Seed Germination of Sunflower as a Case Study for the Risk Assessment and Management of Transgenic Plants Used for Environmental Remediation in South Korea
Abstract
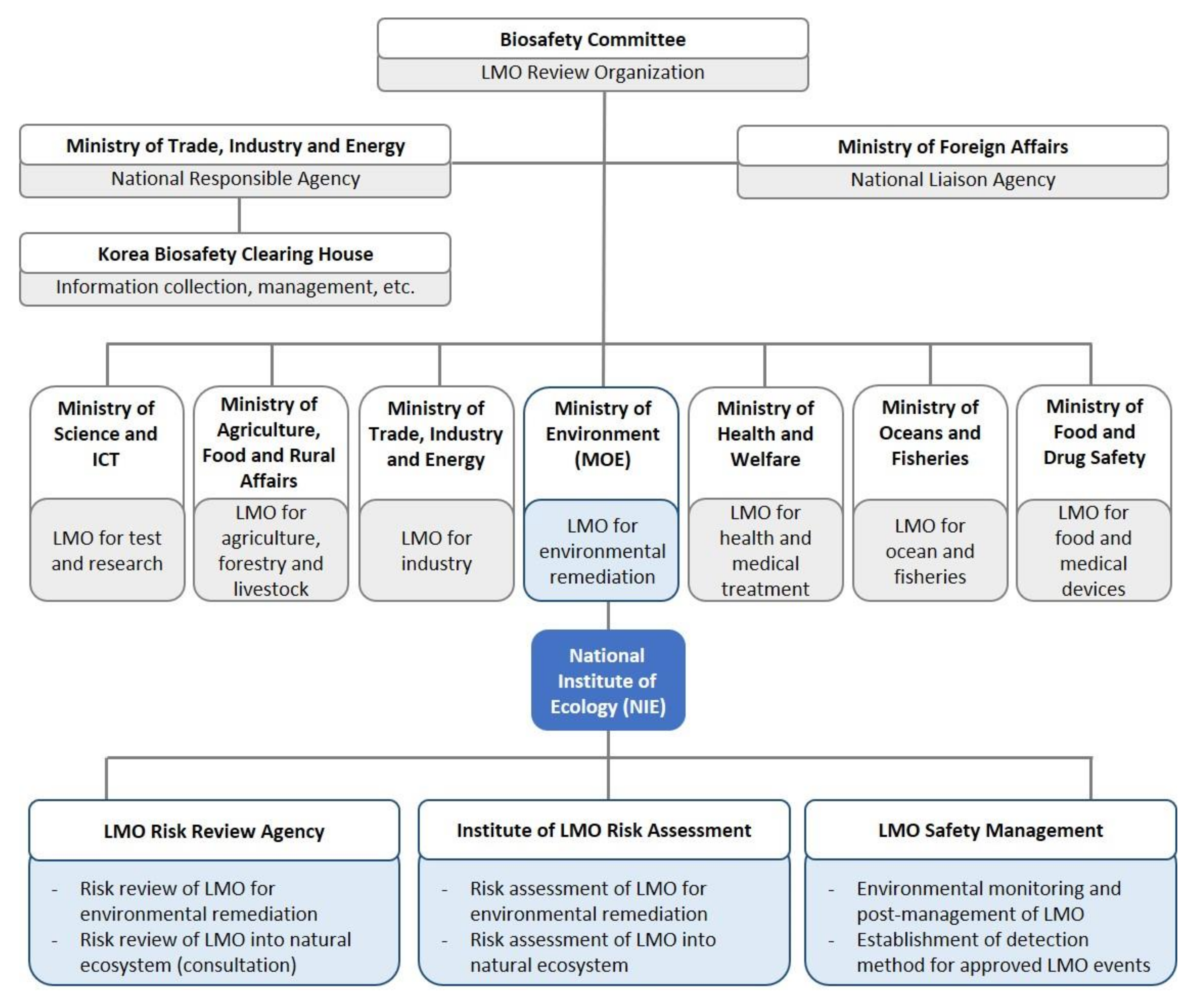
1. Introduction
2. Materials and Methods
2.1. Plant Materials
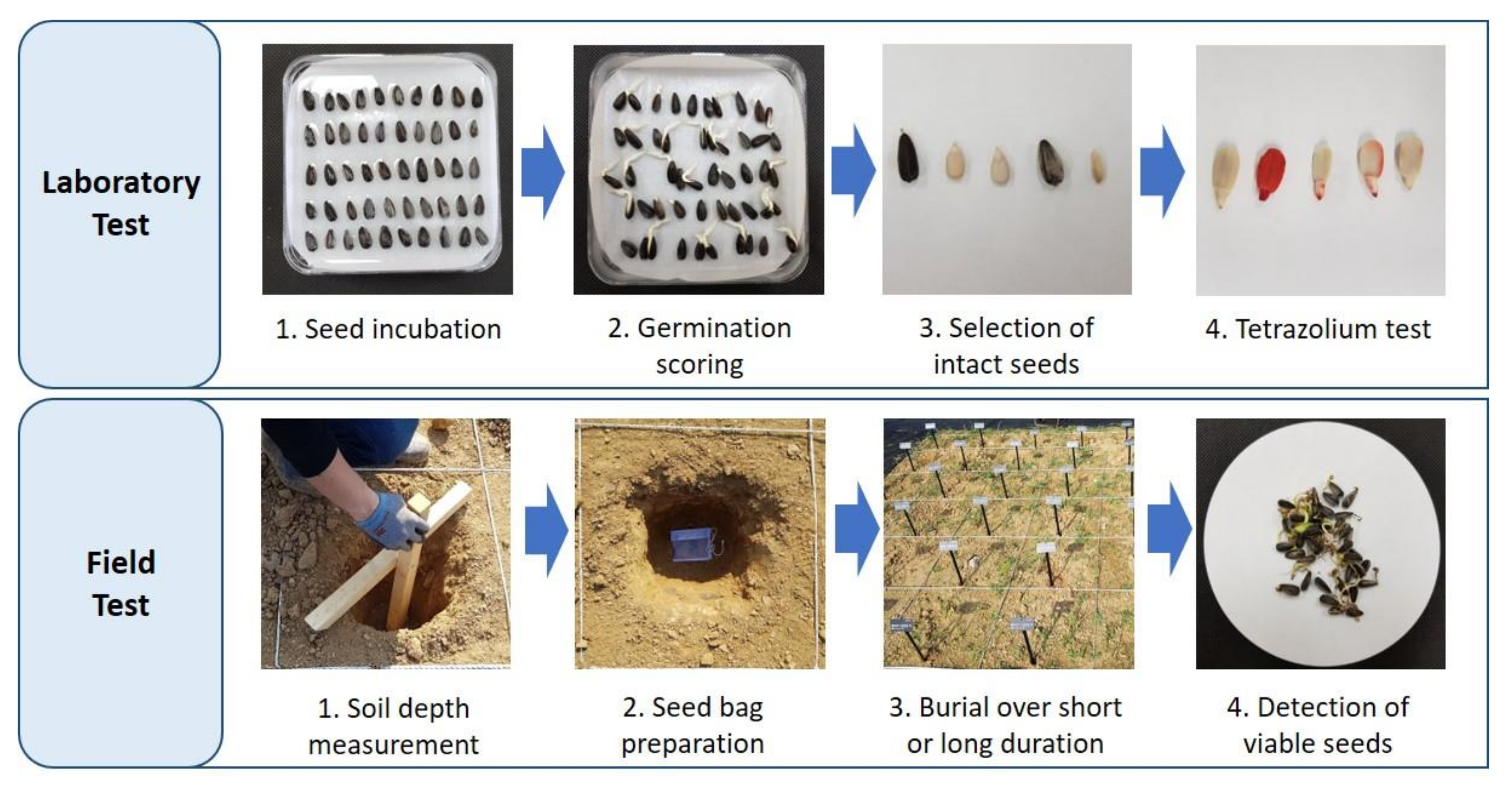
2.2. Seed Germination Test
2.3. Seed Burial Experiment
2.4. Soil Physico-Chemical Properties
2.5. Statistical Analysis
3. Results
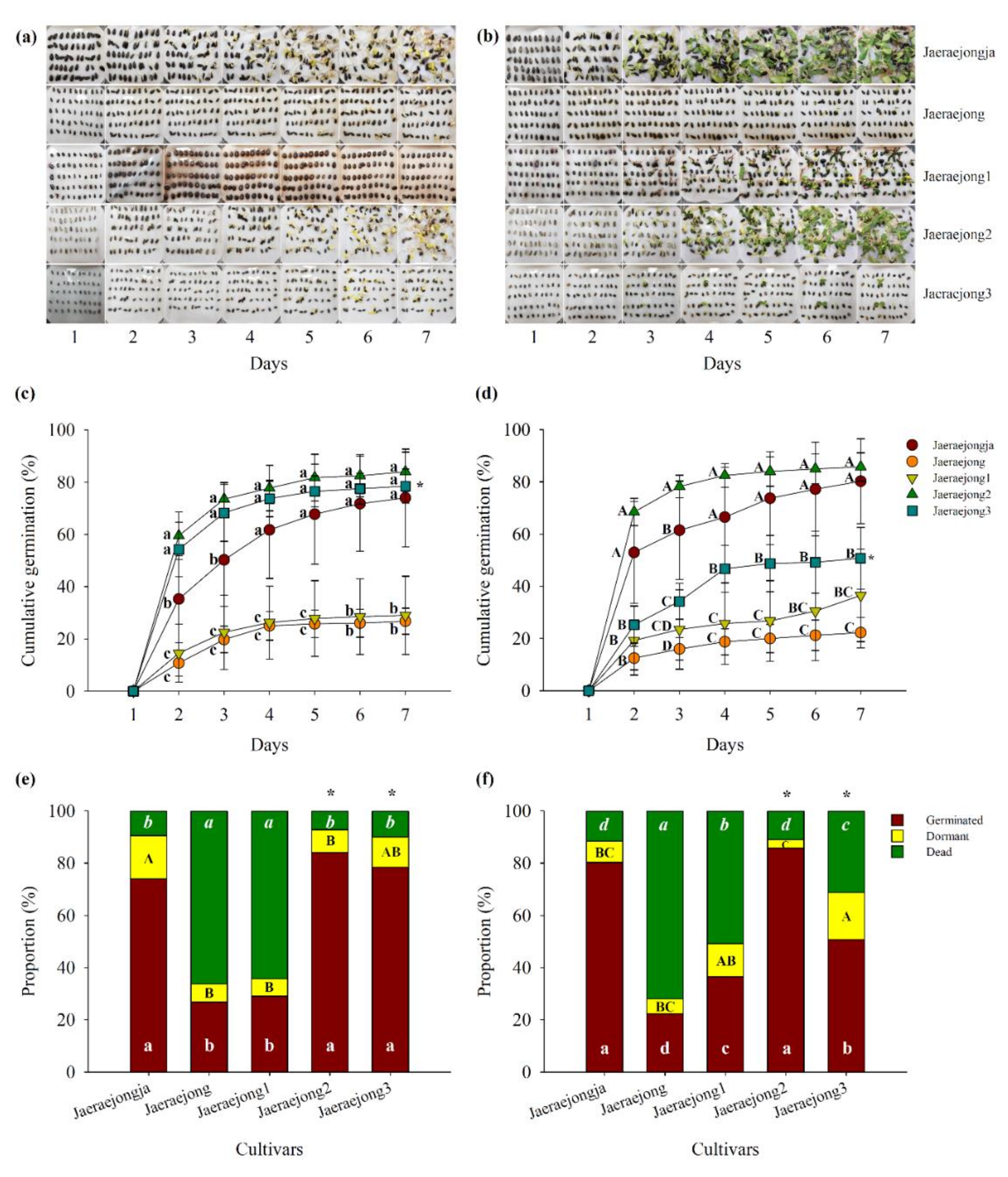
3.1. Seed Germination at Different Controlled Temperatures
