Agron. Crop Sci. 2006, 192, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Brink, G.E.; Sistani, K.R.; Oldham, J.L.; Kingery, W.E.; Johnson, B. Broiler Litter Application Rate Effects on Bermudagrass Nutrient Uptake and Phosphorus Level of Soils Differing in Application History. J. Sustain. Agric. 2008, 31, 79–94. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; Sollenberger, L.E.; Mathews, B.W.; Scholberg, J.M.; Santos, H.Q. Nutrient Cycling in Warm-Climate Grasslands. Crop Sci. 2007, 47, 915–928. [Google Scholar] [CrossRef]
- Dou, Z.; Galligan, D.T.; Ramberg, C.F.; Meadows, C.; Ferguson, J.D. A Survey of Dairy Farming in Pennsylvania: Nutrient Management Practices and Implications. J. Dairy Sci. 2001, 84, 966–973. [Google Scholar] [CrossRef]
- Higgins, S.; Schellberg, J.; Bailey, J.S. Improving productivity and increasing the efficiency of soil nutrient management on grassland farms in the UK and Ireland using precision agriculture technology. Eur. J. Agron. 2019, 106, 67–74. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Tong, B.X.; Wu, Z.G.; Ma, W.Q.; Ma, L. Dietary manipulation to reduce nitrogen and phosphorus excretion by dairy cows. Livest. Sci. 2019, 228, 61–66. [Google Scholar] [CrossRef]
- Wei, S.; Bai, Z.H.; Qin, W.; Wu, Z.G.; Jiang, R.F.; Ma, L. Nutrient use efficiencies, losses, and abatement strategies for peri-urban dairy production systems. J. Environ. Manag. 2018, 228, 232–238. [Google Scholar] [CrossRef]
| Forage System | Seeding Rate (PLS †) kg ha−1 | |
|---|---|---|
| Grass monoculture | ||
| Tall Fescue ‘BarOptima Plus E34’ | 15.0 15.0 | |
| Tall Fescue ‘Fawn’ | ||
| Legume monoculture | ||
| Alfalfa ‘AmeriStand 403T Plus’ | 20.0 12.0 5.0 | |
| Red clover ‘Freedom’ | ||
| White clover ‘Barblanca’ | ||
| Grass–legume mixtures | Grass (25%) | Legumes (75%) |
| 1) Tall Fescue ‘BarOptima Plus E34’ + Alfalfa ‘Ameristand 403T Plus’ | 5.0 | 11.3 |
| 2) Tall Fescue ‘BarOptima Plus E34’ + Red clover ‘Freedom’ | 5.0 | 9 |
| 3) Tall Fescue ‘BarOptima Plus E34’ + White clover ‘Barblanca’ | 5.0 | 3.8 |
| Grass (50%) | Legumes (50%) | |
