Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Methods
2.2.1. Biochar Preparation
2.2.2. Characterizations of Seaweed-Based Biochar
2.3. Batch Adsorption Experiment
2.4. Isotherm and Kinetic Analysis
3. Results and Discussion
3.1. Ultimate and Proximate Analysis
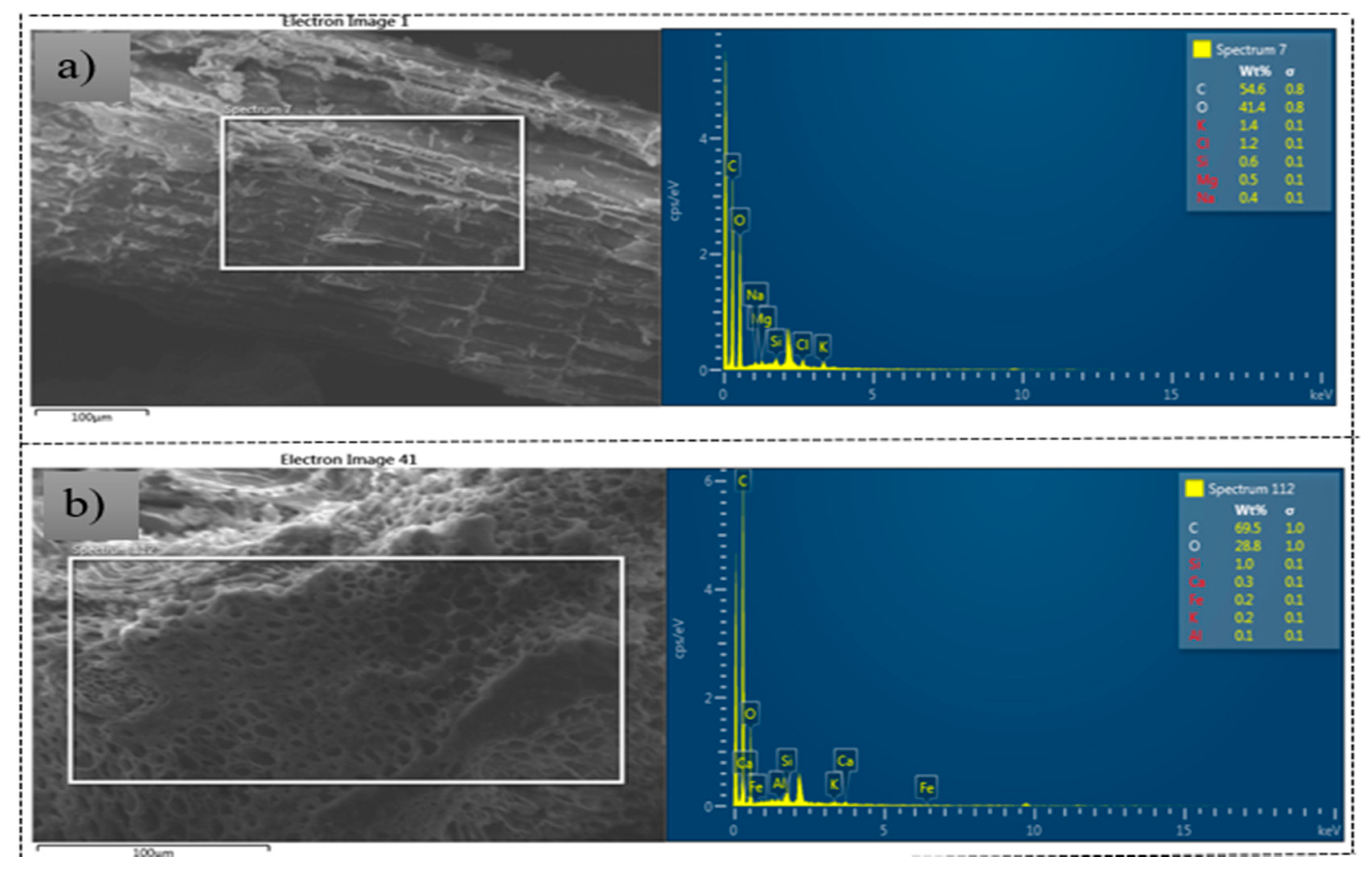
3.2. Microstructure Analysis of the Seaweed-Based Biochar
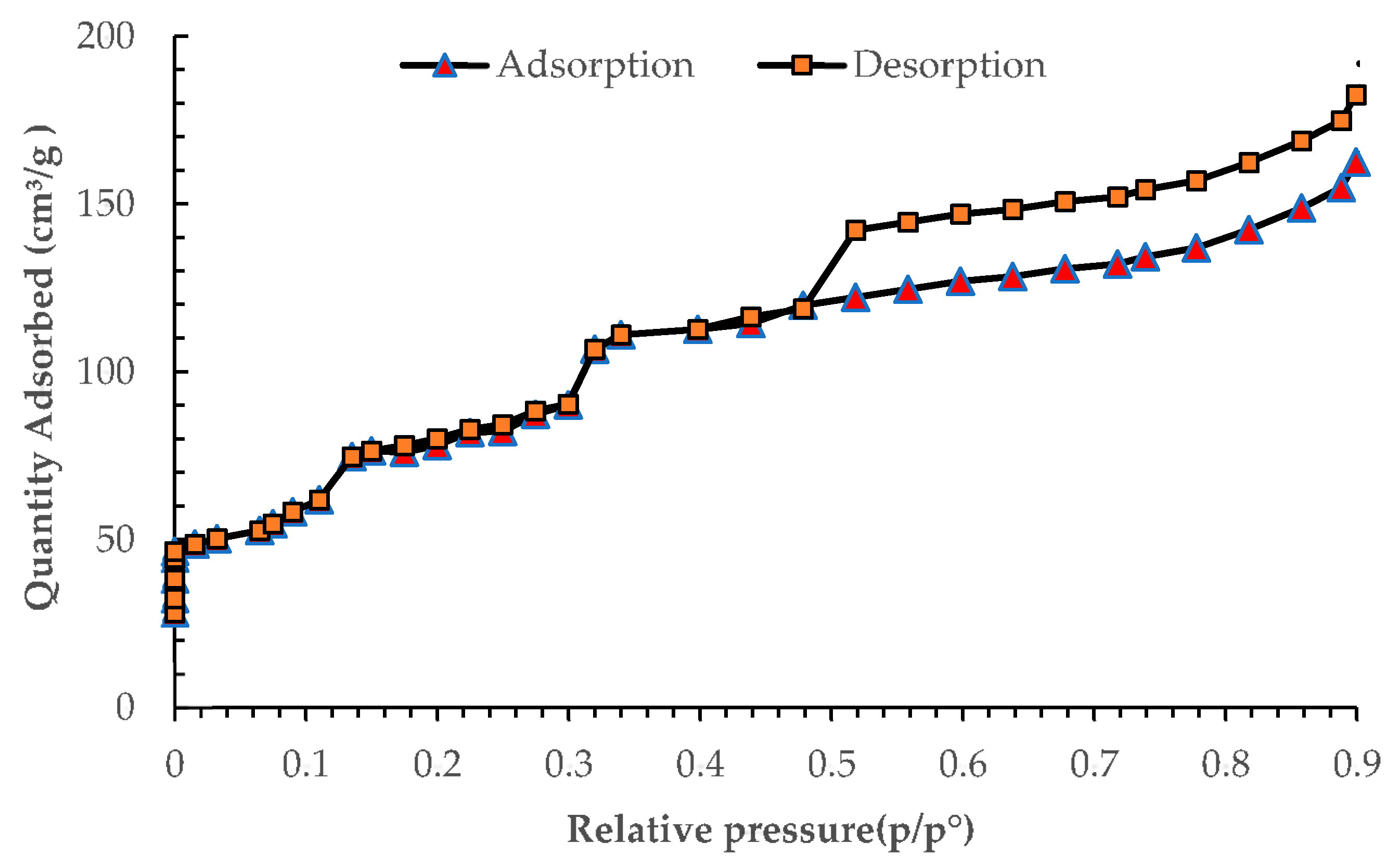
3.3. BET Surface Area and Pore Size Analysis of the Seaweed-Based Biochar
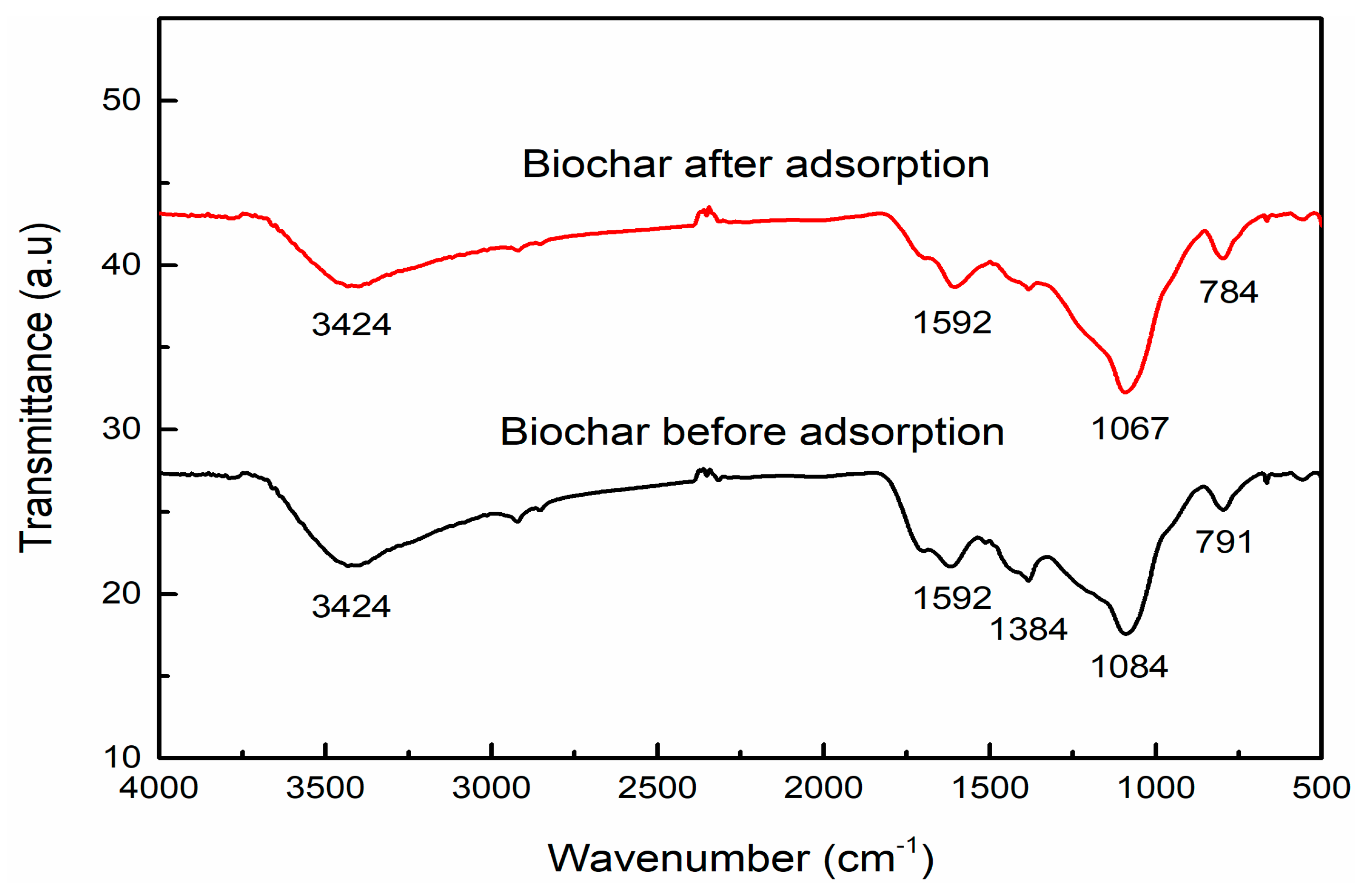
3.4. Functional Group Analysis of the Seaweed-Based Biochar
3.5. Adsorption Studies of MB Using Seaweed-Based Biochar
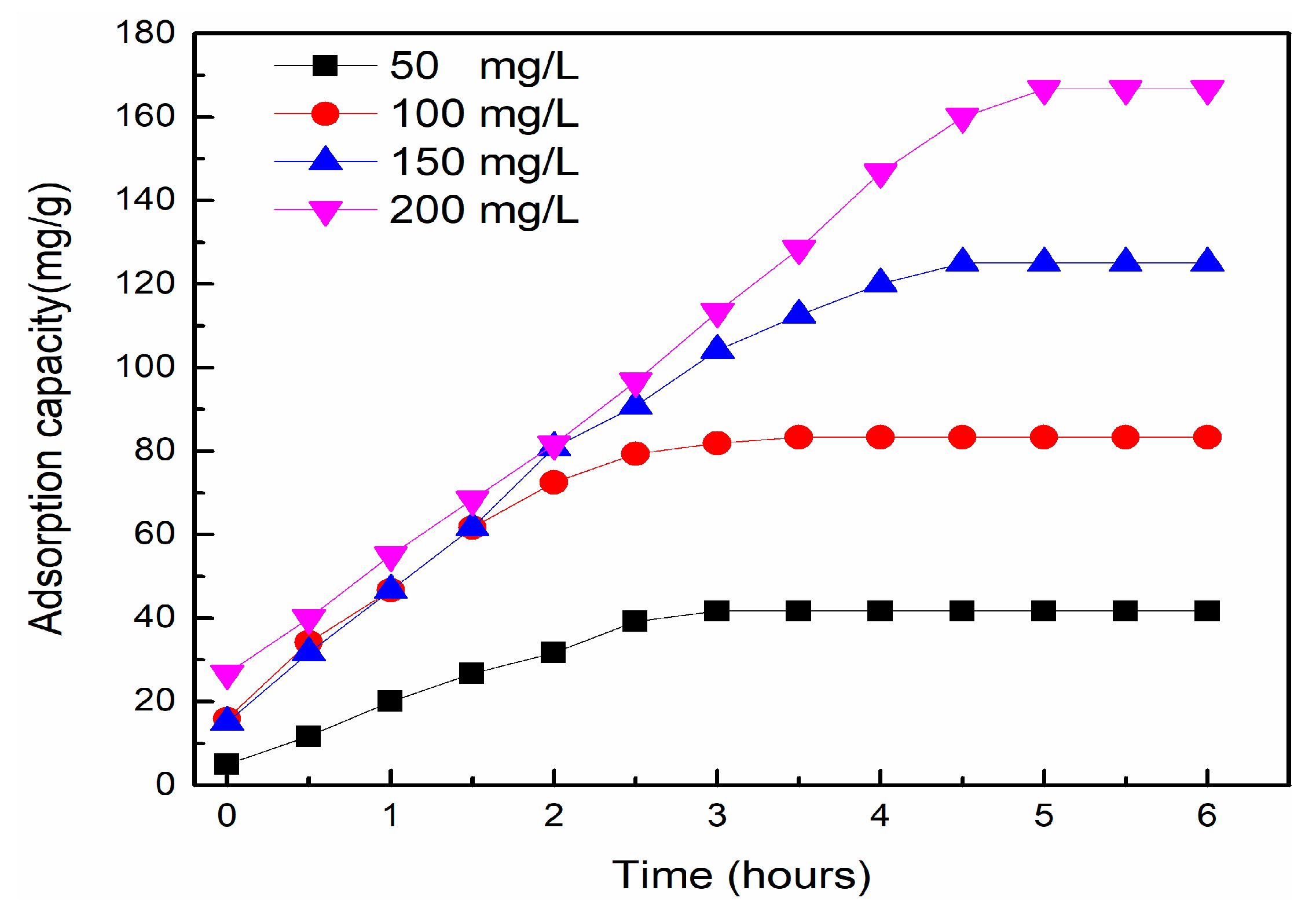
3.5.1. Influence of Initial MB Concentrations
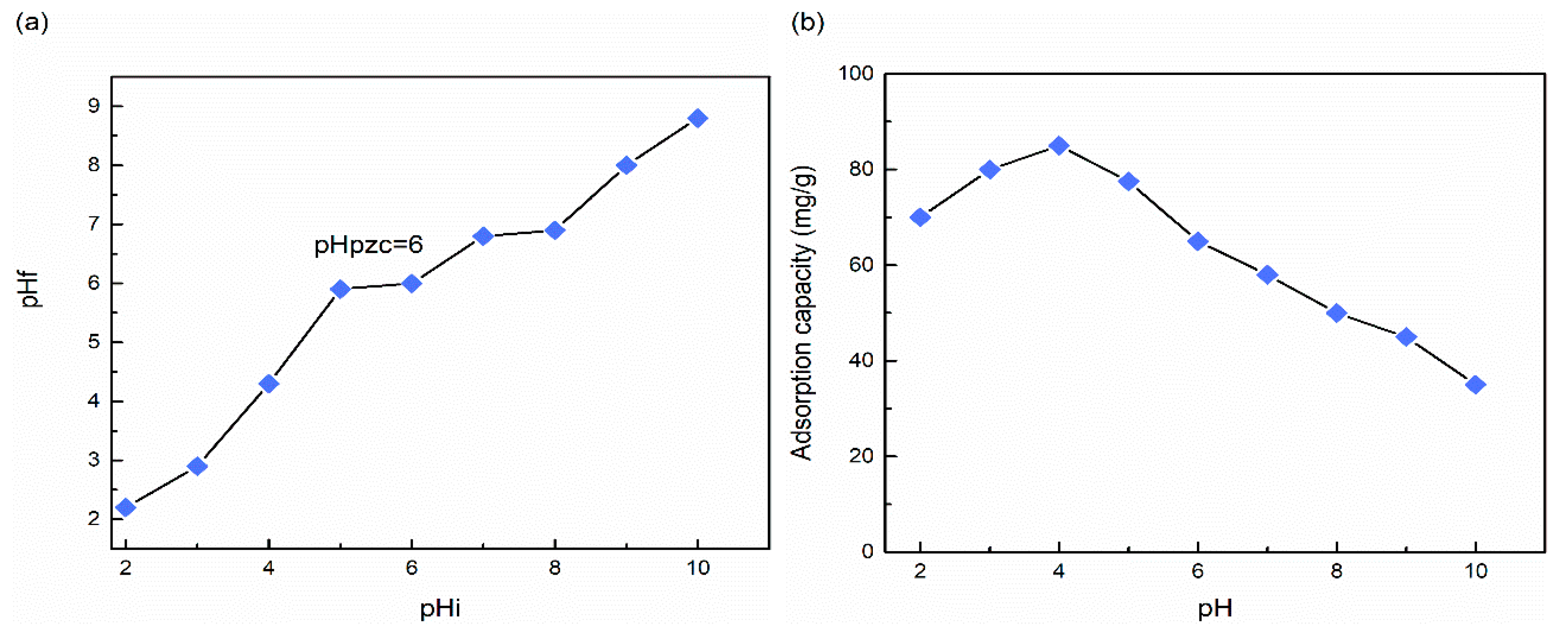
3.5.2. Influence of Solution pH
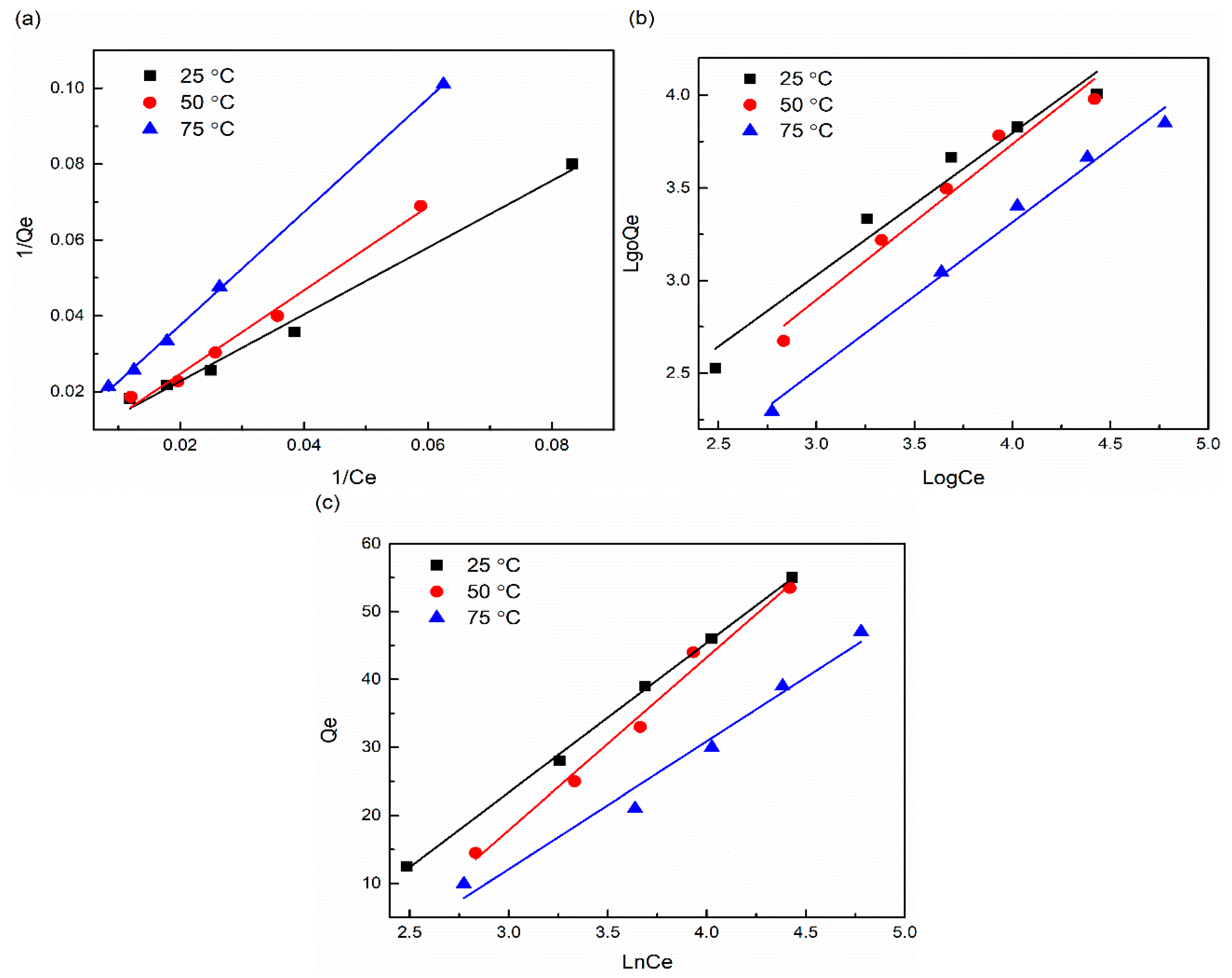
3.6. Adsorption Isotherm Analysis
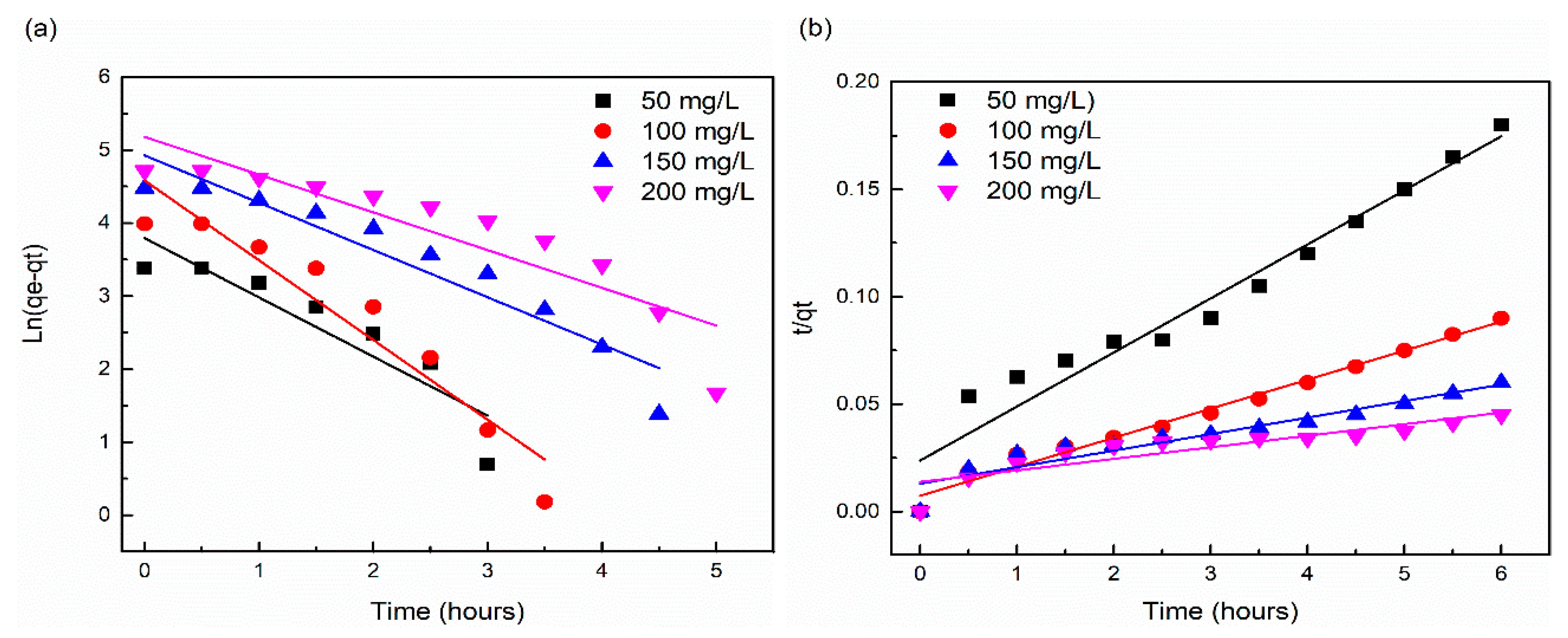
3.7. Adsorption Kinetic Analysis
3.8. Performance Assessment of Biochar Compared to Literature-Based Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Banerjee, S.; Gautam, R.K.; Jaiswal, A.; Chattopadhyaya, M.C.; Sharma, Y.C. Rapid scavenging of methylene blue dye from a liquid phase by adsorption on alumina nanoparticles. RSC Adv. 2015, 5, 14425–14440. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Choi, S.-P.; Thiruvenkatachari, R.; Shim, W.-G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigment. 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, O.; Holmes, S.M. Novel and modified materials for wastewater treatment applications. J. Mater. Chem. 2008, 18, 2751–2761. