Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations
Abstract
:1. Introduction
| Product | Use | Ref. | |
|---|---|---|---|
| Agar | Food ingredient, fruit preserves, hydrocolloids, clarifying brewing agent, paper industry, and others | [13] | |
| Alginate | Food additive, medical, pharmaceutical, paper, cosmetic and fertilizer industries, textile printing | [1] | |
| Antioxidants | Preservatives in cosmetic, chemical, food, and pharmaceutical industries | [14] | |
| Astaxanthin | Food supplement as food dye additive and antioxidant | [15] | |
| Beta-carotene and carotenoids | Precursor for vitamin A and supplement for vitamin C, food additive as coloring agent, and antioxidant | [16] | |
| Bioenergy and biofuels | Biodiesel, bioethanol, biogas, biohydrogen, biomethane, aviation gas, biobutanol, biosyngas, bio-oil, gasoline, solid fuel, jet fuel | [15] | |
| Biochar | Agricultural and sorbent uses, combustion | [13] | |
| Biorefinery | Various chemicals and biofuels | [14] | |
| Biosorbents | Ion exchange materials that bind strongly heavy metal ions | [1] | |
| Carragen or carrageenan | Pet food, food additive, gels, toothpaste | [1] | |
| Catalysts | Catalytic properties | [14] | |
| Chemicals | Industrial and medicinal uses | [1] | |
| Conditioners | Chemical, cosmetic, and farming industries | [15] | |
| Digester residue | Compost or vermicompost | [13] | |
| Extraction of | hydrocolloids or gums | Food industry, phytocolloids such as agar, alginate, and carrageenan | [14] |
| lipids carbohydrates starch and cellulose | Biogas, biodiesel, gasoline, jet fuel, alcohols, renewable hydrocarbons | ||
| minerals and trace elements | Food supplements, glass production, metallurgy | ||
| proteins | Fertilizers, industrial enzymes, animal/fish feeds, surfactants, bioplastics | ||
| Feed | Animal food | [15] | |
| Fertilizers | N-, P-, and K-rich fertilizers | [1] | |
| Phytosterol | Food supplements | [15] | |
| Pigments | Natural colorants in paper and textile industries | [14] | |
| Production | Cosmetic | Water-binding agents and antioxidants, “skin foods” | [15] |
| Food and drink | Nori, kombu, wakame, cheese, soup, noodles, pasta, wine, tea, others | ||
| Fruit and vegetable preservatives | Food industry | ||
| Glass | Glass industry | ||
| Paper pulp supplements | Paper industry | ||
| Textile | Textile industry | ||
| Therapeutic materials | Pharmaceutical industry | ||
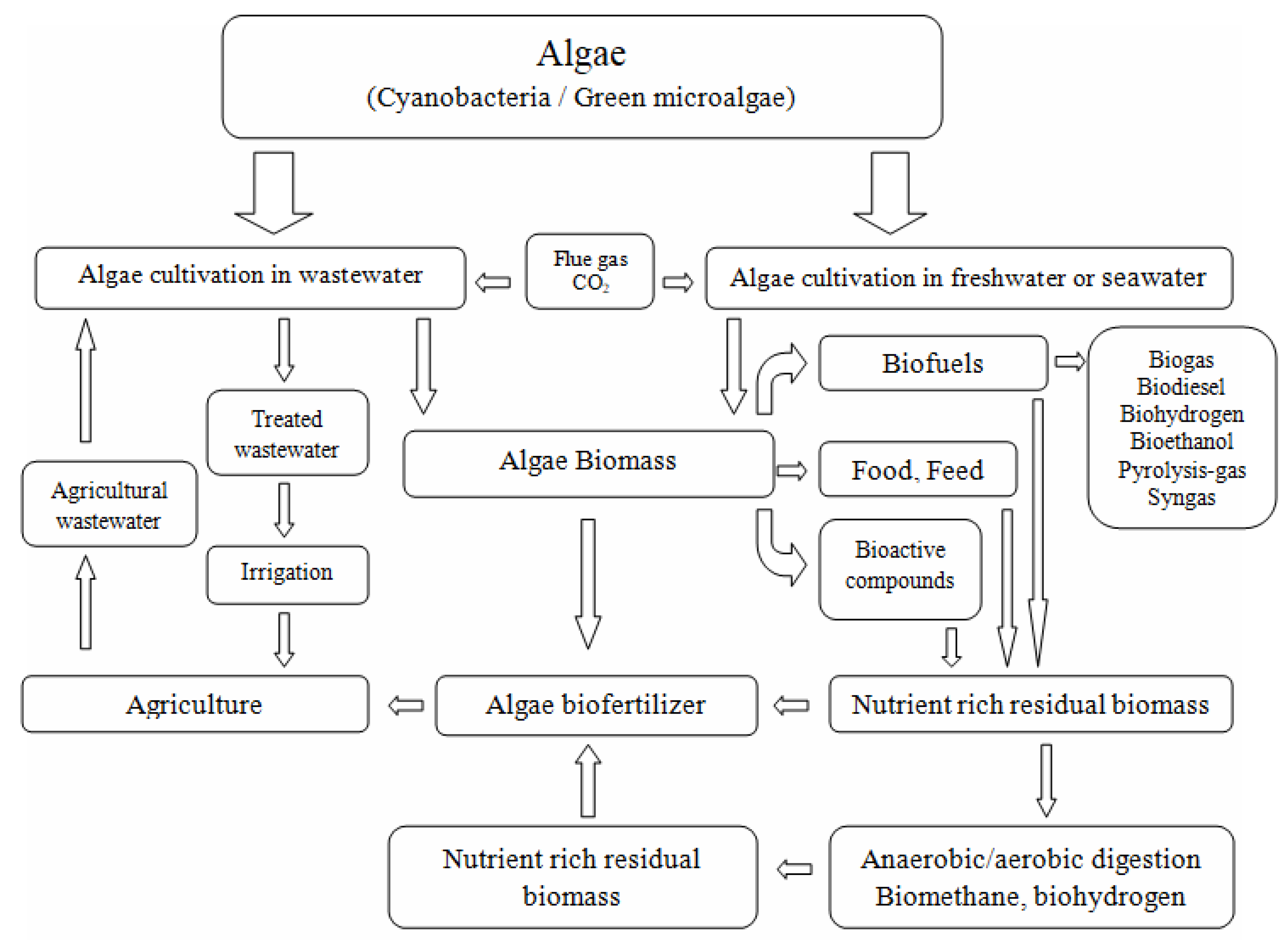
2. Microalgal Biomass as a Source of Biofuels
3. Systems of Microalgae Species Cultivation for Biofuel
4. Strengths and Weaknesses of Different Technologies for Producing and Utilizing Microalgal Biomass
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deviram, G.; Mathimani, T.; Anto, S.; Ahamed, T.S.; Ananth, D.A.; Pugazhendhi, A. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- Kamani, M.H.; Eş, I.; Lorenzo, J.M.; Remize, F.; Roselló-Soto, E.; Barba, F.J.; Clark, J.H.; Khaneghah, A.M. Advances in plant materials, food by-products, and algae conversion into biofuels: Use of environmentally friendly technologies. Green Chem. 2019, 21, 3213–3231. [Google Scholar] [CrossRef]
- Patil, S.; Prakash, G.; Lali, A.M. Reduced chlorophyll antenna mutants of Chlorella saccharophila for higher photosynthetic efficiency and biomass productivity under high light intensities. J. Appl. Phycol. 2020, 32, 1559–1567. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Tolboom, S.N.; Carrillo-Nieves, D.; de Jesús Rostro-Alanis, M.; de la Cruz Quiroz, R.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldivar, R. Algal-based removal strategies for hazardous contaminants from the environment—A review. Sci. Total. Environ. 2019, 665, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, J.R. Chapter 1—Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes, C.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SundarRajan, P.; Gopinath, K.P.; Greetham, D.; Antonysamy, A.J. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J. Clean. Prod. 2019, 210, 445–458. [Google Scholar] [CrossRef]
- Nawaz, T.; Rahman, A.; Pan, S.; Dixon, K.; Petri, B.; Selvaratnam, T. A review of landfill leachate treatment by microalgae: Current status and future directions. Processes 2020, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Biogas Upgrading by Microalgae: Strategies and Future Perspectives. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M., Wang, Z., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A review of algae-based produced water treatment for biomass and biofuel production. Water 2020, 12. 2351. [Google Scholar] [CrossRef]
- Sahu, S.K.; Mantri, V.A.; Zheng, P.; Yao, N. Chapter 1 Algae Biotechnology. Current Status, Potential and Impediments. In Encyclopedia of Marine Biotechnology; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Gomez Villa, H.; Voltolina, D.; Nieves, M.; Pina, P. Biomass production and nutrient budget in outdoor cultures of Scenedesmus obliquus (chlorophyceae) in artificial wastewater, under the winter and summer conditions of Mazatla´ n, Sinaloa, Mexico. Vie Et Milieu 2005, 55, 121–126. [Google Scholar]
- Mùnoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Gorecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Mùnoz, R.; Köllner, C.; Guieysse, B. Biofilm photobioreactors for the treatment of industrial wastewaters. J. Hazard. Mater. 2009, 161, 29–34. [Google Scholar] [CrossRef]
- Yewalkar, S.N.; Dhumal, K.N.; Sainis, J.K. Chromium (VI)-reducing Chlorella spp. isolated from disposal sites of paper-pulp and electroplating industry. J. Appl. Phycol. 2007, 19, 459–465. [Google Scholar]
- Tarlan, E.; Dilek, F.B.; Yetis, U. Effectiveness of algae in the treatment of a wood-based pulp and paper industry wastewater. Bioresour. Technol. 2002, 84, 1–5. [Google Scholar] [CrossRef]
- Acuner, E.; Dilek, F.B. Treatment of tectilon yellow 2G by Chlorella vulgaris. Process. Biochem. 2004, 39, 623–631. [Google Scholar] [CrossRef]
- Essam, T.; Magdy, A.A.; El Tayeb, O.; Mattiasson, B.; Guieysse, B. Solar-based detoxification of phenol and p-nitrophenol by sequential TiO2 photocatalysis and photosynthetically aerated biological treatment. Water Res. 2007, 41, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.A.C.; Raposo, M.F.J.; Castro, P.M.L.; Morais, R.M. Biodegradation of p-chlorophenol by a microalgae consortium. Water Res. 2004, 38, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Valderramaa, L.T.; Del Campoa, C.M.; Rodrigueza, C.M.; de-Bashan, L.E.; Bashan, Y. Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyte Lemna minuscule. Water Res. 2002, 36, 4185–4192. [Google Scholar] [CrossRef]
- Tien, C.J. Biosorption of metal ions by freshwater algae with different surface characteristics. Process. Biochem. 2002, 38, 605–613. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Scoparo, C.H.G.; Queiroz, M.I.; Franco, T.T. Biotransformations of carbon dioxide in photobioreactors. Energy Convers. Manag. 2010, 51, 894–900. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Rahman, M.A. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control. 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Thyagarajan, T.; Puri, M.; Vongsvivut, J.; Barrow, C.J. Evaluation of Bread Crumbs as a Potential Carbon Source for the Growth of Thraustochytrid Species for Oil and Omega-3 Production. Nutrients 2014, 6, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.; Kim, K.; Kim, J.; Han, J.; Yang, J. Use of organic waste from brewery industry for high-density cultivation of docosahexaenoic acid-rich microalga Aurantochytrium sp. KRS101. Bioresour. Technol. 2012, 129, 351–359. [Google Scholar] [CrossRef]
- Unagul, P.; Assantachai, C.; Phadungruengluij, S.; Suphantharika, M.; Tanticharoen, M.; Verduyn, C. Coconut water as a medium additive for the production of docosahexaenoic acid (C22:6 n3) by Schizochytrium mangrovei Sk-02. Bioresour. Technol. 2007, 98, 281–287. [Google Scholar] [CrossRef]
- Hong, W.; Yu, A.; Heo, S.; Oh, B.; Kim, C.; Sohn, J.; Yang, J.W.; Kondo, A.; Seo, J.W. Production of lipids containing high levels of docosahexaenoic acid from empty palm fruit bunches by Aurantiochytrium sp. KRS101. Bioprocess. Biosyst. Eng. 2013, 36, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chan, G.Y.S.; Jiang, B.L.; Lan, C.Y. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Jedynak, P.; Burczyk, J.; Borowski, S.; Kaszycki, P.; Hałat-Łaś, M.; Kędra, M.; Mungunkhuyag, K.; Malec, P. Use of microalgae for treatment of post-fermentation effluent from biogas production. Przemysł Chem. 2018, 97, 2106–2109. [Google Scholar]
- Yan, C.; Zheng, Z. Performance of photoperiod and light intensity on biogas upgrade and biogas effluent nutrient reduction by the microalgae Chlorella sp. Bioresour. Technol. 2013, 139, 292–299. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total. Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. J. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Walsh, M.J.; van Gerber Doren, L.; Sills, D.L.; Archibald, I.; Beal, C.M.; Lei, X.G.; Greene, C.H. Algal food and fuel coproduction can mitigate greenhouse gas emissions while improving land and water-use efficiency. Environ. Res. Lett. 2016, 11, 114006. [Google Scholar] [CrossRef]
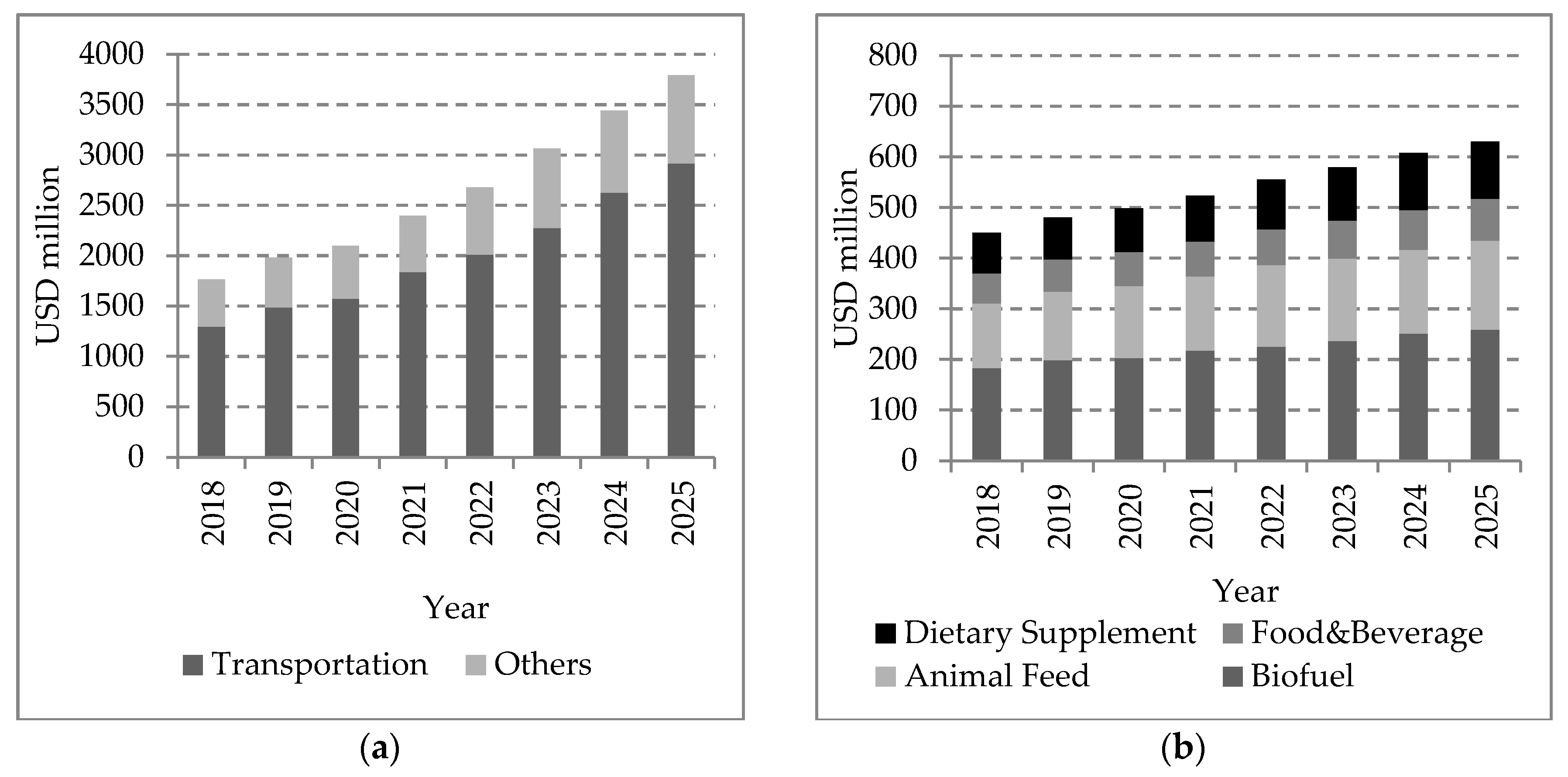
- Market Analysis Report. Algae Biofuel Market Size, Share & Trend Analysis By Application (Transportation, Others), By Region (North America, Europe, Asia Pacific, ROW), By Country, And Segment Forecasts, 2018–2025. Grand View Research. 2017. Available online: https://www.grandviewresearch.com/industry-analysis/algae-biofuel-market (accessed on 4 August 2020).
- Market Analysis Report. Biodiesel Market Analysis by Feedstock [Vegetable Oils (Canola Oil, Soybean Oil, Palm Oil, Corn Oil), Animal Fats (Poultry, Tallow, White Grease)], By Application (Fuel, Power Generation), And Segment Forecasts, 2018–2025. Grand View Research. 2017. Available online: https://www.grandviewresearch.com/industry-analysis/biodiesel-market (accessed on 5 August 2020).
