Formulation of Biochar-Based Phosphorus Fertilizer and Its Impact on Both Soil Properties and Chickpea Growth Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Analysis of Experimental Soil
2.2. Chemical Analysis of Biochar Used for Enrichment
2.3. Preparation and Enrichment of Biochar
2.4. Incubation Experiment
2.5. Pot Trial
2.6. Statistical Analysis
3. Results
3.1. Impact of P-Enriched Biochar on Selected Properties of Incubated Soil
3.2. Impact of P-Enriched Biochar on Growth, Yield and Nodulation of Chickpea
3.2.1. Crop Growth
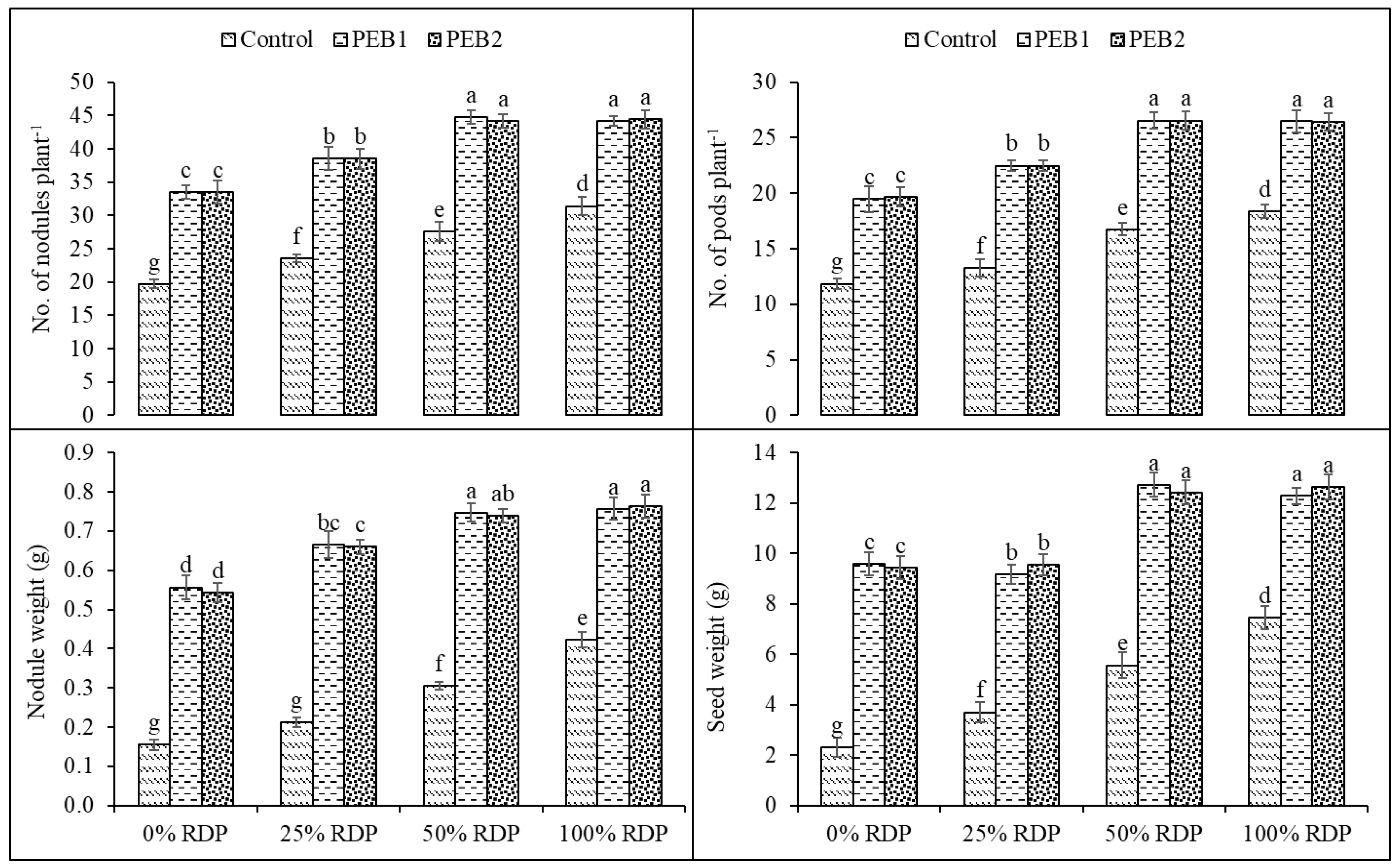
3.2.2. Nodulation
3.2.3. Yield
3.3. Impact of P-Enriched Biochar on Physiological Parameters
3.4. Impact of P-Enriched Biochar on Chemical Parameters
3.5. Impact of P-Enriched Biochar on Post-Harvest Soil Characters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2017. [Google Scholar]
- Scholz, R.W.; Wellmer, F.W. Approaching a dynamic view on the availability of mineral resources: What we may learn from the case of phosphorus? Glob. Environ. Chang. 2013, 23, 11–27. [Google Scholar] [CrossRef]
- Ashley, K.; Cordell, D.; Mavinic, D. A brief history of phosphorus: From the philosopher’s stone to nutrient recovery and reuse. Chemosphere 2011, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Scavia, D.; Allan, J.; Arend, K.; Bartell, S.; Beletsky, D.; Bosch, N.; Brandt, S.B.; Briland, R.D.; Daloğlu, I.; DePinto, J.V.; et al. Assessing and addressing the re-eutrophication of Lake Erie: Central basin hypoxia. J. Great Lakes Res. 2014, 40, 226–246. [Google Scholar] [CrossRef]
- Bell, L.C.; Black, C.A. Transformation of dibasic calcium phosphate di-hydrate and octa-calcium phosphate in slightly acid and alkaline soils. Soil Sci. Soc. Am. J. 2013, 34, 583–587. [Google Scholar] [CrossRef]
- Mitran, T.; Mani, P.K. Effect of organic amendments on rice yield trend, phosphorus use efficiency, uptake, and apparent balance in soil under long-term rice-wheat rotation. J. Plant Nutr. 2017, 40, 1312–1322. [Google Scholar] [CrossRef]
- Fertiliser Working Party. Phasing-Out the Use of Highly Soluble Phosphorus Fertilisers in an Environmentally Sensitive Areas of South West and Western Australia; Minister of the Environment: Western Australia, Australia, 2007; pp. 5–6. [Google Scholar]
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Sanders, J.L.; Murphy, L.S.; Noble, A.; Melgar, R.J.; Perkins, J. Improving phosphorus use efficiency with polymer technology. Procedia Eng. 2012, 46, 178–184. [Google Scholar] [CrossRef]
- Guelfi, D.R.; Chagas, W.F.T.; Lacerda, J.R.; Chagas, R.M.R.; de Souza, T.L.; Andrade, A.B. Monoammonium phosphate coated with polymers and magnesium for coffee plants. Cienc. Agrotecnol. 2018, 42, 139–147. [Google Scholar] [CrossRef]
- Erro, J.; Urrutia, O.; Baigorri, R.; Fuentes, M.; Zamarreño, A.M.; Garcia-Mina, J.M. Incorporation of humic-derived active molecules into compound NPK granulated fertilizers: Main technical difficulties and potential solutions. Chem. Biol. Technol. Agric. 2016, 3, 1–15. [Google Scholar] [CrossRef][Green Version]