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Patnam, V.R.; Sikdar, A.; Moulik, S.K. Removal of Some Common Textile Dyes from Aqueous Solution Using Fly Ash. J. Chem. Eng. Data 2010, 55, 5653–5657. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Zhu, Z. Geopolymeric adsorbents from fly ash for dye removal from aqueous solution. J. Colloid Interface Sci. 2006, 300, 52–59. [Google Scholar] [CrossRef]
- Marković, S.; Stanković, A.; Lopicic, Z.R.; Lazarević, S.; Stojanović, M.; Uskokovic, D.P. Application of raw peach shell particles for removal of methylene blue. J. Environ. Chem. Eng. 2015, 3, 716–724. [Google Scholar] [CrossRef]
- Khan, M.I.; Min, T.K.; Azizli, K.A.; Sufian, S.; Ullah, H.; Man, Z. Effective removal of methylene blue from water using phosphoric acid based geopolymers: Synthesis, characterizations and adsorption studies. RSC Adv. 2015, 5, 61410–61420. [Google Scholar] [CrossRef]
- Huong, P.T.; JiTae, K.; Altahtamouni, T.M.; Tri, N.L.M.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. J. Water Process. Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xie, G.; Hu, X.; Cai, X.; Zhao, Y.; Hu, X.; Jin, Q.; Fu, X.; Tan, X.; Liang, C.; et al. Coupling of kenaf Biochar and Magnetic BiFeO3 onto Cross-Linked Chitosan for Enhancing Separation Performance and Cr(VI) Ions Removal Efficiency. Int. J. Environ. Res. Public Health 2020, 17, 788. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.-L.; Zhang, J.; Zheng, L.; Chen, D.; Wu, Z.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Wang, H.; et al. Coconut-fiber biochar reduced the bioavailability of lead but increased its translocation rate in rice plants: Elucidation of immobilization mechanisms and significance of iron plaque barrier on roots using spectroscopic techniques. J. Hazard. Mater. 