- Fu, W.; Nelson, D.R.; Mystikou, A.; Daakour, S.; Salehi-Ashtiani, K. Advances in microalgal research and engineering development. Curr. Opin. Biotechnol. 2019, 59, 157–164. [Google Scholar] [CrossRef]
- Yadav, G.; Sen, R. Sustainability of Microalgal Biorefinery: Scope, Challenges, and Opportunities. In Sustainable Energy Technology and Policies; Green Energy and, Technology, De, S., Bandyopadhyay, S., Assadi, M., Mukherjee, D., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Webster, A.; Gardner, J. Aligning technology and institutional readiness: The adoption of innovation. Technology Anal. Strateg. Manag. 2019, 31, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, H.I.; Pinto, C.A.; Unal, R.; Keating, C.B.; Britcher, C. Supporting technology selection via portfolio readiness level and technology forecasting. In Proceedings of the International Annual Conference of the American Society for Engineering Management; Huntsville, Philadelphia, PA, USA, 24–26 October 2019. [Google Scholar]
- Bates, C.A.; Clausen, C. Engineering Readiness: How the TRL Figure of Merit Coordinates Technology Development. Eng. Stud. 2020, 12, 9–38. [Google Scholar] [CrossRef]
- Paskuliakova, A.; Tonry, S.; Touzet, N. Phycoremediation of landfill leachate with chlorophytes: Phosphate a limiting factor on ammonia nitrogen removal. Water Res. 2016, 99, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, L.; Zheng, W.; Meng, Q.; Shen, C.; Zhang, G. Oscillating membrane photoreactor combined with salt-tolerated Chlorella pyrenoidosa for landfill leachates treatment. Bioresour. Technol. 2018, 269, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.T.; Senthilnathan, J.; Nagendra, S.M.S. Application of the phycoremediation process for tertiary treatment of landfill leachate and carbon dioxide mitigation. J. Water Process. Eng. 2019, 28, 322–330. [Google Scholar] [CrossRef]
- El Ouaer, M.; Turki, N.; Kallel, A.; Halaoui, M.; Trabelsi, I.; Hassen, A. Recovery of landfill leachate as culture medium for two microalgae: Chlorella sp. and Scenedesmus sp. Environ. Dev. Sustain. 2020, 22, 2651–2671. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Samsudin, S. Biomass production of Chlorella sp., Scenedesmus sp., and Oscillatoria sp. in nitrified landfill leachate. Waste Biomass Valoriz 2017, 8, 2301–2311. [Google Scholar] [CrossRef]
- Reis, C.; Loures, C.; Castro, H.; Rós, P.; Santos, J.; Filho, H.; Silva, M. Microalgae assisted bioremediation of landfill leachate using a biocoil reactor: Evaluation of operational conditions using taguchi experimental design. Br. J. Environ. Clim Chang. 2016, 6, 299–308. [Google Scholar] [CrossRef]
- Pereira, S.F.; Goncalves, A.L.; Moreira, F.C.; Silva, T.F.; Vilar, V.J.; Pires, J.C. Nitrogen removal from landfill leachate by microalgae. Int. J. Mol. Sci. 2016, 17, 1926. [Google Scholar] [CrossRef] [Green Version]
- Casazza, A.A.; Rovatti, M. Reduction of nitrogen content in landfill leachate using microalgae. Desalin. Water Treat 2018, 127, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Quan, X.; Zhong, N.; Zhang, Z.; Lu, C.; Li, G.; Cheng, Z.; Yang, L. High-efficiency nutrients reclamation from landfill leachate by microalgae Chlorella vulgaris in membrane photobioreactor for bio-lipid production. Bioresour. Technol. 2018, 266, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, Z.T.; Övez, S. Growing fresh water microalgae in high ammonium landfill leachate. Am. J. Mech. Appl. 2018, 6, 50–61. [Google Scholar] [CrossRef]
- Richards, R.G.; Mullins, B.J. Using microalgae for combined lipid production and heavy metal removal from leachate. Ecol. Model. 2013, 249, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Dogaris, I.; Loya, B.; Cox, J.; Philippidis, G. Study of landfill leachate as a sustainable source of water and nutrients for algal biofuels and bioproducts using the microalga Picochlorum oculatum in a novel scalable bioreactor. Bioresour. Technol. 2019, 282, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-X.; Tian, G.-M. Preliminary evaluation of a newly isolated microalga Scenedesmus sp. CHX1 for treating landfill leachate. In Third International Conference on Intelligent System Design and Engineering Applications; IEEE: Hong Kong, China, 2013; pp. 1057–1060. [Google Scholar]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae cultivation and harvesting: Growth performance and use of flocculants—A review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Lardon, L.; He´lias, A.; Sialve, B.; Steyer, J.; Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [Green Version]
- Clarens, A.F.; Nassau, H.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Impacts of Algae-Derived Biodiesel and Bioelectricity for Transportation. Environ. Sci. Technol. 2011, 45, 7554–7560. [Google Scholar] [CrossRef]
- Frank, E.D.; Han, J.; Palou-Rivera, I.; Elgowainy, A.; Wang, M.Q. User Manual for algae life-cycle analysis with GREET: Version 0.0; ANL/ESD/11-7; Argonne National Laboratory: Lemont, IL, USA, 2011. [Google Scholar]
- Schultz-Zehden, A.; Matczak, M. (Eds.) SUBMARINER Compendium: An Assessment of Innovative and Sustainable Uses of Baltic Marine Resources; Maritime Institute in Gdansk: Gdansk, Poland, 2012. [Google Scholar]
- ElMekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step toward commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Song, K.; Zhang, P.; Su, Y.; Cheng, J.; Chen, X. Progress of microalgae biofuel’s commercialization. Renew. Sustain. Energy Rev. 2017, 74, 402–411. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant. Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menetrez, M.Y. An overview of algae biofuel production and potential environmental impact. Environ. Sci. Technol. 2012, 46, 7073–7085. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Duong, V.T.; Li, Y.; Nowak, E.; Schenk, P.M. Microalgae Isolation and Selection for Prospective Biodiesel Production. Energies 2012, 5, 1835–1849. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Chu, H.; Wang, Z.; Qi, H. Biofuel production from microalgae: A review. Environ. Chem. Lett. 2020, 18, 285–297. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Otero, M. Comparative Thermogravimetric Assessment on the Combustion of Coal, Microalgae Biomass and Their Blend. Energies 2019, 12, 2962. [Google Scholar] [CrossRef] [Green Version]
- Panahi, H.K.S.; Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Rehan, M.; Nizami, A.-S. Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresour. Technol. Rep. 2019, 7, 100216. [Google Scholar] [CrossRef]
- Feng, R.; Zaidi, A.A.; Zhang, K.; Shi, Y. Optimization of microwave pretreatment for biogas enhancement through anaerobic digestion of microalgal biomass. Period. Polytech. Chem. Eng. 2018, 63, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Córdova, O.; Chamy, R. Chapter 15—Microalgae to Biogas: Microbiological Communities Involved. In Microalgae Cultivation for Biofuels Production; Elsevier: London, UK; San Diego, CA, USA; Oxford, UK, 2020; pp. 227–249. [Google Scholar] [CrossRef]
- Li, F.; Hülsey, M.J.; Yan, N.; Dai, Y.; Wang, C.-H. Co-transesterification of waste cooking oil, algal oil and dimethyl carbonate over sustainable nanoparticle catalysts. Chem. Eng. J. 2020, 405, 127036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Zhang, X.; Han, F.; Tu, W.; Yang, W. Microalgal Hydrogen Production. Small Methods 2020, 4. [Google Scholar] [CrossRef]
- Özçimen, D.; Koçer, A.T.; İnan, B.; Özer, T. Chapter 14—Bioethanol production from microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 373–389. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Larina, O.M.; Sytchev, G.A. Manufacturing gaseous products by pyrolysis of microalgal biomass. Int. J. Hydrog. Energy 2019, 45, 1569–1577. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210. [Google Scholar] [CrossRef]
- Fawzy, M.A. Fatty Acid Characterization and Biodiesel Production by the Marine Microalga Asteromonas gracilis: Statistical Optimization of Medium for Biomass and Lipid Enhancement. Mar. Biotechnol. 2017, 19, 219–231. [Google Scholar] [CrossRef]
- Muto, M.; Nojima, D.; Yue, L.; Kanehara, H.; Naruse, H.; Ujiro, A.; Yoshino, T.; Matsunaga, T.; Tanaka, T. Potential of water surface-floating microalgae for biodiesel production: Floating-biomass and lipid productivities. J. Biosci. Bioeng. 2016, 123, 314–318. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Yuan, C.; Zheng, Y.; Zhang, W.; He, R.; Fan, Y.; Hu, G.-R.; Li, F.-L. Lipid accumulation and anti-rotifer robustness of microalgal strains isolated from Eastern China. J. Appl. Phycol. 2017, 29, 2789–2800. [Google Scholar] [CrossRef] [Green Version]
- Talebi, A.F.; Mohtashami, S.K.; Tabatabaei, M.; Tohidfar, M.; Bagheri, A.; Zeinalabedini, M.; Hadavand Mirzaei, H.; Mirzajanzadeh, M.; Shafaroudi, S.M.; Bakhtiari, S. Fatty acids profiling: A selective criterion for screening microalgae strains for biodiesel production. Algal Res. 2013, 2, 258–267. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, W.G.; Gao, C.F.; Lan, K.; Gao, Y.; Wu, Q.Y. Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J. Chem. Technol. Biotechnol. 2009, 84, 777–781. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.L.; Wu, Q.Y. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Li, D.F.; Ding, K.; Che, R.Q.; Xu, J.W.; Zhao, P.; Li, T.; Ma, H.X.; Yu, X.Y. Production of biomass and lipids by the oleaginous microalgae Monoraphidium sp. QLY-1 through heterotrophic cultivation and photo-chemical modulator induction. Bioresour. Technol. 2016, 211, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar. Drugs 2015, 13, 5111–5127. [Google Scholar] [CrossRef] [Green Version]
- Kusumaningtyas, P.; Nurbaiti, S.; Suantika, G.; Amran, M.B.; Nurachman, Z. Enhanced oil production by the tropical marine diatom Thalassiosira sp. cultivated in outdoor photobioreactors. Appl. Biochem. Biotechnol. 2017, 182, 1605–1618. [Google Scholar] [CrossRef]
- Chen, C.; Lee, M.; Dong, C.; Kit, Y.; Chang, J. Enhanced production of microalgal lipids using a heterotrophic marine microalga Thruastochytrium sp. BM2. Biochem. Eng. J. 2020, 154, 107429. [Google Scholar] [CrossRef]
- Uba, M.O.; Duabe, K.C.P.; Biene, M.A.C.M.; Ortiz, M.K.C.R.; Bennett, R.M.; Dedeles, G.R. Growth and fatty acid profile of Thraustochytrium sp. CR01 using different sugar substitutes. Philippine J. Sci. 2016, 145, 365–371. [Google Scholar]
- Shene, C.; Garces, M.; Vergara, D.; Pena, J.; Claverol, S.; Rubilar, M.; Leyton, A. Production of lipids and proteome variation in a chilean Thraustochytrium striatum strain cultured under different growth conditions. Mar. Biotechnol. 2019, 21, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Kausley, S.B.; Joshi, S.M.; Pandit, A.B. Chapter 27—Process intensification applied to microalgae-based processes and products. In Handbook of Microalgae-Based Processes and Products; Elsevier: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 737–769. [Google Scholar] [CrossRef]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Dahms, H.-U.; Ponnusamy, V.K. Potential of two-stage cultivation in microalgae biofuel production. Fuel 2019, 252, 339–349. [Google Scholar] [CrossRef]
- Fazal, T.; Mushtaq, A.; Rehman, F.; Khan, A.U.; Rashid, N.; Farooq, W.; Rehman, M.S.U.; Xu, J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew. Sustain. Energy Rev. 2018, 82, 3107–3126. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Pei, H.; Pan, J.; Jiang, L.; Hou, Q. Cultivation of microalgae using anaerobically digested ef fl uent from kitchen waste as a nutrient source for biodiesel production. Renew. Energy 2018, 115, 276–287. [Google Scholar] [CrossRef]
