Nitrogen Pulse and Competition Affects Nitrogen Metabolism in Invasive Weed (Amaranthus retroflexus) and Native Crop (Glycine max)
Abstract
1. Introduction
2. Materials and Methods
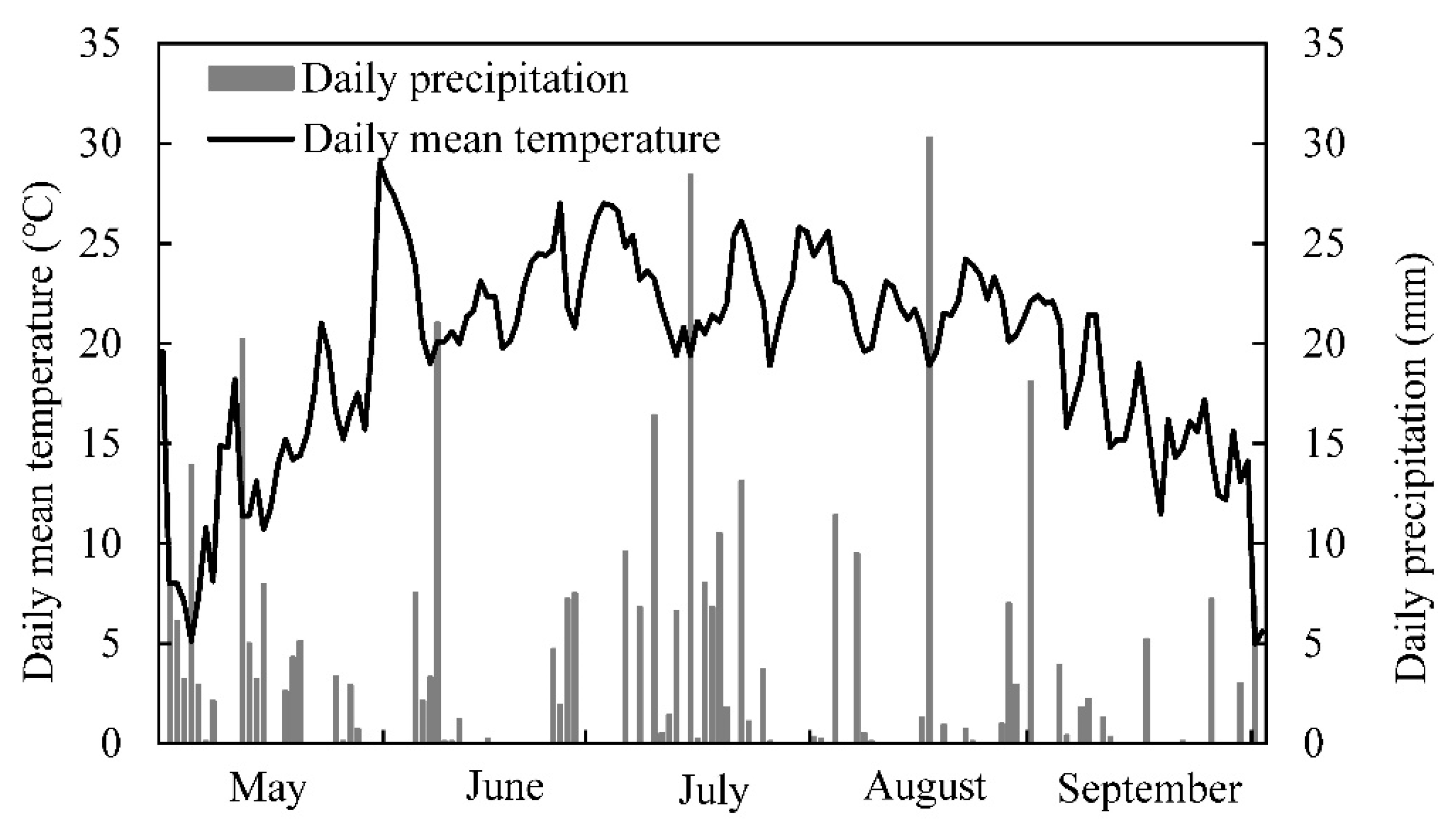
2.1. Experimental Design
2.2. Plant Sampling
2.3. Determination of Nitrate and Ammonium Contents
2.4. NR Extraction and Activity Assays
2.5. GS Extraction and Activity Assays
2.6. GDH Extraction and Activity Assays
2.7. Measurement of Height
2.8. Statistical Analysis
3. Results
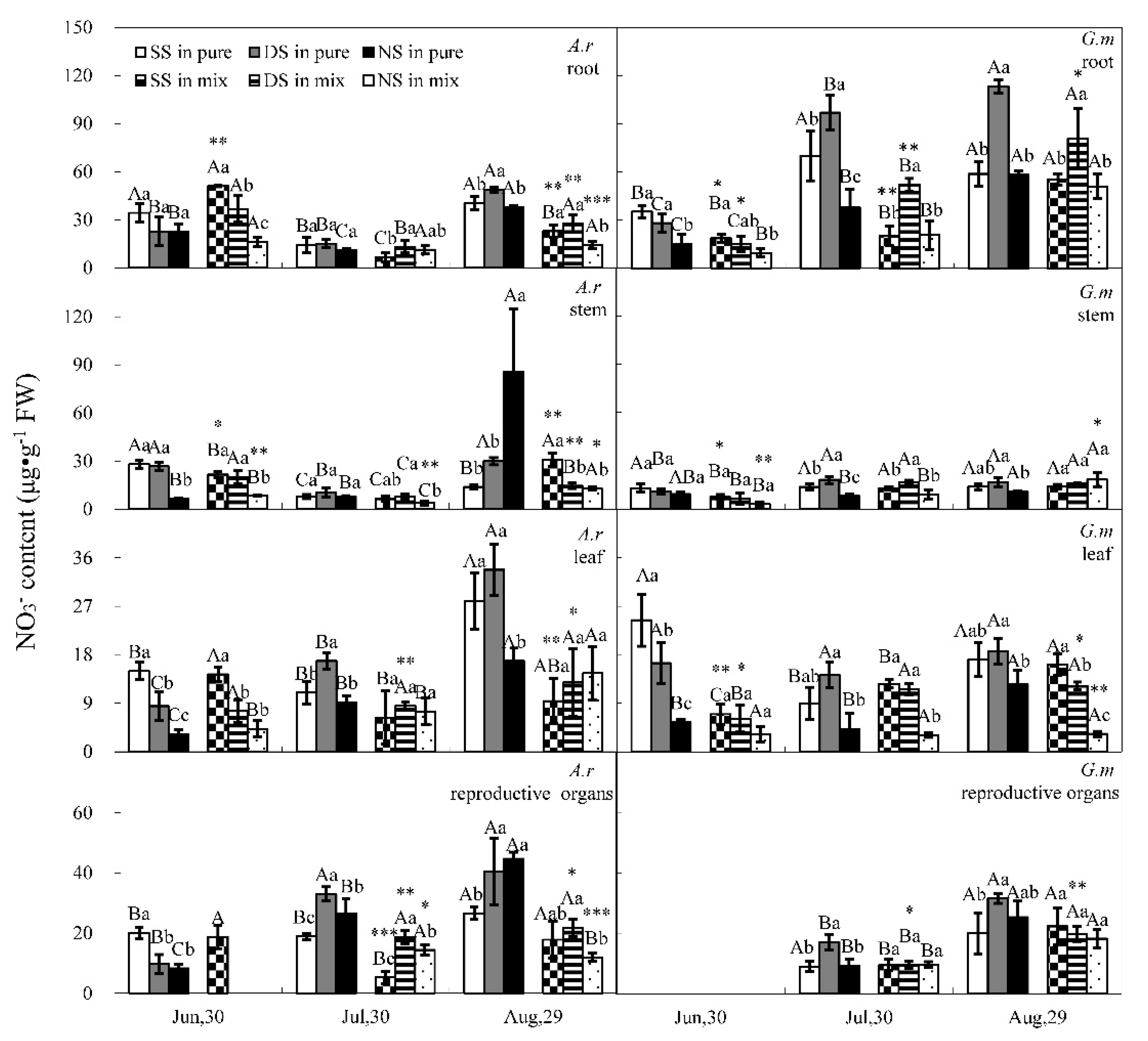
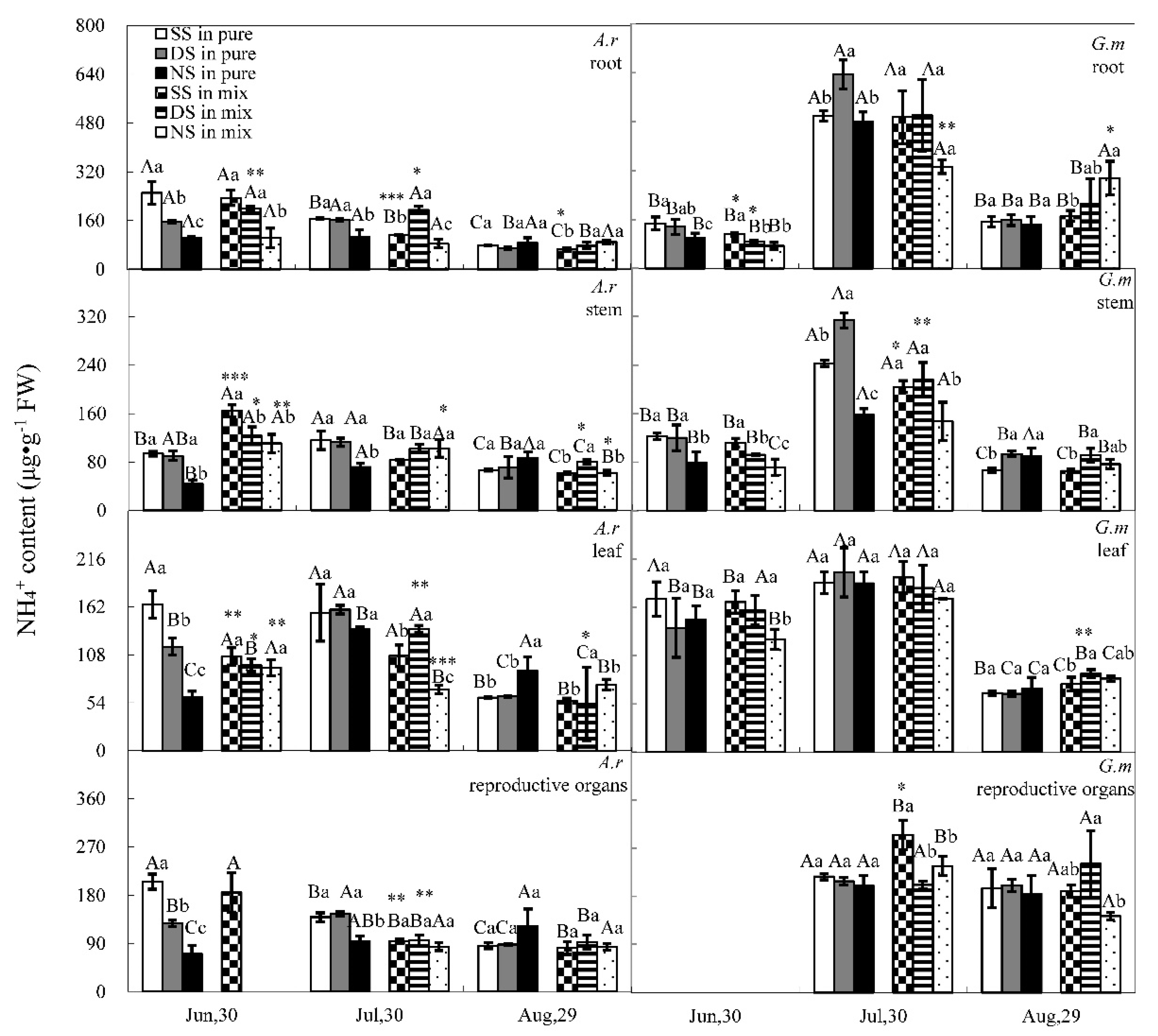
3.1. Nitrate and Ammonium Contents
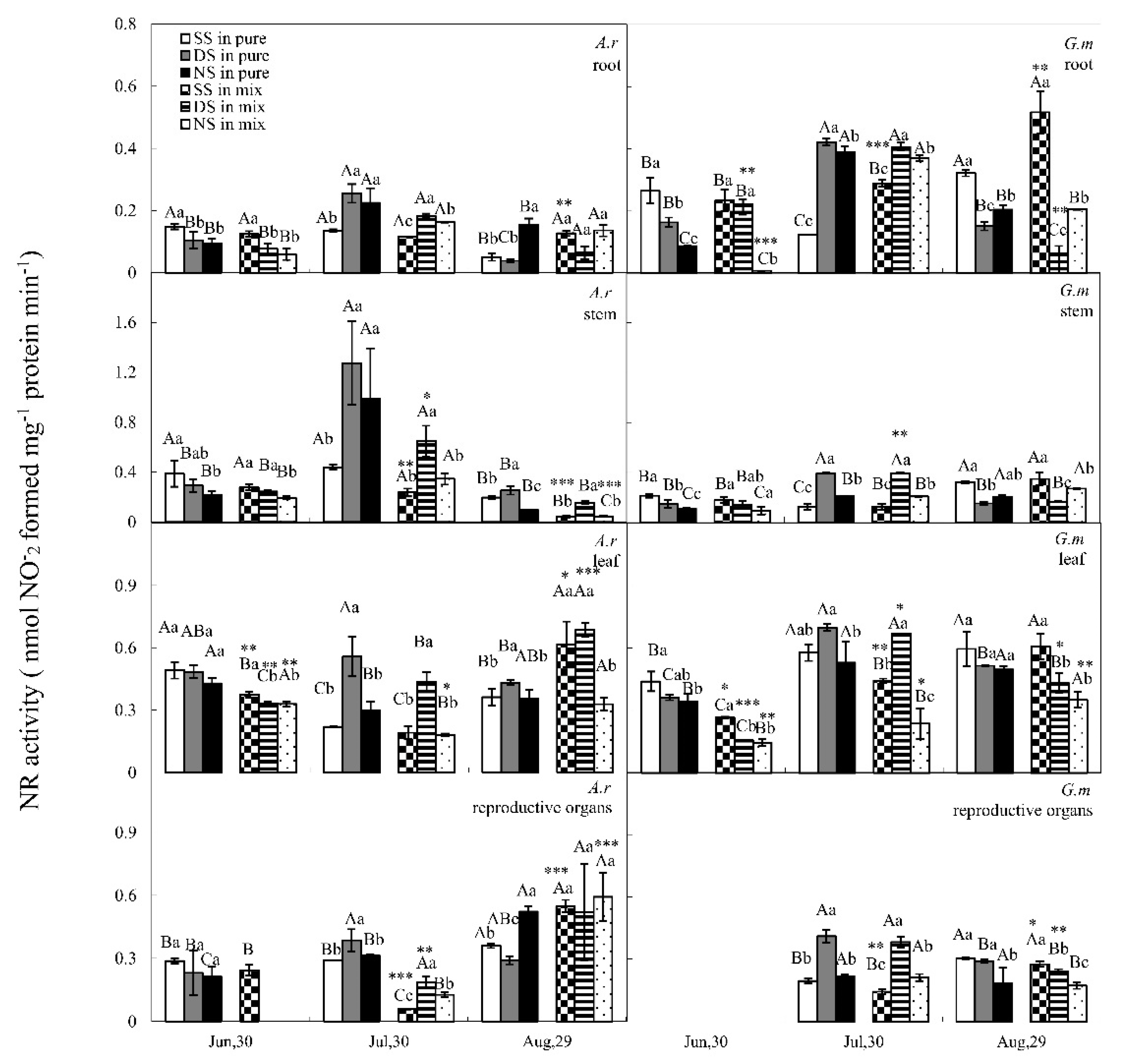
3.2. NR Activity
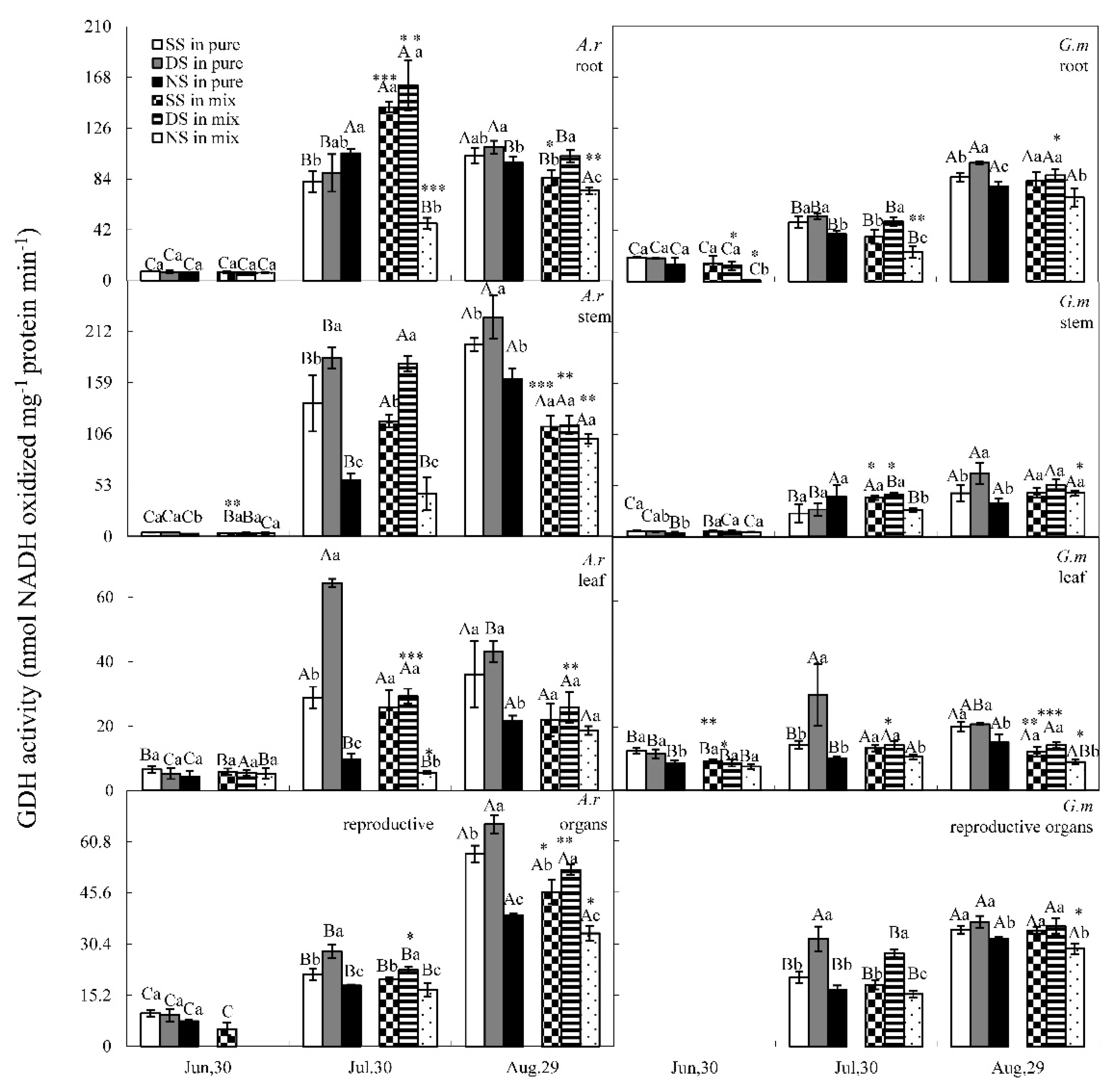
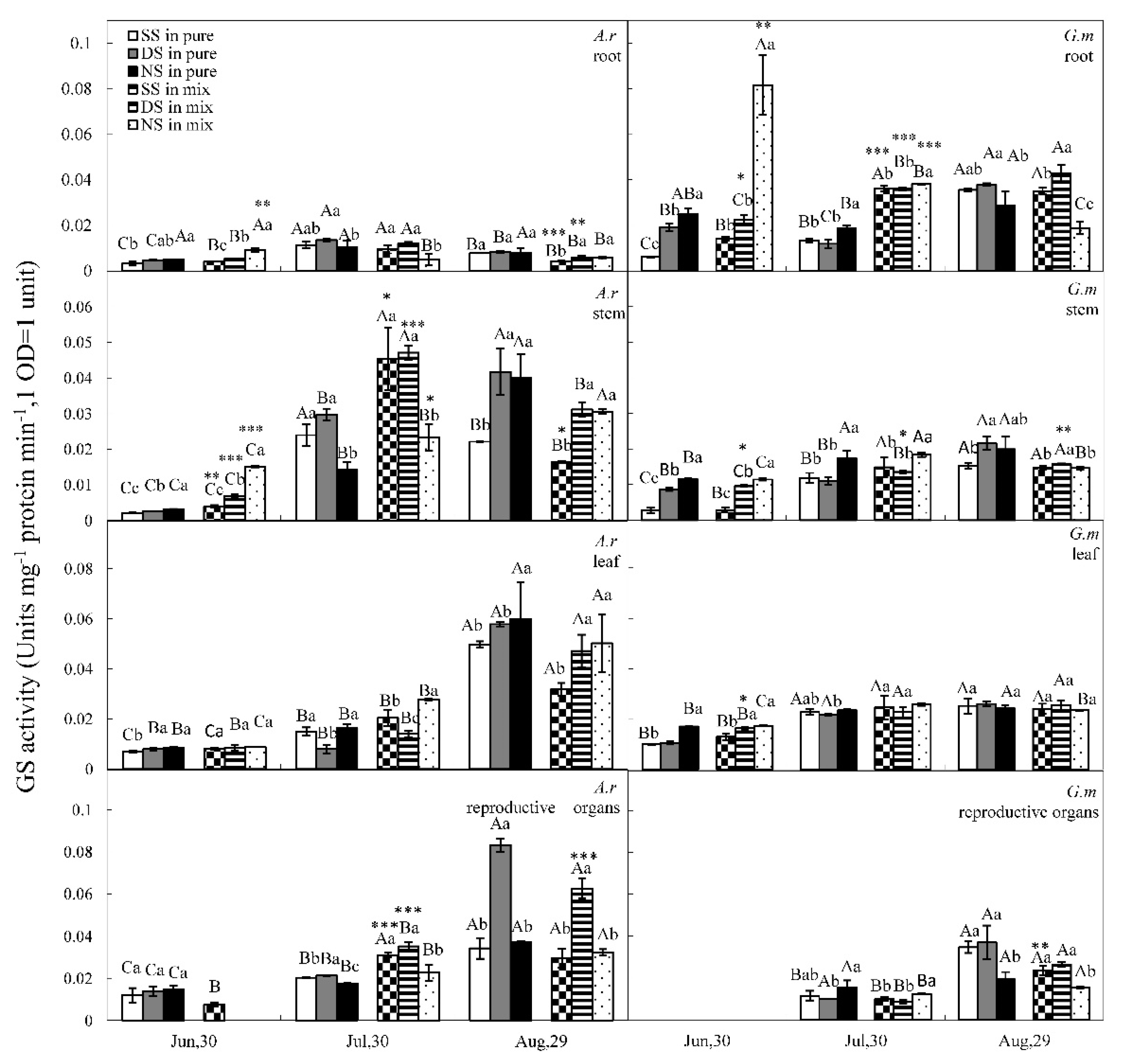
3.3. GS and GDH Activities
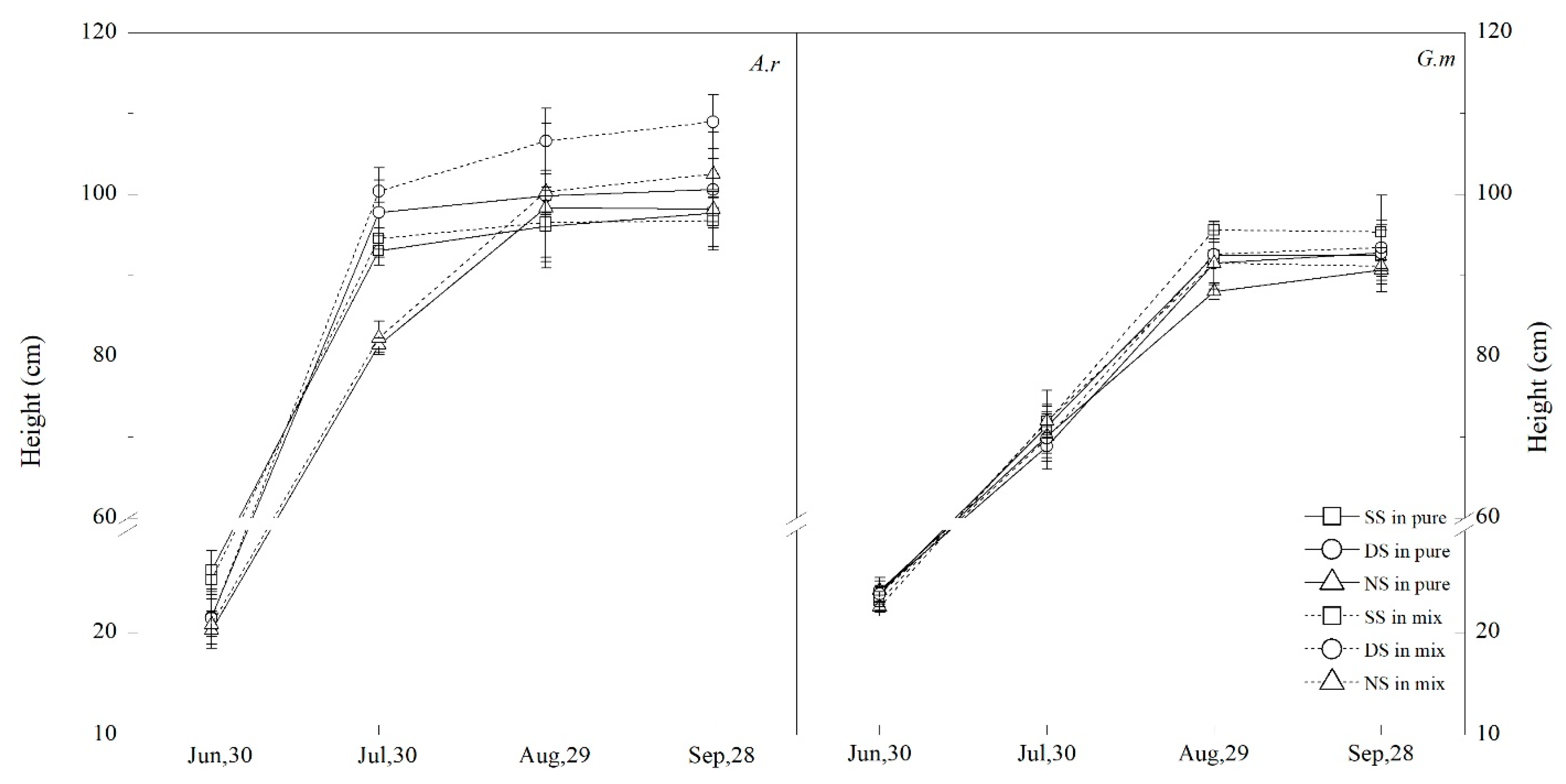
3.4. Plant Height
4. Discussion
4.1. N Pulse
4.2. Competition
Author Contributions
Funding
Conflicts of Interest
References
- Yang, L.H.; Naeem, S. The ecology of resource pulses. Ecology 2008, 89, 619–620. [Google Scholar] [CrossRef]
- Yang, L.H.; Edwards, K.F.; Byrnes, J.E.; Bastow, J.L.; Wright, A.N.; Spence, K.O. A meta-analysis of resource pulse-consumer interactions. Ecol. Monogr. 2010, 80, 125–151. [Google Scholar] [CrossRef]
- Cui, M.Y.; Caldwell, M. A large ephemeral release of nitrogen upon wetting of dry soil and corresponding root responses in the field. Plant Soil 1997, 191, 291–299. [Google Scholar] [CrossRef]
- Jankju-Borzelabad, M.; Griffiths, H. Competition for pulsed resources: An experimental study of establishment and coexistence for an arid-land grass. Oecologia 2006, 148, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Bastow, J.L.; Spence, K.O.; Wright, A.N. What can we learn from resource pulses? Ecology 2008, 89, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; Juliano, S.A. Concurrent effects of resource pulse amount, type, and frequency on community and population properties of consumers in detritus-based systems. Oecologia 2012, 169, 511–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, M.J.; Brown, M.L. Effects of resource pulse magnitude on nutrient availability, productivity, stability, and food web dynamics in experimental aquatic ecosystems. Hydrobiologia 2018, 814, 191–203. [Google Scholar] [CrossRef]
- Miller, T.E.; Buhler, M.L.; Cuellar-Gempeler, C. Species-specific differences determine responses to a resource pulse and predation. Oecologia 2019, 190, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Ding, H.; Li, M.Y.; Qiang, S.; Guo, J.Y.; Han, Z.M.; Huang, Z.G.; Sun, H.Y.; He, S.P.; Wu, H.R.; et al. The distribution and economic losses of alien species invasion to China. Biol. Invasions 2006, 8, 1495–1500. [Google Scholar] [CrossRef]
- Clements, D.R.; DiTommaso, A.; Jordan, N.; Barbara, D.B.; Cardina, J.; Doohan, D.; Mohler, C.L.; Murphy, S.D.; Swanton, C.J. Adaptability of plants invading North American cropland. Agric. Ecosyst. Environ. 2004, 104, 379–398. [Google Scholar] [CrossRef]
- Lu, Z.J.; Ma, K.P. Scale dependent relationships between native plant diversity and the invasion of crofton weed (Eupatorium adenophorum) in southwest China. Weed Sci. 2005, 53, 600–604. [Google Scholar] [CrossRef]
- Sang, W.G.; Zhu, L.; Axmacher, J.C. Invasion pattern of Eupatorium adenophorum Spreng in Southern China. Biol. Invasions 2010, 12, 1721–1730. [Google Scholar] [CrossRef]
- Zhang, F.J.; Guo, J.Y.; Chen, F.X.; Liu, W.X.; Wan, F.H. Identification of volatile compounds released by leaves of the invasive plant croftonweed (Ageratina adenophora, Compositae), and their inhibition of rice seedling growth. Weed Sci. 2012, 60, 205–211. [Google Scholar] [CrossRef]
