Accumulation of Urban Insect Pests in China: 50 Years’ Observations on Camphor Tree (Cinnamomum camphora)
Abstract
1. Introduction
2. Materials and Methods
2.1. The C. camphora-Hosted Insect Pest Dataset
2.1.1. Data Collection
2.1.2. Data Filtering and Compilation
2.2. Pest cumulative Characteristics
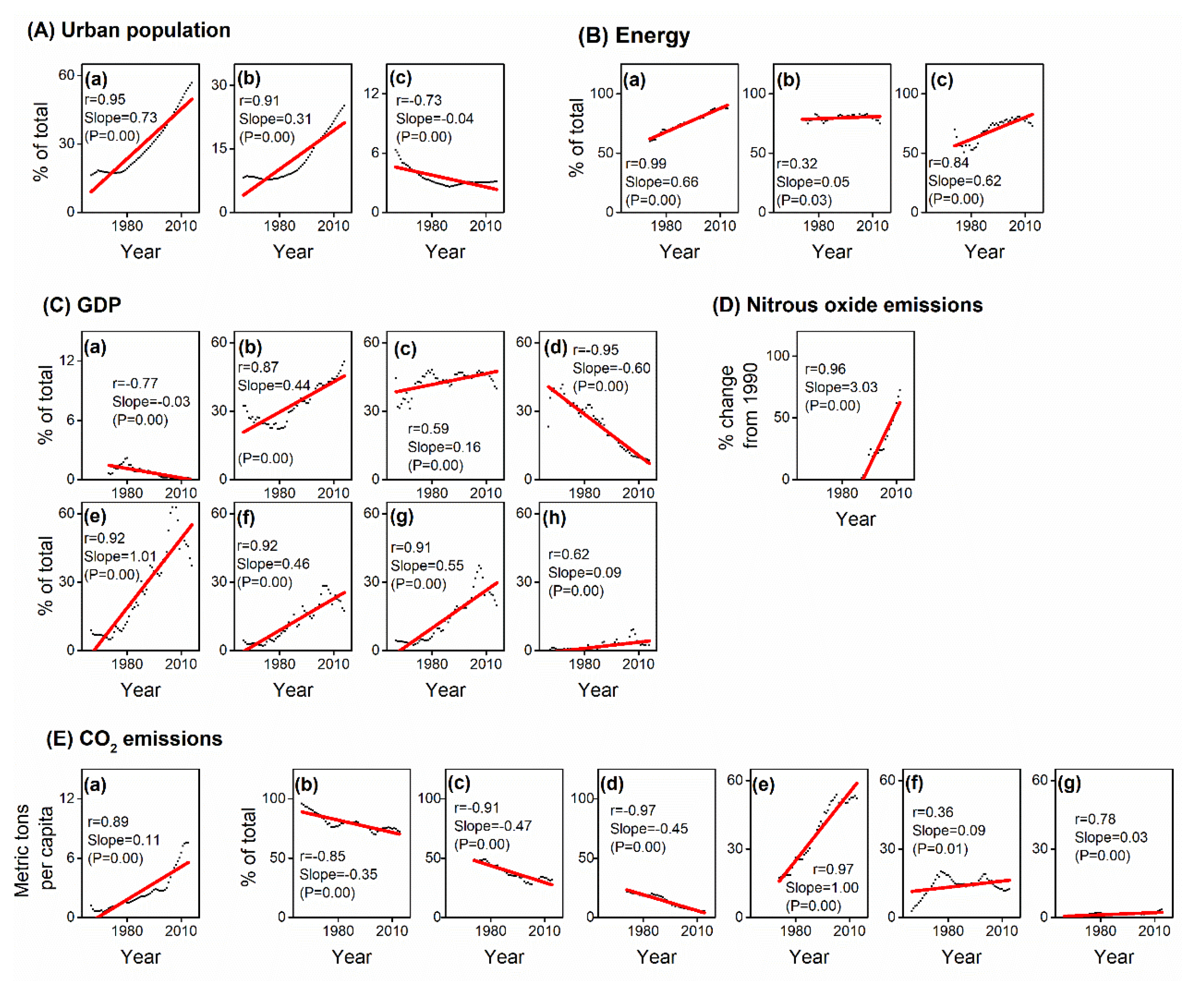
2.3. Socio-Economic Indicators
3. Results
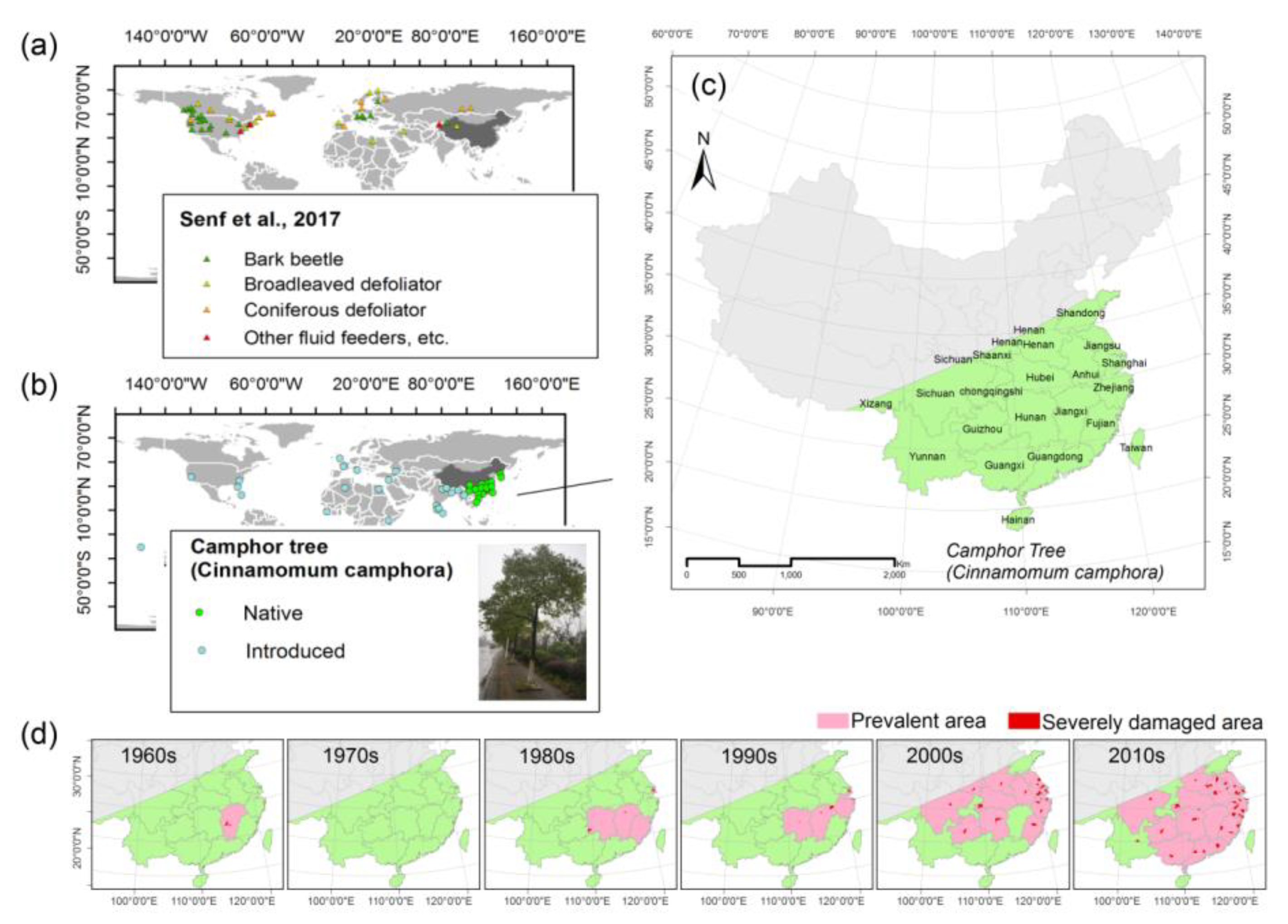
3.1. Insect Pest Spectrum on C. camphora
3.2. Spatial Aggregation
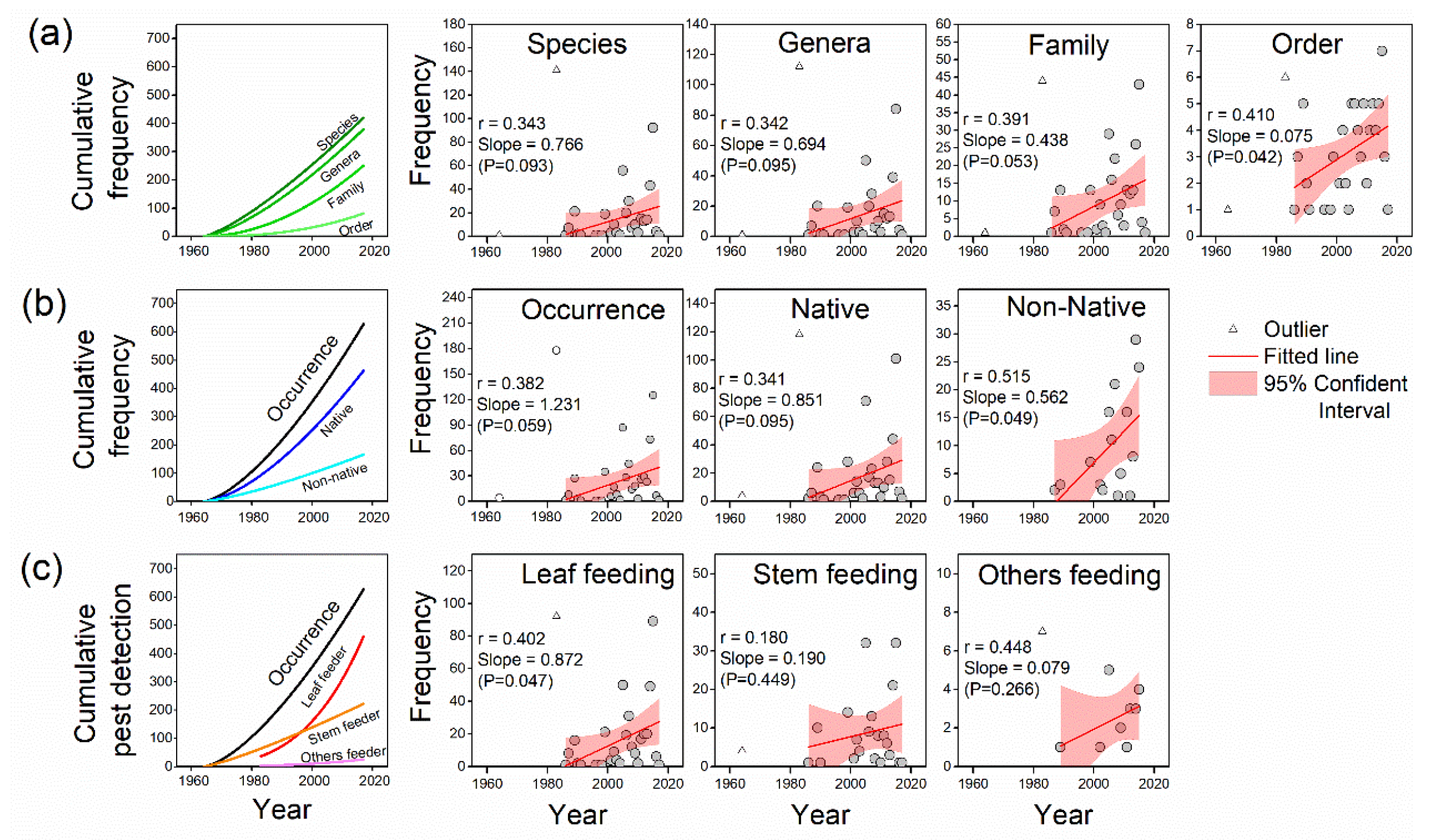
3.3. Temporal Trends
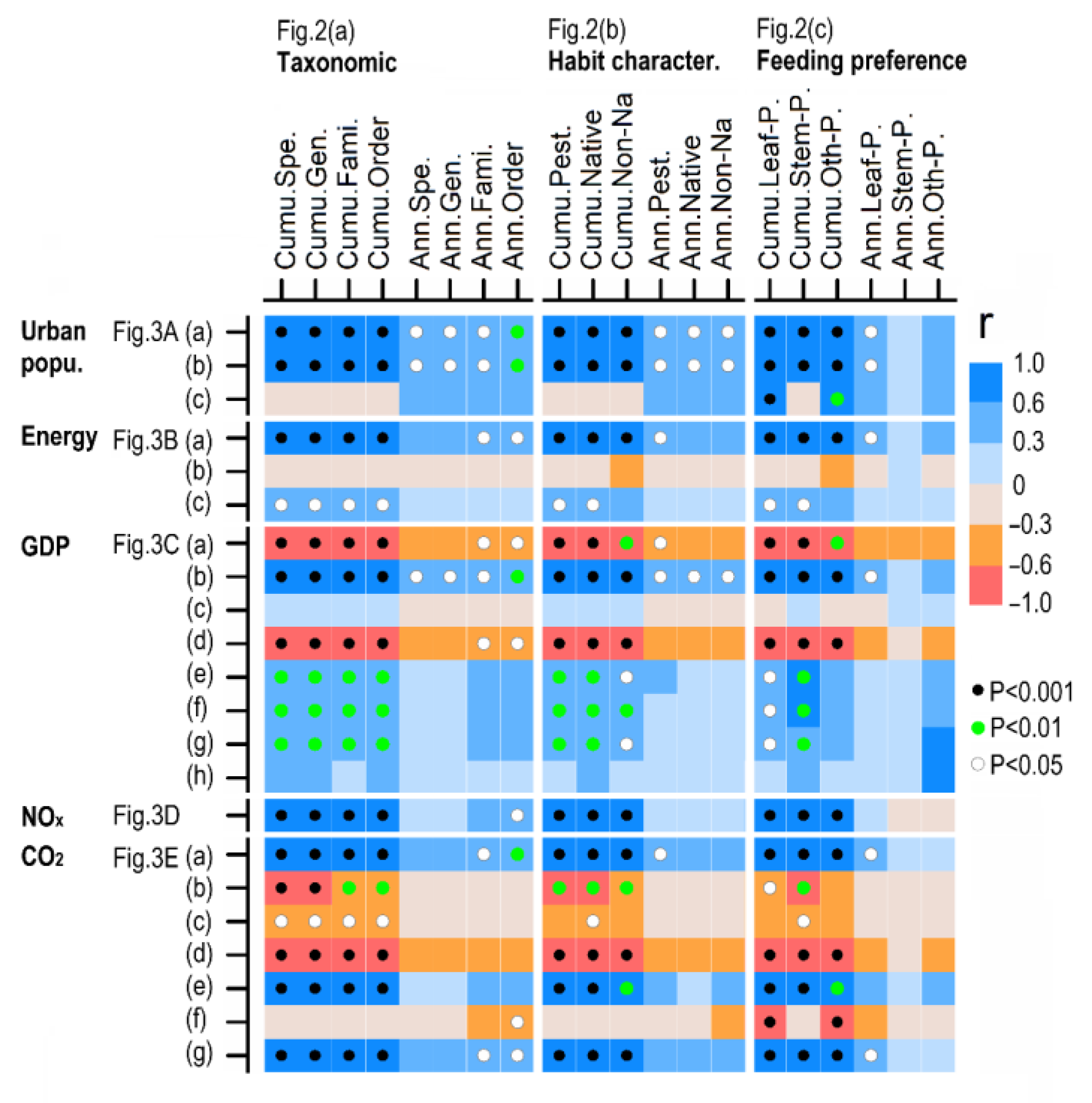
3.4. Causes and Controller Attribution
4. Discussion
4.1. Improved Reference Data for Assessing Insect Pest Outbreak
4.1.1. Field Observational Database
4.1.2. Habitat Characteristics
4.1.3. Types of Feeders
4.2. Accelerated Urban Pest Outbreaks in China Since the 1990s
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V.; Stekolshchikov, A.V.; Söderman, G.; Labina, E.S.; Zverev, V.; Zvereva, E.L. Sap-feeding insects on forest trees along latitudinal gradients in northern Europe: A climate-driven patterns. Glob. Chang. Biol. 2015, 21, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kautz, M.; Meddens, A.J.H.; Hall, R.J.; Arneth, A. Biotic disturbances in Northern Hemisphere forests—A synthesis of recent data, uncertainties and implications for forest monitoring and modelling. Glob. Ecol. Biogeogr. 2017, 26, 533–552. [Google Scholar] [CrossRef]
