Assessment of Fraction and Mobility of Arsenic in Soil Near the Mine Waste Dam
Abstract
1. Introduction
2. Materials and Methods
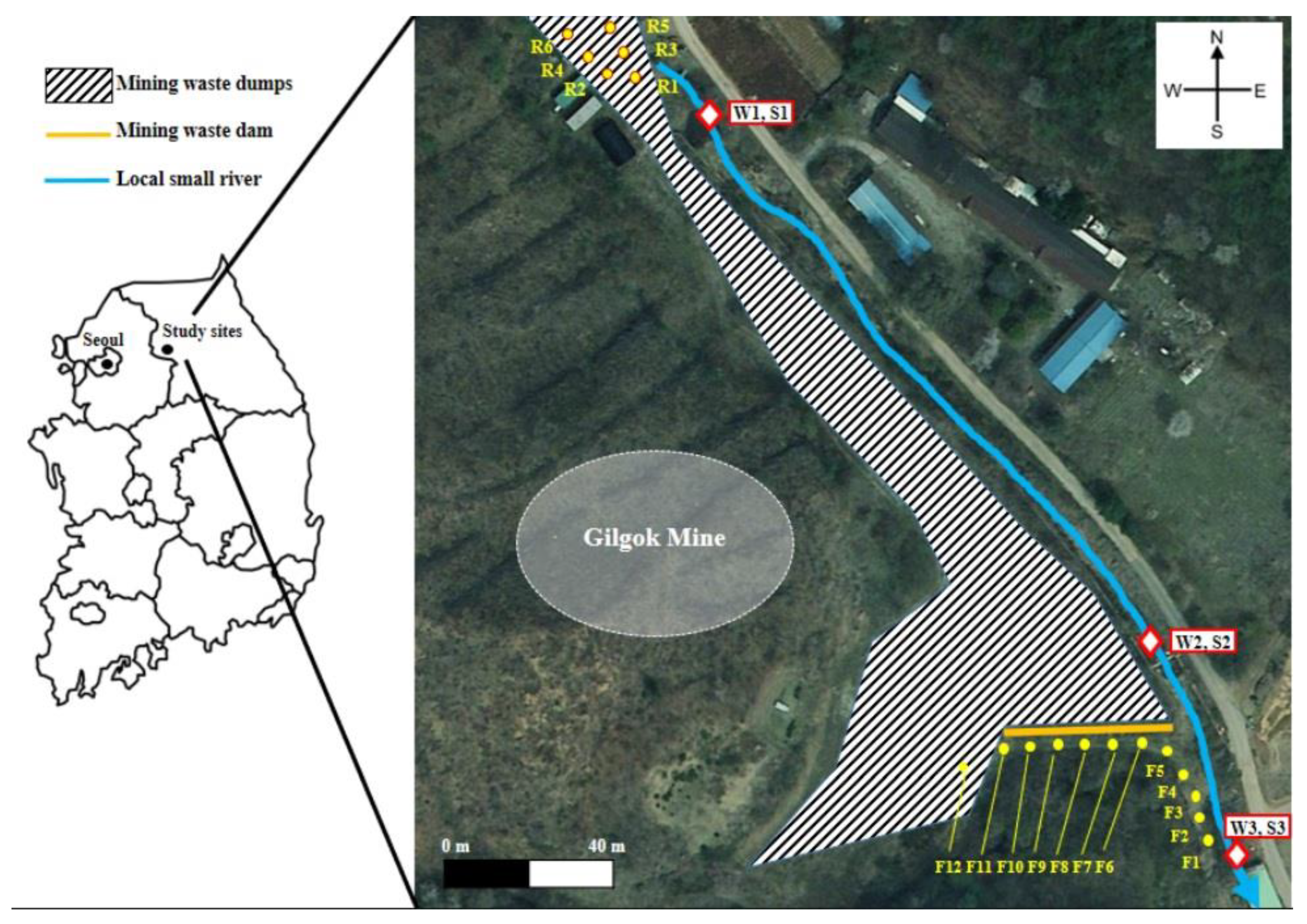
2.1. Site Description
2.2. Samples Analysis
2.3. Sequential and Single Extraction for Chemical Assessment
