How to Choose a Hydrological Recovery Mode for Degraded Semiarid Wetland in China? A Case Study on Restoration of Phragmites australis Saline-Alkaline Wetland
Abstract
:1. Introduction
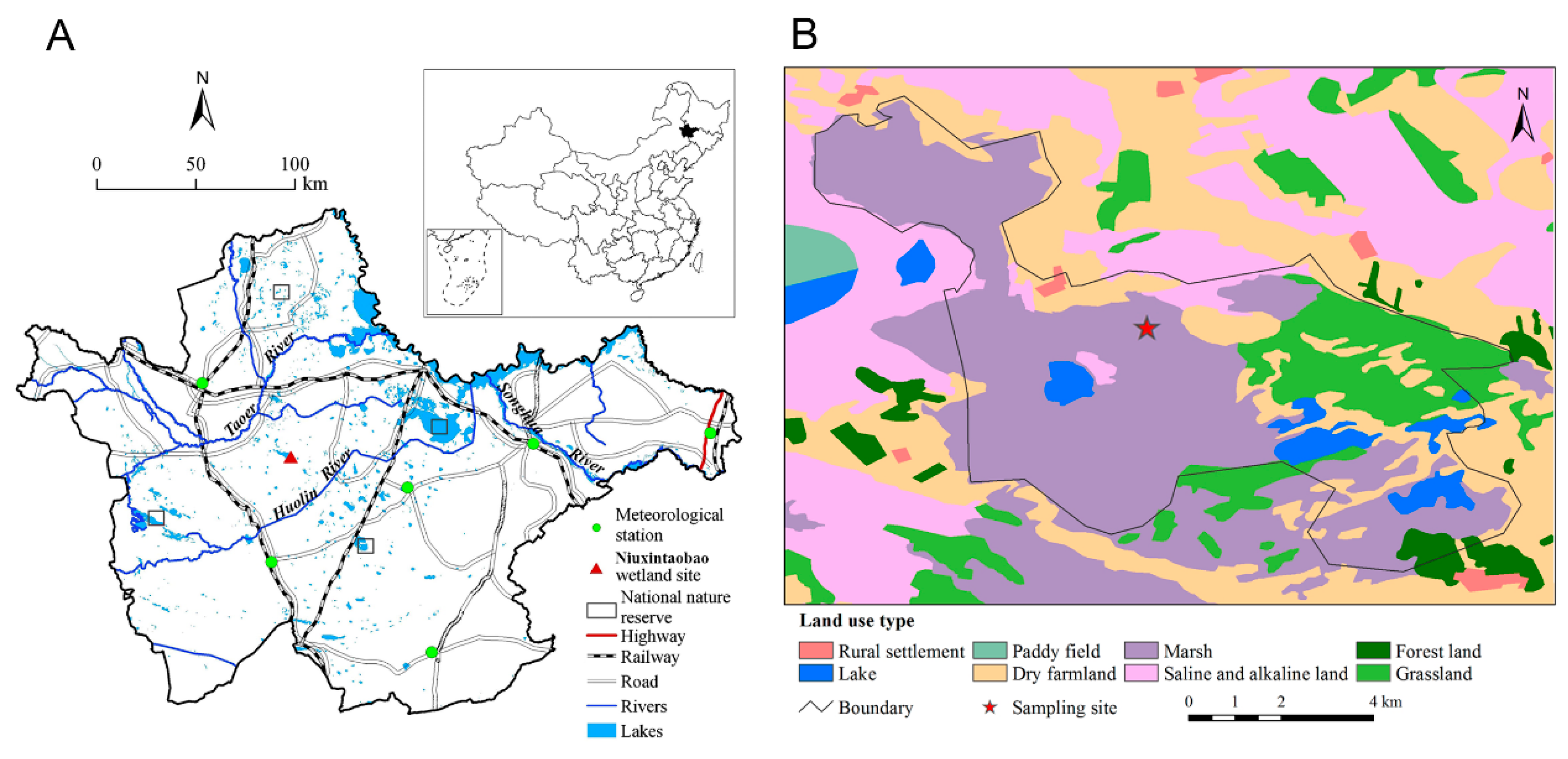
2. Materials and Methods
2.1. Study Materials
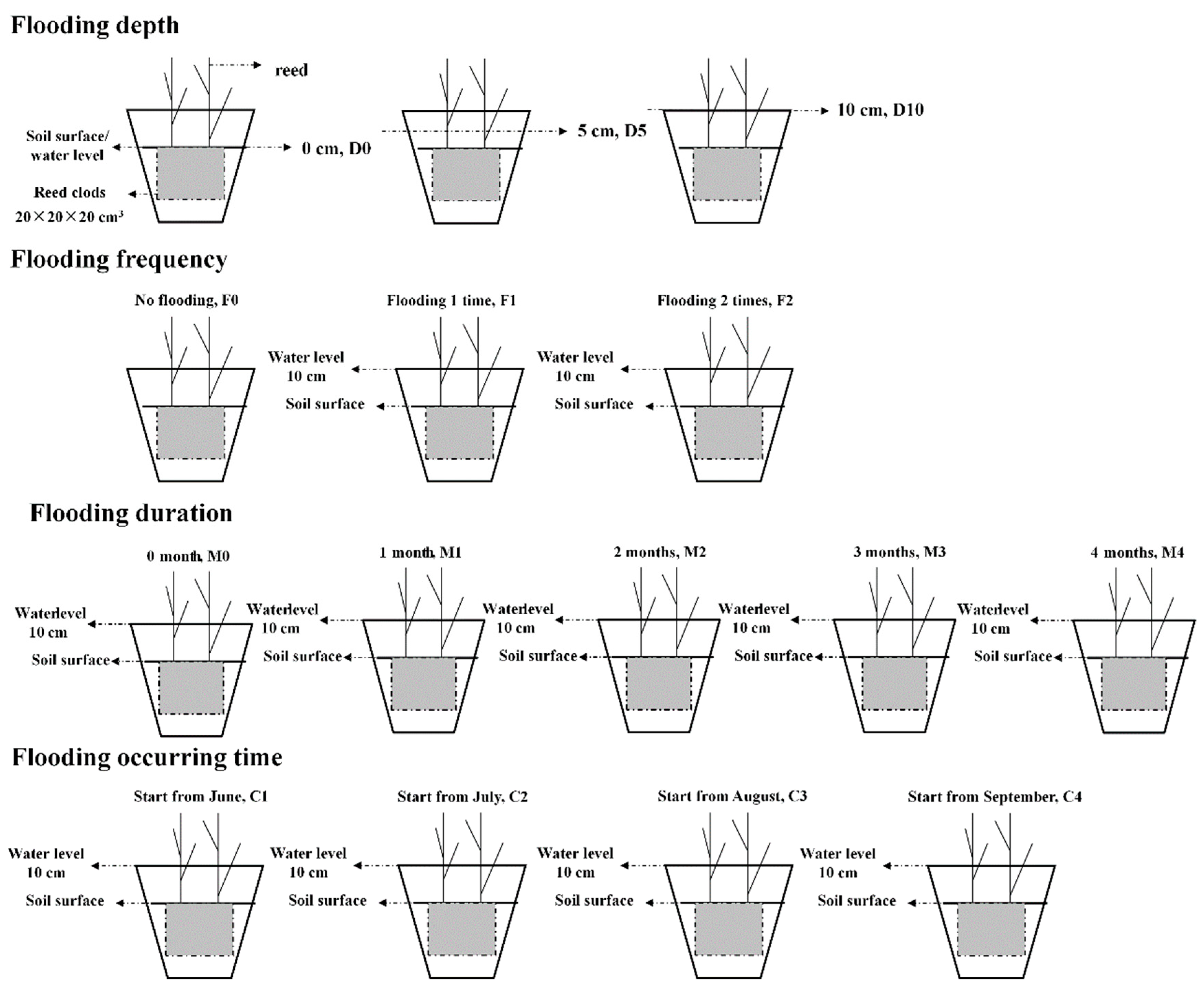
2.2. Flooding Treatments
2.3. Sampling and Determination
2.4. Statistical Analyses
3. Results
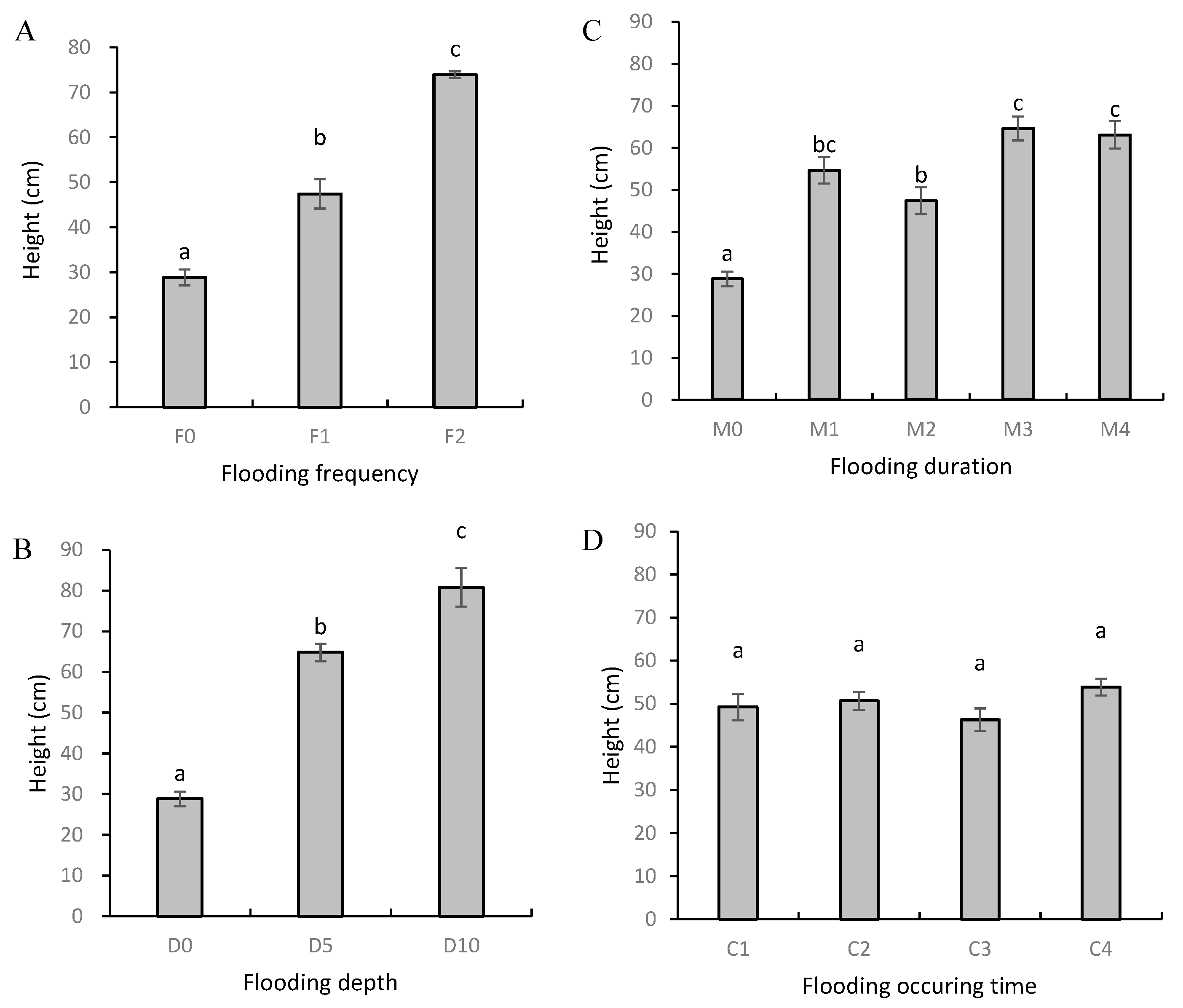
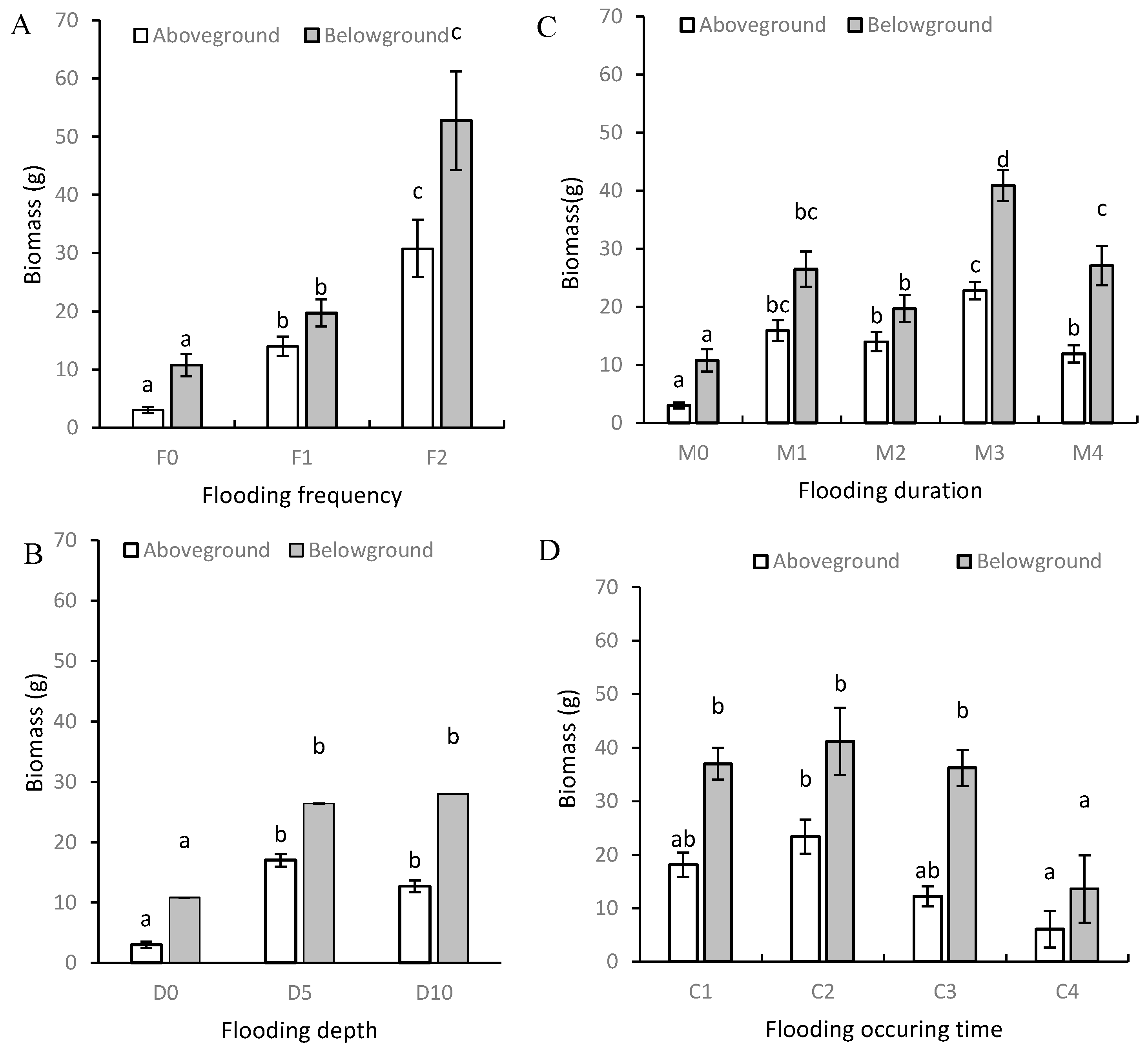
3.1. Plant Height and Biomass
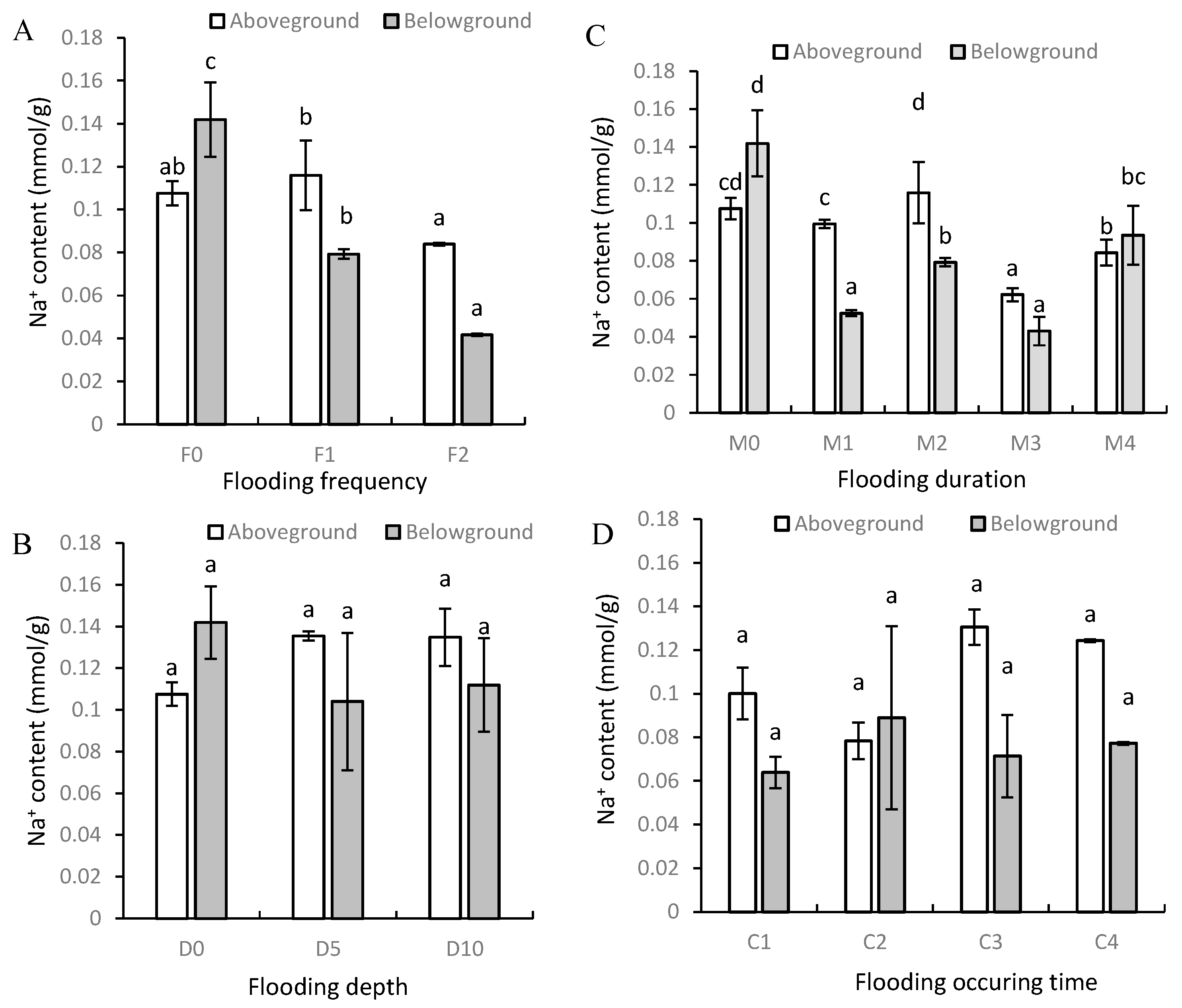
3.2. Na+ and K+ Contents
3.3. Photosynthetic Parameters and WUE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, P.; Mitsch, W.J. Wetland restoration and ecological engineering: International conference of wetland restoration and ecological engineering. Ecol. Eng. 2009, 35, 437–441. [Google Scholar] [CrossRef]
- Bai, J.; Cui, B.; Cao, H.; Li, A.; Zhang, B. Wetlands degradation and ecological restoration. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley and Sons: New York, NY, USA, 2015. [Google Scholar]
- Sullivan, P.L.; Gaiser, E.E.; Surratt, D.; Rudnick, D.T.; Davis, S.E.; Sklar, F.H. Wetland ecosystem response to hydrologic restoration and management: The Everglades and its urban-agricultural boundary (FL, U.S.A.). Wetlands 2014, 34, 1–8. [Google Scholar] [CrossRef]
- Miller, R.L.; Fujii, R. Plant community, primary productivity, and environmental conditions following wetland re-establishment in the Sacramento-San Joaquin Delta, California. Wetl. Ecol. Manag. 2010, 18, 1–16. [Google Scholar] [CrossRef]