- Sati, H.; Mitra, M.; Mishra, S.; Baredar, P. Microalgal lipid extraction strategies for biodiesel production: A review. Algal Res. 2019, 38. [Google Scholar] [CrossRef]
- Menegazzo, M.L.; Fonseca, G.G. Biomass recovery and lipid extraction processes for microalgae biofuels production: A review. Renew. Sustain. Energy Rev. 2019, 107, 87–107. [Google Scholar] [CrossRef]
- Lee, S.Y.; Khoiroh, I.; Vo, D.N.; Kumar, S.; Loke, P. Show Techniques of lipid extraction from microalgae for biofuel production: A review. Environ. Chem. Lett. 2020, 1–21. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid extraction methods from microalgae: A comprehensive review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Arora, N.; Nanda, M.; Pruthi, V. Different Cell Disruption and Lipid Extraction Methods from Microalgae for Biodiesel Production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M., Wang, Z., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; Castro, J.S.; Couto, E.A.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci. Total. Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.-L.; Zhao, X.-Y.; Cao, J.-P. Biomass thermochemical conversion: A review on tar elimination from biomass catalytic gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- Clark, J.; Deswarte, F. Introduction to chemicals from biomass. In Wiley Series in Renewable Resources; Stevens, C.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Radenahmad, N.; Azad, A.T.; Saghir, M.; Taweekun, J.; Bakar, M.S.A.; Reza, M.S.; Azad, A.K. A review on biomass derived syngas for SOFC based combined heat and power application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Kousheshi, N.; Yari, M.; Paykani, A.; Saberi Mehr, A.; de la Fuente, G.F. Effect of Syngas Composition on the Combustion and Emissions Characteristics of a Syngas/Diesel RCCI Engine. Energies 2020, 13, 212. [Google Scholar] [CrossRef] [Green Version]
- Hirano, A.; Hon-Nami, K.; Kunito, S.; Hada, M.; Ogushi, Y. Temperature effect on continuous gasification of microalgal biomass: Theoretical yield of methanol production and its energy balance. Catal. Today 1998, 45, 399–404. [Google Scholar] [CrossRef]
- Minowa, T.; Sawayama, S. A novel microalgal system for energy production with nitrogen cycling. Fuel 1999, 78, 1213–1215. [Google Scholar] [CrossRef]
- Patil, V.; Tran, K.-Q.; Giselrød, H.R. Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 2008, 9, 1188–1195. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Dote, Y.; Sawayama, S.; Inoue, S.; Minowa, T.; Yokoyama, S.-Y. Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 1994, 73, 1855–1857. [Google Scholar] [CrossRef]
- Minowa, T.; Yokoyama, S.-Y.; Kishimoto, M.; Okakura, T. Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 1995, 74, 1735–1738. [Google Scholar] [CrossRef]
- Aravind, S.; Kumar, P.S.; Kumar, N.S.; Siddarth, N. Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ. Chem. Lett. 2020, 18, 829–849. [Google Scholar] [CrossRef]
- Miao, X.L.; Wu, Q.Y. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q.; Yang, C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zhao, B.; Li, Y. Simulation model of pyrolysis biofuel yield based on algal components and pyrolysis kinetics. Bioenerg. Res. 2014, 7, 1293–1304. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, B.; Zhang, Y.; Huang, Y.; Xu, M. Investigation on Pyrolysis of Low Lipid Microalgae Chlorella vulgaris and Dunaliella salina. Energy Fuel 2014, 28, 95–103. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Ellem, G.; Mcgregor, R.; Herbertson, J. Thermal characterisation of microalgae under slow pyrolysis conditions. J. Anal. Appl. Pyrolysis 2009, 85, 118–123. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Q.; Tu, P. Effects of temperature and holding time on production of renewable fuels from pyrolysis of Chlorella protothecoides. J. Appl. Phycol. 2000, 12, 147–152. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, Y.; Yan, F.; Xiao, B.; Liu, S. Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): Product distribution and bio-oil characterization. Energy 2013, 52, 119–125. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuel 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Demirbas, A. Oily products from mosses and algae via pyrolysis. Energy Sources Part A—Recover. Utilization Environ. Effects 2006, 28, 933–940. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew. Sustain. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhang, Y.; Wu, X.; Gong, X.; Wang, Q. Hydrolysis of Chlorella biomass for fermentable sugars in the presence of HCl and MgCl2. Bioresour. Technol. 2011, 102, 10158–10161. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; Labavitch, J.M.; VanderGheynst, J.S. Virus infection of Chlorella variabilis and enzymatic saccharification of algal biomass for bioethanol production. Bioresour. Technol. 2013, 137, 326–331. [Google Scholar] [CrossRef]
- Lee, S.; Oh, Y.; Kim, D.; Kwon, D.; Lee, C.; Lee, J. Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl. Biochem. Biotechnol. 2011, 164, 878–888. [Google Scholar] [CrossRef]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.S.Y.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Kim, H.M.; Oh, C.H.; Bae, H.-J. Comparison of red microalgae (Porphyridium cruentum) culture conditions for bioethanol production. Bioresour. Technol. 2017, 233, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, M.L.; Zhang, L.; Lee, J.W.; Flynn, T.; Seibert, M.; Greenbaum, E.; Melis, A. Microalgae: A green source of renewable H2. Trends Biotechnol. 2000, 18, 506–511. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.-S. Biohydrogen Production From Renewable Biomass Resources. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. [Google Scholar]
- Veras, T.S.; Mozer, T.S.; César, A.S. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Razu, M.H.; Hossain, F.; Khan, M. Advancement of Bio-hydrogen Production from Microalgae. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Gateway East, Singapore, 2019; pp. 423–462. [Google Scholar]
- Grechanik, V.; Romanova, A.; Naydov, I.; Tsygankov, A. Photoautotrophic cultures of Chlamydomonas reinhardtii: Sulfur deficiency, anoxia, and hydrogen production. Photosynth. Res. 2020, 143, 275–286. [Google Scholar] [CrossRef]
- Fakhimi, N.; Tavakoli, O. Improving hydrogen production using co-cultivation of bacteria with Chlamydomonas reinhardtii microalga. Mater. Sci. Energy Technol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Skjanes, K.; Knutsen, G.; Kӓllqvist, T.; Lindblad, P. H2 production from marine and freshwater species of green algae during sulfur deprivation and considerations for bioreactor design. Int. J. Hydrog. Energy 2008, 33, 511–521. [Google Scholar] [CrossRef]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Z.; Lu, H.; Fu, Y.; Yu, X.; Zhang, W. Sustained hydrogen photoproduction by marine green algae platymonas subcordiformis integrated with in situ hydrogen consumption by an alkaline fuel cell system. J. Biotechnol. 2008, 136, 558–576. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Maitland, G.C.; Hellgardt, K. Parameters affecting the growth and hydrogen production of the green alga Chlamydomonas reinhardtii. Int. J. Hydrog. Energy 2011, 36, 7872–7876. [Google Scholar] [CrossRef]
- Oncel, S.; Sukan, F.V. Effect of light intensity and the light: Dark cycles on the long term hydrogen production of Chlamydomonas reinhardtii by batch cultures. Biomass Bioenergy 2011, 35, 1066–1074. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photosynthesis and anaerobiosis. Bioresour. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Z.; Zhang, W.; Yu, X.; Jin, M. Improved hydrogen photoproduction regulated by carbonylcyanide m-chlorophenylhrazone from marine green alga Platymonas subcordiformis grown in CO2-supplemented air bubble column bioreactor. Biotechnol. Lett. 2008, 30, 877–883. [Google Scholar] [CrossRef]
- Ji, C.F.; Legrand, J.; Pruvost, J.; Chen, Z.A.; Zhang, W. Characterization of hydrogen production by Platymonas Subcordiformis in torus photobioreactor. Int. J. Hydrog. Energy 2010, 35, 7200–7205. [Google Scholar] [CrossRef]
- Ji, C.F.; Yu, X.J.; Chen, Z.A.; Xue, S.; Legrand, J.; Zhang, W. Effects of nutrient deprivation on biochemical compositions and photo-hydrogen production of Tetraselmis subcordiformis. Int. J. Hydrog. Energy 2011, 36, 5817–5821. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel. Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Olguin, E.J. The cleaner production strategy applied to animal production. In Environmental Biotechnology and Cleaner Bioprocesses; Olguín, E.J., Sánchez, G., Hemández, E., Eds.; Taylor and Francis: London, UK, 2000; pp. 107–121. [Google Scholar]
- Yen, H.-W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef]
- Klocke, M.; Mahnert, P.; Mundt, K.; Souidi, K.; Linke, B. Microbial community analysis of a biogas-producing completely stirred tank reactor fed continuously with fodder beet silage as mono-substrate. Syst. Appl. Microbiol. 2007, 30, 139–151. [Google Scholar] [CrossRef]
- Schlüter, A.; Bekel, T.; Diaz, N.N.; Dondrup, M.; Eichenlaub, R.; Gartemann, K.H.; Krahn, I.; Krause, L.; Kromeke, H.; Kruse, O.; et al. The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J. Biotechnol. 2008, 136, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, G.; Rubilar, O.; Azocar, L.; Toro, C.; Cea, M.; Torres, A.; Ribera, A.; Navia, R. Performance of an enzymatic extract in Botrycoccus braunii cell wall disruption. J. Biosci. Bioeng. 2014, 117, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Frigon, J.-C.; Matteau-Lebrun, F.; Hamani Abdou, R.; McGinn, P.J.; O’Leary, S.J.B.; Guiot, S.R. Screening microalgae strains for their productivity in methane following anaerobic digestion. Appl. Energy 2013, 108, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, S.K.; Malik, A.; Vijay, V.K. Comparative evaluation of biomass production and bioenergy generation potential of Chlorella spp. through anaerobic digestion. Appl. Energy 2014, 114, 790–797. [Google Scholar] [CrossRef]
- Ayala-Parra, P.; Liu, Y.; Field, J.A.; Sierra-Alvarez, R. Nutrient recovery and biogas generation from the anaerobic digestion of waste biomass from algal biofuel production. Renew. Energy 2017, 108, 410–416. [Google Scholar] [CrossRef]
- Polakovičová, G.; Kušnír, P.; Nagyová, S.; Mikulec, J. Process integration of algae production and anaerobic digestion. Chem. Eng. Trans. 2012, 29, 1129–1134. [Google Scholar]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation and biogas potential of native algal isolates from soil and wastewater. Bioresour. Technol. 2013, 135, 232–238. [Google Scholar] [CrossRef]
- Vergara-Fernández, A.; Vargas, G.; Alarcón, N.; Velasco, A. Evaluation of marine algae as a source of biogas in a two-stage anaerobic reactor system. Biomass Bioenergy 2008, 32, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Buxy, S.; Diltz, R.; Pullammanappallil, P. Biogasification of marine algae Nannochloropsis oculata. In Materials Challenges in Alternative and Renewable Energy II: Ceramic Transactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 59–67. [Google Scholar]