2.4. Plant Cultivation Experiment for Biological Assessment
2.5. Data Analysis
3. Results and Discussion
3.1. Soil, Water, and Sediment Characteristics
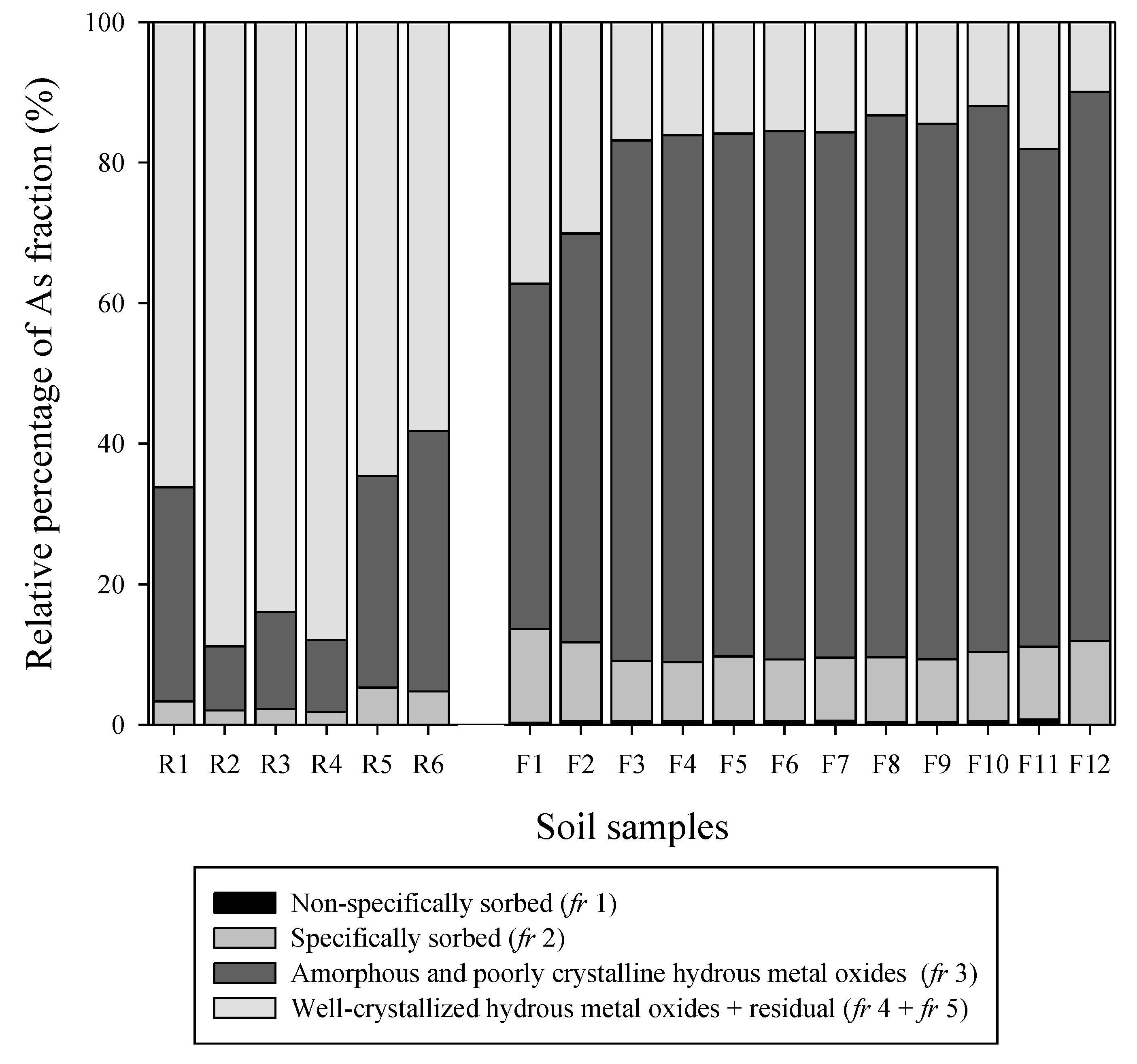
3.2. As Fraction in Soils Near the Mine Waste Duump and Dam
3.3. Chemical Assessment for As Mobility and Bioavailability
