Effects of Different Ages of Robinia pseudoacacia Plantations on Soil Physiochemical Properties and Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area and Soil Sampling
2.2. Measurements of Soil Physiochemical Properties
2.3. PLFA Analysis
2.4. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
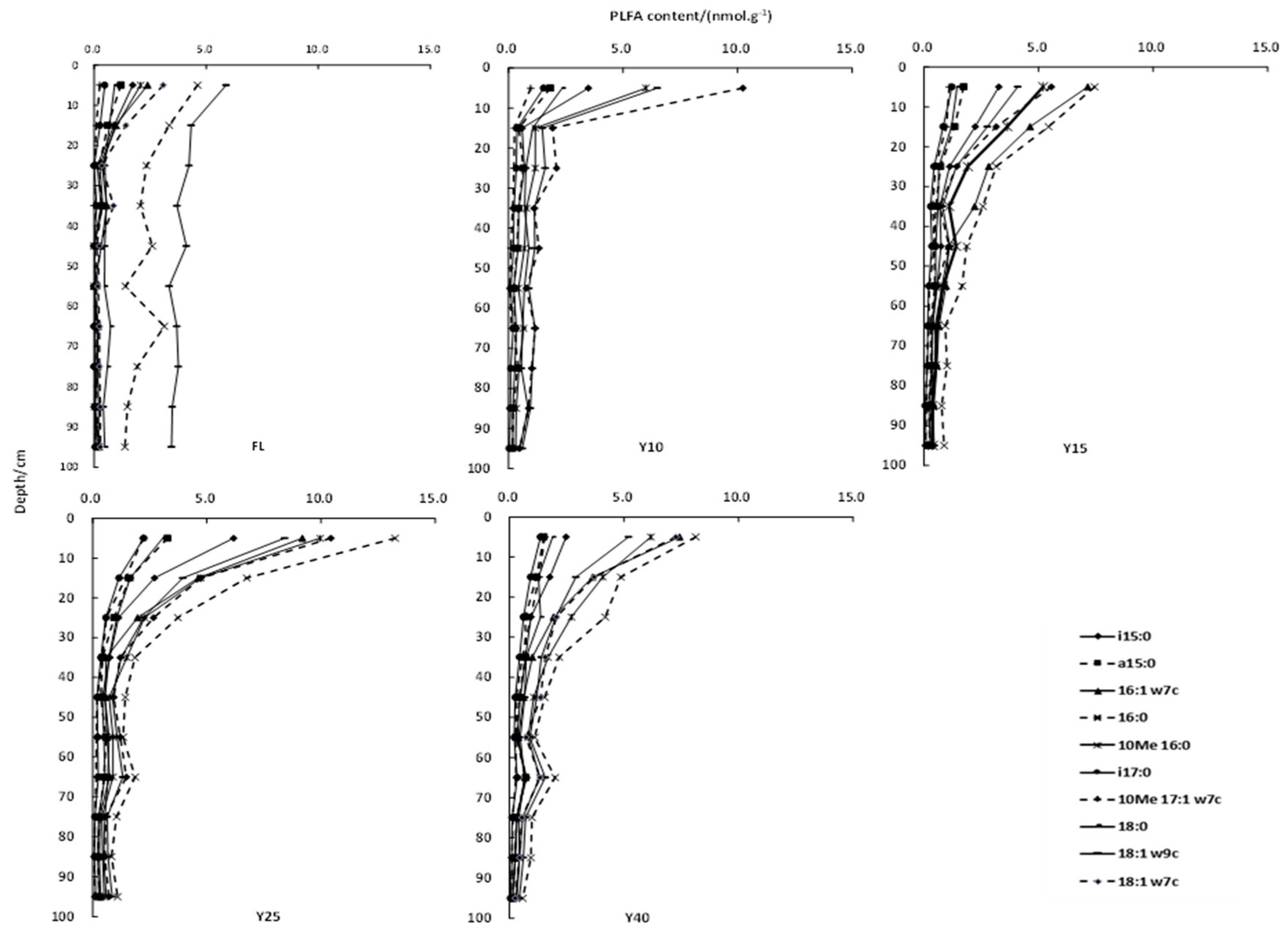
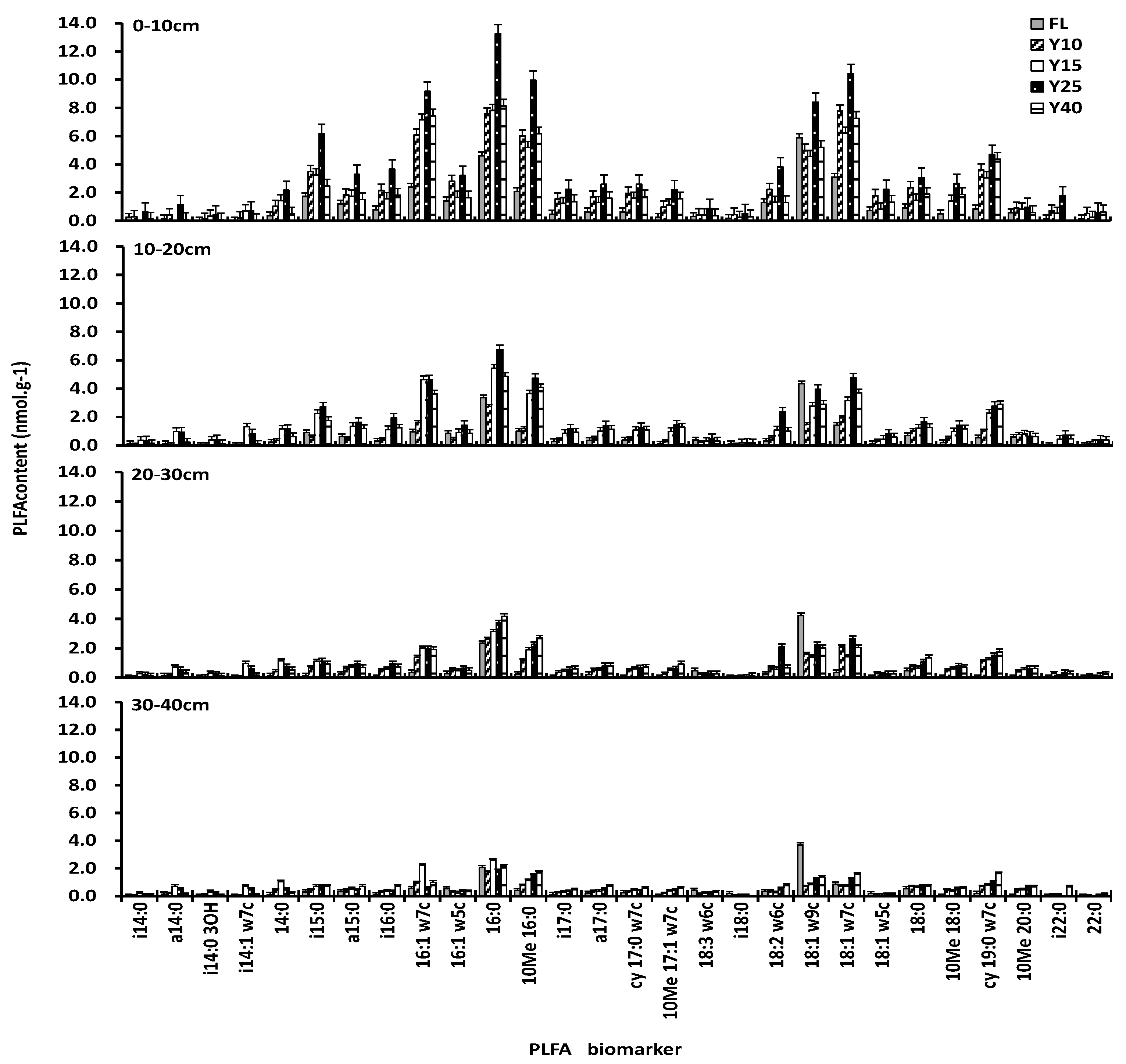
3.2. Soil PLFAs Contents
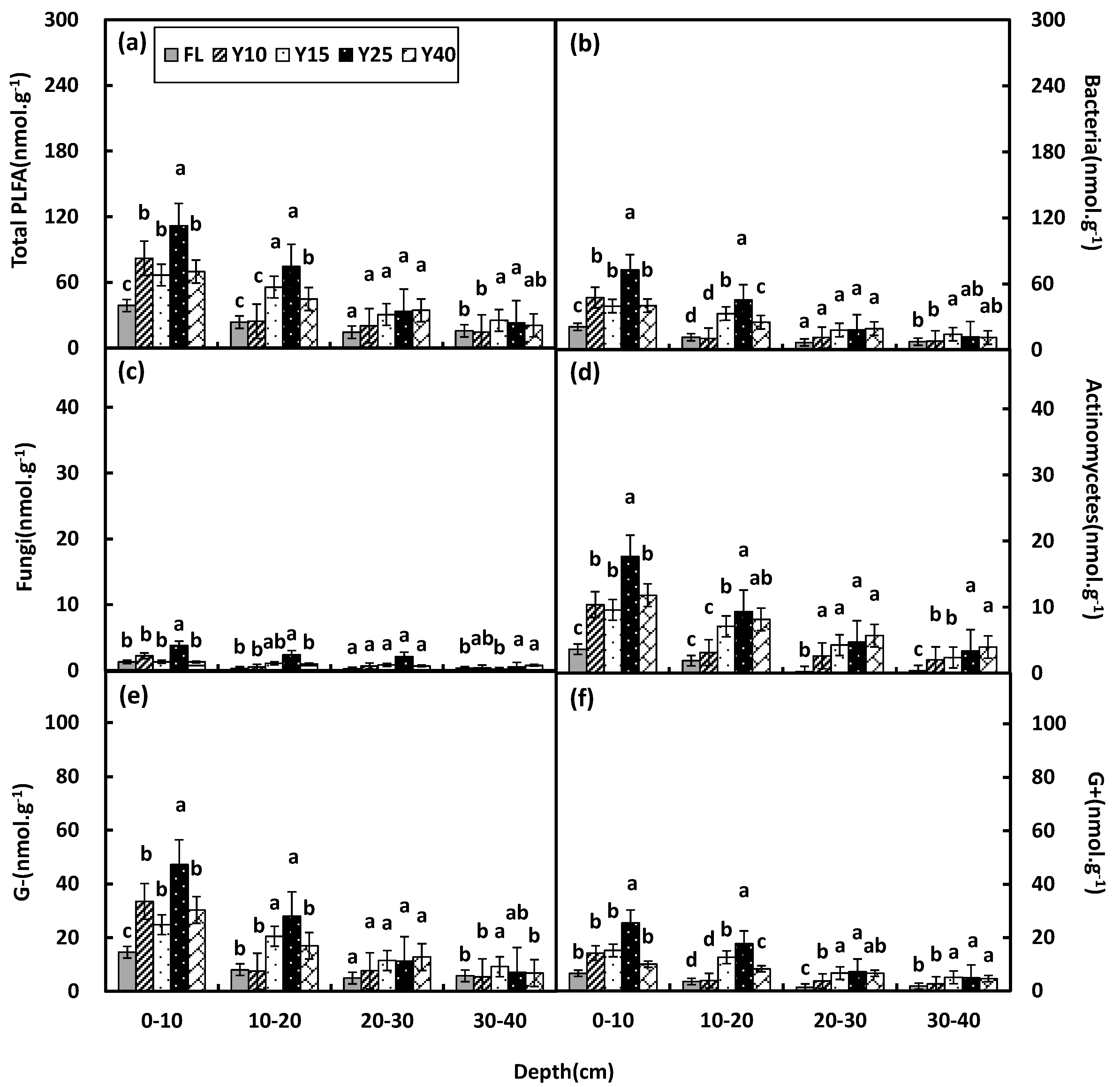
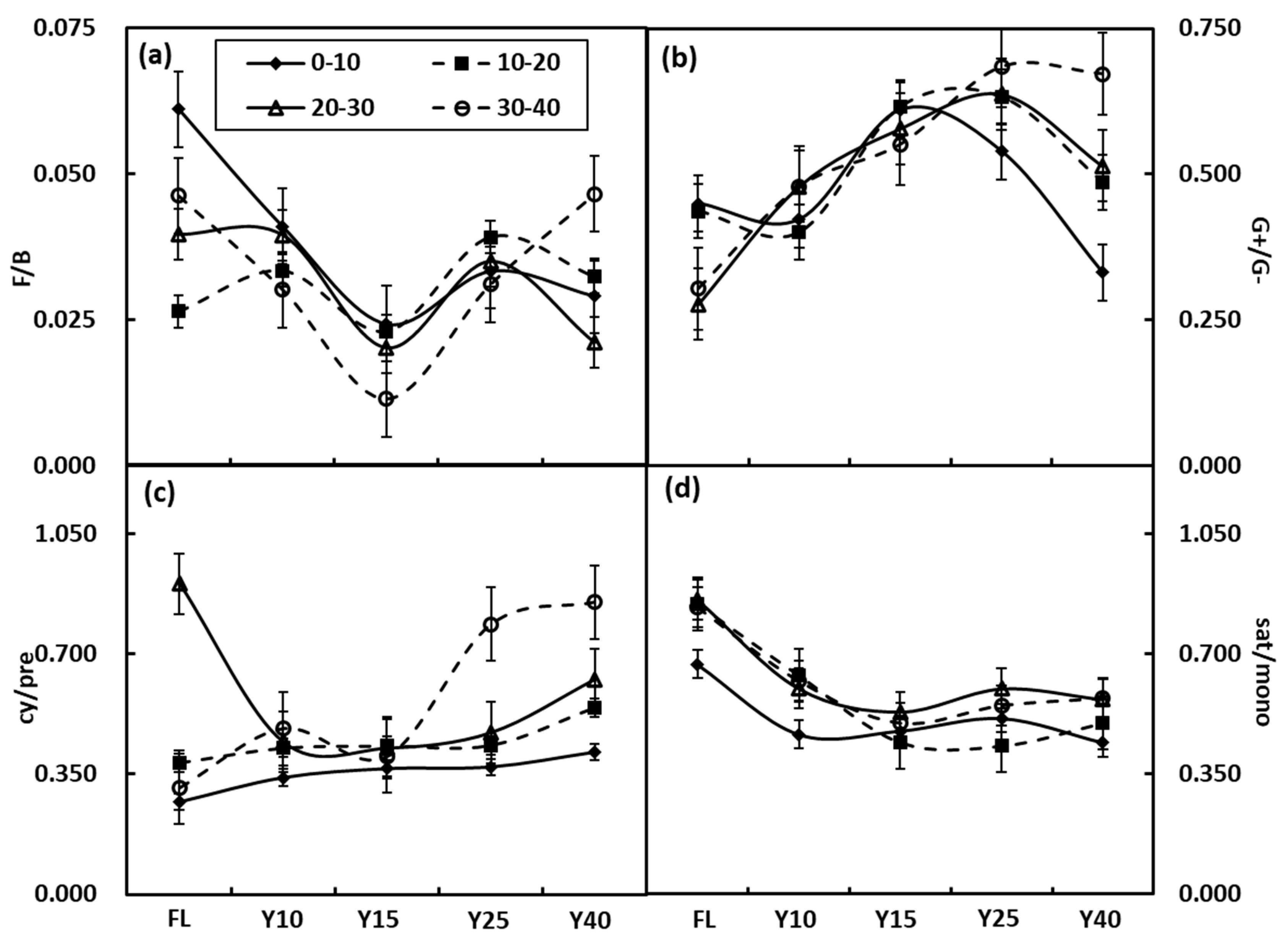
3.3. Soil Microbial Community Structure
3.4. Redundancy Analysis
4. Discussion
4.1. The Effects of Afforestation on Soil Physicochemical Properties
4.2. Positive Effects of Afforestation on Soil Microbial Communities and Their Influencing Factors
4.3. Possible Negative Effects of Afforestation on Soil Ecosystem
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baath, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant-bacteria interactions. Nat. Rev. Microbiol. 2018, 16, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D. Soil Chemical and Biological Changes through the N2 Fixation of Black Locust (Robinia pseudoacacia L.)-A Contribution to the Research of Tree Neophytes. Ph.D. Thesis, Institute of Soil Science and Forest Nutrition of the Georg-August University of Göttingen, Göttingen, Germany, 2005; p. 186. Available online: http://hdl.handle.net/11858/11800-11735-10000-10006-B11850E11854-11856 (accessed on 14 July 2015).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Buzhdygan, O.Y.; Rudenko, S.S.; Kazanci, C.; Patten, B.C. Effect of invasive black locust (Robinia pseudoacacia L.) on nitrogen cycle in floodplain ecosystem. Ecol. Model. 2016, 319, 170–177. [Google Scholar] [CrossRef]
- Cao, Y.S.; Fu, S.L.; Zou, X.M.; Cao, H.L.; Shao, Y.H.; Zhou, L.X. Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. Eur. J. Soil Biol. 2010, 46, 128–135. [Google Scholar] [CrossRef]
- Cederlund, H.; Wessen, E.; Enwall, K.; Jones, C.M.; Juhanson, J.; Pell, M.; Philippot, L.; Hallin, S. Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Appl. Soil Ecol. 2014, 84, 62–68. [Google Scholar] [CrossRef]
- Chang, E.H.; Chiu, C.Y. Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl. Soil Ecol. 2015, 91, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, L.Y.; Peng, Y.F.; Ding, J.Z.; Li, F.; Yang, G.B.; Kou, D.; Liu, L.; Fang, K.; Zhang, B.B.; et al. Linking microbial C:N:P stoichiometry to microbial community and abiotic factors along a 3500-km grassland transect on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2016, 25, 1416–1427. [Google Scholar] [CrossRef]