- Qin, R.M.; Zheng, Y.L.; Valiente-Banuet, A.; Callaway, R.M.; Barclay, G.F.; Pereyra, C.S.; Feng, Y.L. The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytol. 2013, 197, 979–988. [Google Scholar] [CrossRef]
- Lu, P.; Li, J.X.; Jin, C.G.; Jiang, B.W.; Bai, Y.M. Different growth responses of an invasive weed and a native crop to nitrogen pulse and competition. PLoS ONE 2016, 11, e0156285. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Melbourne, B.A.; Cornell, H.V.; Davies, K.F.; Dugaw, C.J.; Elmendorf, S.; Freestone, A.L.; Hall, R.J.; Harrison, S.; Hastings, A.; Holland, M.; et al. Invasion in a heterogeneous world: Resistance, coexistence or hostile takeover? Ecol. Lett. 2007, 10, 77–94. [Google Scholar] [CrossRef]
- Parepa, M.; Fischer, M.; Bossdorf, O. Environmental variability promotes plant invasion. Nat. Commun. 2013, 4, 1604. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Sun, Y.; Heinz, M.S.; Yan, R.; Zhou, Z.X.; Wang, Y.J.; Yu, F.H. Do invasive alien plants differ from non-invasives in dominance and nitrogen uptake in response to variation of abiotic and biotic environments under global anthropogenic change? Sci. Total Environ. 2019, 672, 634–642. [Google Scholar] [CrossRef]
- Patterson, D.T. Effects of environmental stress on weed/crop interactions. Weed Sci. 1995, 43, 483–490. [Google Scholar] [CrossRef]
- Debouba, M.; Gouia, H.; Suzuki, A.; Ghorbela, M.H. NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedlings. J. Plant Physiol. 2006, 163, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, G.; Schuhmacher, M.I.; Krstić, B.; Rehder, H.; Ziegler, H. Biomass production and nitrate metabolism of Atriplex hortensis L.