- Sikora, L.; Enkiri, N. Efficiency of compost-fertiliser blends compared with fertilizer alone. Soil Sci. 2000, 165, 444–451. [Google Scholar] [CrossRef]
- Cornish, P.S. Research directions: Improving plant uptake of soil phosphorus, and reducing dependency on input of phosphorus fertilisers. Crop Past. Sci. 2009, 60, 190–196. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Chia, C.H.; Munroe, P.; Joseph, S.; Lin, Y. Microscopic characterization of synthetic Terra Preta. Aust. J. Soil Res. 2010, 48, 593–605. [Google Scholar] [CrossRef]
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.P.; Bucheli, T.D. Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem. 2012, 60, 3042–3050. [Google Scholar] [CrossRef]
- Perera, F.P. Environment and cancer: Who are susceptible? Science 1997, 278, 1068–1073. [Google Scholar] [CrossRef]
- Gao, Y.; Collins, C.D. Uptake pathways of polycyclic aromatic hydrocarbons in white clover. Environ. Sci. Technol. 2009, 43, 6190–6195. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, H.; Luo, Y.; Deng, X.; Herbert, S.; Xing, B. Characterization and influence of biochars on nitrous oxide emission from agricultural soil. Environ. Pollut. 2013, 174, 289–296. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; Macêdo, J.L.V.; Blum, W.E.H. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Pandit, B.; Martinsen, V.; Cornelissen, G.; Conte, P.; Kammann, C.I. Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture 2015, 5, 723–741. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202, 183–191. [Google Scholar] [CrossRef]
- Shaaban, M.; Zwieten, L.V.; Bashir, S.; Younas, A.; Núnez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils: Effect of variations in digestion conditions and of organic soil constituents. Soil Sci. 1947, 63, 251–263. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.N. Nitrogen-Inorganic Forms. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; SSSA/ASA: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Leoppert, R.H.; Hallmark, C.T.; Koshy, M.M. Routine procedure for rapid determination of soil carbonates. Soil Sci. Soc. Am. J. 1984, 48, 1030–1033. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Wolf, B. The comprehensive system of leaf analysis and its use for diagonosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Figueredo, N.A.; Costa, L.M.; Melo, L.C.A.; Siebeneichlerd, E.A.; Tronto, J. Characterization of biochars from different sources and evaluation of release of nutrients and contaminants. Agron. Sci. Mag. 2017, 48, 395–403. [Google Scholar] [CrossRef]
- Rashid, A. Mapping Zinc Fertility of Soils Using Indicator Plants and Soils-Analyses. Ph.D. Thesis, University of Hawaii, Honolulu, HI, USA, 1986. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1958; Volume 85, pp. 251–252. [Google Scholar]
