Abstract
The development of efficient, environmentally friendly, low-cost approaches used to boost the growth of microalgae is urgently required to meet the increasing demands for food supplements, cosmetics, and biofuels. In this study, the growth promotion effects of protocatechuic acid (PCA) in the freshwater microalga Euglena gracilis were confirmed for the first time. PCA is a simple phenolic compound derived from natural plants and has a range of biological functions. The highest biomass yield, 3.1-fold higher than that of the control, used at 1.3 g·L−1, was obtained at 800 mg·L−1 of PCA. The yields of the metabolites chlorophyll a, carotenoids, and paramylon in the presence of PCA at 800 mg·L−1 were 3.1, 3.3, and 1.7 times higher than those of the control group, respectively. The highest paramylon yield was achieved at a lower dosage of PCA (100 mg·L−1), which is considered to be feasible for economic paramylon production. The growth and biosynthesis of metabolites stimulated by phytochemicals such as PCA could be an efficient and cost-effective strategy to enhance the productivity of microalgae in large-scale cultivations.
1. Introduction
Microalgae are considered to be one of the most promising feedstocks for the sustainable production of commodities such as biofuels, foods, cosmetics, and pharmaceuticals [1,2]. Euglena gracilis is a freshwater flagellate which absorbs light energy and converts it into chemical energy, besides taking up nutrients from the environment. This alga has considerable potential value because of the compounds it produces, which include vitamins, proteins, β-1,3-glucan (paramylon), essential amino acids, and fatty acids [3]. Paramylon, particularly, shows great potential as a stimulator of the immune system and has anti-tumor and antioxidant effects that may be valuable in biomedical applications [4]. The production of paramylon by E. gracilis must be improved to meet the industrial demands, because it naturally has a low biomass yield. Various approaches for optimizing E. gracilis cultivation have been trialed in the past decade, including modifying the alga strains by genetic engineering [5,6], but technical difficulties remain. Previous studies have successfully addressed the improvement of biomass and metabolite production of microalgae using genetic engineering [7,8]. However, this approach is complicated and costly. The addition of stimulating substances to the culture medium to promote the growth of microalgae has also gained the interest of researchers. Some studies have increased the biomass yield of Scenedesmus spp. and Chlamydomonas spp. by adding chemical fertilizers [9]. However, the use of chemical fertilizers causes several problems, including issues of cost and environmental pollution. Some organic sources, such as glucose, fructose, and maltose, can enhance the growth of microalgae [10,11]. These sources, however, can easily be used by bacteria, which increases the risk of contamination of the medium. Thus, the exploration of more eco-friendly, cheaper, and safer sources to promote microalgae biomass growth is necessary to achieve their economically feasible bio-production.
Some phenolic compounds have been used as growth promoters for microalgae, owing to their wide distribution and antibacterial effects. Enhanced biomass production after exposure to different phenolic compounds has been reported for several algal species, including Chlorella vulgaris and Ankistrodesmus braunii [12,13]. In our previous research, we found that ferulic acid had positive effects on the growth of E. gracilis, increasing its growth by 2.5 times with respect to that of the control group when supplied at 500 mg·L−1 [14]. Protocatechuic acid (PCA) (3, 4-dihydroxybenzoic acid) has been detected in the ferulic acid metabolic pathway in some microorganisms including Margarinomyces heteromorpha, Margarinomyces mutabilis, and Pullularia pullulans [15,16]. We therefore suspected that PCA could also be involved in the ferulic acid metabolism of E. gracilis, possibly leading to the promotion of growth.
PCA, a dihydroxybenzoic acid derived from fruits, green tea, and some kinds of Chinese herb, has a variety of biological effects because of its antioxidant activity. Previous researchers have reported that PCA has many benefits for humans and animals, such as anti-inflammatory, anti-hyperglycemic, and potential cancer chemo-preventive activities related to the chelation of transition metals, scavenging of free radicals by donating hydrogen atoms, and upregulation of enzymes related to the neutralization of free radicals [17]. A recent study found that PCA prevents cognitive impairment caused by amyloid β-induced Alzheimer’s disease by inhibiting oxidative stress and inflammation and can therefore be regarded as a protective agent against Alzheimer’s disease [18]. These potential pharmacological benefits in humans and animals have been reported both in vitro and in vivo. However, the effects of PCA on microalgae has not yet been fully explored.