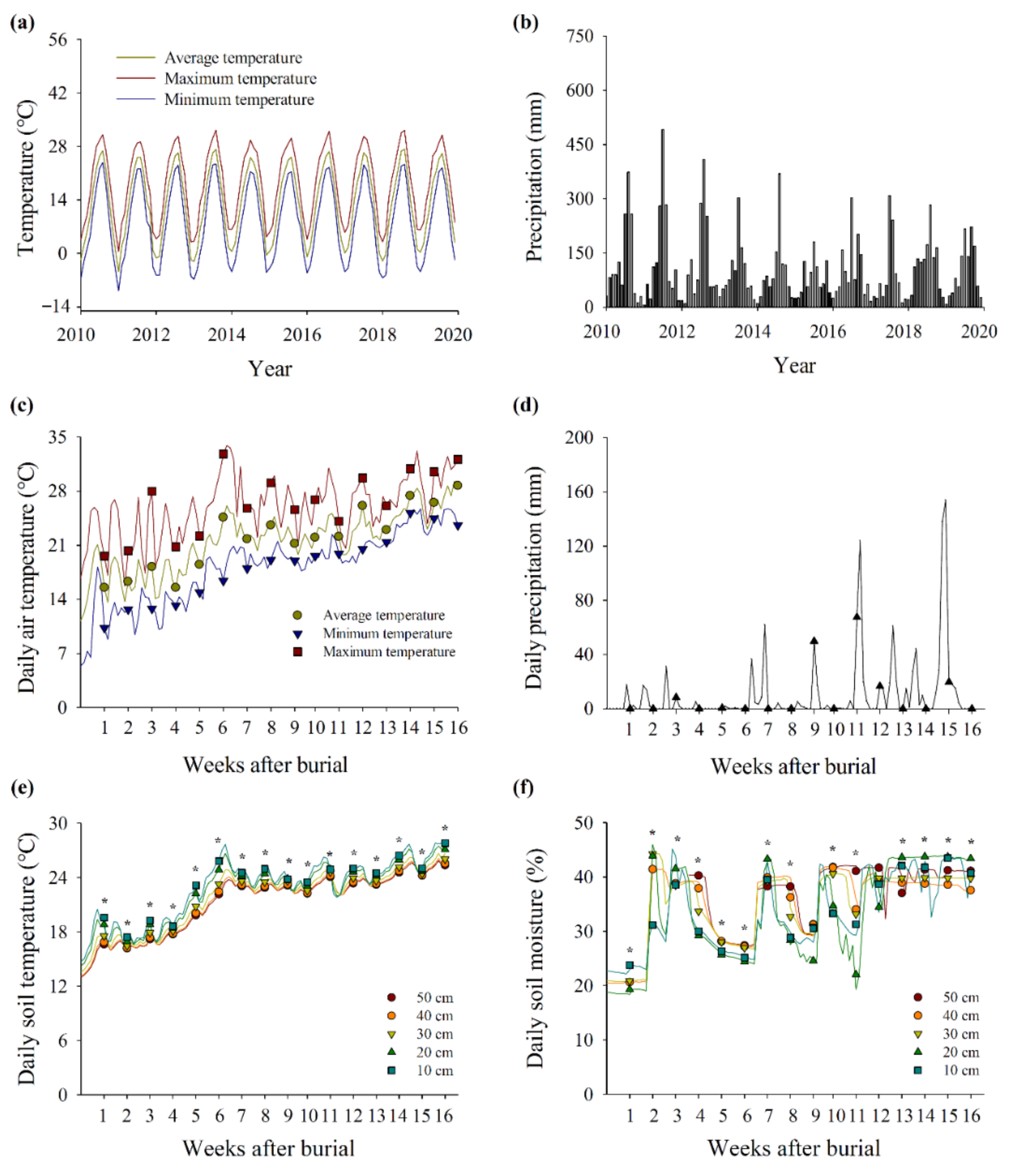
3.2. Viability of Seeds Buried at Various Soil Depths
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Key, S.; Ma, J.K.; Drake, P.M. Genetically modified plants and human health. J. R. Soc. Med. 2008, 101, 290–298. [Google Scholar] [CrossRef]
- United Nations. Cartagena Protocol on Biosafety to the Convention on Biological Diversity; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2000. [Google Scholar]
- MOTIE. Transboundary Movement, etc. of Living Modified Organisms Act (Act No. 6448 of Mar. 28, 2001, as