- Wen, B.L.; Liu, X.T.; Li, X.J.; Yang, F.Y.; Li, X.Y. Restoration and rational use of degraded saline reed wetlands: A case study in western Songnen Plain, China. Chin. Geogra Sci. 2012, 22, 1–11. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, X.T.; Li, X.J.; Lin, J.; Wen, B. Growth and physiological response of organs of Phragmites australis to different water compensation in degraded wetlands. Wetl. Sci. 2012, 10, 23–31, (English edition of Chinses Journal). [Google Scholar]
- Manivannan, P.; Jaleel, C.A.; Kishorekumar, A.; Sankar, B.; Somasundaram, R.; Sridharan, R.; Panneerselvam, R. Changes in antioxidant metabolism of Vigna unguiculate (L.) Walp. by propiconazole under water deficit stress. Colloids Surf. B Biointerfaces 2007, 57, 69–74. [Google Scholar] [CrossRef]
- Yang, F.Y.; Li, X.J.; Liu, X.T.; Sun, L. Recovery pattern of degradation saline-alkaline reed wetland in the Songnen Plain. Wetl. Sci. 2009, 7, 306–313, (In Chinese with English Abstract). [Google Scholar]
- Epting, R.J.; Robison, C.P.; Reddi, R.C. Gauge Record Hydrologic Statistics: Indicators for Lake Classification. Environ. Bioindic. 2008, 3, 193–204. [Google Scholar] [CrossRef]
- Barrett, R.; Nielsen, D.L.; Croome, R. Associations between the plant communities of floodplain wetlands, water regime and wetland type. River Res. Appl. 2010, 26, 866–876. [Google Scholar] [CrossRef]
- Toth, L.A. Restoration response of relict broadleaf marshes to increased water depths. Wetlands 2010, 30, 263–274. [Google Scholar] [CrossRef]
- Jackson, M.B.; Armstrong, W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant. Biol. 1999, 1, 274–287. [Google Scholar] [CrossRef]