(C3 plant) and Amaranthus retroflexus L. (C4 plant) in cultures at different levels of nitrogen supply. Oecologia 1987, 72, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; SkŁodowska, M. Nickel-induced changes in nitrogen metabolism in wheat shoots. J. Plant Physiol. 2009, 166, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S. (Ed.) Mordern Plant Physiology; Higher Education Press: Beijing, China, 2002; pp. 219–220. (In Chinese) [Google Scholar]
- Chaffei, C.; Gouia, H.; Ghorbel, M.H. Nitrogen metabolism in tomato plants under cadmium stress. J. Plant Nutr. 2003, 26, 1617–1634. [Google Scholar]
- Li, X.J.; Zhang, H.J.; Ni, H. Review on the biological characters and control of redroot pigweed (Amaranthus retroflexus). Pestic. Sci. Adm. 2004, 25, 13–16. (In Chinese) [Google Scholar]
- Weber, E.; Sun, S.G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef]
- Nagata, T. Studies on the differentiation of soybeans in the world, with special regard to that in Southeast Asia.2. Origin of culture and paths of dissemination of soybeans, as considered by the distributions of their summer versus autumn soybean habit and plant habit. Crop Sci. Soc. Jpn. 1959, 28, 79–82. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- O’Neal, D.; Joy, K.D. Glutamine synthetase of pea leaves: Purification, stabilization and pH optima. Arch. Biochem. Biophys. 1973, 159, 113–122. [Google Scholar] [CrossRef]
- Groat, R.G.; Vance, C.P. Root nodule enzyme of ammonia assimilation in alfalfa (Medicago sativa L.). Developmental patterns and response to applied nitrogen. Plant Physiol. 