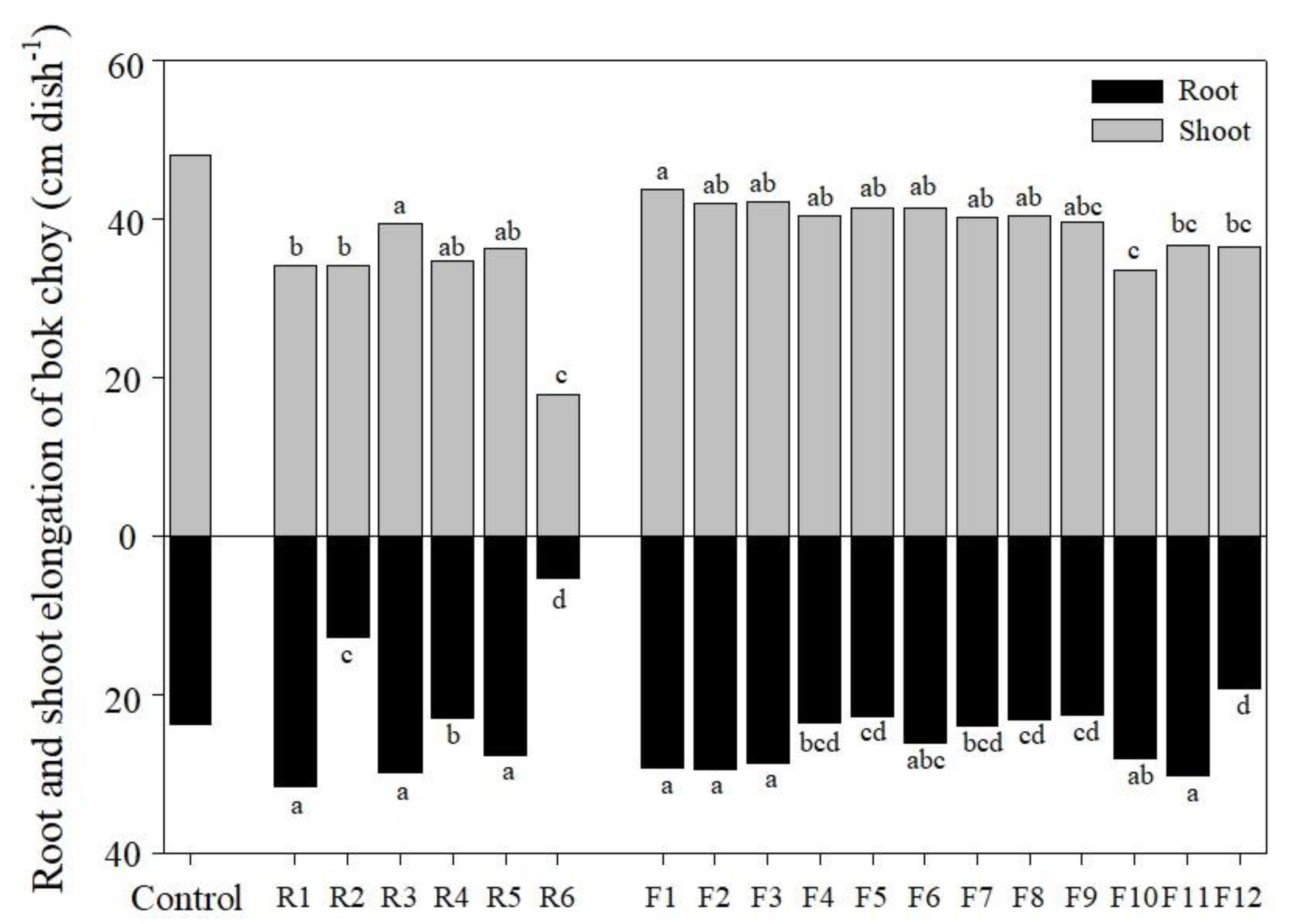
3.4. Plant Cultivation Experiment for Biological Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- KMoTIE. Annual Report of Environmental Status in Mining Areas; Ministry of Trade, Industry, and Energy of the Korea: Sejong, Korea, 2010.