- Li, X.Y.; Wen, B.L.; Yang, F.; Hartley, A.; Li, X. Effects of alternate flooding-drought conditions on degenerated Phragmites australis salt marsh in Northeast China. Restor. Ecol. 2017, 25, 810–819. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, G.; Liu, Q. The study of salt-affected soils in the Yellow River delta based on remote sensing. J. Remote Sens. 2001, 5, 46–52. [Google Scholar]
- Li, Y.Y.; Zhao, K.; Ding, Y.L.; Ren, J.H. An empirical method for soil salinity and moisture inversion in west of Jilin. In 2013 the International Conference on Remote Sensing, Environment and Transportation Engineering (RSETE 2013), Nanjing, China, 26 July 2013; Atlantis Press: Paris, France, 2013; pp. 19–21. [Google Scholar]
- Qiu, S.W.; Wang, X.K.; Zhang, S.Q.; Lian, Y.; Zhang, Z.Q.; Zhu, J.H. The evolution of the large paleolake in Songnen Plain and its formation. Quat. Sci. 2012, 32, 1011–1021, (In Chinese with English Abstract). [Google Scholar]
- Yang, B.G.; Sun, Z.C.; Lv, J.F. The changes of Songhua-Liao River system. Geogr. Res. 1983, 2, 48–56, (Chinese with English Abstract). [Google Scholar]
- Liu, X. Management on Degraded Land and Agricultural Development in the Songnen Plain; Science Press: Beijing, China, 2001; p. 64. [Google Scholar]
- Wang, Z.; Huang, N.; Luo, L.; Li, X.; Ren, C.; Song, K.; Chen, J.M. Shrinkage and fragmentation of marshes in the West Songnen Plain, China, from 1954 to 2008 and its possible causes. Int. J. Appl. Earth Obs. 2011, 13, 477–486. [Google Scholar] [CrossRef]