2020, 389, 122117. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magnetized Tectona grandis sawdust as a novel adsorbent: Preparation, characterization, and utilization for the removal of methylene blue from aqueous solution. Cellulose 2020, 27, 2613–2635. [Google Scholar] [CrossRef]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef]
- Guo, H.; Bi, C.; Zeng, C.; Ma, W.; Yan, L.; Li, K.; Wei, K. Camellia oleifera seed shell carbon as an efficient renewable bio-adsorbent for the adsorption removal of hexavalent chromium and methylene blue from aqueous solution. J. Mol. Liq. 2018, 249, 629–636. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Modified durian seed as adsorbent for the removal of methyl red dye from aqueous solutions. Appl. Water Sci. 2014, 5, 407–423. [Google Scholar] [CrossRef]
- Ding, Z.; Wan, Y.; Xin, H.; Wang, S.; Zimmerman, A.R.; Gao, B. Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: Importance of physicochemical properties. J. Ind. Eng. Chem. 2016, 37, 261–267. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Jalali, R.; Ghafourian, H.; Asef, Y.; Davarpanah, S.; Sepehr, S. Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater. 2002, 92, 253–262. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.; Muñoz, J. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- Ibrahim, W.M. Biosorption of heavy metal ions from aqueous solution by red macroalgae. J. Hazard. Mater. 