This study aimed to investigate the effects of PCA on the growth and metabolism of E. gracilis. Cellular growth, morphology, and the accumulation of metabolites such as photosynthetic pigments and paramylon were examined.
2. Materials and Methods
2.1. Microorganism and Culture Conditions
E. gracilis Klebs strain (NIES-48) was obtained from the National Institute for Environmental Studies, Japan, and grown in Cramer–Myers (CM) medium with the following composition (mg·L−1): (NH4)2HPO4, 1000; KH2PO4, 1000; MgSO4·7H2O, 200; CaCl2·2H2O, 20; FeSO4·7H2O, 3; MnCl2·4H2O, 1.8; CoSO4·7H2O, 1.5; ZnSO4·7H2O, 0.4; Na2MoO4·2H2O, 0.2; CuSO4·5H2O, 0.02; Vitamin B12, 0.0005; and Thiamine HCl, 0.1. The initial pH of the medium was 6.9. The CM medium, except for the organic substrates, was sterilized by autoclaving at 121 °C for 20 min. The organic ingredients in the medium and a PCA (Wako, Japan) stock solution were separately sterilized using 0.45 μm filters (Nalgene, Thermo Fisher Scientific, Waltham, USA).
E. gracilis was incubated until it reached the exponential growth phase, at which time 10 mL aliquots of cell suspension were inoculated into Erlenmeyer flasks. Different concentrations of PCA solution were added to the CM medium, with a final culture volume of 100 mL. The final concentrations of PCA for the treatment groups were 0, 10, 100, 500, and 800 mg·L−1. The cultures were incubated at 25 ± 1 °C with a 5000 light intensity (12/12 h light–dark cycle). The flasks were shaken three times per day by hand to ensure that light was provided equally to all cells and to prevent the cells from clustering.
2.2. Growth Measurement
Cell number was determined using a counting chamber (Hirschmann, Thoma, Germany) with a light microscope (Motic, BA210, Fukuoka, Japan). For cell counting, 300 μL of the sample was taken from the cell culture, and then, 20 μL of ethanol was added to fix the cells. The cell dry weight was measured at day 24, at the end of the log phase. To measure the cell dry weight, 5 mL of the culture was centrifuged at 5000 rpm for 10 min. Cell pellets were re-suspended in distilled water and centrifuged at 5000 rpm for 10 min to remove residual salts, followed by drying in an oven (AVO-250 N, As one, Fukuoka, Japan) at 80 °C overnight. Finally, the cells were transferred to a desiccator and cooled to room temperature. The cell dry weight was determined by calculating the difference between the final weight and the initial weight. The pH of the culture filtrate was regularly measured using a pH meter (LAQUA-2103AL, Horiba, Japan). One milliliter of cell suspension was filtered through 0.45 μm filter paper (Advantec, GC-50, Fukuoka, Japan), and the filtrate was used for pH determination.
Specific growth rate (μ) was estimated using Equation (1), and the doubling time (DT) was calculated using Equation (2).
where N1 and N2 represent the cell densities (cells·mL−1) at the beginning (t1) and at the end (t2) of the exponential period, respectively.
μ = (ln (N2) − ln (N1))/(t2 − t1)
DT = (ln2)/μ
2.3. Analysis of Cell Morphology
To determine the growth status of the microalgae, morphological observations of the microalgae cells were performed using an optical microscope (Motic, BA210). More than 100 cells were recorded using the software (Motic Image Plus 2.2S) at the sixth hour of the light period. The image processing software Image J (open source) was used to quantify cell morphology by particle analysis.