| 4) Tall Fescue ‘BarOptima Plus E34’ + Alfalfa ‘Ameristand 403T Plus’ | 7.5 | 10.0 |
| 5) Tall Fescue ‘BarOptima Plus E34’ + Red clover ‘Freedom’ | 7.5 | 6.0 |
| 6) Tall Fescue ‘BarOptima Plus E34’ + White clover ‘Barblanca’ | 7.5 | 2.5 |
| Grass (75%) | Legumes (25%) | |
| 7) Tall Fescue ‘BarOptima Plus E34’ + Alfalfa ‘Ameristand 403T Plus’ | 11.3 | 5.0 |
| 8) Tall Fescue ‘BarOptima Plus E34’ + Red clover ‘Freedom’ | 11.3 | 3.0 |
| 9) Tall Fescue ‘BarOptima Plus E34’ + White clover ‘Barblanca’ | 11.3 | 1.3 |
| Grass (25%) | Legumes (75%) | |
| 10) Tall Fescue ‘Fawn’ + Alfalfa ‘Ameristand 403T Plus’ | 5.0 | 11.3 |
| 11) Tall Fescue ‘Fawn’ + Red clover ‘Freedom’ | 5.0 | 9 |
| 12) Tall Fescue Fawn + White clover ‘Barblanca’ | 5.0 | 3.8 |
| Grass (50%) | Legumes (50%) | |
| 13) Tall Fescue ‘Fawn’ + Alfalfa ‘Ameristand 403T Plus’ | 7.5 | 10.0 |
| 14) Tall Fescue ‘Fawn’ + Red clover ‘Freedom’ | 7.5 | 6.0 |
| 15) Tall Fescue’ Fawn’ + White clover ‘Barblanca’ | 7.5 | 2.5 |
| Grass (75%) | Legumes (25%) | |
| 16) Tall Fescue ‘Fawn’ + Alfalfa ‘Ameristand 403T Plus’ | 11.3 | 5.0 |
| 17) Tall Fescue’ Fawn’ + Red clover ‘Freedom’ | 11.3 | 3.0 |
| 18) Tall Fescue ‘Fawn’ + White clover ‘Barblanca’ | 11.3 | 1.3 |
| Forage System | Year | SE | P-Value ‡ | |
|---|---|---|---|---|
| 2017 | 2018 | |||
| Grass monoculture | -----Mg DM ha−1--- | |||
| Tall fescue cv. BarOptima Plus E34 | 8.4a † | 12.6ab | 1.1 | 0.001 |
| Tall fescue cv. Fawn | 8.4a | 12.2abc | 1.1 | 0.004 |
| Legume monoculture | ||||
| Alfalfa cv. Ameristand 403T Plus | 5.1cde | 3.0g | 1.1 | 0.163 |
| Red clover cv. Freedom | 5.5bcde | 3.7g | 1.1 | 0.179 |
| White clover cv. Barblanca | 4.0e | 4.5g | 1.1 | 0.721 |
| Grass–legume mixtures | ||||
| 1) Tall fescue cv. BarOptima Plus E34 (25) Alfalfa cv. Ameristand 403T Plus (75) § | 7.0abcd | 9.4ef | 1.1 | 0.061 |
| 2) Tall fescue cv. BarOptima Plus E34 (25) Red clover cv. Freedom (75) | 7.4abc | 9.6def | 1.1 | 0.105 |
| 3) Tall fescue cv. BarOptima Plus E34 (25) White clover cv. Barblanca (75) | 5.6bcde | 10.4bcdef | 1.1 | 0.001 |