- Park, S.; Li, Y. Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae biomass residue and lipid waste. Bioresour. Technol. 2012, 111, 42–48. [Google Scholar] [CrossRef]
- Campbell, J.; Lobell, D.; Field, C. Greater transportation energy and GHG offsets from bioelectricity than ethanol. Science 2009, 324, 1055–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börjesson, P.; Berglund, M. Environmental systems analysis of biogas systems -part I: Fuel-cycle emissions. Biomass Bioenergy 2006, 30, 469–485. [Google Scholar] [CrossRef]
- Ubando, A.T.; Rivera, D.R.T.; Chen, W.H.; Culaba, A.B. A comprehensive review of life cycle assessment (LCA) of microalgal and lignocellulosic bioenergy products from thermochemical processes. Bioresour. Technol. 2019, 291, 121837. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Hemalatha, M.; Chakraborty, D.; Chatterjee, S.; Ranadheer, P.; Kona, R. Algal biorefinery models with self-sustainable closed loop approach: Trends and prospective for blue-bioeconomy. Bioresour. Technol. 2019, 295, 122128. [Google Scholar] [CrossRef]
- Mishra, S.; Roy, M.; Mohanty, K. Microalgal bioenergy production under zero-waste biorefinery approach: Recent advances and future perspectives. Bioresour. Technol. 2019, 292, 122008. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Comparison of Growth Rate and Nutrient Content of Five Microalgae Species Cultivated in Greenhouses. Plants 2019, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.M.A.; Kassim, A.K.; Shokravi, Z.; Jakarni, F.M.; Liu, H.Y.; Zaini, N. Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: A review. Renew. Sustain. Energy Rev. 2020, 119, 109621. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of microalgae and cyanobacteria: Effect of operating conditions on growth and biomass composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef] [PubMed]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguine, O.; Polle, J.E.W. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Sakuragi, K.; Makino, H. Extraction Techniques in Sustainable Biofuel Production: A Concise Review. Fuel Process. Technol. 2019, 193, 295–303. [Google Scholar] [CrossRef]
- Piasecka, A.; Nawrocka, A.; Wiącek, D.; Krzemińska, J. Agro-industrial by-product in photoheterotrophic and mixotrophic culture of Tetradesmus obliquus: Production of ω3 and ω6 essential fatty acids with biotechnological importance. Sci Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Young, Y.; Sang, C.; Sim, J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Marquez-Rocha, F.J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 2004, 3, 21–34. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Ichige, E.; Tanaka, H. Regulating the ratio of photoautotrophic to heterotrophic metabolic activities in photoheterotrophic culture of Euglena gracilis and its application to alpha-tocopherol production. Biotechnol. Lett. 2002, 24, 953–958. [Google Scholar] [CrossRef]
- Huang, G.H.; Chen, F.; Wei, D.; Zhang, X.W.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.Y.; Lee, J.Y.; Ahn, C.Y.; Oh, H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, 71–74. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kaom, C.-J.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2016, 92, 925–936. [Google Scholar] [CrossRef]
- De Swaaf, M.; Sijtsma, L.; Pronk, J. High-cell-density fed-batch cultivation of the docosahexaenoic acid producing microalga Crypthecodinium cohnii. Biotechnol. Bioeng. 2003, 81, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kuei-Ling, Y.; Jo-Shu, C. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour. Technol. 2012, 105, 120–127. [Google Scholar]
- Caporgno, M.P.; Haberkorn, I.; Böcker, L.; Mathys, A. Cultivation of Chlorella protothecoides under different growth modes and its utilisation in oil/water emulsions. Bioresour. Technol. 2019, 288, 121476. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.J.; Joun, J.; Hong, M.E.; Patel, A.K. Split mixotrophy: A novel cultivation strategy to enhance the mixotrophic biomass and lipid yields of Chlorella protothecoides. Bioresour. Technol. 2019, 291, 121820. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Qin, Q.; Yan, S.; Huang, J.L.; Liu, K.; Zhou, S.-B. Biodiesel production from Chlorella vulgaris under nitrogen starvation in autotrophic, heterotrophic, and mixotrophic cultures. J. Appl. Phycol. 2019, 31, 1589–1596. [Google Scholar] [CrossRef]
- Li, T.; Kirchhoff, H.; Gargouri, M.; Feng, J.; Cousins, A.B.; Pienkos, P.T.; Gang, D.R.; Chen, S. Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res. 2016, 19, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Chi, Z.; Lucker, B.; Chen, S. Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. Bioresour. Technol. 2012, 103, 484–488. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Pang, N.; Gu, X.; Chen, S.; Kirchhoff, H.; Lei, H.; Rojec, S. Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew. Sustain. Energy Rev. 2019, 112, 450–460. [Google Scholar] [CrossRef]
- Chen, Y.H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, G.; Yousuf, A.; Pollio, A.; Steyer, J.-P. Microalgae Cultivation Systems. In Microalgae Cultivation for Biofuels Production; Elsevier: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; pp. 11–29. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; del Mejia-da-Silva, L.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, H.; Zhong, Y.; Zhang, C.; Shen, Z.; Sang, W.; Yan, G.; Zhou, X. The effect of bacterial contamination on the heterotrophic cultivation of Chlorella pyrenoidosa in wastewater from the production of soybean products. Water Res. 2012, 46, 5509–5516. [Google Scholar] [CrossRef] [PubMed]
- Marudhupandia, T.; Sathishkumara, R.; Kumara, T.T.A. Heterotrophic cultivation of Nannochloropsis salina for enhancing biomass and lipid production. Biotechnol. Rep. 2016, 10, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, R.; Arora, S.; Rohit, M.V.; Mohan, S.V. Lipid metabolism in response to individual short chain fatty acids during mixotrophic mode of microalgal cultivation: Influence on biodiesel saturation and protein profile. Bioresour. Technol. 2015, 188, 169–176. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Yu, H.F.; Jia, S.R.; Dai, Y.J. Growth characteristics of the cyanobacterium Nostoc flagelliformein photoautotrophic, mixotrophic and heterotrophic cultivation. J. Appl. Phycol. 2009, 21, 127–133. [Google Scholar] [CrossRef]
- Bailey, R.; DiMasi, D.; Hansen, J.; Mirrasoul, P.; Ruecker, C.; Kaneko, T.; Barclay, W. Enhanced Production of Lipids Containing Polyenoic Fatty Acid by Very High Density Cultures of Eukaryotic Microbes in. Fermentors Patent No.: US 6,607,900 B2, 19 August 2003. [Google Scholar]
- Anand, P.; Saxena, R.K. A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. New Biotechnol. 2011, 29, 199–205. [Google Scholar] [CrossRef]
- Jiang, Y.; Yoshida, T.; Quigg, A. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol. Biochem. 2012, 54, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Reverdatto, S.; Beilinson, V.; Nielsen, N.C. A multisubunit acetyl coenzyme A carboxylase fromsoybean. Plant Physiol. 1999, 119, 961–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.W.; Liu, W.J.; Hu, D.X.; Wang, X.; Balamurugan, S.; Alimujiang, A.; Yang, W.D.; Liu, J.S.; Li, H.Y. Identification of a malonyl CoA-acyl carrier protein transacylase and its regulatory role infatty acid biosynthesis in oleaginous microalga Nannochloropsis oceanic. Biotechnol. Appl. Biochem. 2016, 64, 620–626. [Google Scholar] [CrossRef]
- Fan, J.; Andre, C.; Xu, C. A chloroplast pathway for the de novo biosynthesis of triacylglycerol inChlamydomonas reinhardtii. FEBS Lett. 2011, 585, 1985–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Han, J.; Sang, M.; Li, A.; Wu, H.; Yin, S.; Zhang, C. De novo transcriptomic analysis of anoleaginous microalga: Pathway description and gene discovery for production of next-generationbiofuels. PLoS ONE 2012, 7, e35142. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.K.; Carbonaro, N.; Park, R.; Miller, S.M.; Thorpe, I.; Li, Y. Current advances in molecular, biochemical, and computational modeling analysis of microalgal triacylglycerol biosynthesis. Biotechnol. Adv. 2016, 34, 1046–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Show, K.-Y.; Yan, Y.-G.; Lee, D.-J. Biohydrogen production from algae: Perspectives, challenges, and prospects. In Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2019; pp. 325–343. [Google Scholar] [CrossRef]
- Dasgupta, C.N.; Gilbert, J.J.; Lindblad, P.; Heidorn, T.; Borgvang, S.A.; Skjanes, K.; Das, D. Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int. J. Hydrog. Energy 2010, 35, 10218–10238. [Google Scholar] [CrossRef]
- Miyake, J.; Miyake, M.; Asada, Y. Biotechnological hydrogen production: Research for efficient light energy conversion. J. Biotechnol. 1999, 70, 89–101. [Google Scholar] [CrossRef]
- Ni, F.M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrog. Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrog. Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.S. Hydrogenases for biological hydrogen production. Bioresour. Technol. 2011, 102, 8423–8431. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.; Aila, M.; Bangwal, D.P.; Kaul, S.; Garg, M.O. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M. The possibility of using algal cultures in wastewater treatment processes. Water Sewage 2009, 9, 9–12. [Google Scholar]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Chini Zittelli, G.; Sampietro, G. Energy balance of algal biomass production in a 1-ha ‘‘Green Wall Panel’’ plant: How to produce algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, D.P.; Falk, S.; Trick, C.G.; Huner, N.P.A. Growth at low temperature mimics high-light acclimation in Chlorella vulgaris. Plant Physiol. 1994, 105, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Raslavicius, L.; Semenov, V.G.; Chernova, N.I.; Kersys, A.; Kopeyka, A.K. Producing transportation fuels from algae: In search of synergy. Renew. Sustain. Energy Rev. 2014, 40, 133–142. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Oswald, W.J. Ponds in the twenty-first century. Water Sci. Technol. 1995, 31, 1–8. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Physiological and technological considerations for optimising mass algal cultures. J. Appl. Phycol. 2000, 12, 201–206. [Google Scholar] [CrossRef]
- Mùnoz, R.; Kollner, C.; Guieysse, B.; Mattiasson, B. Photosynthetically oxygenated salicylate biodegradation in a continuous stirred tank photobioreactor. Biotechnol. Bioeng. 2004, 87, 797–803. [Google Scholar] [CrossRef]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- van Harmelen, T.; Oonk, H. Microalgae Biofixation Processes: Applications and Potential Contributions to Greenhouse Gas Mitigation Options; International Network on Biofixation of CO2 and Greenhouse Gas Abatement with Microalgae: Apeldoom, The Netherlands, 2006. [Google Scholar]
- Wang, B.; Li, Y.; Wu, N.; Lan, C. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, T.J. Production of algae in conjunction with wastewater treatment. In NREL—AFOSR Workshop on Algal Oil for Jet Fuel Production; Arlington, VA, USA, 2008; Available online: https://www.researchgate.net/profile/John_Benemann2/publication/229039925_Overview_Algae_oil_to_biofuels/links/54171cbf0cf2218008bed0aa/Overview-Algae-oil-to-biofuels.pdf (accessed on 8 August 2020).