Amended up to Act No. 15181 of Dec. 12, 2017); Ministry of Trade, Industry and Energy: Sejong-si, Korea, 2017. Available online: https://elaw.klri.re.kr/kor_mobile/viewer.do?hseq=46334&type=lawname&key=Transboundary+Movement%2C+Etc.+of+Living+Modified+Organisms+Act (accessed on 21 September 2020).
- Lee, J.R.; Choi, W.; Jo, B.H.; Kim, I.R.; Moon, J.C.; Shin, S.Y.; Seol, M.A.; Eum, S.J.; Song, H.R. Ministry of Environment’s Guide to Safety Management of Living Modified Organisms; The Ministry of Environment National Institute of Ecology: Seocheon, Korea, 2015.
- Nam, K.H.; Han, S.M.; Lee, J.R.; Park, J.H.; Choi, W.; Jung, Y.J.; Kim, D.W.; Lim, H.S.; Yoo, S.H.; Kim, I.R.; et al. Establishment of LMO Risk Assessment System and Operation of the Institute of LMO Risk Assessment under the Jurisdiction of the Ministry of Environment; National Institute of Ecology: Seocheon-gun, Korea, 2019. [Google Scholar]
- KBCH. Import and Export Status; Korea Biosafety Clearing House: Daejeon, Korea, 2020; Available online: https://www.biosafety.or.kr/sub/info.do?m=030202&s=kbch (accessed on 21 September 2020).