- Pagter, M.; Bragato, C.; Brix, H. Tolerance and physiological responses of Phragmites australis to water deficit. Aquat. Bot. 2005, 81, 285–299. [Google Scholar] [CrossRef]
- Mauchamp, A.; Mehty, M. Submergence-induced damage of photosynthetic apparatus in Phragmites australis. Environ. Exp. Bot. 2004, 51, 227–235. [Google Scholar] [CrossRef]
- Vretare, V.; Weisner, S.E.B.; Strand, J.A.; Granéli, W. Phenotypic plasticity in Phragmites australis as a functional response to water depth. Aquat. Bot. 2001, 69, 263–274. [Google Scholar] [CrossRef]
- Engloner, A.I. Annual growth dynamics and morphological differences of reed (Phragmites australis [Cav.] Trin. Ex Steudel) in relation to water supply. Flora 2004, 199, 256–262. [Google Scholar] [CrossRef]
- Wang, X.H.; Tong, S.Z.; Lu, X.G. The ecological characteristics dynamic of Phragmites australis community in wetland of Semi-arid areas-A case of Momoge Wetland. Wetl. Sci. 2008, 6, 386–391, (In Chinese with English Abstract). [Google Scholar]
- Li, X.Y.; Qi, M.M.; Li, C.; Li, X.J.; Liu, X.T.; Wang, Y.; Lin, J. Effects of flooding occurrence time and duration on P. australis growth and soil physical-chemical characteristics in degraded inland marshes. Res. Soil Water Conserv. 2016, 23, 83–89, (In Chinese with English abstract). [Google Scholar]
- Sloey, T.M.; Howard, R.J.; Hester, M.W. Response of Schoenoplectus acutus and Schoenoplectus californicus at different life-history stages to hydrologic regime. Wetlands 2016, 36, 37–46. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, J.J.; Zhang, Y.T.; Ma, C.S. Physiological responses and adaptive strategies of wheat seedlings to salt and alkali stresses. Soil Sci. Plant. Nutr. 2009, 55, 680–684. [Google Scholar] [CrossRef]
