Sustainable Production from Shale Gas Resources through Heat-Assisted Depletion
Abstract
1. Introduction
2. Limitations of Macroscopic Equations
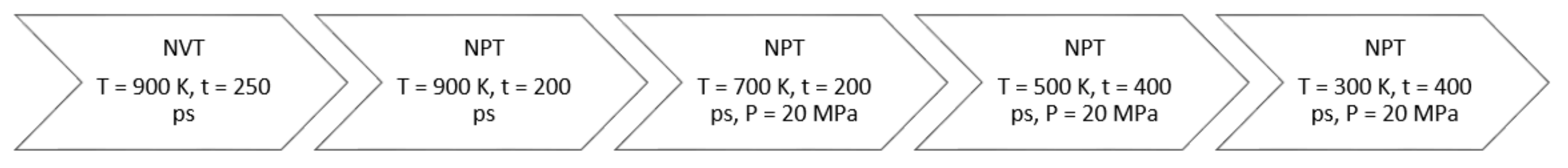
3. Molecular Simulation Approach
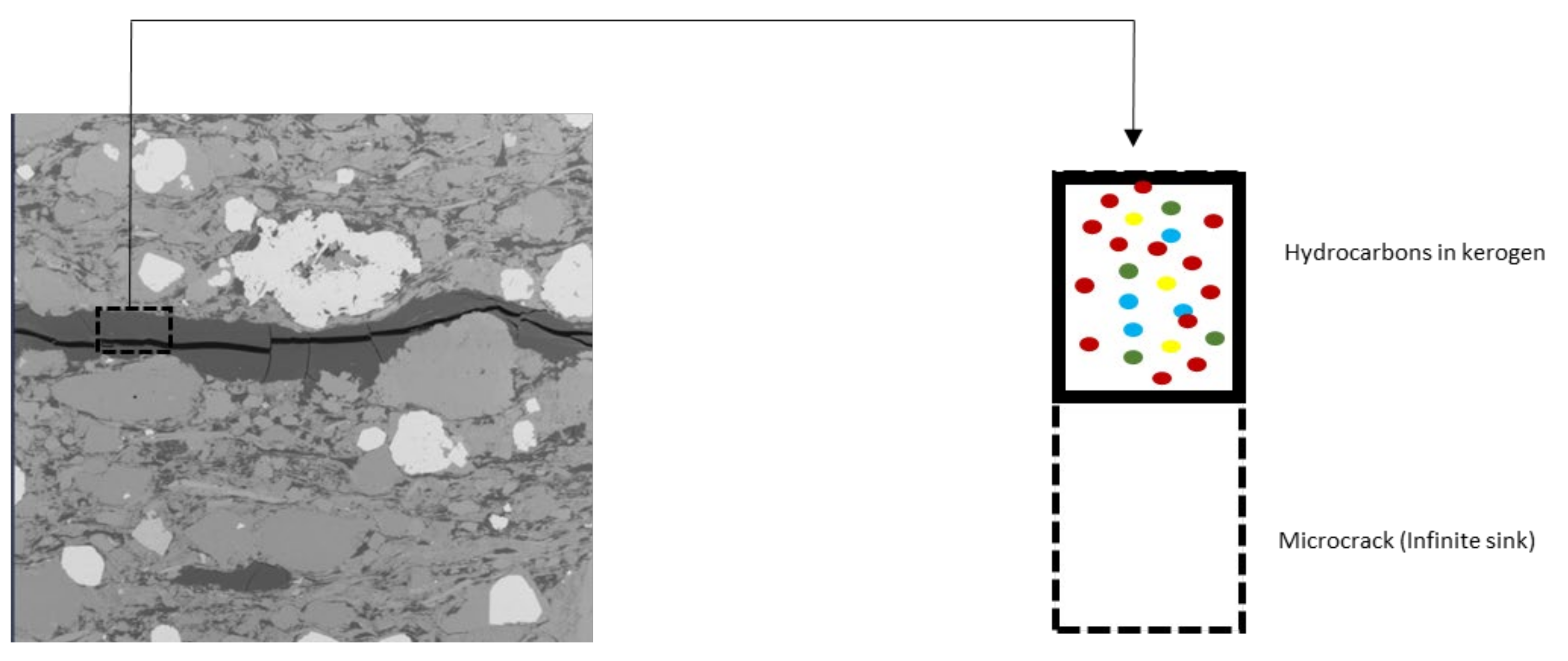
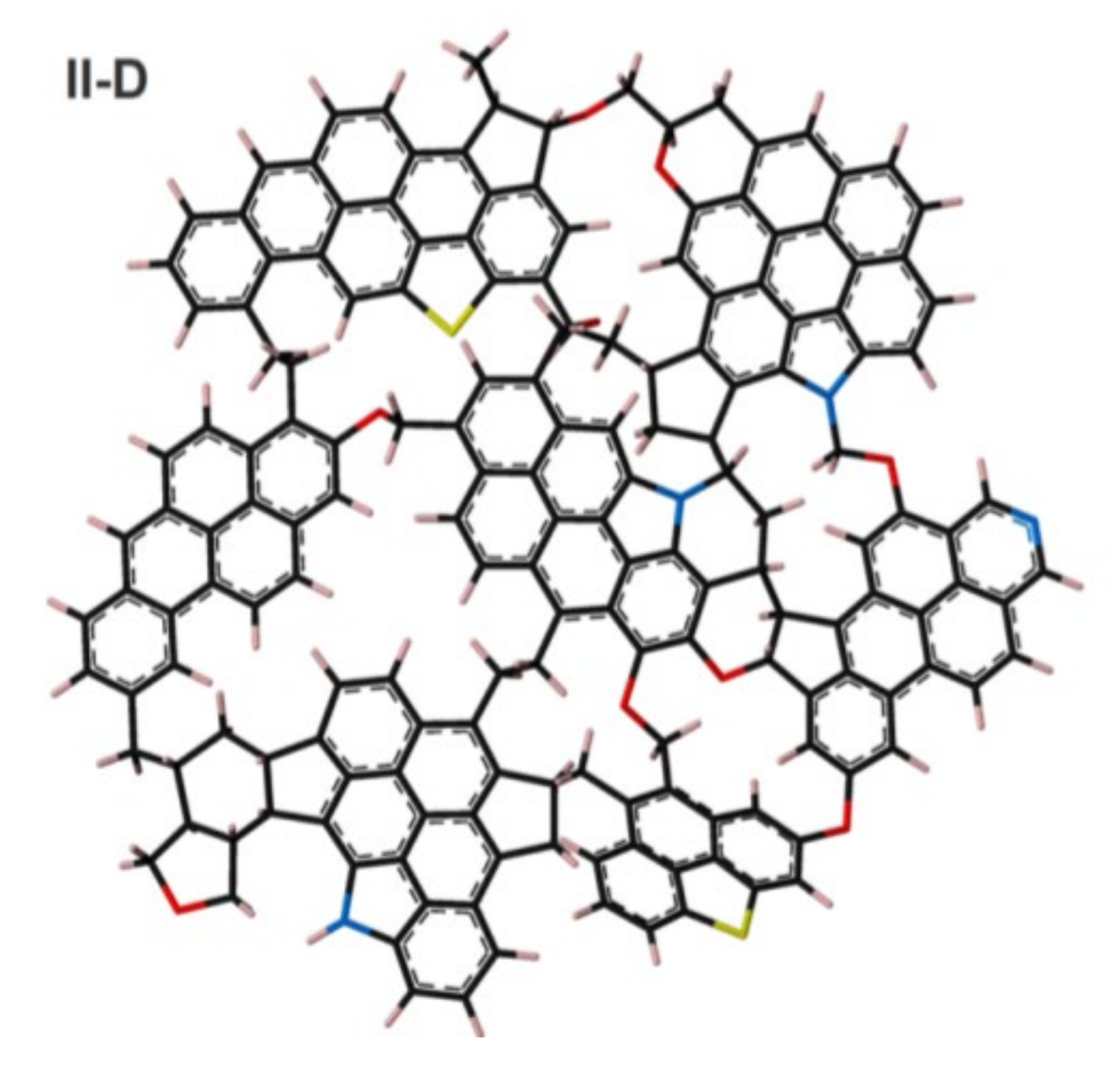
4. Kerogen Models
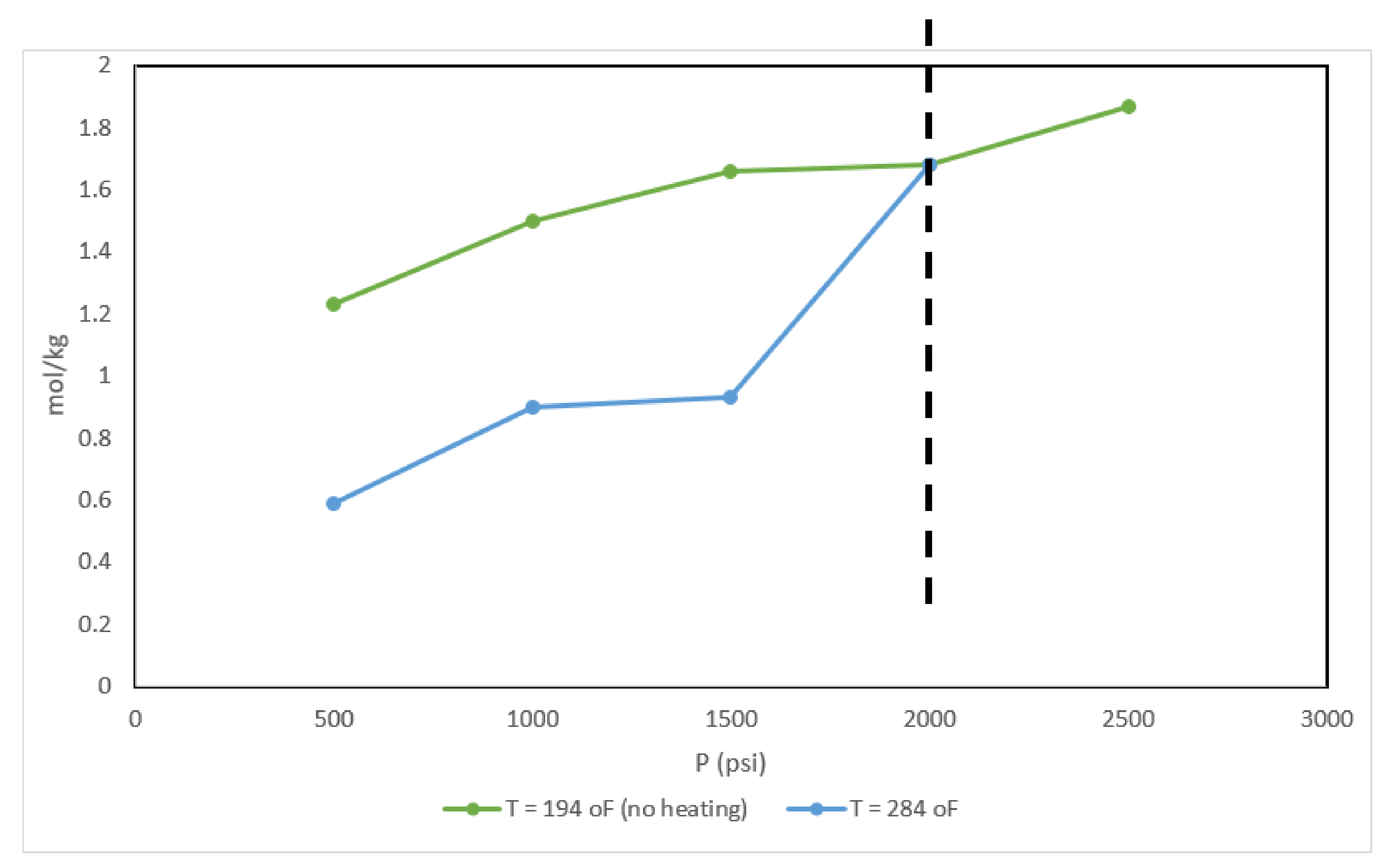
5. Results and Discussions
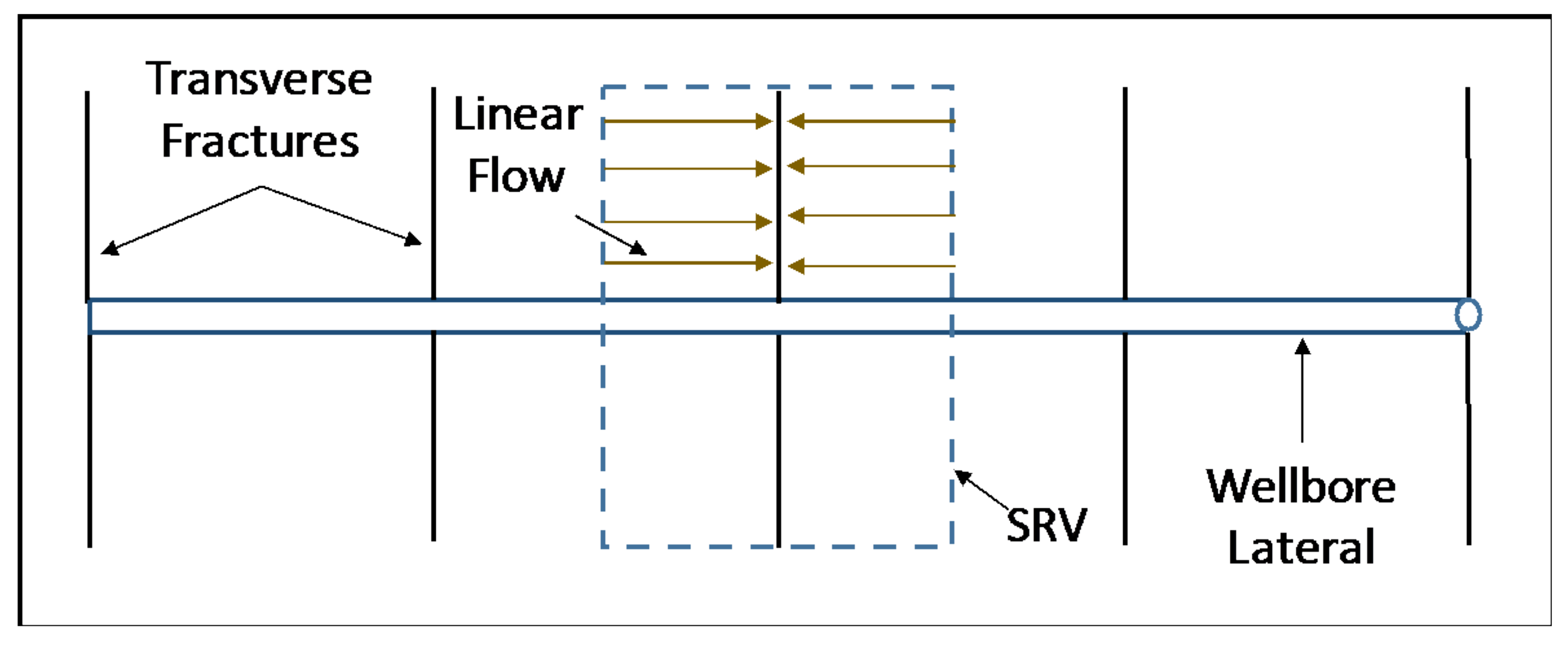
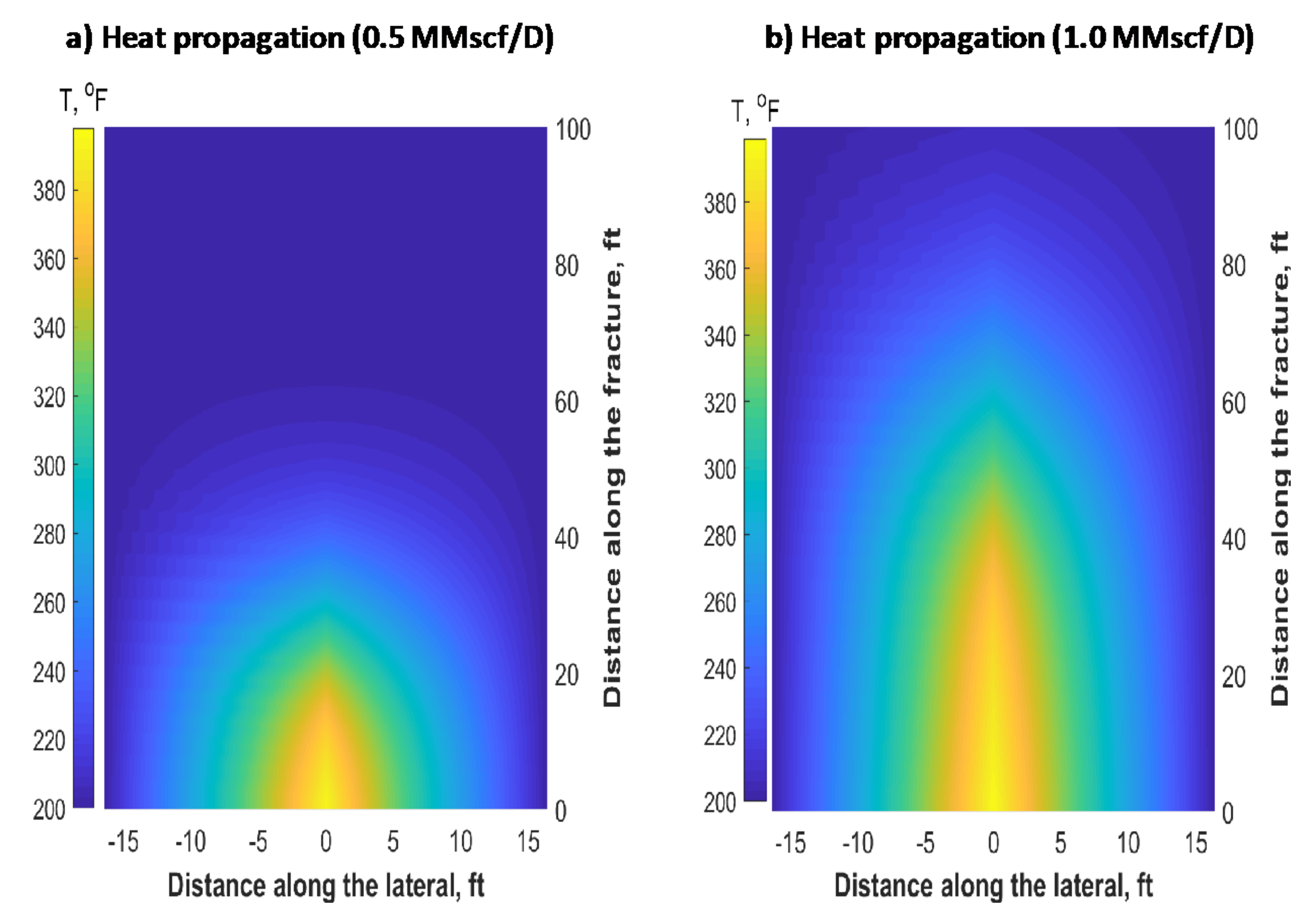
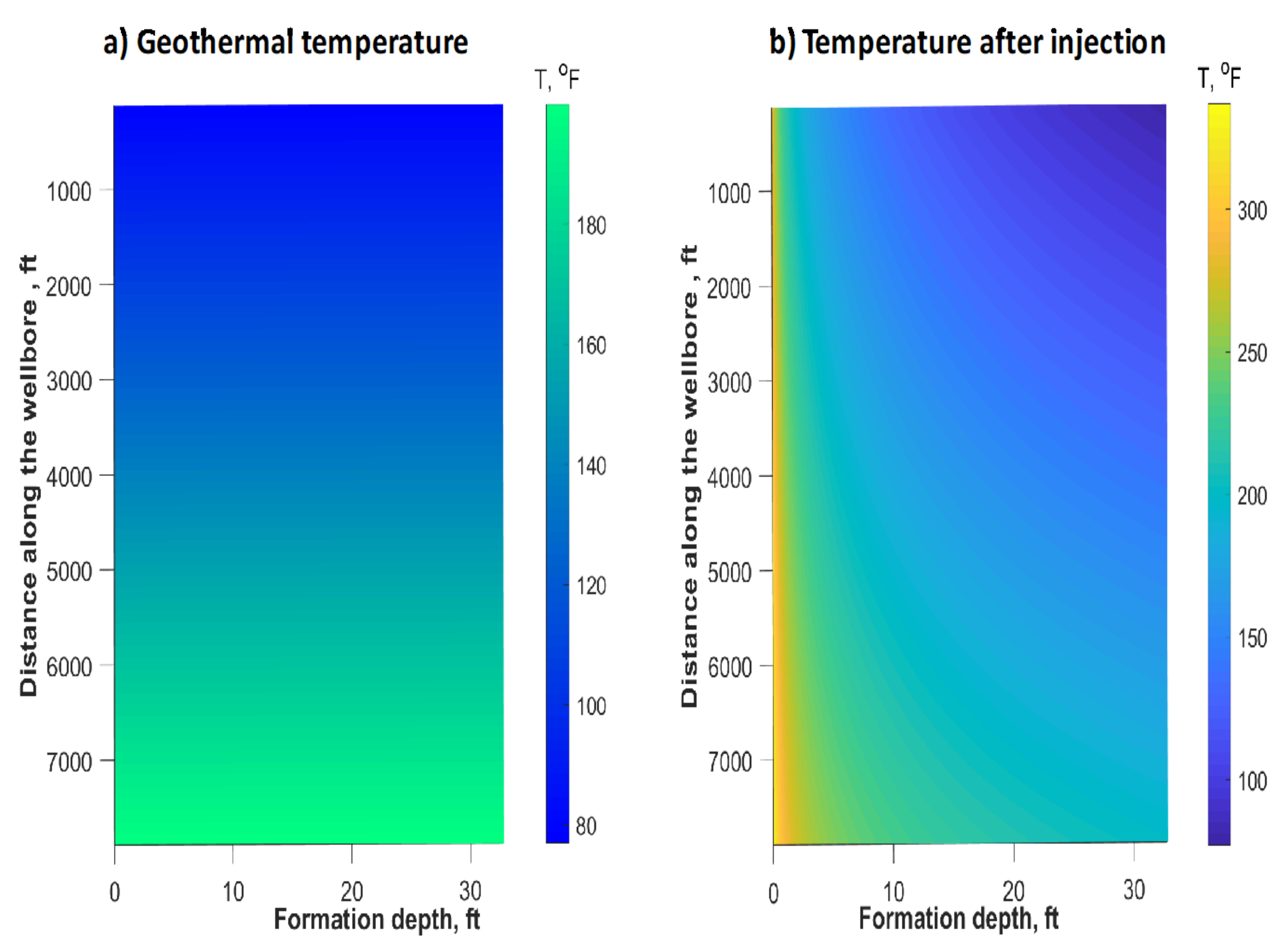
6. Reservoir Scale Heat Transfer Model
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Pressure (psi) | |
| Temperature (°F) | |
| Mole fraction (mole/mole) | |
| Fugacity (MPa) | |
| Specific heat capacity, kJ/(kg·°C) | |
| Thermal conductivity, kJ/(s·m·°C) | |
| Overall heat transfer coefficient, kJ/(s·m2·°C) |
Appendix A
References
- Ambrose, R.J.; Hartman, R.C.; Diaz-Campos, M.; Akkutlu, I.Y.; Sondergeld, C.H. Shale Gas-in-Place Calculations Part I: New Pore-Scale Considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Ertekin, T.; King, G.A.; Schwerer, F.C. Dynamic gas slippage: A unique dual-mechanism approach to the flow of gas in tight formations. SPE Form. Eval. 1986, 1, 43–52. [Google Scholar]
- Javadpour, F. Nanopores and Apparent Permeability of Gas Flow in Mudrocks (Shales and Siltstone). J. Can. Petrol. Technol. 2009, 48, 16–21. [Google Scholar] [CrossRef]