- Kim, C.G.; Yi, H.; Park, S.; Yeon, J.E.; Kim, D.Y.; Kim, D.I.; Lee, K.H.; Lee, T.C.; Paek, I.S.; Yoon, K.W.; et al. Monitoring the occurrence of genetically modified soybean and maize around cultivated fields and at a grain receiving port in Korea. J. Plant Biol. 2006, 49, 218–223. [Google Scholar] [CrossRef]
- Lee, B.; Kim, C.G.; Park, J.Y.; Park, K.W.; Kim, H.J.; Yi, H.; Jeong, S.C.; Yoon, W.K.; Kim, H.M. Monitoring the occurrence of genetically modified soybean and maize in cultivated fields and along the transportation routes of the Incheon port in South Korea. Food Control 2009, 20, 250–254. [Google Scholar] [CrossRef]
- Park, K.W.; Lee, B.; Kim, C.G.; Kim, D.Y.; Park, J.Y.; Ko, E.M.; Jeong, S.C.; Choi, K.H.; Yoon, W.K.; Kim, H.M. Monitoring the occurrence of genetically modified maize at a grain receiving port and along transportation routes in the Republic of Korea. Food Control 2010, 21, 456–461. [Google Scholar] [CrossRef]
- Lee, J.R.; Lim, H.S.; Kim, I.R.; Choi, W.; Park, J.H.; Jung, Y.J.; Kim, D.W.; Eum, S.J.; Seol, M.A.; Hwang, J.E. Study on Environmental Monitoring and Post-Management of LMO; National Institute of Ecology: Seocheon-gun, Korea, 2018. [Google Scholar]
- KBCH. Biosafety Vol.18 No.2; Korea Biosafety Clearing House: Daejeon, Korea, 2017. [Google Scholar]
- KBCH. Biosafety Vol.18 No.3; Korea Biosafety Clearing House: Daejeon, Korea, 2017. [Google Scholar]
- Conner, A.J.; Glare, T.R.; Nap, J.P. The release of genetically modified crops into the environment. Plant J. 2003, 33, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Ammann, K.; Jacot, Y.; Al Mazyad, P.R. Weediness in the light of new transgenic crops and their potential hybrids. J. Plant Dis. Prot. 2000, 17, 19–29. [Google Scholar]
- Raybould, A. The bucket and the searchlight: Formulating and testing risk hypotheses about the weediness and invasiveness potential of transgenic crops. Environ. Biosaf. Res. 2010, 9, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Moody-Weis, J.; Alexander, H.M. The mechanisms and consequences of seed bank formation in wild sunflowers (Helianthus annuus). J. Ecol. 2007, 95, 851–864. [Google Scholar] [CrossRef]
- Warwick, S.I.; Beckie, H.J.; Hall, L.M. Gene flow, invasiveness, and ecological impact of genetically modified crops. Ann. N. Y. Acad. Sci. 2009, 1168, 72–99. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Ventura, L.; Donà, M.; Macovei, A.; Carbonera, D.; Buttafava, A.; Mondoni, A.; Rossi, G.; Balestrazzi, A. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 2012, 60, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ning, F.; Hu, X.; Wang, W. Genetic modification for improving seed vigor is transitioning from model plants to crop plants. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Rugh, C.L.; Senecoff, J.F.; Meagher, R.B.; Merkle, S.A. Development of transgenic yellow poplar for mercury phytoremediation. Nat. Biotechnol. 1998, 16, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Huysen, T.; Terry, N.; Pilson-Smits, E.A.H. Exploring the selenium phytoremediation potential of transgenic Indian mustard overexpressing ATP sulfurylase or cystathionine-γ-synthase. Int. J. Phytoremed. 2004, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Eapen, S.; D’souza, S.F. Prospects of genetic engineering of plants for phytoremediation of toxic metals. Biotechnol. Adv. 2005, 23, 97–114. [Google Scholar] [CrossRef]
- Hussein, H.S.; Ruiz, O.N.; Terry, N.; Daniell, H. Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: Enhanced root uptake, translocation to shoots and volatilization. Environ. Sci. Technol. 2007, 41, 8439–8446. [Google Scholar] [CrossRef]
- Peuke, A.D.; Rennenberg, H. Phytoremediation: Molecular biology, requirements for application, environmental protection, public attention and feasibility. EMBO Rep. 2005, 6, 497–501. [Google Scholar] [CrossRef]