- Haslam, S.M. Biological flora of the British Isles Phragmites communis Trin. J. Ecol. 1972, 60, 585–610. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotox. Environ. Safe. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Vivian, L.M.; Godfree, R.C.; Colloff, M.J.; Mayence, C.E.; Marshall, D.J. Wetland plant growth under contrasting water regimes associated with river regulation and drought: Implications for environmental water management. Plant. Ecol. 2014, 215, 997–1011. [Google Scholar] [CrossRef]
- Casanova, M.T.; Brock, M.A. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities. Plant. Ecol. 2000, 147, 237–250. [Google Scholar] [CrossRef]
- Tartari, A.; Forlani, G. Osmotic adjustments in a psychrophilic alga, Xanthonema sp. (Xanthophyceae). Environ. Exp. Bot. 2008, 63, 342–350. [Google Scholar] [CrossRef]
- Zhu, G.L.; Deng, X.W.; Zuo, W.N. Determination of free proline in plants. Plant. Physiol. Commun. 1983, 1, 35–37. [Google Scholar]
- Li, X.Y.; Mu, C.S.; Lin, J.X.; Wang, Y.; Li, X.J. Effect of alkaline potassium and sodium salts on growth, photosynthesis, ions absorption and solutes synthesis of wheat seedlings. Exp. Agr. 2014, 50, 144–157. [Google Scholar] [CrossRef]
- Ashraf, M.; Rahmatullah; Ahmad, R.; Bhatti, A.S.; Afzal, M.; Sarwar, A.; Maqsood, M.A.; Kanwal, S. Amelioration of salt stress in Sugar cane (Saccharum officinarum L.) by supplying potassium and siliconin hydroponics. Pedosphere 2010, 20, 153–162. [Google Scholar] [CrossRef]