- Little, T.M.; Hills, F.J. Agricultural Experimentation: Design and Analysis (No. 630.72 L5); Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Morales, M.M.; Comerford, N.; Guerrini, I.A.; Falcaand, N.P.S.; Reeves, J.B. Sorption and desorption of phosphate on biochar and biochar–soil mixtures. Soil Use Manag. 2013, 29, 306–314. [Google Scholar] [CrossRef]
- Pan, H.; Eberhardt, T.L. Characterization of fly ash from the gasification of wood and assessment for its application as a soil amendment. Bioresources 2011, 6, 3987–4004. [Google Scholar]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219, 162–167. [Google Scholar] [CrossRef]
- Gao, S.; Hoffman-Krull, K.; Bidwell, A.L.; DeLuca, T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016, 233, 43–54. [Google Scholar] [CrossRef]
- Magrini, K.A.; Czernik, S.; Pilath, H.M.; Evans, R.J.; Ching, P.; Leventhal, J. Biomass derived, carbon sequestering, designed fertilizers. Ann. Environ. Sci. 2009, 3, 217. [Google Scholar]
- Liu, Y.X.; Wu, W.X.; Shi, D.Z.; Zhong, Z.K.; Yang, M. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar]
- El Sharkawi, H.M.; Tojo, S.; Chosab, T.; Malhatcamd, F.M.; Youssef, A.M. Biochar-ammonium phosphate as an uncoated-slow release fertilizer in sandy soil. Biomass Bioenerg. 2018, 117, 154–160. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Knicker, H. “Black nitrogen”—An important fraction in determining the recalcitrance of charcoal. Org. Geochem. 2010, 41, 947–950. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Alling, V.; Hale, S.E.; Martinsen, V.; Mulder, J.; Smebye, A.; Breedveld, G.D.; Cornelissen, G. The role of biochar in retaining nutrients in amended tropical soils. J. Plant Nutr. Soil Sci. 2014, 177, 671–680. [Google Scholar] [CrossRef]
- Mendes, D.O.; Zafra, D.L.; Vassilev, N.B.; Silva, I.R.; Ribeiro, J.I.; Costaa, M.D. Biochar enhances Aspergillus niger rock phosphate solubilization by increasing organic acid production and alleviating fluoride toxicity. Appl. Environ. Microbiol. 2014, 80, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Danesh, S. Nano-fertilizers and their roles in sustainable agriculture. Int. J. Agric. Crop Sci. 2013, 5, 2229–2232. [Google Scholar]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar reduces short-term nitrate leaching from a horizon in an apple orchard. J. Environ. Qual. 2013, 42, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lemke, R.L.; Zhong, Z.; Campbell, C.A.; Zentner, R.P. Can pulse crops play a role in mitigating greenhouse gases from North American agriculture? Agron. J. 2007, 99, 1719–1725. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. Glob. Chang. Biol. Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by C-14 labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Lehmann, J.; Abiven, S.; Kleber, M.; Zimmerman, A.R. Persistence of biochar in soil. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan, Tailor & Francis Group: Washington, DC, USA, 2015; pp. 235–281. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Chan, K.