- Sakhaee-Pour, A.; Bryant, S. Gas Permeability of Shale. SPE Reserv. Eval. Eng. 2012, 15, 401–409. [Google Scholar] [CrossRef]
- Alafnan, S.F.K.; Akkutlu, I.Y. Matrix-Fracture Interactions during Flow in Organic Nanoporous Materials under Loading. Transp. Porous Med. 2018, 121, 69–92. [Google Scholar] [CrossRef]
- Alafnan, S. Pore Network Modeling Study of Gas Transport Temperature Dependency in Tight Formations. ACS Omega 4, 9778–9783. [CrossRef]
- Belyadi, H.; Fathi, E.; Belyadi, F. Hydraulic Fracturing in Unconventional Reservoirs: Theories, Operations, and Economic Analysis; Gulf Professional Publishing: Houston, TX, USA, 2019. [Google Scholar]
- Singh, H.; Javadpour, F. Langmuir slip-Langmuir sorption permeability model of shale. Fuel 2016, 164, 28–37. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, W.W.; Wang, X.Q.; Fan, Y.R.; An, C.J. Sorption of Phenanthrene onto Diatomite under the Influences of Solution Chemistry: A Study of Linear Sorption based on Maximal Information Coefficient. J. Environ. Inform. 2019, 34, 33–54. [Google Scholar] [CrossRef]
- Yu, W.; Sepehrnoori, K. Numerical Model for Shale Gas and Tight Oil Simulation. In Shale Gas and Tight Oil Reservoir Simulation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 11–70. [Google Scholar]