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nanochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Beachama, T.A.; Mora Maciaa, V.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/ 3 following EMS and UV induced mutagenesis. Biotech. Rep. 2015, 7, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noraini, M.Y.; Ong, H.C.; Badrul, M.J.; Chong, W.T. A review on potential enzymatic reaction for biofuel production from algae. Renew. Sustain. Energy Rev. 2014, 39, 24–34. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Dudek, M.; Grala, A. Acquisition feasibility and methane fermentation effectiveness of biomass of microalgae occurring in eutrophicated aquifers on the example of The Vistula Lagoon. Int. J. Green Energy 2016, 13, 395–407. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Krzemieniewski, M. Efficiency of methane fermentation of waste microalgae biomass (WMAB) collected in processes of reclamation of eutrophicated water reservoirs. Environ. Earth Sci. 2016, 75, 525. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Dębowski, M. The Use of Algae Biomass as a Substrate in the Methane Fermentation Process; UWM: Olsztyn, Poland, 2013. [Google Scholar]
- Guo, L. Doing battle with the green monster of Taihu Lake. Science 2007, 317, 1166. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source State Key. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- Qin, B. Lake eutrophication: Control countermeasures and recycling exploitation. Ecol. Eng. 2009, 35, 1569–1573. [Google Scholar] [CrossRef]
- Dębowski, M.; Grala, A.; Zieliński, M.; Dudek, M. Efficiency of the methane fermentation process of macroalgae biomass originating from puck bay. Arch. Environ. Prot. 2012, 38, 99–107. [Google Scholar]
- Dębowski, M.; Zieliński, M.; Rokicka, M.; Kupczyk, K. The possibility of using macroalgae biomass from natural reservoirs as a substrate in the methane fermentation process. Int. J. Green Energy 2015, 12, 970–977. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kupczyk, K.; Rokicka, M.; Hajduk, A. Effect of taxonomic diversification of microalgae harvested from eutrophicated reservoirs on the chemical composition of biomass and effectiveness of methane fermentation. Environ. Prog. Sustain. Energy 2015, 34, 858–865. [Google Scholar] [CrossRef]
- AquaFUELs. Report on Biology and Biotechnology of Algae with Indication of Criteria for Strain Selection. 2011. Available online: http://www.aquafuels.eu/deliverables.html (accessed on 8 August 2020).
- Ryan, D.; Jennifer, M.; Christopher, K.; Nicholas, G.; Eric, T. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing Through Dewatering for Downstream Conversion; Technical Report NREL/TP-5100-64772; National Renewable Energy Laboratory: Golden, CO, USA, 2016; pp. 1–119. [Google Scholar]
- Gouveia, L. Microalgae as a Feedstock for Biofuels; Springer: Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2011. [Google Scholar]
- Mustapa, N.S.; Abu Mansor, M.S.; Serri, N.A. Design and development of centred-light photobioreactor for microalgae cultivation system. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012009. [Google Scholar] [CrossRef]
- Rebolledo-Oyarce, J.; Mejía-López, J.; García, G.; Rodríguez-Córdova, L.; Sáez-Navarrete, C. Novel photobioreactor design for the culture of Dunaliella tertiolecta—Impact of color in the growth of microalgae. Bioresour. Technol. 2019, 289, 121645. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.; Zhu, M.; Yu, C.; Cao, Y.; Zhang, D.; Zhou, G. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis. Bioresour. Technol. 2013, 136, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Lill, J.-O.; Salovius-Lauren, S.; Harju, L.; Rajander, J.; Saarela, K.-E.; Lindroos, A. Temporal changes in elemental composition in decomposing filamentous algae (Cladophora glomerata and Pilayella littoralis) determined with PIXE and PIGE. Sci. Total. Environ. 2012, 414, 646–652. [Google Scholar] [CrossRef]
- Ziolkowska, J.R.; Simon, L. Recent developments and prospects for algae-based fuels in the US. Renew. Sustain. Energy Rev. 2014, 29, 847–853. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]

| Sector | Use | Ref. | |
|---|---|---|---|
| Wastewater treatment | Nitrogen and phosphorus removal from municipal wastewater | [17] | |
| Biodegradation of sparingly degradable pollutants | [18,19] | ||
| Treatment of organic wastewater | [20] | ||
| Treatment of hard-to-manage wastewater produced by | timber and paper industry | [21,22] | |
| textile industry | [23] | ||
| phenol industry | [24,25] | ||
| Ethanol and citric acid production | [26] | ||
| Removal of heavy metals (copper, nickel, lead) from wastewater | [19,27] | ||
| Gas treatment | Reducing emissions of carbon dioxide and other pollutants (nitrogen and sulfur oxides) from waste and exhaust gases | [28,29] | |
| Waste management | Use of waste glycerol as a carbon source in heterotrophic cultivation | [30] | |
| Microalgae cultivation using industrial waste or low-value feedstocks, such as | breadcrumbs | [31] | |
| brewer’s spent yeast | [32] | ||
| coconut water | [33] | ||
| empty palm fruit bunches | [34] | ||
| Leachate treatment | Biodegradation of landfill leachates | [35] | |
| Neutralization of degraded effluent from anaerobic fermentation of sewage sludge | [36] | ||
| Biogas upgrading | Biological sequestration of CO2 with photosynthetic microalgae (photosynthesis allows producing biogas with 94% methane content) | [37] | |
| Microalgal Strains/Biomass | Cultivation System and Operation Mode | Cultivation Time (days) | Algal Growth, in g·dm−3 (Dry Basis) or cells·cm−3 | Biomass Productivity (mg·dm−3·day−1) | Ref. |
|---|---|---|---|---|---|
| Chlamydomonas sp. SW13aLS | 250 cm3 flask; batch | 30 | No growth | No growth | [50] |
| Chlorella pyrenoidosa FACHB-28 | 30 dm3 membrane Define if appropriate.; batch | 30 | 0.41–0.63 g·dm−3 | 60–80 | [51] |
| Chlorella pyrenoidosa NCIM 2738 | 3 dm3 tubular PBR; batch | 18 | 2.9 g·dm−3 | 260 | [52] |
| Chlorella sp. (isolated from a clean lagoon) | 500 cm3 flasks; batch | 28 | 3 × 107cells·cm−3 | n.a. | [53] |
| Chlorella sp. (isolated from leachate) | 350 cm3 flasks; batch | 14 | 0.09–0.43 g·dm−3 | 18–66 | [54] |
| Chlorella sp. (marine) | 24 dm3 tubular PBR; batch | 3 | 2.2–2.6 × 106cells·cm−3 | n.a. | [55] |
| Chlorella vulgaris CCAP 211/11B | 1 dm3 flasks; batch | 10 | 0.81–1.71 g·dm−3 | 20–110 | [56] |
| Chlorella vulgaris CCAP 211 | 2 dm3 vertical PBR; batch | 28 | 2.10 g·dm−3 | 63.8 | [57] |
| Chlorella vulgaris FACHB-31 | Membrane PBR with two 2 dm3 chambers; batch | 8 | 0.95 g·dm−3 | 240 | [58] |
| Chlorella vulgaris FACHB-31 | 2 dm3 tubular PBR; batch | 8 | 0.66 g·dm−3 | 150 | [58] |
| Chlorella vulgaris FACHB-31 | 3 dm3 membrane PBRs; batch | 12 | 2.13 g·dm−3 | n.a. | [58] |
| Chlorella vulgaris FACHB-31 | 3 dm3 tubular PBR; batch | 2 | No growth | No growth | [58] |
| Chlorella vulgaris and Chlamydomonas reinhardii | 500 cm3 bottles; batch | 28–60 | 0.46–1.5 g·dm−3 | n.a. | [59] |
| Chlorella vulgaris and Chlamydomonas reinhardii | 200 dm3 open raceway pond; 5 runs; batch | 32–54 | 0.68–1.03 g·dm−3 | 160–440 | [59] |
| Nannochloropsis gaditana,Pavlova lutheri, Tetraselmis chuii, and Chetoceros muelleri | 2.5 and 12.5 dm3 cylindrical PBR; batch | 10 | Up to 9 × 106cells·cm−3 | n.a. | [60] |
| Oscillatoria sp. (isolated from leachate) | 350 cm3 flasks; batch | 14 | 0.43–0.81 g·dm−3 | 44–107 | [54] |
| Picochlorum oculatum UTEX LB 1998 | 150 dm3 horizontal bioreactor; fed-batch; 3 cycles | 18–37 | 1.5–1.9 g·dm−3 (1.2–1.7 × 109cells·cm−3) | 37–55 | [61] |
| Scenedesmus sp. (isolated from leachate) | 350 cm3 flasks; batch | 14 | 0.16–0.24 g·dm−3 | 37–46 | [54] |
| Scenedesmus sp. CHX1 | 250 cm3 flasks; batch | 20 | 0.22 g·dm−3 | 37.5 | [62] |
| Projects/Research Institutes | Focal Area | Ref. |
|---|---|---|
| Algae Innovation Center, Green Centre, Denmark | Demonstration and test projects concerning algae cultivation; an assessment of biomass viability was conducted on the site (in Rødsand II) | [67] |
| Algenol, USA | Production of bioethanol and pigments in a raceway pond, and closed and semi-closed bioreactors | [68] |