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of green-waste biochar as a soil amendment. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Steven, F.V.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrate. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.; Yang, J.E. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lehmaan, J.; Sohi, S.P.; Thies, J.E.; Neil, B.O.; Turjillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black crabon effects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Zavalloni, C.G.; Alberti, G.; Biasiol, S.; Vedove, G.D.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial mineralization of biochar and wheat straw mixture in soil: A short-term study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Brodowski, S.; John, B.; Flessa, H.; Amelung, W. Aggregate-occulated black carbon in soil. Eur. J. Soil Sci. 2006, 57, 539–546. [Google Scholar] [CrossRef]
- Chang, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and antibiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Kumar, K. Effect of organic sources on phosphorus fractions and available phosphorus in Typic Haplustept. J. Indian Soc. Soil Sci. 2014, 62, 80–83. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; Neill, B.O.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Schmidt, H.P.; Cornelissen, G.; Cowie, A. Biochar from “Kon Tiki” flame curtain and other kilns: Effects of nutrient enrichment and kiln type on crop yield and soil chemistry. PLoS ONE 2017, 12, e0176378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Pignatello, J.J. Effects of post-pyrolysis air oxidation of biomass chars on adsorption of neutral and ionizable compounds. Environ. Sci. Technol. 2016, 50, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Tesfahun, W. Effects of biochar in soil chemical and biological property and mitigating climate change: Review. Civ. Environ. Res. 2018, 10, 58–61. [Google Scholar]
- Jat, R.S.; Ahlawat, I. Effect of vermicompost, biofertilizer and phosphorus on growth, yield and nutrient uptake by gram (Cicer arietinum) and their residual effect on fodder maize (Zea mays). Indian J. Agric. Sci. 2004, 74, 359–361. [Google Scholar]
- Kouas, S.; Labidi, N.; Debez, A.; Abdelly, C. Effect of P on nodule formation and N fixation in bean. Agron. Sustain. Dev. 2005, 25, 389–393. [Google Scholar] [CrossRef]
- Jebara, M.; Aouani, M.E.; Payre, H.; Drevon, J. Nodule conductance varied among common bean (Phaseolus vulgaris) genotypes under phosphorus deficiency. J. Plant Physiol. 2005, 162, 309–315. [Google Scholar] [CrossRef]
- Fairhurst, T.; Witt, C. Rice: A Practical Guide to Nutrient Management. Singapore and Los Baños; Potash and Phosphate Institute & Phosphate Institute of Canada and International Rice Research Institute Philippines: Singapore, 2002; pp. 1–45. [Google Scholar]
- Kumar, B.V.; Sreenivasulu, M. Integrated Nutrient Management. Sci Tech: The Hindu. India’s National Newspaper, 12 August 2004. [Google Scholar]