| 4) Tall fescue cv. BarOptima Plus E34 (50) Alfalfa cv. Ameristand 403T Plus (50) | 5.5bcde | 10.6bcdef | 1.1 | 0.001 |
| 5) Tall fescue cv. BarOptima Plus E34 (50) Red clover cv. Freedom(50) | 6.9abcd | 11.9abcde | 1.1 | 0.001 |
| 6) Tall fescue cv. BarOptima Plus E34 (50) White clover cv. Barblanca (50) | 5.2cde | 13.2a | 1.1 | <0.001 |
| 7) Tall fescue cv. BarOptima Plus E34 (75) Alfalfa cv. Ameristand 403T Plus (25) | 5.6bcde | 11.5abcde | 1.1 | <0.001 |
| 8) Tall fescue cv. BarOptima Plus E34 (75) Red clover cv. Freedom (25) | 6.6abcde | 12.1abcd | 1.1 | <0.001 |
| 9) Tall fescue cv. BarOptima Plus E34 (75) White clover cv. Barblanca (25) | 6.4abcde | 11.1abcdef | 1.1 | 0.001 |
| 10) Tall fescue cv. Fawn (25) Alfalfa cv. Ameristand 403T Plus (75) | 5.2cde | 10.5bcdef | 1.1 | <0.001 |
| 11) Tall fescue cv. Fawn (25) Red clover cv. Freedom (75) | 6.6abcde | 8.8f | 1.1 | 0.097 |
| 12) Tall fescue cv. Fawn (25) White clover cv. Barblanca (75) | 4.5de | 11.4abcde | 1.1 | <0.001 |
| 13) Tall fescue cv. Fawn (50) Alfalfa cv. Ameristand 403T Plus (50) | 8.1ab | 10.2bcdef | 1.1 | 0.135 |
| 14) Tall fescue cv. Fawn (50) Red clover cv. Freedom (50) | 5.1cde | 11.1abcdef | 1.1 | <0.001 |
| 15) Tall fescue cv. Fawn (50) White clover cv. Barblanca (50) | 6.0bcde | 11.7abcde | 1.1 | <0.001 |
| 16) Tall fescue cv. Fawn (75) Alfalfa cv. Ameristand 403T Plus (25) | 7.5abc | 10.7abcdef | 1.1 | 0.012 |
| 17) Tall fescue cv. Fawn (75) Red clover cv. Freedom (25) | 7.4abc | 12.0abcde | 1.1 | 0.001 |
| 18) Tall fescue cv. Fawn (75) White clover cv. Barblanca (25) | 7.3abc | 9.8cdef | 1.1 | 0.048 |
| Grouped Forage Systems | Year | SE | P-Value ‡ | |
|---|---|---|---|---|
| 2017 | 2018 | |||
| --------Mg DM ha−1------- | ||||
| Grass | 8.6a † | 12.7a | 0.8 | 0.003 |
| Legume | 5.0b | 3.8c | 0.8 | 0.312 |
| Grass (25) Legume (75) § | 6.1b | 10.1b | 0.8 | <0.001 |
| Grass (50) Legume (50) | 6.1b | 11.5ab | 0.8 | <0.001 |
| Grass (75) Legume (25) | 6.8ab | 12.7a | 0.8 | <0.001 |
| Forage System | Year | SE | P-Value ‡ | |
|---|---|---|---|---|
| 2017 | 2018 | |||
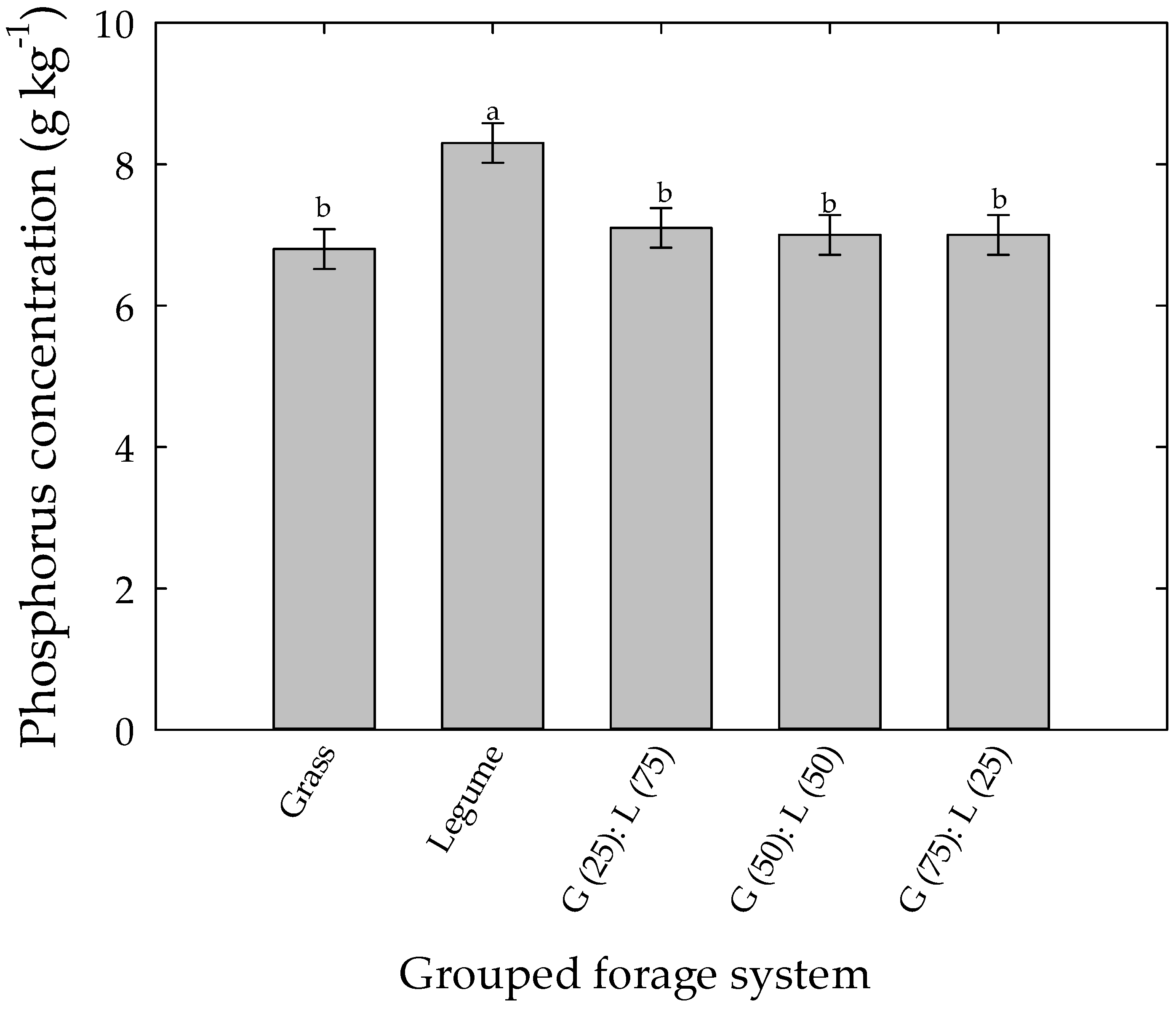