- Beudert, B.; Bässler, C.; Thorn, S.; Noss, R.; Schröder, B.; Dieffenbach-Fries, H.; Foullois, N.; Müller, J. Bark beetles increase biodiversity while maintaining drinking water quality: Bark beetles, biodiversity and drinking water. Conserv. Lett. 2015, 8, 272–281. [Google Scholar] [CrossRef]
- Johnson, M.T.J.; Munshi-South, J. Evolution of life in urban environments. Science 2017, 358, eaam8327. [Google Scholar] [CrossRef]
- Paine, T.D.; Millar, J.G.; Daane, K.M. Accumulation of pest insects on Eucalyptus in California: Random process or smoking gun. J. Econ. Entomol. 2010, 103, 1943–1949. [Google Scholar] [CrossRef]
- Meineke, E.K.; Dunn, R.R.; Frank, S.D. Early pest development and loss of biological control are associated with urban warming. Biol. Lett. 2014, 10, 20140586. [Google Scholar] [CrossRef]
- Kowarik, I.; Hiller, A.; Planchuelo, G.; Seitz, B.; von der Lippe, M.; Buchholz, S. Emerging urban forests: Opportunities for promoting the wild side of the urban green infrastructure. Sustainability 2019, 11, 6318. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; Becker, J.; Peter, S.; Heller, S.; Hölker, F. Long-term comparison of attraction of flying insects to streetlights after the transition from traditional light sources to light-emitting diodes in urban and peri-urban settings. Sustainability 2019, 11, 6198. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landsc. Ecol. 2017, 32, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.H.; Song, S. Rural–urban migration and urbanization in China: Evidence from time-series and cross-section analyses. China Econ. Rev. 2003, 14, 386–400. [Google Scholar] [CrossRef]