- Bañuelos, G.; Terry, N.; LeDuc, D.L.; Pilon-Smits, E.A.H.; Markey, B. Field trial of transgenic Indian mustard plants shoes enhanced phytoremediation of selenium-contaminated sediment. Environ. Sci. Technol. 2005, 39, 1771–1777. [Google Scholar] [CrossRef]
- Bañuelos, G.; Danika, L.; LeDuc, D.L.; Pilon-Smits, E.A.H.; Terry, N. Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ. Sci. Technol. 2007, 41, 599–605. [Google Scholar] [CrossRef]
- James, C.A.; Xin, G.; Doty, S.L.; Muiznieks, I.; Newman, L.; Strand, S.E. A mass balance study of the phytoremediation of perchloroethylene-contaminated groundwater. Environ. Pollut. 2009, 157, 2564–2569. [Google Scholar] [CrossRef]
- Legault, E.K.; James, C.A.; Stewart, K.; Muiznieks, I.; Doty, S.L.; Strand, S.E. A field trail of TCE phytoremediation by genetically modified poplars expressing cytochrome P450 2E1. Environ. Sci. Technol. 2017, 51, 6090–6099. [Google Scholar] [CrossRef] [PubMed]
- Hur, M.; Kim, Y.; Song, H.R.; Kim, J.M.; Choi, Y.I.; Yi, H. Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl. Environ. Microbiol. 2011, 77, 7611–7619. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.; Kim, S.; Choi, Y.I.; Song, W.Y.; Park, J.; Youk, E.S.; Jeong, S.C.; Martinoia, E.; Noh, E.W.; Lee, Y. Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere 2013, 90, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Dushenkov, S.; Vasudev, D.; Kapulnik, Y.; Gleba, D.; Fleisher, D.; Ting, K.C.; Ensley, B. Removal of uranium from water using terrestrial plants. Environ. Sci. Technol. 1997, 31, 3468–3474. [Google Scholar] [CrossRef]
- January, M.C.; Cutright, T.J.; Van Keulen, H.; Wei, R. Hydroponic phytoremediation of Cd, Cr, Ni, As, and Fe: Can Helianthus annuus hyperaccumulate multiple heavy metals? Chemosphere 2008, 70, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V. Sunflower (Helinathus annuus L.)—A potential crop for environmental industry. Helia 2007, 30, 167–174. [Google Scholar]
- Alexander, H.M.; Schrag, A.M. Role of soil seed banks and newly dispersed seeds in population dynamics of the annual sunflower, Helianthus annuus. J. Ecol. 2003, 91, 987–998. [Google Scholar] [CrossRef]
- Fernández-Martínez, J.M.; Pérez-Vich, B.; Velasco, L. Sunflower. In Oil Crops; Vollmann, J., Rajcan, I., Eds.; Springer: New York, NY, USA, 2009; pp. 155–232. [Google Scholar]
- Gupta, R.K.; Das, S.K. Physical properties of sunflower seeds. J. Agric. Eng. Res. 1997, 66, 1–8. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing, 2010 ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2010. [Google Scholar]
- KMA. Synoptic Weather Observation; Korea Meteorological Administration: Seoul, Korea, 2020; Available online: https://data.kma.go.kr/data/grnd/selectAsosRltmList.do (accessed on 21 September 2020).
- KMA. Statistics by Condition; Korea Meteorological Administration: Seoul, Korea, 2020; Available online: https://data.kma.go.kr/climate/RankState/selectRankStatisticsDivisionList.do?pgmNo=179 (accessed on 21 September 2020).
- NIAST. Methods of Soil and Plant Analysis; National Institute of Agricultural Science and Technology: Suwon, Korea, 2000. [Google Scholar]
- Mercer, K.L.; Shaw, R.G.; Wyse, D.L. Increased germination of diverse crop-wild hybrid sunflower seeds. Ecol. Appl. 2006, 16, 845–854. [Google Scholar] [CrossRef][Green Version]
- Roberts, E.H. Temperature and seed germination. In Plants and Temperature; Long, S.P., Woodword, F.I., Eds.; Symposia of the Society for Experimental Biology, Company of Biologists: Cambridge, UK, 1988; pp. 109–132. [Google Scholar]
- Gay, C.; Corbineau, F.; Côme, D. Effects of temperature and oxygen on seed germination and seedling growth in sunflower (Helianthus annuus L.). Envrion. Exp. Bot. 1991, 31, 193–200. [Google Scholar] [CrossRef]