2.4. Analysis of Photosynthetic Pigments
Photosynthetic pigments, including chlorophyll a, chlorophyll b, and carotenoids, were measured according to the method of Lichtenthaler and Wellburn [19]. To measure the photosynthetic pigments, 5 mL of algae was collected using 0.45 μm filter paper, and the collected cells were washed with distilled water and then ground with glass sands in a mortar, using 80% acetone to extract the pigments. The homogenate extract was filtered again to remove cell debris and glass sands. The extract was collected in a volumetric flask, and the volume was adjusted to 10 mL using 80% acetone. The amount of photosynthetic pigments was determined using a spectrophotometer (UV–vis 1200, Shimadzu, Japan) at wavelengths of 470, 646, and 663 nm. Pigment concentrations were calculated using the following equations:
where Chl a, Chl b, and Cx + c are chlorophyll a, chlorophyll b, and carotenoids concentrations, respectively. Abs663, Abs470, and Abs646 are the absorbances at 663, 470, and 646 nm, respectively.
Chl a = 12.21Abs663 − 2.81Abs646
Chl b = 20.13Abs646 − 5.03Abs663
Cx + c = (1000Abs470 − 3.27Chl a − 104 Chl b)/229
2.5. Paramylon Analysis
To quantify the paramylon content, 50 mL of microalga cells were collected by centrifugation at 5000 rpm for 10 min and washed three times with distilled water to eliminate the influence of residual salts in the medium. The harvested cells were then frozen at −20 °C for 12 h and broken by sonication. The pellets were re-suspended in 0.1% (w/v) sodium dodecyl sulfate and 5% (w/v) Na2EDTA solution. The samples were incubated at 37 °C for 1 h and then centrifuged at 5000 rpm for 15 min. The above steps were repeated until the suspension became translucent. Pellets were washed with distilled water at 70 °C three times, collected on a 0.45 μm fiber filter, and dried overnight at 60 °C in an oven. The paramylon yield was measured gravimetrically.
2.6. Statistical Analysis
All experiments were performed in triplicate, and the results were represented as mean ± standard deviation. Significance was analyzed using the statistical software SPSS version 16.0 (IBM, USA). One-way analysis of variance was used to determine statistical differences in the growth and metabolic parameters for different treatment groups, and Student’s t tests were used for pairwise comparisons. A p-value <0.05 was accepted as statistically significant.
3. Results and Discussion
3.1. Growth Profiles of E. gracilis with PCA Treatment
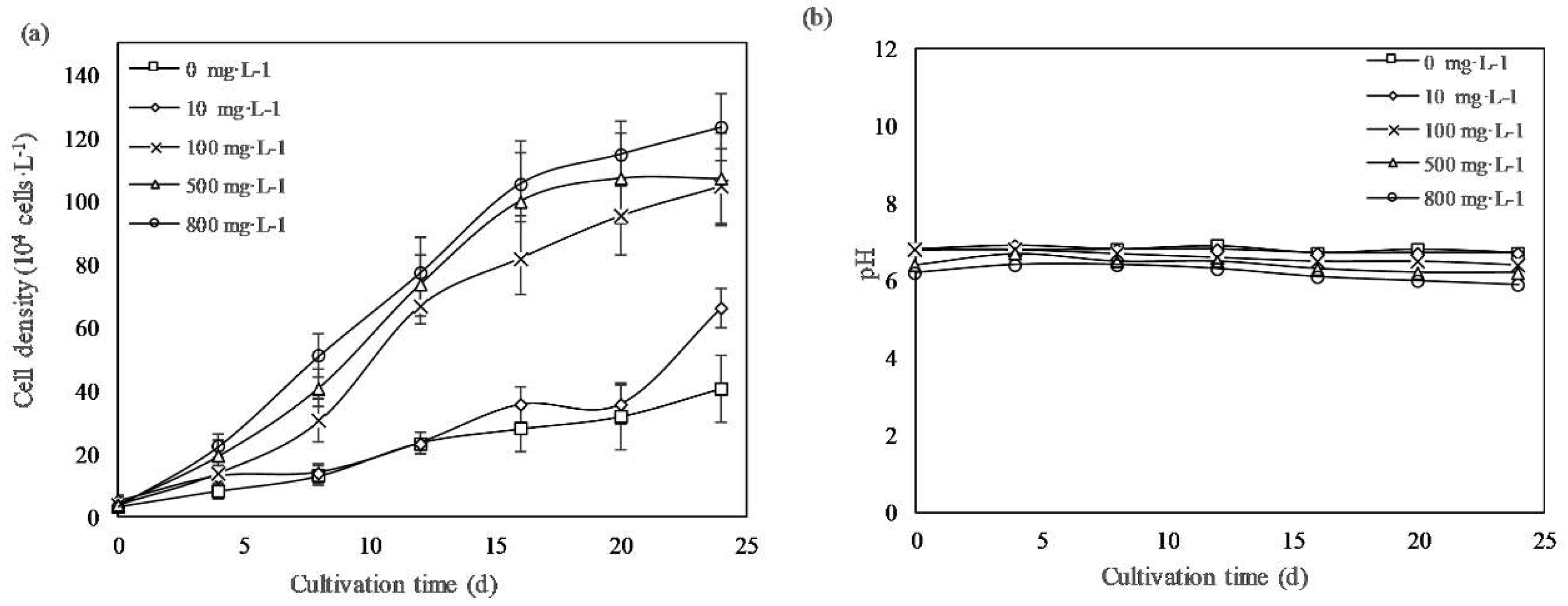
The growth curves of the periodic cultures of E. gracilis under different PCA concentrations was presented in Figure 1. Similar growth trends were observed in all groups, and no apparent lag period were observed at the beginning of the experiment. The cells reached the stationary phase after 24 days of cultivation. PCA promoted growth dose-dependently. The cell densities of cultures treated with PCA concentrations of 800, 500, and 100 mg·L−1 were 3.1-, 2.9-, and 2.4-fold higher than that of the control group, respectively, whereas the 10 mg·L−1 PCA group had values similar to those of the control group.