2011, 192, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Isam, M.; Baloo, L.; Kutty, S.R.M.; Yavari, S. Optimisation and Modelling of Pb (II) and Cu (II) Biosorption onto Red Algae (Gracilaria changii) by Using Response Surface Methodology. Water 2019, 11, 2325. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sustain. Energy Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Zulfani, N. Heavy Metals Capture from Water Sludge by Kenaf Fibre Activated Carbon in Batch Adsorption. J. Ecol. Eng. 2020, 21, 102–115. [Google Scholar] [CrossRef]
- Chung, I.K.; Oak, J.H.; Lee, J.A.; Shin, J.A.; Kim, J.G.; Park, K.-S. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013, 70, 1038–1044. [Google Scholar] [CrossRef]
- Hou, X.; From, N.; Angelidaki, I.; Huijgen, W.J.; Bjerre, A.-B. Butanol fermentation of the brown seaweed Laminaria digitata by Clostridium beijerinckii DSM-6422. Bioresour. Technol. 2017, 238, 16–21. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Li, J.; Li, M.; Lu, C. Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous Mesoporous Mater. 2017, 243, 291–300. [Google Scholar] [CrossRef]
- Harun, N.Y.; Han, T.J.; Vijayakumar, T.; Saeed, A.; Afzal, M. Ash Deposition Characteristics of Industrial Biomass Waste and Agricultural Residues. Mater. Today Proc. 2019, 19, 1712–1721. [Google Scholar] [CrossRef]
- Rathinam, A.; Rao, J.R.; Nair, B.U. Adsorption of phenol onto activated carbon from seaweed: Determination of the optimal experimental parameters using factorial design. J. Taiwan Inst. Chem. Eng. 2011, 42, 952–956. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Nasef, M.M. Physicochemical Characterization of Different Agricultural Residues in Malaysia for Bio Char Production. Int. J. Civ. Eng. Technol. 2019, 10, 213–225. [Google Scholar]