- De Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- De Marco, A.; Arena, C.; Giordano, M.; De Santo, A. Impact of the invasive tree black locust on soil properties of Mediterranean stone pine-holm oak forests. Plant Soil 2013, 372, 473–486. [Google Scholar] [CrossRef]
- De Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.P. Afforestation Drives Soil Carbon and Nitrogen Changes in China. Land Degrad. Dev. 2017, 28, 151–165. [Google Scholar] [CrossRef]
- Du, B.G.; Pang, J.Z.; Hu, B.; Allen, D.E.; Bell, T.L.; Pfautsch, S.; Netzer, F.; Dannenmann, M.; Zhang, S.X.; Rennenberg, H. N-2-fixing black locust intercropping improves ecosystem nutrition at the vulnerable semi-arid Loess Plateau region, China. Sci. Total Environ. 2019, 688, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Duru, M.; Cruz, P.; Ansquer, P.; Navas, M.L. Standing herbage mass: An integrated indicator of management practices for examining how fertility and defoliation regime shape the functional structure of species-rich grasslands. Ecol. Indic. 2014, 36, 152–159. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Federle, T.W.; Dobbins, D.C.; Thortonmanning, J.R.; Jones, D.D. Microbial biomass, activity, and community structure in subsurface soils. Ground Water 1986, 24, 365–374. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fert. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E.; Tunlid, A. Shifts in the structure of soil microbial communities in limed forests as revealed by PLFA analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Frostegård, A.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Meth. 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Fu, X.L.; Yang, F.T.; Wang, J.L.; Di, Y.B.; Dai, X.Q.; Zhang, X.Y.; Wang, H.M. Understory vegetation leads to changes in soil acidity and in microbial communities 27 years after reforestation. Sci. Total Environ. 2015, 502, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hannula, S.E.; Trager, S. Soil fungal guilds as important drivers of the plant richness-productivity relationship. New Phytol. 2020, 226, 947–949. [Google Scholar] [CrossRef]
- Hogberg, M.N.; Hogberg, P.; Myrold, D.D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 2007, 150, 590–601. [Google Scholar] [CrossRef]
- Hyvonen, R.; Persson, T.; Andersson, S.; Olsson, B.; Agren, G.I.; Linder, S. Impact of long-term nitrogen addition on carbon stocks in trees and soils in northern Europe. Biogeochemistry 2008, 89, 121–137. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Chang. 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Jian, S.Q.; Zhao, C.Y.; Fang, S.M.; Yu, K. Effects of different vegetation restoration on soil water storage and water balance in the Chinese Loess Plateau. Agric. For. Meteorol. 2015, 206, 85–96. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, Z.; Bai, W.; Jia, Y.; Wang, N. Assessing the Ecological Success of Restoration by Afforestation on the Chinese Loess Plateau. Restor. Ecol. 2012, 20, 240–249. [Google Scholar] [CrossRef]