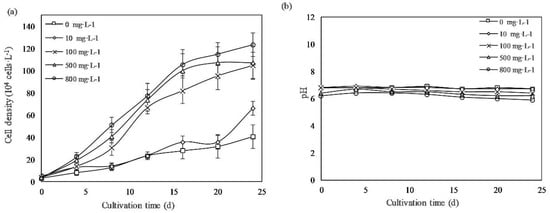
Figure 1.
(a) Growth profiles of Euglena gracilis cells with different concentrations of protocatechuic acid (PCA). (b) pH changes of the culture medium. Values represent the mean of three independent measurements (n = 3), and the error bars indicate the standard deviations (n = 3).
As shown in Figure 1b, the pH of the medium was almost stable within the range of 6–7, which was optimal for E. gracilis, during the culture period [20]. Table 1 summarizes the specific growth rate, DT, and biomass yield under different concentrations of PCA. When the PCA concentration was increased to 800 mg·L−1, the cell-specific growth rate and dry biomass yield reached a maximum of 0.23 d−1 and 1.32 g·L−1, respectively, significantly higher than those of the control group, of 0.14 d−1 and 0.43 g·L−1, respectively.

Table 1.
Specific growth rate, doubling time, and biomass yield of E. gracilis with different concentrations of protocatechuic acid (means ± SD) (n = 3).
This study is the first to report that PCA significantly promoted the growth of E. gracilis concentration-dependently. However, previous research has shown that some phenolic acids, including caffeic acid, p-coumaric acid, sinapic acid, syringic acid, vanillic acids, catechol, and hydroquinone, inhibited the growth of blue algae (Microcystis aeruginosa and -Aphanizomenon flos-aquae) and green algae (Chlorella pyrenoidosa and Scenedesmus obliquus) because they produced many polyphenol-autoxidized products and altered the permeability and lipid/protein ratio of the algae cell membranes, thereby triggering programmed cell death [21,22].
In this study, the positive effect of PCA on the growth of E. gracilis might be attributed to the different type of microalga and the culture conditions established in previous studies. E. gracilis is more resistant to an adverse environment than the abovementioned strains because it exhibits higher tolerance to metals and acids, which adversely affect the growth of algae [23]. Thus, E. gracilis could grow under PCA treatment. However, the phenolic activity would be influenced by the composition of the culture medium. The antioxidant activity of phenolic compounds relates to the number and position of phenolic hydroxyl groups [24]. Phenol hydroxyl can also produce quinone and superoxide anions, especially when the presence of transition metals, particularly Fe and Cu, and a high pH change its chemical properties [25,26]. The transition metals and pH of the medium in this study were different from those of the C and BG-11 medium used in other studies, which tended to be alkaline or to contain large amounts of transition metals. Therefore, different results were observed in this study.