| Algatechnologies, Israel | Production of astaxanthin in closed and semi-closed bioreactors under high light intensity | [69] |
| Algenol Biotech, LLC | An integrated, pilot project involving the photosynthetic production of ethanol and the delivery of a photobioreactor system that can be scaled for commercial operation | [70] |
| BioReal Inc, USA | Production of astaxanthin in an indoor photobioreactor | [71] |
| BioProcess Algae, LLC | A pilot project on growing low-cost algae using renewable CO2, lignocellulosic sugars, and waste heat provided by a co-located ethanol plant | [70] |
| Blue Bio Projekt (IVA Kattegat-Skagerrak) | Finding sustainable ways of exploiting microalgae | [67] |
| Cellana, USA | Production of PUFAs, animal feed, biodiesel, and bio jet fuel in an open-pond bioreactor | [72] |
| Cyanotech, Hawaii | Production of astaxanthin from Spirulina pacifica as a food ingredient in a raceway pond and photobioreactors | [69] |
| IGV Gmbh, Germany | Algae cultivation in a photobioreactor | [67] |
| Kingfisher, Sweden | Tested equipment (including offshore wind parks) for offshore mussel and algae cultivation | [67] |
| Mera Pharmaceuticals Incorporation | Production of astaxanthin from Haematococcus pluvialis in a raceway pond | [73] |
| Muradel Pty Ltd., Australia | Production of biofuels, oleochemicals, biofertilizers, animal feed, and building materials in a raceway pond | [74] |
| Sea6 Energy, India | Production of food additives, biofuel, bioplastic, and animal feed in sea water | [75] |
| Sapphire Energy Inc. USA | A demonstration-scale project involving the construction and operation of a 100-acre algae farm and conversation facility for the production of renewable bio-crude | [70] |
| Solazyme Inc. USA | An integrated pilot project involving heterotrophic algae that can convert cellulosic sugars to diesel fuel | [70] |
| Solix Algadrients Inc., USA | Production of astaxanthin and DHA in enclosed photobioreactors | [76] |
| RWE Power AG, Germany | Flue gas used to grow algae in a demonstration project | [67] |
| Technical Research Centre of Finland | Design and validation of a new integrated “biowaste-to-energy” concept involving algae cultivation and biogas production | [67] |
| University of Warmia and Mazury in Olsztyn, Poland | Cultivation of lipid-rich microalgal biomass as anaerobic digestate valorization technology—a pilot-scale study | [77] |
| Microalgal Strains/Biomass | Type of Culture | Biomass Yield (gDM·dm−3·day−1) | Lipid Yield | Ref. |
|---|---|---|---|---|
| (mg·dm−3·day−1) | ||||
| Asteromonas gracilis | Phototrophic | 0.04 | 8.25 | [89] |
| Botryosphaerella sp. AVFF007 (floating cells) | Phototrophic | 0.16 | 46 | [90] |
| Chaetoceros muelleri F&M-M43 | Phototrophic | 0.07 | 21.8 | [91] |
| Chlamydomonas sp. YQJ-1 | Phototrophic | 0.06 | 20 | [92] |
| Chlamydomonas reinhardtii | Phototrophic | 0.05 | 10 | [93] |
| Chlorella emersonii | Phototrophic | 0.29 | 55 | [93] |
| Chlorella minutissima UTEX 2341 | Phototrophic | 0.02–0.03 | 9.0–10.2 | [94] |
| Chlorella protothecoides | Heterotrophic | 4.0–4.4 | 1881.3–1840.0 | [95] |
| Chlorella protothecoides | Heterotrophic | 2 | 932 | [96] |
| Chlorella vulgaris #259 | Mixotrophic | 0.09–0.25 | 22.0–54.0 | [97] |
| Chlorella vulgaris CCAP 211/11B | Phototrophic | 0.17 | 32.6 | [91] |
| Desmodesmus sp. DZL-4 | Phototrophic | 0.16 | 50 | [92] |
| Dunaliella salina | Phototrophic | 0.05 | 10 | [93] |
| Micractinium sp. IR-4 | Phototrophic | 0.11 | 20 | [92] |
| Monoraphidium sp. QLY-1 | Phototrophic | 0.02 | 11.6 | [98] |
| Monoraphidium sp. QLZ-3 | Phototrophic | 0.03 | 7.2 | [98] |
| Monoraphidium sp. YLY-2 | Phototrophic | 0.01 | 4.9 | [98] |
| Nannochloropsis sp. F&M-M29 | Phototrophic | 0.17 | 37.6 | [91] |
| Pavlova salina CS 49 | Phototrophic | 0.16 | 49.4 | [91] |
| Phaeodactylum tricornutum F&M-M40 | Phototrophic | 0.24 | 44.8 | [91] |
| Scenedesmus obliquus | Mixotrophic | 0.10–0.51 | 11.6–58.6 | [99] |
| Scenedesmus obliquus | Phototrophic | 0.06 | 7.14 | [99] |
| Scenedesmus quadricauda | Phototrophic | 0.19 | 35.1 | [91] |
| Scenedesmus sp. DM | Phototrophic | 0.26 | 53.9 | [91] |
| Scenedesmus sp. F&M-M19 | Phototrophic | 0.21 | 40.8 | [91] |
| Schizochytrium sp. S31 | Phototrophic | 0.88 | 100.7 | [100] |
| Selenastrum sp. XL-3-3 | 0.22 | 130 | [92] | |
| Skeletonema costatum CS 181 | Phototrophic | 0.08 | 17.4 | [91] |
| Tetraselmis suecica F&M-M33 | Phototrophic | 0.32 | 27 | [91] |
| Thalassiosira pseudonana CS 173 | Phototrophic | 0.08 | 17.4 | [91] |
| Thalassiosira sp. | Phototrophic | 0.02–0.03 | 10.4 | [101] |
| Thraustochytrium sp. BM2 | Heterotrophic | 2.13 | 1683 | [102] |
| Thraustochytrium sp. CR01 | Heterotrophic | 2 | 1140 | [103] |
| Thraustochytrium striatum | Heterotrophic | 0.4 | 52 | [104] |
| Microalgal Strains/Biomass | Oil Recovery | Ref. | |
|---|---|---|---|
| Temperature (°C) | Efficiency (%) | ||
| Chlorella | 425 | 35.0 | [129] |
| Chlorella vulgaris | 500 | 49.2 | [130] |
| Chlorella vulgaris | 500 | 41.0 | [131] |
| Chlorella protothecoides | 500 | 55.3 | [132] |
| Chrysophyceae | 450 | 49.4 | [129] |
| Cladophora sp. | 600 | 20.0 | [133] |
| Dunaliella salina | 500 | 55.4 | [130] |
| Lyngbya sp. | 600 | 13.0 | [133] |
| Microcystis sp. | 500 | 54.97 | [134] |
| Spirulina | 425 | 40.6 | [129] |
| Spirulina platensis | 350–500 | 23.0–29.0 | [135] |
| Microalgal Strains/Biomass | Pretreatment | Fermentative Microorganism | Fermentation Condition | Ethanol Production | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Process | Temperature (°C) | Time | pH | Agitation (rpm) | Max | Units | ||||
| Chlamydomonas reinhardtii UTEX 90 | Enzymatic | Saccharomycescerevisiae S288C | SSF | 30 | 40 (h) | - | 160 | 0.235 | (g·g−1 algae) | [141] |
| Chlorella | Chemical (HCI and MgCI2) | Saccharomycescerevisiae Y01 | - | 30 | 48 (h) | - | 200 | 22.60 | (g·dm−3) | [142] |
| Chlorella variabilis | Viral and enzymatic | Escherichiacoli KO11 | - | 35 | 3 (days) | 6.5 | 150 | 0.326 | (g·g−1 carbohydrate consumed) | [143] |
| Chlorella vulgaris | Chemical (H2SO4) | Escherichiacoli SJL2526 | SHF | 37 | - | 7.0 | 170 | 0.4 | (g·g−1 algae) | [144] |
| Chlorella vulgaris FSP-E | Chemical (H2SO4) | Zymomonasmobilis ATCC 29191 | SHF | 30 | 12 (h) | 5–6 | 11.66 | (g·dm−3) | [145] | |
| Chlorococcum infusionum | Chemical (NaOH) | Saccharomycescerevisiae | - | 72 | - | 200 | 0.26 | (g·g−1 algae) | [146] | |
| Chlorococum sp. | Supercritical fluid | Saccharomycesbayanus | - | 30 | 60 (h) | - | 200 | 3.83 | (g·dm−3) | [147] |
| Porphyridium cruemtum | Enzymatic | Saccharomycescerevisiae KCTC 7906 | SSF | 37 | 9 (h) | 4.8 | - | 2.77 | (g·dm−3) (seawater) | [148] |
| 2.98 | (g·dm−3) (freshwater) | |||||||||
| Scenedesmus obliquus CNW-N | Chemical (H2SO4) | Zymomonasmobilis ATCC29191 | SHF | 30 | 4 (h) | 6 | - | 8.55 | (g·dm−3) | [145] |
| Microalgal Strains/Biomass | Hydrogen Yield (cm3·dm−3) | Ref. |
|---|---|---|
| Chlamydomonas reinhardtii | 5.2 | [158] |
| Chlamydomonas reinhardtii | 210.9 | [159] |
| Chlamydomonas reinhardtii | 120.0 | [155] |
| Chlorella sp. | 150.0 | [160] |
| Platymonas Subcordiformis | 50.0 | [161] |
| Platymonas Subcordiformis | 157.7 | [162] |
| Tetraselmis Subcordiformis | 55.8 | [163] |
| Microalgal Strains/Biomass | Methane Fermentation Condition | Biogas Yield (cm3·g−1 VS) | Methane Yield (cm3·g−1 VS) | Ref. | |
|---|---|---|---|---|---|
| T (°C) | Time (days) | ||||
| Arthrospira platensis | 38 | 32 | 481 | 293.41 | [170] |
| Botrycoccus braunii (pretreated biomass) | 30 | 45 | – | 521 | [171] |
| Botryococcus braunii | 35 | 34–50 | – | 343–370 | [172] |
| Chlamydomonas reinhardtii | 38 | 32 | 587 | 387.42 | [170] |
| Chlamydomonas sp. | 35 | 34–50 | – | 333 | [172] |
| Chlorella kessleri | 38 | 32 | 335 | 217.75 | [170] |
| Chlorella minutissima | 36 | – | 340 | 166.12 | [173] |
| Chlorella pyrenoidosa | 36 | – | 464 | 264.71 | [173] |
| Chlorella sorokiniana | 30 | 42 | – | 298 | [174] |
| Chlorella sorokiniana (pretreated biomass) | 30 | 42 | – | 388 | [174] |
| Chlorella sorokiniana | 40–41 | 71 | 248 | 212 | [175] |
| Chlorella vulgaris | 36 | – | 369 | 195.64 | [173] |
| Chroococcus sp. | 36 | 30 | 487 | 267.36 | [176] |