- Appel, A.G.; Hu, X.P.; Zhou, J.; Qin, Z.; Zhu, H.; Chang, X.; Wang, Z.; Liu, X.; Liu, M. Observations of the biology and ecology of the black-winged termite, Odontotermes formosanus Shiraki (Termitidae: Isoptera), in Camphor, Cinnamomum camphora (L.) (Lauraceae). Psyche J. Entomol. 2012, 2012, 123102. [Google Scholar]
- Huang, H.; Wu, S.; Huang, J.; Wu, S.; Gao, L.; Yang, L. Diagnosis and damage of weevil pest Pagiophloeus tsushimanus on camphor tree (in Chinese). J. Zhejiang A&F Univ. 2011, 31, 764–767. [Google Scholar]
- Senf, C.; Seidl, R.; Hostert, P. Remote sensing of forest insect disturbances: Current state and future directions. Int. J. Appl. Earth Obs. Geoinf. 2017, 60, 49–60. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Aukema, J.E.; McCullough, D.G.; Von Holle, B.; Liebhold, A.M.; Britton, K.; Frankel, S.J. Historical accumulation of nonindigenous forest pests in the continental United States. BioScience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Cummins, K.W. Trophic relations of aquatic insects. Annu. Rev. Entomol. 1973, 18, 183–206. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Pregitzer, K.S.; Wright, I.J.; Oleksyn, J.; Machado, J.L. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008, 11, 793–801. [Google Scholar] [CrossRef]
- Katsaruware, R.; Mafongoya, P.; Gubba, A. Responses of insect pests and plant diseases to changing and variable climate: A review. J. Agric. Sci. 2017, 9, 160–168. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, J.; Chen, F.; Wu, G.; Ge, F. How does atmospheric elevated CO2 affect crop pests and their natural enemies? Case histories from China. Insect Sci. 2011, 18, 393–400. [Google Scholar] [CrossRef]