1981, 67, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, F.A.; Garbutt, K.; Reekie, E.R.; Williama, W.E. Using growth analysis to interpret competition between a C3 and a C4 annual under ambient and elevated CO2. Oecologia 1989, 79, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, R.E.; Brandt, R.N.; Janzen, H.H.; Entz, T.; Grant, C.A.; Derksen, D.A. Differential response of weed species to added nitrogen. Weed Sci. 2003, 51, 532–539. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Brandt, R.N. Nitrogen fertilizer rate effects on weed competitiveness is species dependent. Weed Sci. 2008, 56, 743–747. [Google Scholar] [CrossRef]
- Di, W.; Jin, X.J.; Ma, C.M.; Gong, Z.P.; Dong, S.K.; Zhang, L. Effects of nitrogen application on yield and nitrogen accumulation in soybean. J. Nucl. Sci. Technol. 2010, 24, 612–617. (In Chinese) [Google Scholar]
- Wang, S.Q.; Han, X.Z.; Qiao, Y.F.; Yan, J.; Li, X.H. Soybean (Glycine max L.) growth and nitrogen fixation as affected by different N supplying modes. Soybean Sci. 2009, 5, 859–862. (In Chinese) [Google Scholar]
- Nelson-Schreiber, B.M.; Schweitzer, L.E. Limitations on leaf nitrate reductase activity during flowering and podfill in soybean. Plant Physiol. 1986, 80, 454–458. [Google Scholar] [CrossRef]
- Geisseler, D.; Doane, T.A.; Horwath, W.R. Determining potential glutamine synthetase and glutamate dehydrogenase activity in soil. Soil Biol. Biochem. 2009, 41, 1741–1749. [Google Scholar] [CrossRef]
- Helling, R.E. Pathway choice in glutamate synthesis in Escherichia coli. J. Bacteriol. 1998, 180, 4571–4573. [Google Scholar] [CrossRef]
- Baars, J.J.; Op den Camp, J.M.H.; van der Drift, C.; Van Griensven, L.J.L.D.; Vogels, G.D. Regulation of nitrogen-metabolizing enzymes in the commercial mushroom Agaricus bisporus. Curr. Microbiol. 1995, 31, 345–350. [Google Scholar] [CrossRef]