- Wang, X.; Wang, S.; Hagan, P.; Cao, C. Lacustrine Shale Gas: Case study from the Ordos Basin; Gulf Professional Publishing: Houston, TX, USA, 2017. [Google Scholar]
- Pini, R. Interpretation of net and excess adsorption isotherms in microporous adsorbents. Microporous Mesoporous Mater. 2014, 187, 40–52. [Google Scholar] [CrossRef]
- Menon, P.G. Adsorption at high pressures. Chem. Rev. 1968, 68, 277–294. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L. Fundamentals of high pressure adsorption. Langmuir 2009, 25, 13461–13466. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Ritter, J.A.; Yang, R.T. An extended Langmuir model for adsorption of gas mixtures on heterogeneous surfaces. Langmuir 1990, 6, 660–664. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press And Distributed By World Scientific Publishing Co: London, UK, 1998; Volume 2. [Google Scholar]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Piñeiro, Á.; Brocos, P.; Amigo, A.; Gracia-Fadrique, J.; Lemus, M.G. Extended Langmuir Isotherm for Binary Liquid Mixtures. Langmuir 2001, 17, 4261–4266. [Google Scholar] [CrossRef]
- Van Assche, T.R.C.; Baron, G.V.; Denayer, J.F.M. An explicit multicomponent adsorption isotherm model: Accounting for the size-effect for components with Langmuir adsorption behavior. Adsorption 2018, 24, 517–530. [Google Scholar] [CrossRef]