| Grass monoculture | ------P g kg−1------ | |||
| Tall fescue cv. BarOptima Plus E34 | 8.5cdef † | 5.3c | 0.44 | <0.001 |
| Tall fescue cv. Fawn | 8.1def | 5.4bc | 0.44 | <0.002 |
| Legume monoculture | ||||
| Alfalfa cv. Ameristand 403T Plus | 10.9a | 5.9abc | 0.44 | <0.002 |
| Red clover cv. Freedom | 9.0bcd | 6.9a | 0.44 | <0.002 |
| White clover cv. Barblanca | 11.3a | 6.3ab | 0.44 | <0.002 |
| Grass–legume mixtures | ||||
| 1) Tall fescue cv. BarOptima Plus E34 (25) Alfalfa cv. Ameristand 403T Plus (75)§ | 9.0bcd | 5.6bc | 0.44 | <0.002 |
| 2) Tall fescue cv. BarOptima Plus E34 (25) Red clover cv. Freedom (75) | 8.9bcde | 5.2c | 0.44 | <0.002 |
| 3) Tall fescue cv. BarOptima Plus E34 (25) White clover cv. Barblanca (75) | 8.8bcdef | 5.5bc | 0.44 | <0.002 |
| 4) Tall fescue cv. BarOptima Plus E34 (50) Alfalfa cv. Ameristand 403T Plus (50) | 9.6b | 5.7bc | 0.44 | <0.002 |
| 5) Tall fescue cv. BarOptima Plus E34 (50) Red clover cv. Freedom(50) | 8.6cdef | 5.1c | 0.44 | <0.002 |
| 6) Tall fescue cv. BarOptima Plus E34 (50) White clover cv. Barblanca (50) | 7.8f | 5.3c | 0.44 | <0.002 |
| 7) Tall fescue cv. BarOptima Plus E34 (75) Alfalfa cv. Ameristand 403T Plus (25) | 8.5cdef | 5.7bc | 0.44 | <0.002 |
| 8) Tall fescue cv. BarOptima Plus E34 (75) Red clover cv. Freedom (25) | 9.0bcd | 5.9abc | 0.44 | <0.002 |
| 9) Tall fescue cv. BarOptima Plus E34 (75) White clover cv. Barblanca (25) | 8.9bcde | 5.5bc | 0.44 | <0.002 |
| 10) Tall fescue cv. Fawn (25) Alfalfa cv. Ameristand 403T Plus (75) | 9.3bc | 5.4bc | 0.44 | <0.002 |
| 11) Tall fescue cv. Fawn (25) Red clover cv. Freedom (75) | 7.9ef | 5.4bc | 0.44 | <0.002 |
| 12) Tall fescue cv. Fawn (25) White clover cv. Barblanca (75) | 8.7bcdef | 5.0c | 0.44 | <0.002 |
| 13) Tall fescue cv. Fawn (50) Alfalfa cv. Ameristand 403T Plus (50) | 8.2def | 5.6bc | 0.44 | <0.002 |
| 14) Tall fescue cv. Fawn (50) Red clover cv. Freedom (50) | 8.0ef | 5.6bc | 0.44 | <0.002 |
| 15) Tall fescue cv. Fawn (50) White clover cv. Barblanca (50) | 8.9bcde | 5.4bc | 0.44 | <0.002 |
| 16) Tall fescue cv. Fawn (75) Alfalfa cv. Ameristand 403T Plus (25) | 7.9ef | 5.1c | 0.44 | <0.002 |