The mechanism by which PCA promotes the growth of E. gracilis might be related to its chemical characteristics and the species of microalga. The structure of PCA is unstable, and the compound is easily autoxidized, since it has two hydroxyl groups in the ortho position on the aromatic ring. This instability can lead to the formation of quinone and subsequent damage to the microalga cells by reactive oxygen species (ROS) [21]. The antioxidant defense system of the microalga and the expression of related genes would be stimulated to prevent the oxidative stress caused by ROS. Previous studies have demonstrated that the production of lipids in the biomass was 26% greater than that in the control and that superoxide dismutase enzyme activity was enhanced when Dunaliella salina was exposed to phenol. The growth of C. vulgaris increased when the cells were subjected to oxidative stress [27,28]. These results suggest that triggering the antioxidant system could promote the growth of algae.
PCA may interact with endogenous plant hormones to enhance growth. The effect of plant hormones and their analogs on the promotion of the growth of various algae has previously been investigated. The biomass productivity of E. gracilis was enhanced by over 20% by the indole-3-acetic acid (IAA)-producing bacterium Vibrio natriegens [29]. In this case, the growth promotion of E. gracilis could be due to IAA-induced growth that counteracted the decarboxylation of IAA [30]. Previous research has shown that caffeic acid, ferulic acid, chlorogenic acid, and other polyphenols can synergistically work with IAA in the Avena curvature test. The elongation of coleoptile sections promoted by caffeic acid [31] indicates that phenolic compounds could interact with endogenous IAA and affect growth. Both phenols and IAA oxidase are widely distributed in plants. Thus, these interactions might exist in microalgae and stimulate their growth.