- Zulfiqar, M.; Sufian, S.; Mansor, N.; Rabat, N.E. Synthesis and characterization of TiO2-based nanostructures via fluorine-free solvothermal method for enhancing visible light photocatalytic activity: Experimental and theoretical approach. J. Photochem. Photobiol. A Chem. 2020, 112834. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Rabat, N.E.; Mansor, N. Photocatalytic degradation and adsorption of phenol by solvent-controlled TiO2 nanosheets assisted with H2O2 and FeCl3: Kinetic, isotherm and thermodynamic analysis. J. Mol. Liq. 2020, 308, 112941. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh. Fiz. Chim. 1941, 15, 296–332. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Bin Johan, R. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; IntechOpen: Rijeka, Croatia, 2017; p. 1. [Google Scholar]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, W.; Xu, W.; Liu, Y. Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine. Chem. Eng. J. 2018, 339, 468–478. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; IBI: Philadelphia, PA, USA, 2012. [Google Scholar]
- Rafiq, M.K.; Bachmann, R.T.; Shang, Z.; Joseph, S.; Long, R. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed]
- Yahia, M.B.; Torkia, Y.B.; Knani, S.; Hachicha, M.A.; Khalfaoui, M.; Lamine, A.B. Models for Type VI Adsorption Isotherms from a Statistical Mechanical Formulation. Adsorpt. Sci. Technol. 2013, 31, 341–357. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process. Technol. 2017, 160, 55–63. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, G.; Wei, D.; Yan, L.; Xue, X.; Shi, S.; Wei, Q. Preparation and utilization of anaerobic granular sludge-based biochar for the adsorption of methylene blue from aqueous solutions. J. Mol. Liq. 2014, 198, 334–340. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalination Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M. Adsorption of Congo Red Dye from Aqueous Solutions Using Roots of Eichhornia Crassipes: Kinetic and Equilibrium Studies. Energy Procedia 2014, 50, 862–869. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers 2020, 12, 430. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interfaces 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Chowdhury, S.; Samsudin, M.F.R.; Siyal, A.A.; Omar, A.A.; Ahmad, T.; Sufian, S. Effect of organic solvents on the growth of TiO2 nanotubes: An insight into photocatalytic degradation and adsorption studies. J. Water Process. Eng. 2020, 37, 101491. [Google Scholar] [CrossRef]
- Ahmed, M.; Okoye, P.; Hummadi, E.; Hameed, B. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (Gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğılı, H.; Sayğılı, G.A.; Koyuncu, F.; Yılmaz, C. Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J. Clean. Prod. 2017, 144, 260–265. [Google Scholar] [CrossRef]
| Models | Equilibrium | Linearized | Ref. |
|---|---|---|---|
| Langmuir | [36] | ||
| Freundlich | [37] | ||
| Temkin | [38] | ||
| Pseudo-first order | [39] | ||
| Pseudo-second order | [40] |
| Prepared Samples | Proximate Analysis, wt.% | Ultimate Analysis, wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile | Fixed Carbon | C | H | N | S | O | H/C | O/C | |