- Lu, B.B.; Yuan, Y.Z.; Zhang, C.F.; Ou, J.Q.; Zhou, W.; Lin, Q.H. Modulation of key enzymes involved in ammonium assimilation and carbon metabolism by low temperature in rice (Oryza sativa L.) roots. Plant Sci. 2005, 169, 295–302. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ. Exp. Bot. 2011, 71, 399–408. [Google Scholar] [CrossRef]
- Gan, Y.B.; Chen, J.; Qiu, Z.M.; Stulen, I. Effects of N fertilizer application at different growth stages on N uptake and N-fixation of soybeans. Chin. J. Oil Crop Sci. 1996, 18, 45–47. (In Chinese) [Google Scholar]
- Huang, J.Z. Effects of photoperiod on the phenological development of redroot pigweed (Amaranthus retroflexus L.). Can. J. Plant Sci. 2000, 80, 929–938. [Google Scholar] [CrossRef]
- Rajcan, I.; AghaAlikhani, M.; Swanton, C.J.; Tollenaar, M. Development of redroot pigweed is influenced by light spectral quality and quantity. Crop Sci. 2002, 42, 1930–1936. [Google Scholar] [CrossRef]
- Zhou, D.W.; Wang, T.H.; Valentine, I. Phenotypic plasticity of life-history characters in response to different germination timing in two annual weeds. Can. J. Bot. 2005, 83, 28–36. [Google Scholar] [CrossRef]
- Jannink, J.L.; Jordan, N.R.; Orf, J.H. Feasibility of selection for high weed suppressive ability in soybean: Absence of tradeoffs between rapid initial growth and sustained later growth. Euphytica 2001, 120, 291–300. [Google Scholar] [CrossRef]
- Shurtleff, J.L.; Coble, H.D. The interations of soybean (Glycine max) and five weed species in the greenhouse. Weed Sci. 1985, 33, 669–679. [Google Scholar] [CrossRef]
- Légère, A.; Schreiber, M.M. Competition and canopy architecture as affected by soybean (Glycine max) row width and density of redroot pigweed (Amaranthus retroflexus). Weed Sci. 1989, 37, 84–92. [Google Scholar] [CrossRef]