- Liebhold, A.M.; McCullough, D.G.; Blackburn, L.M.; Frankel, S.J.; Von Holle, B.; Aukema, J.E. A highly aggregated geographical distribution of forest pest invasions in the USA. Divers. Distrib. 2013, 19, 1208–1216. [Google Scholar] [CrossRef]
- Hanks, L.M.; Denno, R.F. Natural enemies and plant water relations influence the distribution of an armored scale insect. Ecology 1993, 74, 1081–1091. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Yamanaka, T.; Roques, A.; Augustin, S.; Chown, S.L.; Brockerhoff, E.G.; Pyšek, P. Global compositional variation among native and non-native regional insect assemblages emphasizes the importance of pathways. Biol. Invasions 2016, 18, 893–905. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Schwartzberg, E.G.; Raffa, K.F.; Reich, P.B.; Lindroth, R.L. Experimental climate warming alters aspen and birch phytochemistry and performance traits for an outbreak insect herbivore. Glob. Chang. Biol. 2015, 21, 2698–2710. [Google Scholar] [CrossRef]
- Youngsteadt, E.; Dale, A.G.; Terando, A.J.; Dunn, R.R.; Frank, S.D. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Chang. Biol. 2015, 21, 97–105. [Google Scholar] [CrossRef]
- Rand, T.A.; Louda, S.M. Invasive insect abundance varies across the biogeographic distribution of a native host plant. Ecol. Appl. 2006, 16, 877–890. [Google Scholar] [CrossRef][Green Version]
- Edney-Browne, E.; Brockerhoff, E.G.; Ward, D. Establishment patterns of non-native insects in New Zealand. Biol. Invasions 2018, 20, 1657–1669. [Google Scholar] [CrossRef]