- Kim, M.S.; Park, M.J.; Yang, J.H.; Lee, S.H. Human health risk assessment for toxic trace elements in the Yaro mine and reclamation options. Int. J. Environ. Res. Public Health 2019, 16, 5077. [Google Scholar] [CrossRef] [PubMed]
- Koo, N.; Lee, S.H.; Kim, J.G. Arsenic mobility in the amended mine tailings and its impact on soil enzyme activity. Environ. Geochem. Health 2012, 34, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Min, H.G.; Kim, J.G.; Lee, S.R. Estimating arsenic mobility and phytotoxicity using two different phosphorous fertilizer release rates in soil. Agronomy 2019, 9, 111. [Google Scholar] [CrossRef]
- Benvenuti, M.; Mascaro, I.; Corsini, F.; Lattanzi, P.; Parrini, P.; Tanelli, G. Mine waste dumps and heavy metal pollution in abandoned mining district of Boccheggiano (Southern Tuscany, Italy). Environ. Geol. 1997, 30, 238–243. [Google Scholar] [CrossRef]
- Choong, T.S.Y.; Chuah, T.G.; Robiah, Y.; Koay, F.L.G.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Lottermoser, B. Mine Waste: Characterization, Treatment and Environmental Impacts; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 3-540-48629-1. [Google Scholar]
- Favas, P.J.C.; Martino, L.E.; Prasad, M.N.V. Abandoned mine land reclamation—Challenges and opportunities (Holistic Approach). In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., Favas, P.J.C., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–31. ISBN 9780128129869. [Google Scholar]
- Kim, H.J.; Park, B.K.; Kong, S.H.; Lee, J.Y.; Ok, Y.S.; Jun, S.H. Fraction and geoaccumulation assessment index of heavy metals in abandoned mines wastes. J. Soil Groundw. Environ. 2005, 10, 75–80. [Google Scholar]
- Kim, M.S.; Kim, Y.S.; Min, H.G.; Kim, J.G.; Koo, N. Pine forest soil characteristics and major soil impact factors for natural regeneration. Korean J. Soil Sci. Fertil. 2017, 50, 179–186. [Google Scholar] [CrossRef][Green Version]
- Kossoff, D.; Hudson-Edwards, K.A.; Dubbin, W.E.; Alfredsson, M. Major and trace metal mobility during weathering of mine tailings: Implications for floodplain soils. Appl. Geochem. 2012, 27, 562–576. [Google Scholar] [CrossRef]
- Gao, Y.; Bradshaw, A.D. The containment of toxic wastes: II. Metal movement inn leachate and drainage at Parc leadn/zinc mine, North Wales. Environ. Pollut. 1995, 90, 379–382. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Barlow, B.; Lemmer, N.; Waanders, F. Geochemical speciation of metal ions in the leachate of tailings treated with synthetic rain water. In Proceedings of the International Conference on Advances in Science, Engineering, Technology & Waste Management, Parys, South Africa, 27–28 November 2017; pp. 19–23. [Google Scholar]
- Kim, S.; Devineni, N.; Lall, U.; Kim, H.S. Sustainable development of water resources: Spatio-temporal analysis of water stress in South Korea. Sustainability 2018, 10, 3795. [Google Scholar] [CrossRef]
- Kang, G.C.; Ahn, N.K.; Oh, J.I.; Kim, T.H. Stability investigation of the large size heap of coal associated wastes. J. Eng. Geol. 2005, 15, 133–144. [Google Scholar]
- Mine Reclamation Corporation (MIRECO). Safety Evaluation and Development of Maintenance Technique for Tailing Dam; Technical Report (Research); MIRECO: Wonju, Korea, 2016; pp. 1–172. [Google Scholar]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Effect of redox potential and pH on arsenic speciation and solubility in contaminated soil. Environ. Sci. Technol. 1991, 25, 1414–1419. [Google Scholar] [CrossRef]