- Gruhn, P.; Goletti, F.; Yudelman, M. Integrated Nutrient Management, Soil Fertility, and Sustainable Agriculture: Current Issues and Future Challenges, 2020; International Food Policy Research Institute: Washington, DC, USA, 2000; pp. 1–3. [Google Scholar]
- Hossaini, M.A.; Hamid, A. Influence of N and P fertilizer application on root growth, leaf photosynthesis and yield performance of groundnut. J. Agric. Res. 2007, 32, 369–374. [Google Scholar] [CrossRef]
- Burman, U.; Garg, B.K.; Kathju, S. Effect of phosphorus application on cluster bean under different intensities of water stress. J. Plant Nutr. 2009, 32, 668–680. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Nielsen, S.; Joseph, S.D.; Huang, D.; Thomas, T. A combination of biochar–mineral complexes and compost improves soil bacterial processes, soil quality, and plant properties. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lusiba, S.G.; Odhiambo, J.J.O.; Ogola, J.B.O. Effect of biochar and phosphorus fertilizer application on soil fertility: Soil physical and chemical properties. Arch. Agron. Soil Sci. 2016, 63, 477–490. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Ulyett, J.; Sakrabani, R.; Kbblewhite, M.G.; Hann, M. Impact of biochar addition on water retention, nitrification and carbon dioxide evolution from two sandy soils. Eur. J. Soil Sci. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Xu, H.X.; Weng, X.Y.; Yang, Y. Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. J. Plant Physiol. 2007, 54, 741–748. [Google Scholar] [CrossRef]
- Sarker, B.C.; Karmokerand, J.L.; Rashid, P. Effects of phosphorus deficiency on anatomical structures in maize (Zea mays L.). Bangladesh J. Bot. 2010, 9, 57–60. [Google Scholar] [CrossRef]
- Ahmad, M.; Wang, X.; Hilger, T.H.; Luqman, M.; Nazli, F.; Hussain, A.; Zahir, Z.A.; Latif, M.; Saeed, Q.; Malik, H.A.; et al. Evaluating Biochar-Microbe Synergies for Improved Growth, Yield of Maize, and Post-Harvest Soil Characteristics in a Semi-Arid Climate. Agronomy 2020, 10, 1055. [Google Scholar] [CrossRef]
- Meena, K.N.; Pareek, R.G.; Jat, R.S. Effect of phosphorus and bio-fertilizers on yield and quality of chickpea. Ann. Agric. Res. 2005, 22, 388–390. [Google Scholar]
- Singh, R.; Prasad, K. Effect of vermin-compost, Rhizobium and DAP on growth, yield and nutrient uptake by chickpea. J. Food Legumes 2008, 21, 112–114. [Google Scholar]
- Ogola, A.H.; Odhiambo, G.D.; Okalebo, J.R.; Muyekho, F.N. Influence of phosphorus on selected desmodium growth and nodulation parameters. ARPN J. Agric. Biol. Sci. 2012, 7, 294–301. [Google Scholar]
- Sulieman, S.; Tran, L.S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Balai, K.; Jajoria, M.; Verma, R.; Deewan, P.; Bairwa, S.K. Nutrient content, uptake, quality of chickpea and fertility status of soil as influenced by fertilization of phosphorus and zinc. J. Pharmacogn. Phytochem. 2017, 6, 392–398. [Google Scholar]