- Dale, A.G.; Frank, S.D. The effects of urban warming on herbivore abundance and street tree condition. PLoS ONE 2014, 9, e102996. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Ge, F. Plant–aphid interactions under elevated CO2: Some cues from aphid feeding behavior. Front. Plant Sci. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, S.; Zhou, D. Prevalent vegetation growth enhancement in urban environment. Proc. Natl. Acad. Sci. USA 2016, 113, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dahlhausen, J.; Schütze, G.; Perkins, D.; Rötzer, T.; Caldentey, J.; Koike, T.; van Con, T.; et al. Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 2017, 7, 15403. [Google Scholar] [CrossRef]
- Meineke, E.K.; Dunn, R.R.; Sexton, J.O.; Frank, S.D. Urban warming drives insect pest abundance on street trees. PLoS ONE 2013, 8, e59687. [Google Scholar] [CrossRef]
- Merckx, T.; Van Dyck, H. Urbanization-driven homogenization is more pronounced and happens at wider spatial scales in nocturnal and mobile flying insects. Glob. Ecol. Biogeogr. 2019, 28, 1440–1455. [Google Scholar] [CrossRef]
- United Nations, Population Division. World Urbanization Prospects: The 2014 Revision; United Nations, Department of Economic and Social Affairs: New York, NY, USA, 2015. [Google Scholar]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Gordo, O.; Sanz, J.J. Temporal trends in phenology of the honey bee Apis mellifera (L.) and the small white Pieris rapae (L.) in the Iberian Peninsula (1952–2004). Ecol. Entomol. 2006, 31, 261–268. [Google Scholar] [CrossRef]