- Kieft, T.L.; Wilch, E.; Oconnor, K.; Ringelberg, D.B.; White, D.C. Survival and phospholipid fatty acid profiles of surface and subsurface bacteria in natural sediment microcosms. Appl. Environ. Microb. 1997, 63, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Garcia-Fayos, P.; Hu, S.; Jiao, J.Y. The effect of Robinia pseudoacacia afforestation on soil and vegetation properties in the Loess Plateau (China): A chronosequence approach. For. Ecol. Manag. 2016, 375, 146–158. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Lazzaro, L.; Mazza, G.; d’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Landi, S.; Lastrucci, L.; Pastorelli, R.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total Environ. 2018, 622, 1509–1518. [Google Scholar] [CrossRef]
- Li, X.G.; Jousset, A.; de Boer, W.; Carrion, V.J.; Zhang, T.L.; Wang, X.X.; Kuramae, E.E. Legacy of land use history determines reprogramming of plant physiology by soil microbiome. ISME J. 2019, 13, 738–751. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of soil microbial community dynamics to Robinia pseudoacacia L. afforestation in the loess plateau: A chronosequence approach. Plant Soil 2017, 423, 327–338. [Google Scholar] [CrossRef]
- Liu, Z.S.; Hu, B.; Bell, T.L.; Flemetakis, E.; Rennenberg, H. Significance of mycorrhizal associations for the performance of N-2-fixing Black Locust (Robinia pseudoacacia L.). Soil Biol. Biochem. 2020, 145, 107776. [Google Scholar] [CrossRef]
- Macdonald, C.A.; Thomas, N.; Robinson, L.; Tate, K.R.; Ross, D.J.; Dando, J.; Singh, B.K. Physiological, biochemical and molecular responses of the soil microbial community after afforestation of pastures with Pinus radiata. Soil Biol. Biochem. 2009, 41, 1642–1651. [Google Scholar] [CrossRef]
- Malcolm, G.M.; Bush, D.S.; Rice, S.K. Soil nitrogen conditions approach preinvasion levels following restoration of nitrogen-fixing black locust (Robinia pseudoacacia) stands in a pine-oak ecosystem. Restor. Ecol. 2008, 16, 70–78. [Google Scholar] [CrossRef]
- Mendhama, D.S.; Sankaranb, K.V.; O’Connella, A.M.; Grove, T.S. Eucalyptus globulus harvest residue management effects on soil carbon and microbial biomass at 1 and 5 years after plantation establishment. Soil Biol. Biochem. 2002, 34, 1903–1912. [Google Scholar] [CrossRef]
- Moore-Kucera, J.; Dick, R.P. PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microb. Ecol. 2008, 55, 500–511. [Google Scholar] [CrossRef]