- Sadiq, M. Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Water Air Soil Pollut. 1997, 93, 117–136. [Google Scholar] [CrossRef]
- Koo, N.; Kim, M.S.; Hyun, S.H.; Kim, J.G. Effects of the incorporation of phosphorus and iron into arsenic-spiked artificial soils on root growth of lettuce using response surface methodology. Commun. Soil Sci. Plant Anal. 2013, 44, 1259–1271. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.Y.; Park, H.; Yun, J.; Kim, J.G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 2011, 161, 1–7. [Google Scholar] [CrossRef]
- Sadiq, M.; Zaidi, T.H.; Mian, A.A. Environmental behavior of arsenic in soils: Theoretical. Water Air Soil Pollut. 1983, 20, 369–377. [Google Scholar] [CrossRef]
- Kim, M.S.; Min, H.G.; Lee, S.H.; Kim, J.G. The effects of various amendments on trace element stabilization in acidic, neutral and alkali soil with similar pollution index. PLoS ONE 2016, 11, e0166335. [Google Scholar] [CrossRef]
- Kim, M.S.; Min, H.G.; Koo, N.; Park, J.S.; Lee, S.H.; Bak, G.I.; Kim, J.G. The effectiveness of spent coffee grounds and its biochar on the amelioration of heavy metals-contaminated water and soil using chemical and biological assessment. J. Environ. Manag. 2014, 146, 130. [Google Scholar] [CrossRef]
- Bowell, R.J. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 1994, 126, 157–167. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 961–1010. ISBN 9780891188254. [Google Scholar]
- NIAST. Method of SOIL and Plant Analysis; National Institute of Agricultural Science and Technology, Rural Development Administration: Suwon, Korea, 2000. [Google Scholar]
- ISO. Soil Quality-Extraction of Trace Elements Soluble in Aqua Regia; ISO 11466; International Organization of Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Narwal, R.P.; Singh, B.R.; Salbu, B. Association of Cd, Zn, Cu, and Ni with components in naturally heavy metal rich soils studied by parallel and sequential extractions. Commun. Soil Sci. Plant Anal. 1999, 30, 1209–1230. [Google Scholar] [CrossRef]
- Lee, S.H.; Ji, W.H.; Lee, W.S.; Koo, N.; Koh, I.H.; Kim, M.S.; Park, J.S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sofianska, E.; Michailidis, K. Chemical assessment and fractionation of some heavy metals and arsenic in agricultural soils of the mining affected Drama plain, Macedonia, northern Greece. Environ. Monit. Assess. 2015, 101. [Google Scholar] [CrossRef]
- Hsu, W.M.; His, H.C.; Huang, Y.T.; Liao, C.S.; Hseu, Z.Y. Partitioning of arsenic in soil-crop systems irrigated using groundwater: A case study of rice paddy soils in southwestern Taiwan. Chemosphere 2012, 86, 606–613. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.Y.; Liu, C.; Li, F.; Xu, X.; Wang, Q. Arsenic availability in rice from a mining area: Is amorphous iron oxide bound arsenic a source or sink? Environ. Pollut. 2015, 199, 95–101. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R.; Kondo, T.; Huq, S.M.I.; Kawai, S. Comparison of extractability of Cd, Cu, Pb and Zn with sequential extraction in contaminated and non-contaminated soils. Int. J. Environ. Sci. Technol. 2007, 4, 169–176. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 14, 1409–1416. [Google Scholar] [CrossRef]