- Michalec, L.; Lísal, M. Molecular simulation of shale gas adsorption onto overmature type II model kerogen with control microporosity. Mol. Phys. 2017, 115, 1086–1103. [Google Scholar] [CrossRef]
- Zhao, H.; Lai, Z.; Firoozabadi, A. Sorption Hysteresis of Light Hydrocarbons and Carbon Dioxide in Shale and Kerogen. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hu, H. Methane adsorption comparison of different thermal maturity kerogens in shale gas system. Chin. J. Geochem. 2014, 33, 425–430. [Google Scholar] [CrossRef]
- Pang, Y.; He, Y.; Chen, S. An innovative method to characterize sorption-induced kerogen swelling in organic-rich shales. Fuel 2019, 254, 115629. [Google Scholar] [CrossRef]
- Fianu, J.; Gholinezhad, J.; Hassan, M. Comparison of Temperature-Dependent Gas Adsorption Models and Their Application to Shale Gas Reservoirs. Energy Fuels 2018, 32, 4763–4771. [Google Scholar] [CrossRef]
- Luo, S.; Nasrabadi, H.; Lutkenhaus, J.L. Effect of confinement on the bubble points of hydrocarbons in nanoporous media. AIChE J. 2016, 62, 1772–1780. [Google Scholar] [CrossRef]
- Walton, J.P.R.B.; Quirke, N. Capillary condensation: A molecular simulation study. Mol. Simulat. 1989, 2, 361–391. [Google Scholar] [CrossRef]