- Singh, V.; Thenua, O.V.S.; Shivay, Y.S. Effect of phosphorus management on productivity of sunflower (Helianthus annuus L.). Prog. Res. Int. J. 2017, 12, 348–352. [Google Scholar]
| DAP: Biochar (w/w) | Extractable Phosphorus (mgkg−1) | Nitrogen (%) | Organic Matter (%) | EC (dSm−1) | pH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEB1 | PEB2 | PEB1 | PEB2 | PEB1 | PEB2 | PEB1 | PEB2 | PEB1 | PEB2 | |
| 0:0 (control) | 3.81 ± 0.09 d | 3.87 ± 0.09 d | 0.07 ± 0.03 c | 0.07 ± 0.02 d | 0.55 ± 0.05 de | 0.55 ± 0.07 de | 0.58 ± 0.02 g | 0.58 ± 0.01 g | 8.09 ± 0.09 a | 8.08 ± 0.03 a |
| 0:100 | 3.92 ± 0.04 d | 3.91 ± 0.09 d | 0.11 ± 0.04 c | 0.11 ± 0.05 c | 1.07 ± 0.08 a | 1.09 ± 0.03 a | 0.72 ± 0.02 f | 0.73 ± 0.02 f | 7.81 ± 0.01 d | 7.82 ± 0.02 d |
| 25:75 | 5.41 ± 0.35 c | 5.57 ± 0.12 bc | 0.60 ± 0.03 b | 0.61 ± 0.01 b | 0.98 ± 0.01 ab | 0.97 ± 0.01 ab | 0.96 ± 0.03 e | 0.91 ± 0.01 e | 7.88 ± 0.01 b–d | 7.87 ± 0.02 cd |
| 50:50 | 7.87 ± 0.83 a | 7.92 ± 0.09 a | 0.94 ± 0.03 a | 0.95 ± 0.03 a | 0.84 ± 0.02 bc | 0.85 ± 0.04 b | 1.08 ± 0.02 d | 1.07 ± 0.06 d | 7.96 ± 0.03 bc | 7.97 ± 0.03 bc |
| 75:25 | 8.50 ± 0.33 a | 8.30 ± 0.43 a | 0.96 ± 0.02 a | 0.96 ± 0.02 a | 0.68 ± 0.06 cd | 0.66 ± 0.08 de | 1.27 ± 0.01 bc | 1.22 ± 0.03 c | 7.98 ± 0.01 b | 7.97 ± 0.02 bc |
| 100:0 | 6.54 ± 0.29 b | 6.33 ± 0.22 bc | 0.93 ± 0.03 a | 0.93 ± 0.02 a | 0.50 ± 0.06 e | 0.52 ± 0.07 de | 1.35 ± 0.04 a | 1.37 ± 0.02 ab | 7.97 ± 0.02 bc | 7.97 ± 0.02 bc |
| DAP: Biochar (w/w) | Extractable Phosphorus (mgkg−1) | Nitrogen (%) | Organic Matter (%) | EC (dS m−1) | pH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEB1 | PEB2 | PEB1 | PEB2 | PEB1 | PEB2 | PEB1 | PEB2 | PEB1 | PEB2 | |
| 0:0 (control) | 3.95 ± 0.02 e | 3.96 ± 0.02 e | 0.07 ± 0.03 c | 0.07 ± 0.02 c | 0.53 ± 0.10 de | 0.52 ± 0.07 e | 0.58 ± 0.07 f | 0.56 ± 0.08 f | 8.09 ± 0.09 a | 8.08 ± 0.03 a |
| 0:100 | 4.17 ± 0.05 d | 4.18 ± 0.05 d | 0.12 ± 0.05 c | 0.11 ± 0.05 c | 1.07 ± 0.03 a | 1.00 ± 0.01 ab | 0.72 ± 0.01 e | 0.73 ± 0.02 e | 7.81 ± 0.01 d | 7.82 ± 0.02 d |
| 25:75 | 6.01 ± 0.03 c | 6.02 ± 0.03 c | 0.80 ± 0.03 b | 0.81 ± 0.01 b | 0.91 ± 0.01 bc | 0.93 ± 0.04 bc | 0.91 ± 0.01 d | 0.92 ± 0.01 d | 7.88 ± 0.01 b–d | 7.87 ± 0.02 cd |
| 50:50 * | 9.98 ± 0.02 a | 9.99 ± 0.02 a | 1.14 ± 0.03 a | 1.15 ± 0.03 a | 0.85 ± 0.02 c | 0.84 ± 0.04 c | 1.08 ± 0.03 c | 1.08 ± 0.05 c | 7.96 ± 0.03 bc | 7.97 ± 0.03 bc |
| 75:25 | 10.06 ± 0.04 a | 10.05 ± 0.04 a | 1.162 ± 0.02 a | 1.16 ± 0.02 a | 0.65 ± 0.03 de | 0.66 ± 0.02 d | 1.26 ± 0.02 b | 1.22 ± 0.02 b | 7.98 ± 0.01 b | 7.97 ± 0.02 bc |
| 100:0 | 8.13 ± 0.02 b | 8.18 ± 0.02 b | 1.13 ± 0.03 a | 1.13 ± 0.02 a | 0.53 ± 0.01 de | 0.52 ± 0.02 de | 1.39 ± 0.03 a | 1.37 ± 0.02 a | 7.97 ± 0.02 bc | 7.97 ± 0.02 bc |
| Treatment | Plant Height (cm) | No. of Primary Branches | Length (cm) | Weight (g) | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Fresh | Dry | |||||
| Root | Shoot | Root | Shoot | |||||