- Bakker, R.M.; Bakker, J.P.; Grandin, U.; Kalamees, R.; Milberg, P.; Poschlod, P.; Thompson, K.; Willems, J.H. Seed size, shape and vertical distribution in the soil: Indicators of seed longevity. Funct. Ecol. 1998, 12, 834–842. [Google Scholar] [CrossRef]
- Tanveer, A.; Tasneem, M.; Khaliq, A.; Javaid, M.M.; Chaudhry, M.N. Influence of seed size and ecological factors on the germination and emergence of field bindweed (Convolvulus arvensis). Planta Daninha 2013, 31, 39–51. [Google Scholar] [CrossRef]
- Leishman, M.R.; Westoby, M. The role of large seed size in shaded conditions: Experimental evidence. Funct. Ecol. 1994, 8, 205–214. [Google Scholar] [CrossRef]
- Khalifa, F.M.; Schneiter, A.A.; El Tayeb, E.I. Temperature-germination responses of sunflower (Helianthus annuus L.) genotypes. Helia 2000, 23, 97–104. [Google Scholar]
- Roberts, H.A. Seed banks in soils. Adv. Appl. Biol. 1981, 6, 1–55. [Google Scholar]
- Murdoch, A.J.; Ellis, R.H. Dormancy, viability and longevity. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 183–214. [Google Scholar]
- Traba, J.; Azcárate, F.M.; Peco, B. From what depth do seeds emerge? A soil seed bank experiment with Mediterranean grassland species. Seed Sci. Res. 2004, 14, 297–303. [Google Scholar] [CrossRef]
- Vleeshouwers, L.M. Modelling the effect of temperature, soil penetration resistance, burial depth and seed weight on pre-emergence growth of weeds. Ann. Bot. 1997, 79, 553–563. [Google Scholar] [CrossRef]
- Benvenuti, S.; Macchia, M.; Miele, S. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Sci. 2001, 49, 528–535. [Google Scholar] [CrossRef]
- Pekrun, C.; Hewitt, J.D.J.; Lutman, P.J.W. Cultural control of volunteer oilseed rape (Brassica napus). J. Agric. Sci. 1998, 130, 155–163. [Google Scholar] [CrossRef]
- Umurzokov, M.; Jia, W.; Cho, K.M.; Khaitov, B.; Sohn, S.I.; Cho, J.W.; Park, K.W. Persistence, Viability and Emergence Rate of Canola (Brassica napus L) in Korean Soil. Weed Turfgrass Sci. 2019, 8, 309–318. [Google Scholar]
- Benvenuti, S.; Macchia, M. Effect of hypoxia on buried weed seed germination. Weed Res. 1995, 35, 343–351. [Google Scholar] [CrossRef]
- Karssen, C.M. Environmental conditions and endogenous mechanisms involved in secondary dormancy of seeds. Isr. J. Plant Sci. 1980, 29, 45–64. [Google Scholar]
- Gulden, R.H.; Thomas, A.G.; Shirtliffe, S.J. Secondary dormancy, temperature, and burial depth regulate seedbank dynamics in canola. Weed Sci. 2004, 52, 382–388. [Google Scholar] [CrossRef]
- Chantre, G.R.; Sabbatini, M.R.; Orioli, G.A. Effect of burial depth and soil water regime on the fate of Lithospermum arvense seeds in relation to burial time. Weed Res. 2009, 49, 81–89. [Google Scholar] [CrossRef]
- Kremer, R.J. Management of weed seed banks with microorganisms. Ecol. Appl. 1993, 3, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Squartini, A.; Concheri, G.; Stevanato, P.; Zanin, G.; Masin, R. Weed seed decay in no-till field and planted riparian buffer zone. Plants 2020, 9, 293. [Google Scholar] [CrossRef]
- Blaney, C.S.; Kotanen, P.M. Persistence in the seed bank: The effects of fungi and invertebrates on seeds of native and exotic plants. Ecoscience 2002, 9, 509–517. [Google Scholar] [CrossRef]
- Wagner, M.; Mitschunas, N. Fungal effects on seed bank persistence and potential applications in weed biocontrol: A review. Basic Appl. Ecol. 2008, 9, 191–203. [Google Scholar] [CrossRef]
- Seiwa, K.; Watanabe, A.; Saitoh, T.; Kannu, H.; Akasaka, S. Effects of burying depth and seed size on seedling establishment of Japanese chestnuts, Castanea crenata. For. Ecol. Manag. 2002, 164, 149–156. [Google Scholar] [CrossRef]
- Ahmed, T.A.M.; Mutwali, E.M.; Salih, E.A. The Effect of Seed Size and Burial Depth on the Germination, Growth and Yield of Sunflower (Helianthus annus L.). Am. Sci. Res. J. Eng. Technol. Sci. 2019, 53, 75–82. [Google Scholar]