- Lopes, C.; Herva, M.; Franco-Uría, A.; Roca, E. Multicorrelation models and uptake factors to estimate extractable metal concentrations from soil and metal in plants in pasturelands fertilized with manure. Environ. Pollut. 2012, 166, 17–22. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.W.; Lee, J.U.; Lee, J.S.; Cook, J. Assessment of as and heavy metal contamination in the vicinity of duckum Au-Ag mine, Korea. Environ. Geochem. Health 2002, 24, 213–225. [Google Scholar] [CrossRef]
- USEPA. Synthetic Precipitation Leaching Procedure (SPLP); Method 1312; EPA: Washington, DC, USA, 1994. [Google Scholar]
- Shu, W.S.; Ye, Z.H.; Lan, C.Y.; Zhang, Z.Q.; Wong, M.H. Acidification of lead/zinc mine tailings and its effect on heavy metal mobility. Environ. Int. 2001, 26, 389–394. [Google Scholar] [CrossRef]
- Eriksson, N.; Destouni, G. Combined effects of dissolution kinetics, secondary mineral precipitation, and preferential flow on copper leaching from mining waste rock. Water Resour. Res. 1997, 33, 471–483. [Google Scholar] [CrossRef]
- Pellegrini, S.; Garcia, G.; Penas-Castejon, J.M.; Vignozzi, N.; Costantini, E.A.C. Pedogenesis in mine tails affects macroporosity, hydrological properties, and pollutant flow. CATENA 2016, 136, 3–16. [Google Scholar] [CrossRef]
- Anderson, M.; Ferguson, J.; Gavis, J. Arsenate adsorption on amorphous aluminium hydroxide. J. Colloid Interface Sci. 1976, 54, 391–399. [Google Scholar] [CrossRef]
- Pierce, M.L.; Moore, C.B. Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res. 1982, 16, 1247–1253. [Google Scholar] [CrossRef]
- Ladeira, A.C.Q.; Ciminelli, V.S.T. Adsorption and desorption of arsenic on an oxisol and its constituents. Water Res. 2004, 38, 2087–2094. [Google Scholar] [CrossRef]
- Kim, J.Y.; Davis, A.P.; Kim, K.W. Stabilization of available arsenic in highly contaminated mine tailing using iron. Environ. Sci. Technol. 2003, 37, 189–195. [Google Scholar] [CrossRef]
- DeSisto, S.L.; Jamieson, H.E.; Parsons, M.B. Arsenic mobility in weathered gold mine tailings under a low-organic soil cover. Environ. Earth Sci. 2017, 76, 773. [Google Scholar] [CrossRef]
- Beiyuan, J.; Li, J.S.; Tsang, D.C.W.; Wang, L.; Poon, C.S.; Li, X.D.; Fendorf, S. Fate of arsenic before and after chemical enhanced washing of an arsenic containing soil in Hong-Kong. Sci. Total Environ. 2017, 599–600, 679–688. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Smith, E.; Weber, J.; Naidu, R.; Ress, M.; Rofe, A.; Kuchel, T.; Sansom, L. Effect of soil ageing on in vivo arsenic bioavailability in two dissimilar soils. Chemosphere 2008, 71, 2180–2186. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ma, L.Q. Arsenic uptake by two hyperaccumulator ferns from four arsenic contaminated soils. Water Air Soil Pollut. 2005, 168, 71–89. [Google Scholar] [CrossRef]
- Kim, W.I.; Kim, J.J.; Yoo, J.H.; Kim, J.Y.; Kee, J.H.; Paik, M.K.; Kim, R.Y.; Im, G.J. Arsenic fractionation and bioavailability in paddy soils near closed mines in Korea. Korea J. Soil Sci. Fertil. 2010, 43, 914–922. [Google Scholar]
- Thomas, G.W.; Hargrov, W.L. The chemistry of soil acidity. In Soil Acidity and Liming, 2nd ed.; Adams, F., Ed.; ASA Monogr 12; ASA: Madison, WI, USA, 1984. [Google Scholar]
- Corwin, D.L.; David, A.; Goldberg, S. Mobility of arsenic in soil from the rocky mountain arsenal area. J. Contam. Hydrol. 1999, 39, 35–58. [Google Scholar] [CrossRef]