| 0% RDP | 21.60 ± 0.25 g | 4 ± 0.04 d | 12.33 ± 0.49 f | 23.38 ± 0.32 g | 2.63 ± 0.07 g | 12.88 ± 0.05 g | 1.05 ± 0.03 h | 5.13 ± 0.10 g |
| 25% RDP | 23.31 ± 0.35 f | 4 ± 0.13 cd | 14.75 ± 0.10 e | 25.73 ± 0.20 f | 3.56 ± 0.43 f | 15.10 ± 0.08 f | 1.43 ± 0.17 g | 6.13 ± 0.07 f |
| 50% RDP | 27.36 ± 0.46 e | 5 ± 0.14 cd | 18.20 ± 0.10 d | 29.50 ± 0.45 e | 4.50 ± 0.31 e | 18.59 ± 0.33 e | 1.80 ± 0.12 f | 7.47 ± 0.11 e |
| 100% RDP | 29.80 ± 0.10 d | 5 ± 0.03 c | 20.57 ± 0.49 c | 32.36 ± 0.45 d | 5.22 ± 0.24 e | 21.61 ± 0.41 d | 2.61 ± 0.12 e | 8.61 ± 0.15 d |
| 0% RDP + PEB1 | 33.33 ± 0.49 c | 6 ± 0.16 b | 21.10 ± 0.10 c | 33.38 ± 0.37 c | 6.10 ± 0.07 d | 24.11 ± 0.08 c | 3.05 ± 0.03 d | 9.65 ± 0.03 c |
| 0% RDP + PEB2 | 33.43 ± 0.40 c | 6 ± 0.40 b | 21.18 ± 0.20 c | 33.39 ± 0.33 c | 6.06 ± 0.05 d | 23.91 ± 0.07 c | 3.03 ± 0.02 d | 9.57 ± 0.11 c |
| 25% RDP + PEB1 | 37.67 ± 0.20 b | 7 ± 0.46 a | 23.20 ± 0.20 b | 39.47 ± 0.25 b | 7.47 ± 0.44 c | 26.26 ± 0.31 b | 3.74 ± 0.22 c | 10.51 ± 0.12 b |
| 50% RDP + PEB1 | 42.36 ± 0.46 a | 8 ± 0.53 a | 27.61 ± 0.11 a | 44.66 ± 0.15 a | 9.94 ± 0.38 a | 32.62 ± 0.47 a | 4.63 ± 0.16 ab | 12.71 ± 0.39 a |
| 100% RDP + PEB1 | 42.59 ± 0.51 a | 8 ± 0.17 a | 27.20 ± 0.19 a | 44.56 ± 0.38 a | 9.16 ± 0.16 b | 32.64 ± 0.28 a | 4.57 ± 0.08 b | 12.35 ± 0.55 a |
| 25% RDP + PEB2 | 37.30 ± 0.26 b | 7 ± 0.28 a | 23.21 ± 0.28 b | 39.61 ± 0.29 b | 7.46 ± 0.21 c | 25.38 ± 0.57 b | 3.73 ± 0.10 c | 10.39 ± 0.13 b |
| 50% RDP + PEB2 | 42.23 ± 0.15 a | 8 ± 0.22 a | 27.34 ± 0.40 a | 44.63 ± 0.20 a | 9.84 ± 0.13 ab | 33.17 ± 0.27 a | 4.92 ± 0.06 a | 12.27 ± 0.11 a |
| 100% RDP + PEB2 | 42.58 ± 0.36 a | 8 ± 0.21 a | 27.21 ± 0.28 a | 44.61 ± 0.29 a | 9.17 ± 0.06 b | 32.58 ± 0.37 a | 4.59 ± 0.03 ab | 12.44 ± 0.28 a |
| Treatment | Physiological Parameters | Chemical Parameters | ||||
|---|---|---|---|---|---|---|
| Photosynthetic Rate (µmol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | Chlorophyll Contents (mmol cm−2 s−1) | Stomatal Conductance (µMol m−2 s−1) | Shoot Nitrogen (%) | Shoot Phosphorus (%) | |
| 0% RDP | 30.43 ± 0.41 g | 5.13 ± 0.04 e | 1.34 ± 0.04 f | 220 ± 0.94 g | 0.53 ± 0.03 g | 0.65 ± 0.03 f |
| 25% RDP | 31.88 ± 0.61 f | 5.24 ± 0.03 e | 1.37 ± 0.02 ef | 227 ± 0.90 f | 0.64 ± 0.04 f | 0.84 ± 0.03 e |
| 50% RDP | 34.22 ± 0.30 e | 5.77 ± 0.10 d | 1.45 ± 0.04 de | 236 ± 0.75 e | 0.75 ± 0.03 e | 1.26 ± 0.02 d |
| 100% RDP | 38.54 ± 0.34 d | 6.16 ± 0.13 c | 1.55 ± 0.03 d | 241 ± 0.88 d | 0.85 ± 0.02 d | 1.45 ± 0.04 c |
| 0% RDP + PEB1 | 41.15 ± 0.26 c | 7.16 ± 0.03 b | 2.35 ± 0.03 c | 258 ± 0.76 c | 0.95 ± 0.02 c | 1.65 ± 0.04 b |
| 0% RDP + PEB2 | 41.21 ± 0.59 bc | 7.12 ± 0.10 b | 2.37 ± 0.02 c | 258 ± 1.4 c | 0.96 ± 0.03 bc | 1.64 ± 0.03 b |
| 25% RDP + PEB1 | 42.57 ± 0.28 b | 7.32 ± 0.05 b | 3.05 ± 0.03 b | 264 ± 0.33 b | 1.05 ± 0.03 b | 1.73 ± 0.02 b |
| 50% RDP + PEB1 | 44.34 ± 0.37 a | 7.64 ± 0.04 a | 3.34 ± 0.04 a | 276 ± 1.02 a | 1.36 ± 0.03 a | 2.04 ± 0.03 a |
| 100% RDP + PEB1 | 44.87 ± 0.67 a | 7.76 ± 0.06 a | 3.38 ± 0.05 a | 277 ± 1.81 a | 1.34 ± 0.03 a | 2.05 ± 0.03 a |