- Wongkoblap, A.; Do, D.D.; Birkett, G.; Nicholson, D. A critical assessment of capillary condensation and evaporation equations: A computer simulation study. J. Colloid Interface Sci. 2011, 356, 672–680. [Google Scholar] [CrossRef]
- Jiang, J.; Sandler, S.I. Capillary phase transitions of linear and branched alkanes in carbon nanotubes from molecular simulation. Langmuir 2006, 22, 7391–7399. [Google Scholar] [CrossRef]
- Singh, S.K.; Sinha, A.; Deo, G.; Singh, J.K. Vapor-Liquid Phase Coexistence, Critical Properties, and Surface Tension of Confined Alkanes. J. Phys. Chem. C 2009, 113, 7170–7180. [Google Scholar] [CrossRef]
- Didar, B.R.; Akkutlu, I.Y. Pore-Size Dependence of Fluid Phase Behavior and Properties in Organic-Rich Shale Reservoirs. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar] [CrossRef]
- Pitakbunkate, T.; Balbuena, P.B.; Moridis, G.J.; Blasingame, T.A. Effect of confinement on pressure/volume/temperature properties of hydrocarbons in shale reservoirs. SPE J. 2016, 21, 621–634. [Google Scholar] [CrossRef]
- Bui, K.; Akkutlu, I.Y. Hydrocarbons Recovery from Model-Kerogen Nanopores. Soc. Petrol. Engs. 2017, 22, 854–862. [Google Scholar] [CrossRef]
- Curtis, E.M. Influence of thermal maturity on organic shale microstructure. (n.d.). Available online: http://www.ogs.ou.edu/MEETINGS/Presentations/2013Shale/2013ShaleCurtis.pdf (accessed on 9 March 2019).