- Brooks, D.R.; Bater, J.E.; Clark, S.J.; Monteith, D.T.; Andrews, C.; Corbett, S.J.; Beaumont, D.A.; Chapman, J.W. Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J. Appl. Ecol. 2012, 49, 1009–1019. [Google Scholar] [CrossRef]
- Wu, J.; Hobbs, R. Key issues and research priorities in landscape ecology: An idiosyncratic synthesis. Landsc. Ecol. 2002, 17, 355–365. [Google Scholar] [CrossRef]
- Bai, X.M.; Shi, P.J.; Liu, Y.S. Society: Realizing China’s urban dream. Nature 2014, 509, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lau, N. Increasing heat stress in urban areas of eastern China: Acceleration by urbanization. Geophys. Res. Lett. 2018, 45, 13060–13069. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Huang, J.; Su, B.; Cao, L.; Wang, Y.; Jiang, T.; Fischer, T. Intensity–area–duration analysis of droughts in China 1960–2013. Clim. Dyn. 2017, 48, 151–168. [Google Scholar] [CrossRef]
- Han, W.; Liang, C.; Jiang, B.; Ma, W.; Zhang, Y. Major natural disasters in China, 1985–2014: Occurrence and damages. Int. J. Environ. Res. Public Health 2016, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Yan, Z.; Wang, Y. A spatial cluster analysis of heavy rains in China. Atmos. Ocean. Sci. Lett. 2011, 4, 36–40. [Google Scholar]
- Liu, X.; Wang, S.; Zhou, Y.; Wang, F.; Li, W.; Liu, W. Regionalization and spatiotemporal variation of drought in China based on standardized precipitation evapotranspiration index (1961–2013). Adv. Meteorol. 2015, 2015, 950262. [Google Scholar] [CrossRef]