- Centritto, M.; Loreto, F. Photosynthesis in a changing world: Photosynthesis and abiotic stresses. Agr. Ecosyst. Environ. 2005, 106, 115–117. [Google Scholar] [CrossRef]
- Lessmann, J.M.; Brix, H.; Bauer, V.; Clevering, O.A.; Comıín, F.A. Effect of climatic gradients on the photosynthetic responses of four Phragmites australis populations. Aquat. Bot. 2001, 69, 109–126. [Google Scholar] [CrossRef]
- Gorai, M.; Ennajeh, M.; Song, J.; Khemira, H.; Neffati, M. Changes in leaf gas exchange, water relations, biomass production and solute accumulation in Phragmites australis under hypoxic conditions. Acta Ecol. Sin. 2011, 31, 97–102. [Google Scholar] [CrossRef]
- Zheng, W.J.; Zheng, X.P.; Zhang, C.L. A survey of photosynthetic carbon metabolism in 4 ecotypes of Phragmites australis in northwest China: Leaf anatomy, ultrastructure, and activities of ribulose1, 5- bisphosphate carboxylase, phosphoenolpyruvate carboxylase and glycollate oxidase. Physiol. Plant. 2000, 110, 201–208. [Google Scholar] [CrossRef]
- Gong, C.M.; Bai, J.; Deng, J.M.; Wang, G.X.; Liu, X.P. Leaf anatomy and photosynthetic carbon metabolic characteristic in Phragmites communis in different soil water availability. Plant. Ecol. 2011, 212, 675–687. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Wang, S.M.; Zhang, C.L. Composition and characteristic difference in photosynthetic membranes of two ecotypes of Reed (Phragmites communis L.) from different habitats. Photosynthestica 2003, 41, 97–104. [Google Scholar] [CrossRef]