- Chen, S.; Han, Y.; Fu, C.; Zhang, H.; Zhu, Y.; Zuo, Z. Micro and nano-size pores of clay minerals in shale reservoirs: Implication for the accumulation of shale gas. Sediment. Geol. 2016, 342, 180–190. [Google Scholar] [CrossRef]
- Hou, Y.; He, S.; Wang, J.; Harris, N.B.; Cheng, C.; Li, Y.; Pedersen, P. Preliminary study on the pore characterization of lacustrine shale reservoirs using low pressure nitrogen adsorption and field emission scanning electron microscopy methods: A case study of the Upper Jurassic Emuerhe Formation, Mohe basin, northeastern China. Can. J. Earth Sci. 2015, 52, 294–306. [Google Scholar]
- Wasaki, A.; Akkutlu, I.Y. Permeability of organic-rich shale. SPE J. 2015, 20, 381–384. [Google Scholar] [CrossRef]
- Kou, R.; Alafnan, S.F.K.; Akkutlu, I.Y. Multi-scale Analysis of Gas Transport Mechanisms in Kerogen. Tranp. Porous Med. 2017, 116, 493–519. [Google Scholar] [CrossRef]
- Ungerer, P.; Collell, J.; Yiannourakou, M. Molecular modeling of the volumetric and thermodynamic properties of kerogen: Influence of organic type and maturity. Energy Fuels 2015, 29, 91–105. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Yiannourakou, M.; Ungerer, P.; Leblanc, B.; Rozanska, X.; Saxe, P.; Vidal-Gilbert, S.; Gouth, F.; Montel, F. Molecular simulation of adsorption in microporous materials. Oil Gas Sci. Technol. 2013, 68, 977–994. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 34, 120–131. [Google Scholar] [CrossRef]