| PECS | 7.32 ± 0.02 | 22.50 ± 0.60 | 53.60 ± 1.3 | 16.58 ± 0.30 | 48.60 ± 0.60 | 6.94 ± 0.15 | 1.42 ± 0.08 | 0.76 ± 0.01 | 42.28 ± 1.0 | 0.14 ± 0.040 | 0.87 ± 0.030 |
| BC550 | 1.18 ± 0.04 | 16.10 ± 0.85 | 18.22 ± 1.1 | 64.5 ± 0.60 | 67.6 ± 0.45 | 5.40 ± 0.08 | 1.76 ± 0.10 | 1.24 ± 0.02 | 26 ± 0.80 | 0.08 ± 0.035 | 0.385 ± 0.014 |
| BC450 | 1.60 ± 0.06 | 19.54 ± 0.75 | 26.40 ± 1.25 | 52.47 ± 0.40 | 58 ± 0.30 | 5.75 ± 10 | 1.81 ± 0.09 | 1.36 ± 0.01 | 34 ± 1.1 | 0.099 ± 0.040 | 0.58 ± 0.014 |
| Models | Temperature (°C) | Constant | R2 | RMSE | |
|---|---|---|---|---|---|
| Langmuir | qm (mg g−1) | KL (L mg−1) | |||
| 25 | 107.32 | 0.22 | 0.990 | 7.77 | |
| 50 | 117.25 | 0.17 | 0.992 | 8.20 | |
| 75 | 133.33 | 0.19 | 0.994 | 8.90 | |
| Freundlich | KF (L mg(1−(1/n))/g) | nf | |||
| 25 | 18.24 | 1.456 | 0.956 | 9.65 | |
| 50 | 38.54 | 1.438 | 0.952 | 15.54 | |
| 75 | 56.43 | 1.428 | 0.954 | 10.45 | |
| Temkin | KT (L mg−1) | B (J mol−1) | |||
| 25 | 0.1144 | 84.50 | 0.945 | 7.10 | |
| 50 | 0.1094 | 88.60 | 0.955 | 6.30 | |
| 75 | 0.1165 | 94.50 | 0.932 | 6.80 | |
| C0 (mg/L) | qexp (mg/g) | Pseudo-First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qcal (mg/g) | K1 (h ½) | R2 | RMSE | qcal (mg/g) | K2 (g/mg.h) | R2 | RMSE | ||
| 50 | 33.33 | 34.22 | 0.341 | 0.988 | 0.64 | 36.55 | 0.0321 | 0.995 | 0.58 |
| 100 | 66.66 | 69.34 | 0.568 | 0.966 | 1.73 | 74.55 | 0.0226 | 0.993 | 1.32 |
| 150 | 98.10 | 105.32 | 3.4 | 0.955 | 3.46 | 112.5 | 0.0131 | 0.987 | 1.91 |
| 200 | 120.67 | 132.43 | 6.4 | 0.940 | 6.92 | 138.16 | 0.0036 | 0.982 | 3.5 |
| Adsorbent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| N-doped microporous biochar | 436 | [16] |