| 17) Tall fescue cv. Fawn (75) Red clover cv. Freedom (25) | 8.9bcde | 5.8abc | 0.44 | <0.002 |
| 18) Tall fescue cv. Fawn (75) White clover cv. Barblanca (25) | 8.1def | 5.2c | 0.44 | <0.002 |
| Forage System | Year | SE | P-Value ‡ | Total | |
|---|---|---|---|---|---|
| 2017 | 2018 | ||||
| Grass monoculture | ---------kg P ha−1---- | ||||
| Tall fescue cv. BarOptima Plus E34 | 33.2a † | 34.2abc | 3.7 | 0.829 | 67.4a |
| Tall fescue cv. Fawn | 32.9a | 34.4abc | 3.7 | 0.737 | 67.4a |
| Legume monoculture | |||||
| Alfalfa cv. Ameristand 403T Plus | 25.1abcdef | 7.9e | 3.7 | 0.001 | 32.7e |
| Red clover cv. Freedom | 21.8cdef | 12.5e | 3.7 | 0.035 | 34.3e |
| White clover cv. Barblanca | 22.4bcdef | 13.9e | 3.7 | 0.075 | 35.1de |
| Grass–legume mixtures | |||||
| 1) Tall fescue cv. BarOptima Plus E34 (25) Alfalfa cv. Ameristand 403T Plus (75) § | 27.8abcd | 27.1bcd | 3.7 | 0.887 | 54.9bc |
| 2) Tall fescue cv. BarOptima Plus E34 (25) Red clover cv. Freedom (75) | 30.2abc | 25.8cd | 3.7 | 0.349 | 57.2abc |
| 3) Tall fescue cv. BarOptima Plus E34 (25) White clover cv. Barblanca (75) | 23.3bcdef | 28.8abcd | 3.7 | 0.251 | 49.4c |
| 4) Tall fescue cv. BarOptima Plus E34 (50) Alfalfa cv. Ameristand 403T Plus (50) | 26.2abcdef | 31.3abcd | 3.7 | 0.247 | 57.5abc |
| 5) Tall fescue cv. BarOptima Plus E34 (50) Red clover cv. Freedom(50) | 27.6abcde | 31.0abcd | 3.7 | 0.443 | 58.5abc |
| 6) Tall fescue cv. BarOptima Plus E34 (50) White clover cv. Barblanca (50) | 18.7f | 35.6ab | 3.7 | 0.001 | 54.3bc |
| 7) Tall fescue cv. BarOptima Plus E34 (75) Alfalfa cv. Ameristand 403T Plus (25) | 22.9bcdef | 33.8abc | 3.7 | 0.015 | 56.7abc |
| 8) Tall fescue cv. BarOptima Plus E34 (75) Red clover cv. Freedom (25) | 28.7abc | 37.4a | 3.7 | 0.048 | 66.1ab |
| 9) Tall fescue cv. BarOptima Plus E34 (75) White clover cv. Barblanca (25) | 27.4abcdef | 31.4abcd | 3.7 | 0.360 | 58.7abc |
| 10) Tall fescue cv. Fawn (25) Alfalfa cv. Ameristand 403T Plus (75) | 21.6cdef | 28.2bcd | 3.7 | 0.135 | 49.9c |
| 11) Tall fescue cv. Fawn (25) Red clover cv. Freedom (75) | 24.0bcdef | 23.9d | 3.7 | 0.968 | 47.9cd |