- Tian, Y.; Yan, C.; Jin, Z. Characterization of Methane Excess and Absolute Adsorption in Various Clay Nanopores from Molecular Simulation. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Nicholson, D.; Parsonage, N.G. Computer Simulation and the Statistical Mechanics Ofadsorption; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Aljawad, M.S.; Alafnan, S.; Abu-Khamsin, S. Artificial Lift and Mobility Enhancement of Heavy Oil Reservoirs Utilizing a Renewable Energy-Powered Heating Element. ACS Omega 2019, 4, 20048–20058. [Google Scholar] [CrossRef]
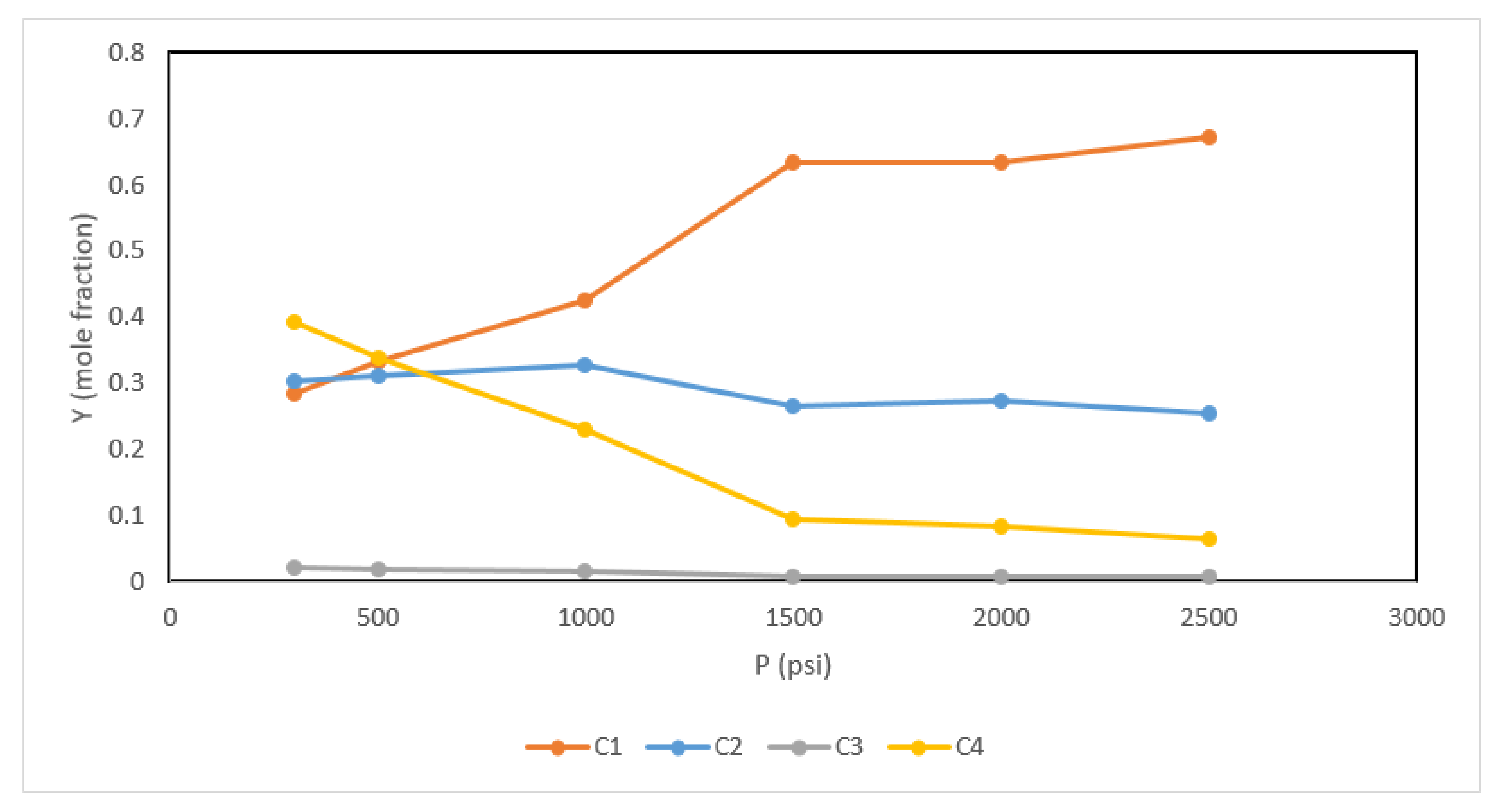
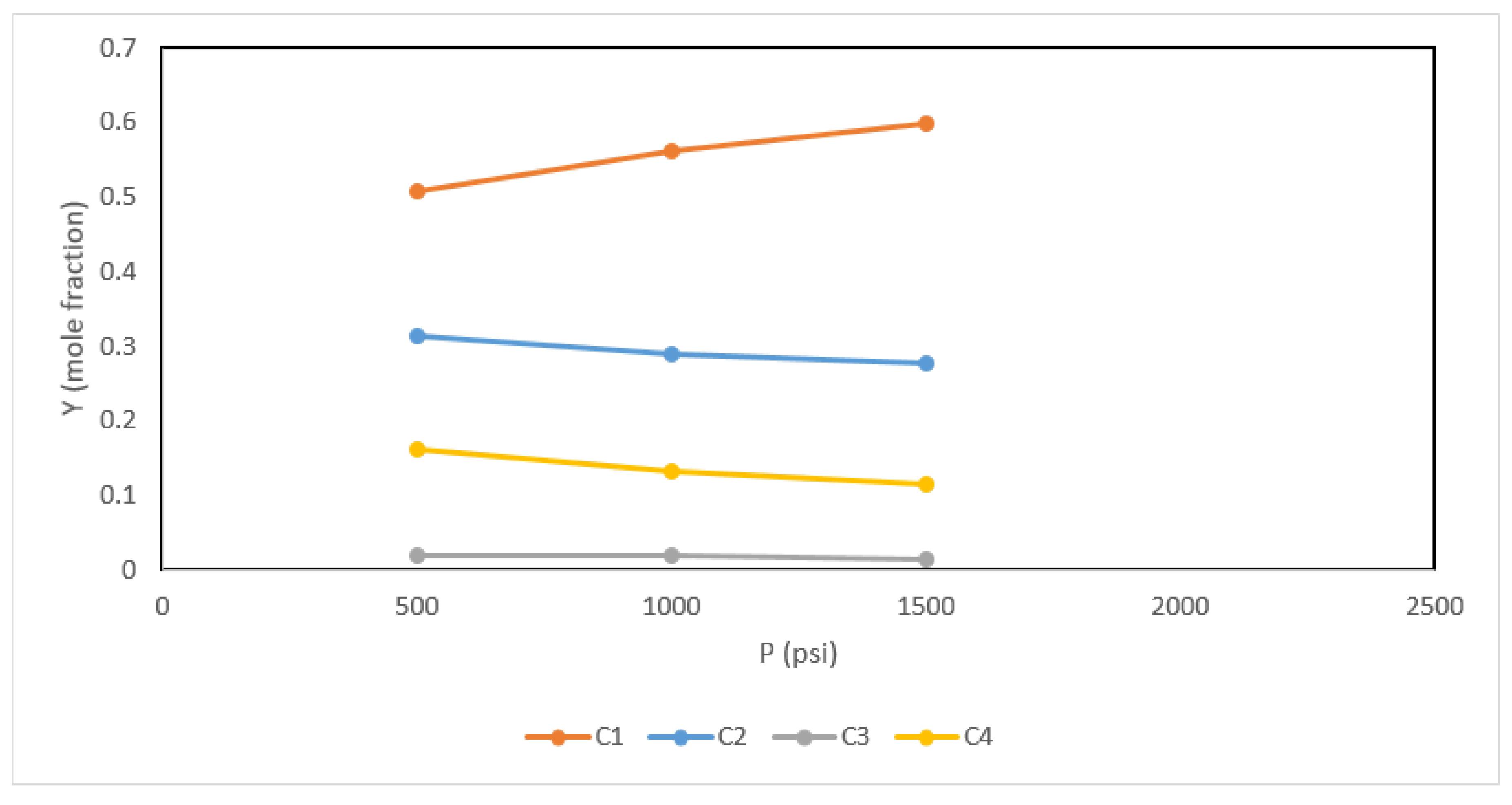
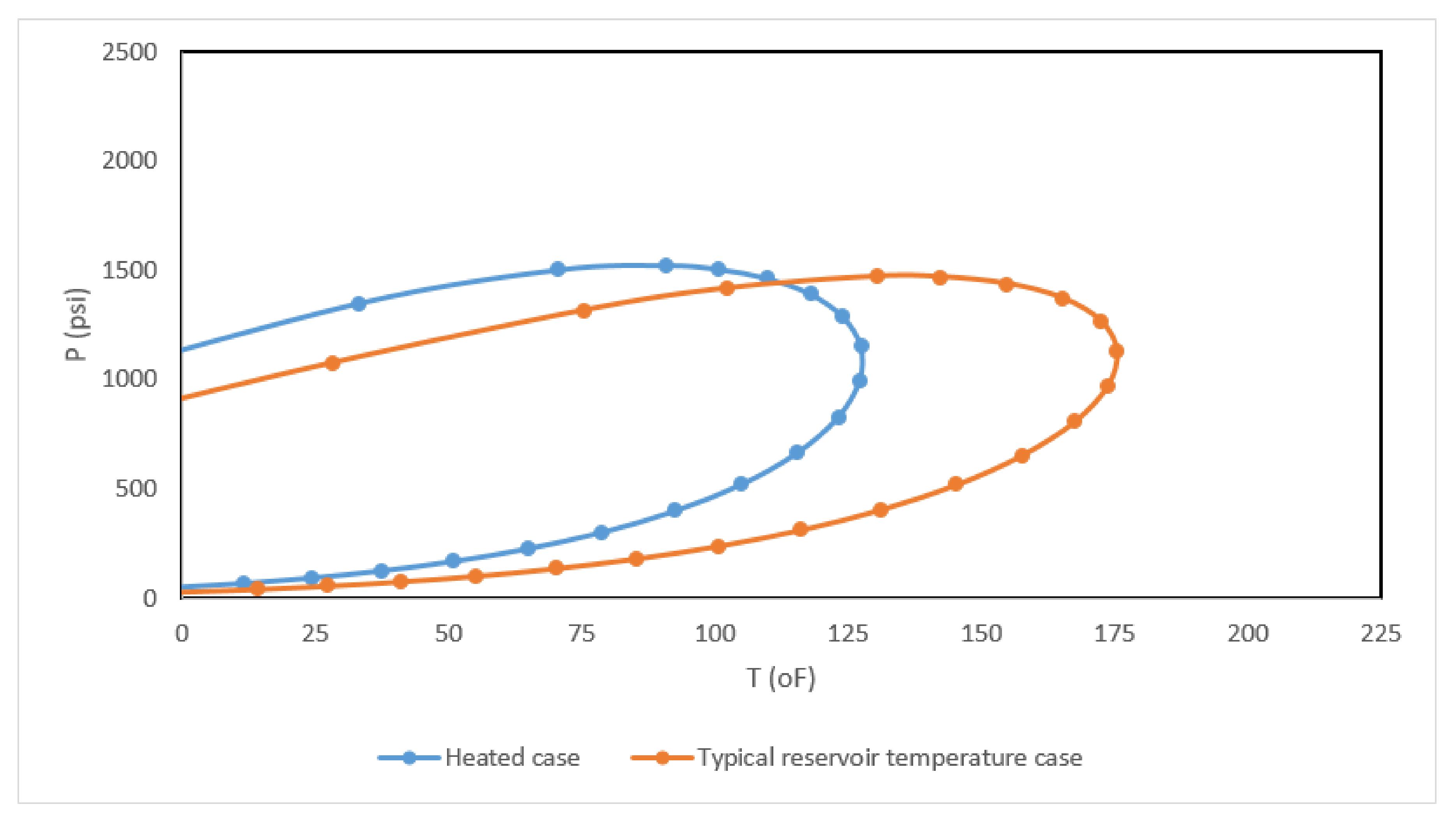
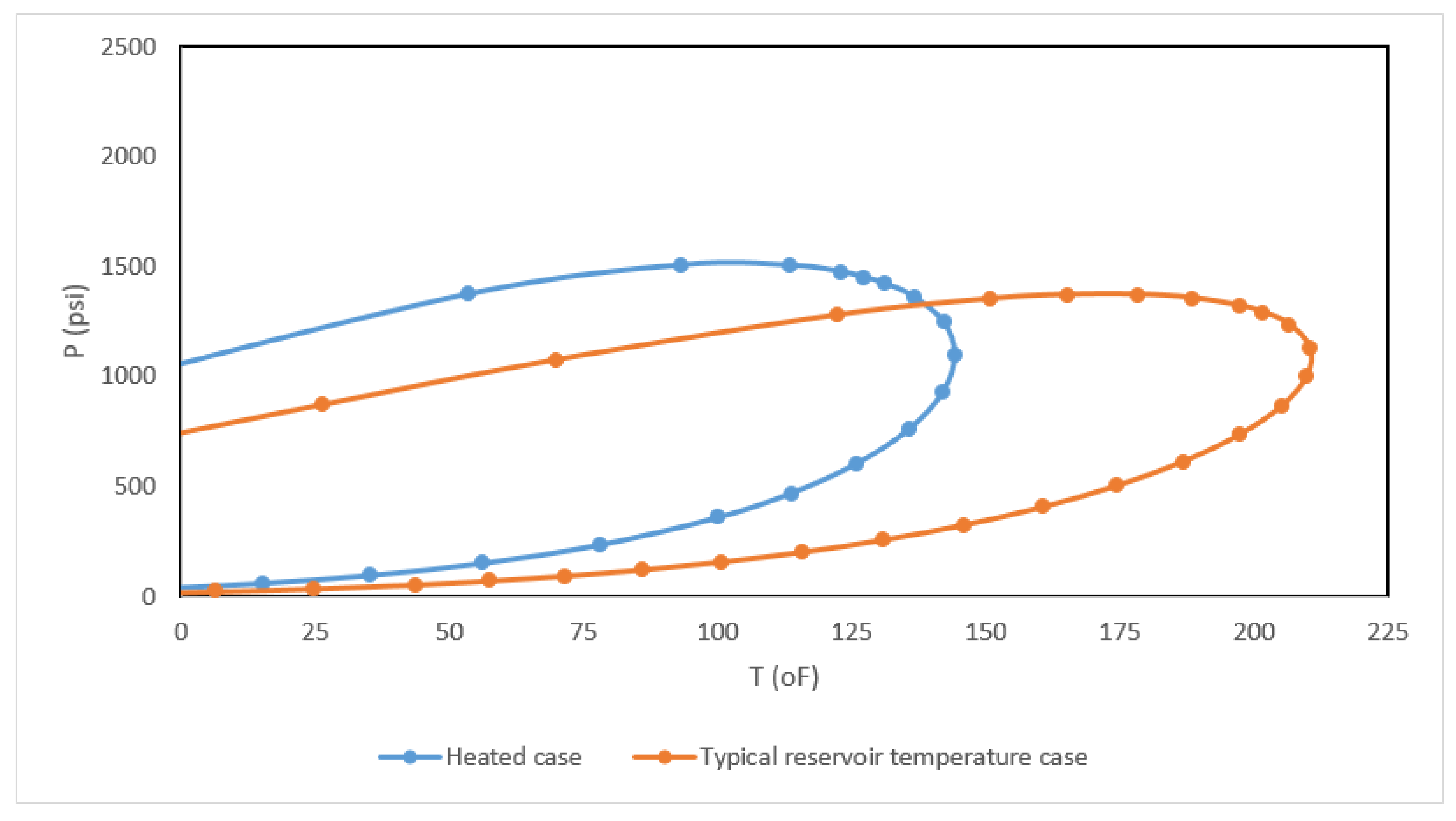


| Component | Composition | Fugacity (psi) |
|---|---|---|
| CH4 | 30% | 1061.1 |
| C2H6 | 15% | 195.7 |
| C3H8 | 20% | 123.4 |
| n-C4H10 | 35% | 106.2 |
| CH4 | C2H6 | C3H8 | n-C4H10 |
|---|---|---|---|
| 67.30% | 25.50% | 0.73% | 6.50% |
| Input Data | SI Unit | Field Unit |
|---|---|---|
| Ambient temperature, | 25 °C | 77 °F |
| Reservoir temperature, | 93.33 °C | 200 °F |
| Gas temperature at injection or element temperature, | 204.4 °C | 400 °F |