- Clevering, O.A.; Lissner, J. Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquat. Bot. 1999, 64, 185–208. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, M.; Tong, S.; Zhang, W.; Wu, H.; Liu, Y.; Lu, X. Differential flooding impacts on Echinochloa caudata and Scirpus planiculmis: Implications for weed control in wetlands. Wetlands 2016, 36, 979–984. [Google Scholar] [CrossRef]

| Flooding Frequency | Flooding Depth | Flooding Duration | Flooding Occurring Time | |
|---|---|---|---|---|
| Height | ** | ** | ** | ns |
| Biomass (A) | ** | ** | ** | ** |
| Biomass (B) | ** | ** | ** | ** |
| Na+ (A) | ** | ns | ** | ns |
| Na+ (B) | ** | ns | ** | ns |
| K+ (A) | ** | * | ** | ** |
| K+ (B) | ** | ns | ** | ns |
| Pn | ** | ** | ** | ** |
| Gs | ** | ** | ** | ** |
| E | ** | ** | ** | ** |
| Water use efficiency (WUE) | ** | ** | ** | ** |
| Pn (μmol CO2 m−2 s−1) | Gs (mmol H2O m−2 s−1) | E (mmol H2O m−2 s−1) | WUE (g/kg) | |
|---|---|---|---|---|
| Flooding Frequency | ||||
| F0 | 14.65 ± 0.65a | 0.19 ± 0.02a | 5.73 ± 0.53a | 2.56 ± 0.13a |
| F1 | 21.54 ± 0.46b | 0.33 ± 0.02b | 8.51 ± 0.39b | 2.93 ± 0.39a |
| F2 | 17.40 ± 0.21c | 0.25 ± 0.01c | 5.53 ± 0.14a | 3.14 ± 0.07b |
| Flooding Depth | ||||
| D0 | 14.65 ± 0.65b | 0.19 ± 0.02c | 5.73 ± 0.53b | 2.56 ± 0.13a |
| D5 | 10.57 ± 0.35a | 0.13 ± 0.00a | 3.81 ± 0.13a | 2.77 ± 0.08a |
| D10 | 10.55 ± 0.33a | 0.15 ± 0.00b | 4.02 ± 0.05a | 2.62 ± 0.08a |
| Flooding Duration | ||||
| M0 | 14.65 ± 2.05c | 0.19 ± 0.05c | 5.73 ± 1.67d | 2.67 ± 0.13b |
| M1 | 8.55 ± 1.36b | 0.03 ± 0.03a | 0.90 ± 0.81a | 11.18 ± 0.58d |
| M2 | 21.54 ± 0.46d | 0.33 ± 0.02d | 8.51 ± 0.39e | 2.93 ± 0.39b |
| M3 | 9.18 ± 0.80b | 0.05 ± 0.01a | 1.61 ± 0.30b | 5.85 ± 0.47c |