- Dowdle, P.R.; Laverman, A.M.; Oremland, R.S. Bacterial dissimilatory reduction of arsenic (V) to arsenic (III) in anoxic sediments. Appl. Environ. Microbiol. 1996, 62, 1664–1669. [Google Scholar] [CrossRef] [PubMed]

| Sample Number | pH | LOI a (%) | Sand (%) | Silt (%) | Clay (%) | Total As Concentration (mg kg−1) |
| R1 | 5.0 | 9 | 67 | 20 | 14 | 1449 |
| R2 | 3.7 | 10 | 85 | 3 | 13 | 1639 |
| R3 | 4.9 | 8 | 80 | 5 | 15 | 1673 |
| R4 | 4.3 | 11 | 76 | 13 | 11 | 2239 |
| R5 | 5.7 | 9 | 76 | 14 | 10 | 3944 |
| R6 | 3.2 | 18 | 79 | 9 | 12 | 4142 |
| F1 | 5.6 | 4.9 | 80 | 2 | 18 | 788 |
| F2 | 5.8 | 4.1 | 79 | 6 | 15 | 1716 |
| F3 | 6.2 | 5.7 | 75 | 9 | 16 | 3418 |
| F4 | 6.2 | 5.7 | 73 | 13 | 14 | 4153 |
| F5 | 6.3 | 5.0 | 75 | 9 | 16 | 4614 |
| F6 | 6.6 | 7.7 | 69 | 16 | 15 | 5899 |
| F7 | 6.7 | 7.9 | 66 | 18 | 16 | 6461 |
| F8 | 6.0 | 4.3 | 74 | 13 | 14 | 6791 |
| F9 | 6.0 | 6.0 | 73 | 13 | 14 | 7755 |
| F10 | 7.2 | 4.7 | 59 | 26 | 15 | 7960 |
| F11 | 7.6 | 4.5 | 65 | 21 | 15 | 7871 |
| F12 | 3.8 | 4.0 | 76 | 1 | 23 | 9079 |
| S1 | 7.9 | 3.7 | 88 | 3 | 9 | 143 |
| S2 | 7.7 | 1.6 | 87 | 4 | 9 | 175 |
| S3 | 7.6 | 4.2 | 89 | 2 | 9 | 47 |
| Sample Number | pH | EC b (us cm−1) | DO c (mg L−1) | Temperature (°C) | As Concentration (ug L−1) | |
| W1 | 8.2 | 92 | 13.5 | 16.0 | 5.9 | |
| W2 | 6.9 | 111 | 9.4 | 16.5 | 7.5 | |
| W3 | 7.2 | 97 | 10.2 | 16.8 | 6.4 | |
| Sample Number | Mehlich-3 | 1N HCl | SBET a | SPLP b |
|---|---|---|---|---|
| R1 | 6 c | 132 b | 7 c | 0.9 bc |
| R2 | 2 d | 56 b | 10 bc | 1.1 bc |
| R3 | 2 d | 81 b | 2 c | 0.6 c |
| R4 | 2 d | 49 b | 4 c | 2.0 a |
| R5 | 32 a | 657 a | 26 ab | 1.7 ab |
| R6 | 27 b | 764 a | 38 a | 1.6 ab |
| F1 | 24 h | 251 e | 30 h | 1.7 g |
| F2 | 103 g | 64 e | 106 h | 9.7 f |
| F3 | 214 f | 1302 d | 236 g | 26.6 e |
| F4 | 277 e | 1637 d | 383 f | 36.3 d |
| F5 | 289 e | 1719 d | 352 f | 40.1 cd |
| F6 | 352 cd | 2870 c | 500 de | 58.5 b |
| F7 | 358 cd | 2664 c | 534 de | 60.7 b |
| F8 | 413 b | 2849 c | 474 e | 40.8 cd |
| F9 | 378 bc | 3053 c | 556 cd | 44.2 c |
| F10 | 523 a | 3527 b | 1376 a | 69.1 a |
| F11 | 382 bc | 3687 b | 962 b | 62.2 b |
| F12 | 329 d | 4630 a | 612 c | 2.0 g |
| R Soil | Mehlich-3 | 1N HCl | SBET a | SPLP b |
|---|---|---|---|---|
| Total As | 0.940 * | 0.957 * | 0.922 * | 0.630 |
| fr 1 | 0.063 | −0.083 | −0.100 | −0.372 |
| fr 2 | 0.971 * | 0.940 * | 0.859 * | 0.288 |
| fr 3 | 0.826 * | 0.841 * | 0.783 | 0.137 |
| F Soil | Mehlich-3 | 1N HCl | SBET | SPLP |
| Total As | 0.890 ** | 0.980 ** | 0.786 * | 0.523 |
| fr 1 | 0.090 | −0.212 | 0.183 | 0.597 * |
| fr 2 | −0.562 | −0.238 | −0.181 | −0.435 |
| fr 3 | 0.687 * | 0.684 * | 0.632 * | 0.395 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Lee, S.-H.; Kim, J.-G. Assessment of Fraction and Mobility of Arsenic in Soil Near the Mine Waste Dam. Sustainability 2020, 12, 1480. https://doi.org/10.3390/su12041480
Kim M-S, Lee S-H, Kim J-G. Assessment of Fraction and Mobility of Arsenic in Soil Near the Mine Waste Dam. Sustainability. 2020; 12(4):1480. https://doi.org/10.3390/su12041480
Chicago/Turabian StyleKim, Min-Suk, Sang-Hwan Lee, and Jeong-Gyu Kim. 2020. "Assessment of Fraction and Mobility of Arsenic in Soil Near the Mine Waste Dam" Sustainability 12, no. 4: 1480. https://doi.org/10.3390/su12041480
APA StyleKim, M.-S., Lee, S.-H., & Kim, J.-G. (2020). Assessment of Fraction and Mobility of Arsenic in Soil Near the Mine Waste Dam. Sustainability, 12(4), 1480. https://doi.org/10.3390/su12041480