| Dunaliella salina | 38 | 32 | 505 | 323.2 | [170] |
| Euglena gracilis | 38 | 32 | 485 | 324.95 | [170] |
| Isochrysis sp. | 35 | 34–50 | – | 408 | [172] |
| Macrocystis pyrifera | 37 | 31 | – | 545 | [177] |
| Nannochloropsis oculata | 35 | 30 | – | 204 | [178] |
| Nannochloropsis salina (lipid extracted biomass) | 37 | 40 | – | 130 | [179] |
| Scenedesmus dimorphus | 35 | 34–50 | – | 397 | [172] |
| Scenedesmus obliquus | 38 | 32 | 287 | 177.94 | [170] |
| Type of Culture | Type | Advantages | Issues | Ref. |
|---|---|---|---|---|
| Photoautotrophic | Closed photobioreactors | Water saving Greater long-term culture maintenance High yield | High cost Temperature control (requires cooling) Maximum light exposure Periodic cleaning | [194] |
| Open ponds | Lower costs Evaporative cooling | Low yield Changes in humidity and temperature Maximum light exposure | [194] | |
| Heterotrophic | Closed photobioreactors | Easy to maintain High biomass concentrations Contamination prevention Utilization of inexpensive lignocellulosic sugars | Competition with other biofuel technologies for feedstock | [194] |
| Mixotrophic | Photobioreactor | Two-route cultivation, easy to bioremediate Uses organic compounds as energy source, provides superior energy recovery and carbon footprint | Design needs to be upgraded to improve operation and economy Rarely used for bio-oil production Not used for biodiesel production | [195,196,197] |
| Photoheterotrophic | Photobioreactor | Fast growth of algae and synthesis of valuable metabolites (i.e., fatty acids) | Requires light as an energy source, unlike mixotrophic cultivation Not used for biodiesel production | [193,196,197] |
| Advantages/Disadvantages of Microalgal Biomass Production and Use | Ref. | ||
|---|---|---|---|
| Culture efficiency | 4–100 tons of dry microalgal biomass·ha−1·year−1, e.g., Tetraselmis suecica 36 tons of dry microalgal biomass·ha−1·year−1 (for comparison, soybean production efficiency is at 2.6 tons of beans·ha−1·year−1) | [235,243,245] | |
| Culture medium | Fresh water | 2.0–5.0 g weight·dm−3 using Chlorella vulgaris ESP-31 strain and a modified Bristol medium or MBL | [203] |
| Salt water | 7 × 107 cells of microalgae·cm−3 using Nannochloropsis salina CCAP849 strain and F/2 culture medium | [257] | |
| High photosynthetic efficiency | 4–10% (0.5–2.2% in the case of higher plants) | [235,242] | |
| Protein concentration in dry weight | 20–60% | [250] | |
| Demand for CO2 during culture (a factor impairing high biomass growth) | ca. 1.83 kg CO2 ·kg−1 of dry biomass grown | [200] | |
| Oxidation | Oxygen release during fermentation | 1.50–1.92 kg O2·kg−1 of the produced biomass | [248,249] |
| Oxidation rate during degradation of organic pollutants | 0.48–1.85 kg O2·m−3·day−1 | ||
| Redfield’s ratio in wastewater-based systems | C:N:P = 106:16:1 | [255] | |
| Bio-oil production | 19 m3·ha−1·year−1 (for comparison, 6.1 m3·ha−1·year−1 from oil palm plantation, 4.3 m3·ha−1·year−1 from sugar cane, 2.4 m3·ha−1·year−1 from maize, and 0.5 m3·ha−1·year−1 from soybean) | [91,241] | |
| Biogas production | 389.07 ± 8.21–420.95 ± 0.95 cm3·g dry matter−1 (with dominating Cyanoprokaryota and subdominating Chlorophyta) | [262] | |
| Use of biogenic compounds | Reduction of nitrogen compound concentration by 60–90% and phosphorus compound concentration by 70–100% in the effluent from manure concentration | [269] | |
| Advantages | Disadvantages |
|---|---|
| Renewable, sustainable, effective, and environmentally friendly biofuel | Incomplete renewable energy resource for biofuels with respect to complete life cycle assessment |
| Transition to low-carbon economy, i.e., from hydrocarbons to carbohydrate, protein, and lipid resources | Insecurity of algae feedstock supply, regional and seasonal availability, and local energy supply |
| No competition or lower risk of competition with feeds and foods | Lack of global monitoring and control of algae fuel production with certification of origin and source |
| Energy security, diversification of fuel supply | Lack of established transparent policy frameworks and instruments (subsidies, mandates and tax credit incentives) |
| Enormous greenhouse gas uptake and superior CO2 capture and sequestration with extra oxygen release while growing | Possible competition with edible algae and biomaterials production |
| Conservation of fossil fuels | Utilization of fossil fuels during algae processing |
| Rural revitalization and social benefits with creation of new jobs and income | High production costs for growing, harvesting, collecting, transporting, storing, and pretreating, as well as the low cost-effectiveness with high initial capital investment |
| Mitigation of negative effects of spiking crude oil prices and reduced dependency on foreign oil imports | The ‘‘biofuel only” production approach is not commercially viable |
| High energy conversion efficiency by photosynthesis | Damage to natural ecosystems (water, soil, biodiversity conservation, eutrophication, pollution) |
| High productivity with rapid growth rate and high growing yield | Disruption of the ecological balance in the already stressed lakes, ponds, seas, and oceans |
| Herbicide or pesticide use is not recommended during cultivation | Harmful algal blooms in global waters |
| Easily cultured and readily and rapidly bioengineered | Odor, potentially dangerous emissions (Cl, CH4, CO2, SOX, NOX, toxic trace elements), ozone depletion, and leaching of hazardous components during disposal and processing |
| Use of oceans, seas, ponds, and low-productive, degraded and contaminated nonarable lands, can grow even in industrial, municipal, and agricultural wastewaters | Utilization of arable land or land-use changes |
| Readily adaptable to a wide range of climatic conditions | Use of genetically modified organisms in algae cultivation and in production of biofuels |
| Reclamation of degraded and contaminated areas and ponds | Unclear utilization of waste products |
| Prevents eutrophication and pollution in aquatic ecosystems | Health problems due to neurotoxic properties of some algae |
| Highly biodegradable resource, quick to bioremediate | Technological problems during thermochemical processing (separation, agglomeration, deposit formation, slagging, fouling, corrosion, erosion) |
| Reduction of algae residues and waste | Restrictions on direct combustion and gasification of algae |
| Plentiful and relatively cheap resource for production of biochemicals, sorbents, fertilizers, building materials, synthesis of some minerals, and recovery of certain elements and compounds | Lack of accepted terminology, methodologies, standards, and classification and certification systems |
| High levels of volatiles, Au, B, Br, Ca, I, Mg, P, Ti, carbohydrates, proteins, lipids, structural organic components, extractives, water-soluble nutrient elements | Insufficient knowledge for the assessment and validation; variability of composition, properties, and quality |
| Low values of C and some trace elements | Algae cultivation occasionally requires high volumes of nutrient-rich water or fertilizers |
| Great reactivity and low initial ignition and combustion temperatures during conversion | Use of extra water during algae processing |
| Reduction of some hazardous emissions (CO2, SOX, NOX, toxic trace elements) by capture and storage of toxic components in ash | Limited practical experience in biofuel production |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. https://doi.org/10.3390/su12239980
Dębowski M, Zieliński M, Kazimierowicz J, Kujawska N, Talbierz S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability. 2020; 12(23):9980. https://doi.org/10.3390/su12239980
Chicago/Turabian StyleDębowski, Marcin, Marcin Zieliński, Joanna Kazimierowicz, Natalia Kujawska, and Szymon Talbierz. 2020. "Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations" Sustainability 12, no. 23: 9980. https://doi.org/10.3390/su12239980
APA StyleDębowski, M., Zieliński, M., Kazimierowicz, J., Kujawska, N., & Talbierz, S. (2020). Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability, 12(23), 9980. https://doi.org/10.3390/su12239980