| 25% RDP + PEB2 | 42.40 ± 0.38 bc | 7.32 ± 0.08 b | 3.06 ± 0.02 b | 264 ± 0.95 b | 1.04 ± 0.03 bc | 1.72 ± 0.02 b |
| 50% RDP + PEB2 | 44.10 ± 0.10 a | 7.63 ± 0.03 a | 3.33 ± 0.03 a | 275 ± 040 a | 1.36 ± 0.02 a | 2.05 ± 0.03 a |
| 100% RDP + PEB2 | 45.09 ± 0.88 a | 7.72 ± 0.08 a | 3.43 ± 0.04 a | 277 ± 2.08 a | 1.34 ± 0.03 a | 2.06 ± 0.03 a |
| Treatments | Extractable Phosphorus (mgkg−1) | Nitrogen (%) | Organic Matter (%) | EC (dSm−1) | pH |
|---|---|---|---|---|---|
| 0% RDP | 4.07 ± 0.05 f | 0.22 ± 0.03 e | 0.53 ± 0.10 b | 0.95 ± 0.02 h | 8.17 ± 0.09 a |
| 25% RDP | 4.55 ± 0.28 ef | 0.28 ± 0.02 e | 0.52 ± 0.07 b | 1.25 ± 0.04 g | 8.13 ± 0.19 ab |
| 50% RDP | 5.15 ± 0.12 de | 0.79 ± 0.05 d | 0.58 ± 0.02 b | 1.44 ± 0.02 f | 8.14 ± 0.40 a |
| 100% RDP | 5.43 ± 0.16 d | 0.89 ± 0.04 cd | 0.56 ± 0.03 b | 1.64 ± 0.03 e | 8.14 ± 0.18 a |
| 0% RDP + PEB1 | 7.12 ± 0.04 c | 1.00 ± 0.05 bc | 0.94 ± 0.03 a | 1.84 ± 0.03 d | 7.98 ± 0.04 bc |
| 0% RDP + PEB2 | 7.27 ± 0.18 c | 0.99 ± 0.04 bc | 0.93 ± 0.02 a | 1.85 ± 0.02 d | 7.97 ± 0.06 cd |
| 25% RDP + PEB1 | 8.36 ± 0.16 b | 1.10 ± 0.04 b | 0.94 ± 0.02 a | 2.24 ± 0.03 c | 7.97 ± 0.09 cd |
| 50% RDP + PEB1 | 9.35 ± 0.22 a | 1.99 ± 0.04 a | 0.96 ± 0.02 a | 2.85 ± 0.02 b | 7.82 ± 0.06 d |
| 100% RDP + PEB1 | 9.43 ± 0.41 a | 2.08 ± 0.05 a | 0.95 ± 0.03 a | 3.15 ± 0.04 a | 7.87 ± 0.06 cd |
| 25% RDP + PEB2 | 8.28 ± 0.14 b | 1.09 ± 0.04 b | 0.97 ± 0.01 a | 2.25 ± 0.03 c | 7.97 ± 0.09 cd |
| 50% RDP + PEB2 | 9.34 ± 0.48 a | 2.01 ± 0.04 a | 0.96 ± 0.03 a | 2.86 ± 0.04 b | 7.97 ± 0.06 cd |
| 100% RDP + PEB2 | 9.53 ± 0.42 a | 2.07 ± 0.03 a | 0.95 ± 0.04 a | 3.17 ± 0.01 a | 7.97 ± 0.06 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wali, F.; Naveed, M.; Bashir, M.A.; Asif, M.; Ahmad, Z.; Alkahtani, J.; Alwahibi, M.S.; Elshikh, M.S. Formulation of Biochar-Based Phosphorus Fertilizer and Its Impact on Both Soil Properties and Chickpea Growth Performance. Sustainability 2020, 12, 9528. https://doi.org/10.3390/su12229528
Wali F, Naveed M, Bashir MA, Asif M, Ahmad Z, Alkahtani J, Alwahibi MS, Elshikh MS. Formulation of Biochar-Based Phosphorus Fertilizer and Its Impact on Both Soil Properties and Chickpea Growth Performance. Sustainability. 2020; 12(22):9528. https://doi.org/10.3390/su12229528
Chicago/Turabian StyleWali, Farman, Muhammad Naveed, Muhammad Asaad Bashir, Muhammad Asif, Zulfiqar Ahmad, Jawaher Alkahtani, Mona S. Alwahibi, and Mohamed Soliman Elshikh. 2020. "Formulation of Biochar-Based Phosphorus Fertilizer and Its Impact on Both Soil Properties and Chickpea Growth Performance" Sustainability 12, no. 22: 9528. https://doi.org/10.3390/su12229528
APA StyleWali, F., Naveed, M., Bashir, M. A., Asif, M., Ahmad, Z., Alkahtani, J., Alwahibi, M. S., & Elshikh, M. S. (2020). Formulation of Biochar-Based Phosphorus Fertilizer and Its Impact on Both Soil Properties and Chickpea Growth Performance. Sustainability, 12(22), 9528. https://doi.org/10.3390/su12229528