- OLeary, M.H. Carbon isotopes in photosynthesis. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Papaioannou, A.; Chatzistathis, T.; Papaioannou, E.; Papadopoulos, G. Robinia pseudoacacia as a valuable invasive species for the restoration of degraded croplands. Catena 2016, 137, 310–317. [Google Scholar] [CrossRef]
- Poschner, J.; Kroppenstedt, R.M.; Fischer, A.; Stackebrandt, E. DNA-DNA Reassociation and Chemotaxonomic Studies on Actinomadura, Microbispora, Microtetraspora, Micropolyspora and Nocardiopsis. Syst. Appl. Microbiol. 1985, 6, 264–270. [Google Scholar] [CrossRef]
- Priha, O.; Grayston, S.J.; Pennanen, T.; Smolander, A. Microbial activities related to C and N cycling and microbial community structure in the rhizospheres of Pinus sylvestris, Picea abies and Betula pendula seedlings in an organic and mineral soil. FEMS Microbiol. Ecol. 1999, 30, 187–199. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 516–520. [Google Scholar] [CrossRef]
- Shaw, E.A.; Denef, K.; Cotrufo, M.F.; Wall, D.H. Tracing Carbon Flow through the Soil Nematode Food Web: Do Long-Term Burning Practices Affect Carbon Trophic Dynamics in the Tallgrass Prairie? J. Nematol. 2012, 44, 490–491. [Google Scholar]
- Shen, J.B.; Yuan, L.X.; Zhang, J.L.; Li, H.G.; Bai, Z.H.; Chen, X.P.; Zhang, W.F.; Zhang, F.S. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Sitzia, T.; Campagnaro, T.; Dainese, M.; Cierjacks, A. Plant species diversity in alien black locust stands: A paired comparison with native stands across a north-Mediterranean range expansion. For. Ecol. Manag. 2012, 285, 85–91. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Spohn, M. Element cycling as driven by stoichiometric homeostasis of soil microorganisms. Basic Appl. Ecol. 2016, 17, 471–478. [Google Scholar] [CrossRef]
- Sul, W.J.; Asuming-Brempong, S.; Wang, Q.; Tourlousse, D.M.; Penton, C.R.; Deng, Y.; Rodrigues, J.L.M.; Adiku, S.G.K.; Jones, J.W.; Zhou, J.Z.; et al. Tropical agricultural land management influences on soil microbial communities through its effect on soil organic carbon. Soil Biol. Biochem. 2013, 65, 33–38. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kanzaki, N.; Tamai, S.; Yamanaka, N.; Futai, K. Does ectomycorrhizal fungal community structure vary along a Japanese black pine (Pinus thunbergii) to black locust (Robinia pseudoacacia) gradient? New Phytol. 2007, 173, 322–334. [Google Scholar] [CrossRef]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.D.; Wang, S.Q.; Hou, Q.C. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau, China. For. Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Thiet, R.K.; Frey, S.D.; Six, J. Do growth yield efficiencies differ between soil microbial communities differing in fungal: Bacterial ratios? Reality check and methodological issues. Soil Biol. Biochem. 2006, 38, 837–844. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Thomas, R.B. A test of a potential short cut in the nitrogen cycle: The role of exudation of symbiotically fixed nitrogen from the roots of a N-fixing tree and the effects of increased atmospheric CO2 and temperature. Plant Soil 1999, 210, 21–32. [Google Scholar] [CrossRef]
- Van der Heijen, M.G.A. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Vasquez-Dean, J.; Maza, F.; Morel, I.; Pulgar, R.; Gonzalez, M. Microbial communities from arid environments on a global scale. A systematic review. Biol. Res. 2020, 53, 1–12. [Google Scholar] [CrossRef]
- Vitkova, M.; Muellerova, J.; Sadlo, J.; Pergl, J.; Pysek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, M.; Tonika, J.; Mullerova, J. Black locust-Successful invader of a wide range of soil conditions. Sci. Total Environ. 2015, 505, 315–328. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.B.; Xue, S. Effect of black locust (Robinia pseudoacacia) on soil chemical and microbiological properties in the eroded hilly area of China’s Loess Plateau. Environ. Earth Sci. 2012, 65, 597–607. [Google Scholar] [CrossRef]
- Williard, K.W.J.; Dewalle, D.R.; Edwards, P.J. Influence of bedrock geology and tree species composition on stream nitrate concentrations in mid-Appalachian forested watersheds. Water Air Soil Pollut. 2005, 160, 55–76. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Tuo, D.F.; Wang, C.; Fu, B.J.; Lv, Y.H.; Liu, G.H. Land use change and stand age regulate soil respiration by influencing soil substrate supply and microbial community. Geoderma 2020, 359, 113991. [Google Scholar] [CrossRef]
- Wu, Y.P.; Ma, B.; Zhou, L.; Wang, H.Z.; Xu, J.M.; Kemmitt, S.; Brookes, P.C. Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis. Appl. Soil Ecol. 2009, 43, 234–240. [Google Scholar] [CrossRef]
- Xu, X.F.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.C.; Yu, G.R.; Sinsabaugh, R.L.; Tang, D.D.; Zhang, X.C.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.; Dou, Y.X.; An, S.S. Plant and soil traits driving soil fungal community due to tree plantation on the Loess Plateau. Sci. Total Environ. 2020, 708, 134560. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Penuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Zhang, R.; Bai, Y.F.; Zhang, T.; Henkin, Z.; Degen, A.A.; Jia, T.H.; Guo, C.C.; Long, R.J.; Shang, Z.H. Driving Factors That Reduce Soil Carbon, Sugar, and Microbial Biomass in Degraded Alpine Grasslands. Rangel. Ecol. Manag. 2019, 72, 396–404. [Google Scholar] [CrossRef]