| Formation specific heat capacity, | 0.879 kJ/(Kg·°C) | 0.2099 Btu(lb·°F) |
| Formation thermal conductivity, | 1.57 × 10−3 kJ/(s·m·°C) | 0.907 Btu/(hr·ft·°F) |
| Gas heat capacity, | 2.18 kJ/(Kg·°C) | 0.52 Btu/(lbm·°F) |
| Gas thermal conductivity, | 5.5 × 10−5 kJ/(s·m·°C) | 0.0318 Btu/(hr·ft·°F) |
| Overall heat transfer coefficient, | 0.1 kJ/(s·m2·°C) | 0.00488 Btu/(hr·ft2·°F) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafnan, S.; Aljawad, M.; Glatz, G.; Sultan, A.; Windiks, R. Sustainable Production from Shale Gas Resources through Heat-Assisted Depletion. Sustainability 2020, 12, 2145. https://doi.org/10.3390/su12052145
Alafnan S, Aljawad M, Glatz G, Sultan A, Windiks R. Sustainable Production from Shale Gas Resources through Heat-Assisted Depletion. Sustainability. 2020; 12(5):2145. https://doi.org/10.3390/su12052145
Chicago/Turabian StyleAlafnan, Saad, Murtada Aljawad, Guenther Glatz, Abdullah Sultan, and Rene Windiks. 2020. "Sustainable Production from Shale Gas Resources through Heat-Assisted Depletion" Sustainability 12, no. 5: 2145. https://doi.org/10.3390/su12052145
APA StyleAlafnan, S., Aljawad, M., Glatz, G., Sultan, A., & Windiks, R. (2020). Sustainable Production from Shale Gas Resources through Heat-Assisted Depletion. Sustainability, 12(5), 2145. https://doi.org/10.3390/su12052145