| 12) Tall fescue cv. Fawn (25) White clover cv. Barblanca (75) | 19.0ef | 28.7bcd | 3.7 | 0.030 | 47.7cd |
| 13) Tall fescue cv. Fawn (50) Alfalfa cv. Ameristand 403T Plus (50) | 32.0ab | 28.7cd | 3.7 | 0.476 | 56.2bc |
| 14) Tall fescue cv. Fawn (50) Red clover cv. Freedom (50) | 19.9def | 32.4abcd | 3.7 | 0.005 | 52.2c |
| 15) Tall fescue cv. Fawn (50) White clover cv. Barblanca (50) | 25.2abcdef | 32.3abcd | 3.7 | 0.111 | 57.5abc |
| 16) Tall fescue cv. Fawn (75) Alfalfa cv. Ameristand 403T Plus (25) | 28.0abcd | 27.4bcd | 3.7 | 0.891 | 55.3bc |
| 17) Tall fescue cv. Fawn (75) Red clover cv. Freedom (25) | 30.8ab | 34.4abc | 3.7 | 0.407 | 65.1ab |
| 18) Tall fescue cv. Fawn (75) White clover cv. Barblanca (25) | 27.8abcd | 26.4cd | 3.7 | 0.754 | 54.3c |
| SEM | 6.2 | ||||
| Grouped Forage Systems | Year | SE | P-Value ‡ | Total | |
|---|---|---|---|---|---|
| 2017 | 2018 | ||||
| -----kg P ha−1---- | kg P ha−1 | ||||
| Grass | 34.5a † | 35.4a | 2.8 | 0.856 | 67.4a |
| Legume | 23.5b | 11.8c | 2.8 | 0.006 | 34.0c |
| Grass (25) Legume (75) § | 24.2b | 27.2b | 2.8 | 0.326 | 51.0b |
| Grass (50) Legume (50) | 24.6b | 31.8ab | 2.8 | 0.019 | 56.0ab |
| Grass (75) Legume (25) | 27.7ab | 31.3ab | 2.8 | 0.856 | 59.4ab |
| SEM | 4.7 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mclennon, E.; Solomon, J.K.Q.; Davison, J. Grass–Legume Forage Systems Effect on Phosphorus Removal from a Grassland Historically Irrigated with Reclaimed Wastewater. Sustainability 2020, 12, 2256. https://doi.org/10.3390/su12062256
Mclennon E, Solomon JKQ, Davison J. Grass–Legume Forage Systems Effect on Phosphorus Removal from a Grassland Historically Irrigated with Reclaimed Wastewater. Sustainability. 2020; 12(6):2256. https://doi.org/10.3390/su12062256
Chicago/Turabian StyleMclennon, Everald, Juan K. Q. Solomon, and Jason Davison. 2020. "Grass–Legume Forage Systems Effect on Phosphorus Removal from a Grassland Historically Irrigated with Reclaimed Wastewater" Sustainability 12, no. 6: 2256. https://doi.org/10.3390/su12062256