| Forest Age (a) | Slope | Altitude (m) | Density (pl hm−2) | Height (m) | Diameter at Breast Height (cm) | Undergrowth Vegetation | Vegetation Coverage (%) |
|---|---|---|---|---|---|---|---|
| 0 (FL a) | N bE74° | 1205 | NA c | NA | NA | NA | NA |
| 10 | E20° | 1276 | 1600 | 6.9 ± 0.4 | 6.4 ± 0.5 | Thalictrum aquilegiifolium, Clematis ochotensis, Lespedeza davurica, Periploca sepium | 55 |
| 15 | W18° | 1338 | 2800 | 7.3 ± 0.2 | 6.9 ± 0.3 | Stipa bungeana, Clematis ochotensis, Melica scabrosa, Artemisia sacrorum, Artemisia capillaris, Periploca sepium | 65 |
| 25 | SW16° | 1352 | 1500 | 9.8 ± 0.9 | 15.3 ± 1.6 | Melica scabrosa, Stipa bungeana, Potentilla bifurca, Artemisia indica, Rosa xanthina, Ostryopsis davidiana, Syringa oblate, Cotinus coggygria | 85 |
| 40 | W18° | 1342 | 1300 | 10.7 ± 0.3 | 17.3 ± 0.2 | Heteropappus altaicus, Stipa bungeana, Artemisia indica, Buxus sinica, Cotoneaster acuminatus, Rosa hugonis, Lonicera hispida | 80 |
| Microbe Type | PLFA Biomarker | Reference |
|---|---|---|
| Bacteria | a14:0, i14:0, i15:0, a15:0, a16:0, i16:0, 16:1ω7c, 16:1ω9c, i17:0, a17:0, cy17:0, 17:1ω9c, i18:0, a18:0, 18:1ω5c, 18:1ω7c, 18:1ω9c, cy19:0, i22:0 | [28] |
| Actinomycetes | 10Me16:0, 10Me17:1ω7c, 10Me18:1ω7c, 10Me18:0, 10Me20:0 | [29] |
| Gram-positive | i14:0, i15:0, a15:0, i16:0, a16:0, i17:0, a17:0, i18:0, i22:0 | [30] |
| Gram-negative | 16:1ω7c, 16:1ω9c, 17:1ω9c, 18:1ω5c, 18:1ω7c, 18:1ω9c, cy17:0, cy19:0 | [30] |
| Fungi | 18:2ω6c, 18:2ω9c | [28,31] |
| Saturated PLFAs | 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0 | [32] |
| Monosaturated PLFAs | i16:1, 16:1ω11c, 16:1ω7c, 16:1ω5c, 17:1ω9c, a17:1, 17:1ω8c, 18:1ω9c, 18:1ω7c, 18:1ω5c, 11Me18:1ω7c | [32] |
| Cyclopropyl PLFAs | cy17:0, cy19:0 | [33] |
| Precursor PLFAs | 16:1ω7c, 18:1ω7c | [33] |
| Depth (cm) | Forest Stand | pH | Moisture (%) | Bulk Density (g.cm−3) | SOC d (g.kg−1) | TN (g.kg−1) | NO3−-N (mg.kg−1) | NH4+-N (mg.kg−1) | TP (g.kg−1) | AP (mg.kg−1) | C/N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | FL | 8.63 ± 0.05ns | 11.8 ± 0.85 b | 1.31 ± 0.05ns | 7.81 ± 0.31 c | 0.61 ± 0.02 c | 6.83 ± 0.04 a | 3.75 ± 0.44 a | 0.77 ± 0.01ns | 5.97 ± 0.52 a | 10.53 ± 0.47 a |
| Y10 a | 8.75 ± 0.05 bns | 10.79 ± 0.60 b,c | 1.16 ± 0.09ns | 5.94 ± 0.48 c | 0.75 ± 0.15 c | 2.21 ± 0.40 b | 0.52 ± 0.09 b | 0.53 ± 0.11ns | 2.27 ± 0.72 b,c | 9.18 ± 0.65 c | |
| Y15 | 8.60 ± 0.06ns | 14.15 ± 1.40 a | 1.09 ± 0.09ns | 6.89 ± 0.24 c | 0.67 ± 0.13 c | 1.47 ± 0.51 b | 0.52 ± 0.18 b | 0.66 ± 0.20ns | 2.04 ± 0.78 c | 9.02 ± 0.10 c | |