- Yanful, M.; Maun, M.A. Effects of burial of seeds and seedlings from different seed sizes on the emergence and growth of Strophostyles helvola. Can. J. Bot. 1996, 74, 1322–1330. [Google Scholar] [CrossRef]
- Mtambalika, K.; Munthali, C.; Gondwe, D.; Missanjo, E. Effect of seed size of Afzelia quanzensis on germination and seedling growth. Int. J. For. Res. 2014. [Google Scholar] [CrossRef]


| Cultivars | Seeds | Seed Category 1 | |||
|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Thickness (mm) | Weight (mg) | ||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Jaeraejongja | 12.3 ± 0.6 a | 6.0 ± 0.9 a | 3.9 ± 0.3 a | 84.5 ± 18.3 a | Large |
| Jaeraejong | 9.5 ± 0.1 b | 4.4 ± 0.6 b | 2.6 ± 0.4 c | 40.8 ± 10.9 c | Medium |
| Jaeraejong1 | 7.9 ± 0.9 d | 4.3 ± 1.0 b | 3.0 ± 0.6 b | 38.1 ± 13.0 c | Small |
| Jaeraejong2 | 9.2 ± 1.1 c | 4.4 ± 0.8 b | 3.1 ± 0.5 b | 49.6 ± 15.9 b | Medium |
| Jaeraejong3 | 6.6 ± 0.7 e | 3.4 ± 0.5 c | 2.4 ± 0.4 d | 20.6 ± 5.2 d | Small |
| Soil Characteristics | p-Value | Soil Depth (cm) | ||
|---|---|---|---|---|
| 5 | 15 | 30 | ||
| Moisture (%) | 0.285 | 12.2 ± 0.3 | 13.3 ± 2.9 | 10.8 ± 0.4 |
| pH | 0.086 | 6.2 ± 0.1 | 6.3 ± 0.1 | 6.4 ± 0.1 |
| EC (ds m−1) | 0.706 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Organic matter (%) | 0.399 | 4.4 ± 0.7 | 4.0 ± 0.2 | 4.8 ± 0.8 |
| Total N (mg kg−1) | 0.330 | 580.9 ± 204.6 | 424.9 ± 185.3 | 363.4 ± 91.0 |
| P2O5 (mg kg−1) | 0.677 | 19.0 ± 7.6 | 23.0 ± 7.7 | 23.6 ± 4.0 |
| CEC (cmol+ kg−1) | 0.479 | 13.7 ± 1.6 | 12.4 ± 0.8 | 12.7 ± 1.3 |
| Exchangeable Ca (cmol+ kg−1) | 0.748 | 6.7 ± 1.0 | 6.4 ± 0.4 | 6.3 ± 0.4 |
| Exchangeable K (cmol+ kg−1) | 0.313 | 0.4 ± 0.3 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Exchangeable Mg (cmol+ kg−1) | 0.978 | 4.5 ± 0.8 | 4.4 ± 0.4 | 4.4 ± 0.3 |
| Exchangeable Na (cmol+ kg−1) | 0.784 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Sand (%) | 0.501 | 56.3 ± 3.1 | 57.8 ± 2.1 | 55.5 ± 1.3 |
| Silt (%) | 0.604 | 26.3 ± 2.0 | 24.6 ± 3.0 | 25.9 ± 0.8 |
| Clay (%) | 0.397 | 17.3 ± 1.2 | 17.7 ± 1.5 | 18.7 ± 0.6 |
| Soil texture | Sandy Loam | Sandy Loam | Sandy Loam | |
| Source | SS 1 | DF 2 | MS 3 | F4 | p5 |
|---|---|---|---|---|---|
| Duration of burial | 103,582.9 | 6 | 17263.8 | 3061.6 | <0.001 |
| Cultivar | 1676.6 | 1 | 1676.6 | 297.3 | <0.001 |
| Depth of burial | 3993.4 | 5 | 798.7 | 141.6 | <0.001 |
| Duration × cultivar | 4293.7 | 6 | 715.6 | 126.9 | <0.001 |
| Duration × depth | 11,482.1 | 30 | 382.7 | 67.8 | <0.001 |
| Cultivar × depth | 446.5 | 5 | 89.3 | 15.8 | <0.001 |
| Duration × cultivar × depth | 5928.5 | 30 | 197.6 | 35.0 | <0.001 |
| Error | 947.3 | 168 | 5.6 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.-H.; Han, S.M. Seed Germination of Sunflower as a Case Study for the Risk Assessment and Management of Transgenic Plants Used for Environmental Remediation in South Korea. Sustainability 2020, 12, 10110. https://doi.org/10.3390/su122310110
Nam K-H, Han SM. Seed Germination of Sunflower as a Case Study for the Risk Assessment and Management of Transgenic Plants Used for Environmental Remediation in South Korea. Sustainability. 2020; 12(23):10110. https://doi.org/10.3390/su122310110
Chicago/Turabian StyleNam, Kyong-Hee, and Sung Min Han. 2020. "Seed Germination of Sunflower as a Case Study for the Risk Assessment and Management of Transgenic Plants Used for Environmental Remediation in South Korea" Sustainability 12, no. 23: 10110. https://doi.org/10.3390/su122310110
APA StyleNam, K.-H., & Han, S. M. (2020). Seed Germination of Sunflower as a Case Study for the Risk Assessment and Management of Transgenic Plants Used for Environmental Remediation in South Korea. Sustainability, 12(23), 10110. https://doi.org/10.3390/su122310110