| Sewage-sludge-derived biochar | 29.85 | [20] |
| Magnetized Tectona Grandis sawdust | 172.41 | [15] |
| Activated carbon from cashew nut shells | 476 | [48] |
| Anaerobic-granular-sludge-based biochar (AGS-BC) | 90.91 | [50] |
| Walnut shell powder | 178.9 | [54] |
| Oxidized weed-based biochar (OWC) | 161.29 | [57] |
| Weed-based biochar (WC) | 39.68 | [57] |
| Biochar from pyrolysis of wheat straw | 12.03 | [51] |
| Seaweed biochar | 133.33 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Siyal, A.A.; Zulfiqar, M.; Bilad, M.R.; Vagananthan, A.; Al-Fakih, A.; Ghaleb, A.A.S.; Almahbashi, N. Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye. Sustainability 2020, 12, 10318. https://doi.org/10.3390/su122410318
Saeed AAH, Harun NY, Sufian S, Siyal AA, Zulfiqar M, Bilad MR, Vagananthan A, Al-Fakih A, Ghaleb AAS, Almahbashi N. Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye. Sustainability. 2020; 12(24):10318. https://doi.org/10.3390/su122410318
Chicago/Turabian StyleSaeed, Anwar Ameen Hezam, Noorfidza Yub Harun, Suriati Sufian, Ahmer Ali Siyal, Muhammad Zulfiqar, Muhammad Roil Bilad, Arvind Vagananthan, Amin Al-Fakih, Aiban Abdulhakim Saeed Ghaleb, and Najib Almahbashi. 2020. "Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye" Sustainability 12, no. 24: 10318. https://doi.org/10.3390/su122410318
APA StyleSaeed, A. A. H., Harun, N. Y., Sufian, S., Siyal, A. A., Zulfiqar, M., Bilad, M. R., Vagananthan, A., Al-Fakih, A., Ghaleb, A. A. S., & Almahbashi, N. (2020). Eucheuma cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye. Sustainability, 12(24), 10318. https://doi.org/10.3390/su122410318