| Y25 | 8.52 ± 0.06ns | 10.05 ± 1.12 b | 1.29 ± 0.10ns | 13.86 ± 0.35 b | 1.27 ± 0.32 b | 1.92 ± 1.28 b | 0.72 ± 0.18 b | 0.61 ± 0.02ns | 3.88 ± 0.85 a,b | 9.30 ± 0.25 c | |
| Y40 | 8.49 ± 0.14ns | 9.57 ± 0.53 b | 1.21 ± 0.11ns | 19.14 ± 0.17 a | 1.96 ± 0.43 a | 2.99 ± 1.41 b | 0.91 ± 0.11 b | 0.65 ± 0.03ns | 5.07 ± 0.89 a | 9.58 ± 0.15 b | |
| 10–20 | FL | 8.76 ± 0.06ns | 12.13 ± 0.63 b | 1.25 ± 0.03ns | 4.52 ± 0.17 b | 0.41 ± 0.03 b | 3.64 ± 0.02 a | 2.81 ± 0.33 a | 0.68 ± 0.01ns | 5.92 ± 0.04 a | 9.64 ± 0.40 a |
| Y10 | 8.74 ± 0.01ns | 11.72 ± 1.07 b | 1.19 ± 0.06ns | 3.93 ± 0.27 b | 0.50 ± 0.09 b | 1.18 ± 0.46 b | 0.39 ± 0.15 b | 0.50 ± 0.11ns | 2.32 ± 0.38 a,b | 7.12 ± 0.48 c | |
| Y15 | 8.73 ± 0.02ns | 14.23 ± 0.80 a | 1.21 ± 0.09ns | 4.24 ± 0.23 b | 0.62 ± 0.13 b | 1.24 ± 0.42 b | 0.35 ± 0.03 b | 0.57 ± 0.02ns | 1.47 ± 0.15 b | 5.59 ± 0.53 d | |
| Y25 | 8.71 ± 0.03ns | 10.03 ± 0.90 bc | 1.23 ± 0.04ns | 6.16 ± 0.13 a | 0.67 ± 0.10 a,b | 1.17 ± 0.66 b | 0.60 ± 0.13 b | 0.57 ± 0.01ns | 1.68 ± 0.26 b | 8.26 ± 0.28 b | |
| Y40 | 8.67 ± 0.04ns | 9.75 ± 0.17 c | 1.11 ± 0.11ns | 6.85 ± 1.19 a | 0.95 ± 0.28 a | 1.37 ± 0.75 b | 0.60 ± 0.09 b | 0.59 ± 0.01ns | 4.84 ± 0.92 a | 8.93 ± 0.24 b | |
| 20–30 | FL | 8.79 ± 0.04ns | 12.85 ± 0.91 a | 1.27 ± 0.01 a | 2.92 ± 0.71 b | 0.34 ± 0.03 a,b | 2.50 ± 0.01 a | 1.02 ± 0.12 a | 0.57 ± 0.01ns | 2.60 ± 0.01 a | 7.67 ± 2.08 b |
| Y10 | 8.72 ± 0.01ns | 11.95 ± 0.79 a | 1.28 ± 0.04 a | 3.80 ± 0.62 a | 0.42 ± 0.06 b | 0.81 ± 0.42 b | 0.41 ± 0.16 b | 0.53 ± 0.06ns | 1.00 ± 0.06 a,b | 8.96 ± 0.35 a | |
| Y15 | 8.73 ± 0.02ns | 12.30 ± 0.94 a | 1.25 ± 0.04 a,b | 2.68 ± 0.44 b | 0.34 ± 0.06 a,b | 0.50 ± 0.14 b | 0.37 ± 0.10 b | 0.55 ± 0.02ns | 0.57 ± 0.20 b | 7.97 ± 0.09 b | |
| Y25 | 8.84 ± 0.03ns | 9.58 ± 0.95 b | 1.15 ± 0.05 b,c | 3.65 ± 0.80 a | 0.44 ± 0.05 a,b | 0.30 ± 0.02 b | 0.42 ± 0.02 b | 0.55 ± 0.02ns | 0.94 ± 0.08 b | 7.72 ± 0.80 b | |
| Y40 | 8.69 ± 0.11ns | 9.08 ± 0.35 b | 1.13 ± 0.02 c | 3.77 ± 0.61 a | 0.59 ± 0.21 a | 0.72 ± 0.53 b | 0.45 ± 0.04 b | 0.56 ± 0.03ns | 2.12 ± 0.13 a | 8.47 ± 0.13 a,b | |
| 30–40 | FL | 8.68 ± 0.11ns | 13.10 ± 0.45 a | 1.25 ± 0.05ns | 1.62 ± 0.76 c | 0.29 ± 0.01 b,c | 1.17 ± 0.01 a | 0.62 ± 0.07 a | 0.56 ± 0.01ns | 0.70 ± 0.01 a | 10.07 ± 2.63 a |
| Y10 | 8.68 ± 0.01ns | 12.47 ± 0.58 a | 1.30 ± 0.15ns | 3.35 ± 0.18 a | 0.36 ± 0.03 a | 0.38 ± 0.09 b | 0.35 ± 0.15 c | 0.53 ± 0.05ns | 0.40 ± 0.23 a | 9.34 ± 0.18 b | |
| Y15 | 8.76 ± 0.04ns | 11.33 ± 0.85 a,b | 1.28 ± 0.05ns | 2.32 ± 0.19 b | 0.28 ± 0.02 c | 0.32 ± 0.06 b | 0.32 ± 0.12 c | 0.54 ± 0.01ns | 0.62 ± 0.15 a | 8.72 ± 0.21 c | |