| M4 | 4.26 ± 1.83a | 0.16 ± 0.04b | 4.10 ± 0.86c | 0.98 ± 0.07a |
| Flooding Occurring Time | ||||
| C1 | 11.62 ± 0.13b | 0.14 ± 0.01b | 4.02 ± 0.14b | 2.95 ± 0.09c |
| C2 | 16.25 ± 0.26d | 0.30 ± 0.00d | 7.51 ± 0.11d | 2.16 ± 0.01a |
| C3 | 9.73 ± 0.15a | 0.08 ± 0.00a | 2.46 ± 0.08a | 4.01 ± 0.09d |
| C4 | 15.58 ± 0.31c | 0.22 ± 0.01c | 6.24 ± 0.18c | 2.51 ± 0.03b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Li, X.; An, S.; Yang, Y.; Zhang, N.; Du, Z. How to Choose a Hydrological Recovery Mode for Degraded Semiarid Wetland in China? A Case Study on Restoration of Phragmites australis Saline-Alkaline Wetland. Sustainability 2020, 12, 10103. https://doi.org/10.3390/su122310103
Yu S, Li X, An S, Yang Y, Zhang N, Du Z. How to Choose a Hydrological Recovery Mode for Degraded Semiarid Wetland in China? A Case Study on Restoration of Phragmites australis Saline-Alkaline Wetland. Sustainability. 2020; 12(23):10103. https://doi.org/10.3390/su122310103
Chicago/Turabian StyleYu, Shuling, Xiaoyu Li, Subang An, Yanli Yang, Na Zhang, and Zhixin Du. 2020. "How to Choose a Hydrological Recovery Mode for Degraded Semiarid Wetland in China? A Case Study on Restoration of Phragmites australis Saline-Alkaline Wetland" Sustainability 12, no. 23: 10103. https://doi.org/10.3390/su122310103
APA StyleYu, S., Li, X., An, S., Yang, Y., Zhang, N., & Du, Z. (2020). How to Choose a Hydrological Recovery Mode for Degraded Semiarid Wetland in China? A Case Study on Restoration of Phragmites australis Saline-Alkaline Wetland. Sustainability, 12(23), 10103. https://doi.org/10.3390/su122310103