- Leite, G.L.D.; Von dos Santos Veloso, R.; Zanuncio, J.C.; Azevedo, A.M.; Silva, J.L.; Wilcken, C.F.; Soares, M.A. Architectural diversity and galling insects on Caryocar brasiliense trees. Sci. Rep. 2017, 7, 16677. [Google Scholar] [CrossRef]
- Ziter, C.; Robinson, E.A.; Newman, J.A. Climate change and voltinism in Californian insect pest species: Sensitivity to location, scenario and climate model choice. Glob. Chang. Biol. 2012, 18, 2771–2780. [Google Scholar] [CrossRef]
| Yi | Parameter * | R2 | F Value | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ai | bij | ||||||||||
| X2 | X6 | X8 | X10 | X13 | X14 | X16 | |||||
| Y1 | 65.21 ± 10.21 | - | - | - | - | - | 4.27 ± 1.49 | 39.11 ± 1.87 | 0.969 | 239.22 | <0.001 |
| Y2 | 43.18 ± 9.55 | - | - | - | - | - | 3.82 ± 1.39 | 36.85 ± 1.75 | 0.970 | 241.42 | <0.001 |
| Y3 | 2.75 ± 6.33 | - | - | - | - | - | - | 28.1 ± 1.32 | 0.968 | 450.6 | <0.001 |
| Y4 | −45.62 ± 6.87 | −1.65 ± 0.49 | - | 1.8 ± 0.32 | - | - | - | 8.96 ± 0.5 | 0.995 | 1067.36 | <0.001 |
| Y5 | −19.02 ± 12.80 | - | - | - | - | 1.19 ± 0.48 | - | - | 0.260 | 6.27 | <0.05 |
| Y6 | −16.75 ± 11.62 | - | - | - | - | 1.08 ± 0.43 | - | - | 0.260 | 6.26 | <0.05 |
| Y7 | −10.68 ± 7.07 | - | - | - | - | 0.75 ± 0.26 | - | - | 0.321 | 8.08 | <0.05 |
| Y8 | 6.37 ± 1.04 | - | - | - | −0.26 ± 0.08 | - | - | - | 0.412 | 11.51 | <0.01 |
| Y9 | 53.09 ± 16.25 | - | - | - | - | - | 6.02 ± 2.37 | 63.27 ± 2.98 | 0.970 | 244.62 | <0.001 |
| Y10 | −110.04 ± 55.68 | - | - | 4.28 ± 1.6 | - | - | 5.19 ± 1.54 | 39.26 ± 3.18 | 0.977 | 209.21 | <0.001 |
| Y11 | 20.21 ± 4.60 | - | - | - | - | - | - | 17.48 ± 0.96 | 0.956 | 329.62 | <0.001 |
| Y12 | −25.24 ± 20.37 | - | - | - | - | 1.74 ± 0.76 | - | - | 0.220 | 5.24 | <0.05 |