| Y25 | 8.84 ± 0.09ns | 9.94 ± 1.28 b,c | 1.24 ± 0.01ns | 2.85 ± 0.37 b | 0.31 ± 0.05 a,b | 0.17 ± 0.75 b | 0.36 ± 0.08 c | 0.55 ± 0.03ns | 0.56 ± 0.06 a | 8.58 ± 0.46 c | |
| Y40 | 8.79 ± 0.03ns | 9.24 ± 0.18 c | 1.23 ± 0.06ns | 3.24 ± 0.42 a | 0.39 ± 0.06 a | 0.57 ± 0.48 b | 0.47 ± 0.11 b | 0.56 ± 0.03ns | 0.57 ± 0.18 a | 8.32 ± 0.07 c | |
| 0–40 | FL | 8.72 ± 0.10ns | 12.47 ± 0.71 a | 1.27 ± 0.05ns | 4.22 ± 2.68 c | 0.41 ± 0.14 c | 3.54 ± 2.42 a | 2.05 ± 1.48 a | 0.64 ± 0.01 a | 3.80 ± 2.60 a | 9.48 ± 1.26 a |
| Y10 | 8.72 ± 0.02ns | 11.73 ± 0.66 a | 1.14 ± 0.22ns | 4.12 ± 0.04 c | 0.45 ± 0.01 c | 1.33 ± 0.12 b | 0.50 ± 0.05 b | 0.52 ± 0.06 b | 1.50 ± 0.68 b | 8.57 ± 0.44 a,b | |
| Y15 | 8.71 ± 0.04ns | 13.03 ± 0.99 a | 1.21 ± 0.05ns | 3.94 ± 0.12 c | 0.48 ± 0.03 c | 0.88 ± 0.10 b | 0.45 ± 0.02 b | 0.58 ± 0.05 a b | 1.22 ± 0.21 b | 7.88 ± 0.55 b | |
| Y25 | 8.73 ± 0.06ns | 9.44 ± 0.57 b | 1.23 ± 0.04ns | 6.59 ± 0.14 b | 0.67 ± 0.02 b | 1.09 ± 0.18 b | 0.53 ± 0.02 b | 0.57 ± 0.01 a b | 1.77 ± 0.55 b | 8.65 ± 0.20 a,b | |
| Y40 | 8.66 ± 0.08ns | 9.41 ± 0.11 b | 1.17 ± 0.01ns | 7.37 ± 0.67 a | 0.97 ± 0.26 a | 0.88 ± 0.24 b | 0.61 ± 0.08 b | 0.59 ± 0.02 a | 3.76 ± 0.77 a | 9.13 ± 0.12 a |
| Variable | Simple Term Effects | Forward Selection Results | ||
|---|---|---|---|---|
| Explains (%) | P | Explains (%) | P | |
| TN | 30.6 | 0.002 | 30.6 | 0.002 |
| SOC | 26.6 | 0.002 | / | / |
| pH | 16.6 | 0.002 | / | / |
| TP | 5.1 | 0.02 | 4.1 | 0.006 |
| AP | 4.8 | 0.006 | / | / |
| NH4+-N | 3.1 | 0.062 | / | / |
| NO3−-N | 2.8 | 0.074 | 3.4 | 0.01 |
| SM | 2.7 | 0.094 | / | / |
| C/N | 1.9 | 0.18 | / | / |
| BD | 1.3 | 0.336 | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Zhang, B.; Liu, J.; Wang, X.; Han, F.; Zhou, J. Effects of Different Ages of Robinia pseudoacacia Plantations on Soil Physiochemical Properties and Microbial Communities. Sustainability 2020, 12, 9161. https://doi.org/10.3390/su12219161
Luo C, Zhang B, Liu J, Wang X, Han F, Zhou J. Effects of Different Ages of Robinia pseudoacacia Plantations on Soil Physiochemical Properties and Microbial Communities. Sustainability. 2020; 12(21):9161. https://doi.org/10.3390/su12219161
Chicago/Turabian StyleLuo, Chaoyi, Bingxue Zhang, Jiang Liu, Xiaoxia Wang, Fengpeng Han, and Jihai Zhou. 2020. "Effects of Different Ages of Robinia pseudoacacia Plantations on Soil Physiochemical Properties and Microbial Communities" Sustainability 12, no. 21: 9161. https://doi.org/10.3390/su12219161
APA StyleLuo, C., Zhang, B., Liu, J., Wang, X., Han, F., & Zhou, J. (2020). Effects of Different Ages of Robinia pseudoacacia Plantations on Soil Physiochemical Properties and Microbial Communities. Sustainability, 12(21), 9161. https://doi.org/10.3390/su12219161