- Tian, Y.H.; Liu, Y.Y.; Zhang, W.Z.; Luo, S.G. Effect of N fertilization at different stage on nitrogenase activity and yield of soybean. J. Northeast Agric. Univ. 2008, 39, 15–19. (In Chinese) [Google Scholar]
| Nitrate Content a | Ammonium Content a | |||||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | RO | Root | Stem | Leaf | RO | |
| A. retroflexus | ||||||||
| N | *** | *** | *** | * | *** | *** | *** | *** |
| C | *** | *** | *** | *** | ns | *** | *** | *** |
| S | *** | *** | *** | *** | *** | *** | *** | ** |
| N × S | *** | *** | *** | *** | ns | *** | *** | *** |
| C × N | * | *** | *** | *** | *** | ns | ** | ** |
| S × C | *** | ns | *** | *** | *** | *** | *** | *** |
| S × N × C | ** | *** | * | ** | ns | *** | *** | *** |
| G. max | ||||||||
| N | *** | *** | *** | * | * | *** | ns | * |
| C | *** | ns | *** | ** | ns | *** | ns | ns |
| S | *** | *** | *** | *** | *** | *** | *** | *** |
| N × S | *** | *** | * | ns | *** | *** | ns | *** |
| C × N | ** | ns | ns | ** | ns | ** | ns | ns |
| S × C | *** | *** | *** | * | *** | *** | ns | * |
| S × N × C | * | ns | *** | * | * | ** | ns | ** |
| NR a | GS a | GDH a | Height | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | RO | Root | Stem | Leaf | RO | Root | Stem | Leaf | RO | ||
| A. retroflexus | |||||||||||||
| N | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| C | ** | *** | ns | ns | ** | *** | ** | *** | ns | *** | *** | *** | ** |
| S | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| N × S | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| C × N | *** | ns | *** | ns | ns | ns | ns | *** | *** | ns | *** | *** | * |
| S × C | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns |
| S × N × C | ns | ns | *** | *** | * | ns | ns | *** | *** | * | *** | * | ns |
| G. max | |||||||||||||