| Y13 | - | - | - | - | - | - | - | - | - | - | >0.05 |
| Y14 | −234.00 ± 102.71 | - | 3.08 ± 1.3 | - | - | - | - | - | 0.337 | 5.57 | <0.05 |
| Y15 | 13.82 ± 10.42 | - | - | - | - | - | 3.41 ± 1.52 | 39.68 ± 1.91 | 0.969 | 232.28 | <0.001 |
| Y16 | −31.09 ± 24.95 | - | - | 2.02 ± 0.72 | - | - | 2.5 ± 0.69 | 18.23 ± 1.42 | 0.978 | 226.31 | <0.001 |
| Y17 | 2.04 ± 0.73 | - | - | - | - | - | - | 2.23 ± 0.15 | 0.933 | 211.39 | <0.001 |
| Y18 | −17.23 ± 11.77 | - | - | - | - | 1.15 ± 0.44 | - | - | 0.280 | 6.834 | <0.05 |
| Y19 | - | - | - | - | - | - | - | - | - | - | >0.05 |
| Y20 | - | - | - | - | - | - | - | - | - | - | >0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Z.; Zhao, M.; Ogbodo, U.S. Accumulation of Urban Insect Pests in China: 50 Years’ Observations on Camphor Tree (Cinnamomum camphora). Sustainability 2020, 12, 1582. https://doi.org/10.3390/su12041582
Xiang Z, Zhao M, Ogbodo US. Accumulation of Urban Insect Pests in China: 50 Years’ Observations on Camphor Tree (Cinnamomum camphora). Sustainability. 2020; 12(4):1582. https://doi.org/10.3390/su12041582
Chicago/Turabian StyleXiang, Zhiyuan, Meifang Zhao, and U. S. Ogbodo. 2020. "Accumulation of Urban Insect Pests in China: 50 Years’ Observations on Camphor Tree (Cinnamomum camphora)" Sustainability 12, no. 4: 1582. https://doi.org/10.3390/su12041582
APA StyleXiang, Z., Zhao, M., & Ogbodo, U. S. (2020). Accumulation of Urban Insect Pests in China: 50 Years’ Observations on Camphor Tree (Cinnamomum camphora). Sustainability, 12(4), 1582. https://doi.org/10.3390/su12041582