| N | *** | * | *** | *** | *** | *** | ** | *** | *** | *** | *** | *** | * |
| C | ** | ns | *** | ** | *** | ns | ** | *** | *** | ns | *** | ** | ns |
| S | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| N × S | *** | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | *** | ns |
| C × N | *** | ns | *** | ns | *** | ns | ns | ns | ns | ns | ** | ns | ns |
| S × C | *** | * | *** | ns | *** | *** | ** | *** | ns | ns | ns | * | ns |
| S × N × C | *** | ns | ** | ns | *** | ns | ns | ns | ns | *** | *** | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Zhou, X.; Lu, P.; Li, Q.; Yang, H.; Feike, T.; Zhang, L.; Guan, J.; Zhao, W.; Liu, H. Nitrogen Pulse and Competition Affects Nitrogen Metabolism in Invasive Weed (Amaranthus retroflexus) and Native Crop (Glycine max). Sustainability 2020, 12, 772. https://doi.org/10.3390/su12030772
Jiang B, Zhou X, Lu P, Li Q, Yang H, Feike T, Zhang L, Guan J, Zhao W, Liu H. Nitrogen Pulse and Competition Affects Nitrogen Metabolism in Invasive Weed (Amaranthus retroflexus) and Native Crop (Glycine max). Sustainability. 2020; 12(3):772. https://doi.org/10.3390/su12030772
Chicago/Turabian StyleJiang, Baiwen, Xue Zhou, Ping Lu, Qi Li, Haonan Yang, Til Feike, Luyang Zhang, Jiunian Guan, Wei Zhao, and Haiying Liu. 2020. "Nitrogen Pulse and Competition Affects Nitrogen Metabolism in Invasive Weed (Amaranthus retroflexus) and Native Crop (Glycine max)" Sustainability 12, no. 3: 772. https://doi.org/10.3390/su12030772
APA StyleJiang, B., Zhou, X., Lu, P., Li, Q., Yang, H., Feike, T., Zhang, L., Guan, J., Zhao, W., & Liu, H. (2020). Nitrogen Pulse and Competition Affects Nitrogen Metabolism in Invasive Weed (Amaranthus retroflexus) and Native Crop (Glycine max). Sustainability, 12(3), 772. https://doi.org/10.3390/su12030772