The promotion of the growth of E. gracilis by PCA could be related to the use of PCA as a carbon source. Aromatic rings can be opened by either ortho-cleavage or meta-cleavage after the formation of a 1, 2-dihydroxybenzoidal moiety [32]. Earlier studies have reported that the phenols selected from olive oil mill and petroleum hydrocarbons are degraded by microalgae [13,33], providing support for the contention that microalgae catabolize aromatic compounds. Earlier research has also shown that PCA is metabolized by Vibrio and is finally used by microorganisms as an exogenous carbon source [34]. Therefore, it has been speculated that PCA may also be used in E. gracilis metabolism during growth.
One or more of the above mechanisms may be a reasonable explanation for the growth promotion of E. gracilis by PCA, leading to a 3.1-times increase in biomass compared with that of the control.
3.2. Cell Morphology Analysis
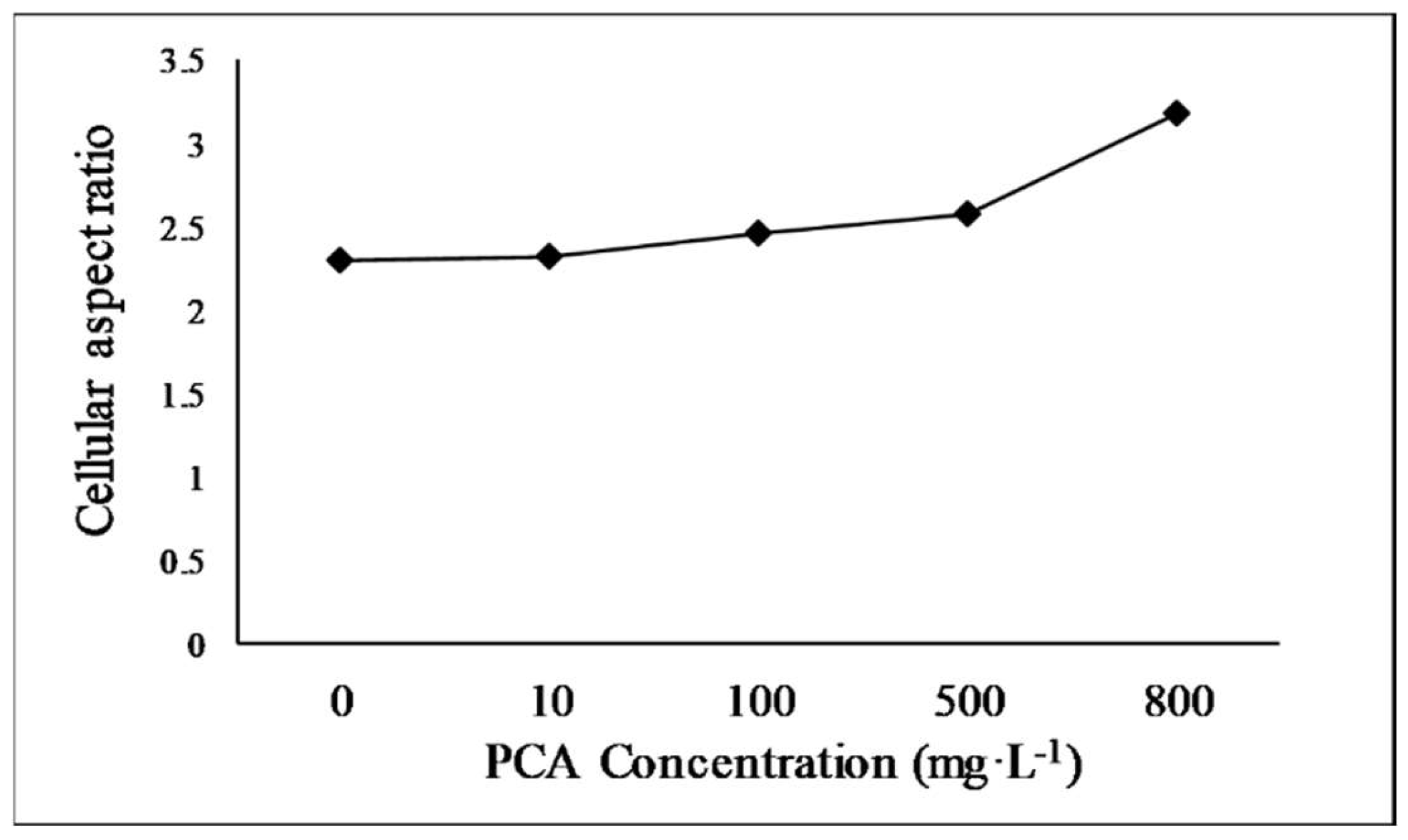
Cell morphology is an essential biological indicator of E. gracilis growth. It reflects the response of the microalga to environmental changes. E. gracilis cells exhibit various morphologies, such as spherical, spindle-shaped, and elongated forms, in response to different stimuli [35]. E. gracilis cells are spindle-shaped and motile in normal, favorable environments. However, the cells become spherical or cystic when exposed to unfavorable conditions [36]. The cell aspect ratio is calculated as the cell length divided by the cell width and indicates the shape of the alga cells. The median cell aspect ratio of the alga cultured under different concentrations of PCA in this research is shown in Figure 2. The cell aspect ratio increased with increasing PCA concentration. At PCA concentrations of 800 mg·L−1, the median cell aspect ratio increased from 2.31 to 3.18 times that of the control, and the cell aspect ratios of the alga treated with other PCA concentrations (10, 100, and 500 mg·L−1) were 2.33, 2.46, and 2.58, respectively. These results are within the range of values previously reported [37,38]. The increases in the aspect ratio with increasing PCA concentration was related to cell elongation, suggesting that PCA exposure provided a suitable environment for E. gracilis growth. The results of the cell morphology analysis are consistent with the promotion of growth.
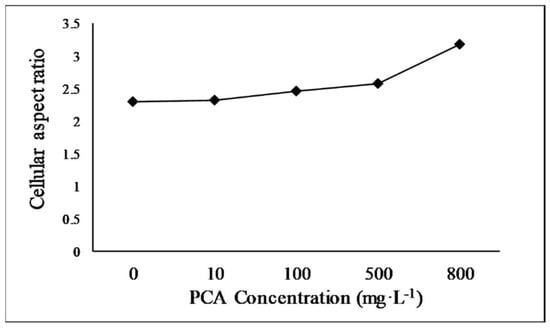
Figure 2.
Median cell aspect ratio of E. gracilis cultured with different concentrations of protocatechuic acid.
3.3. Photosynthetic Pigment Analysis
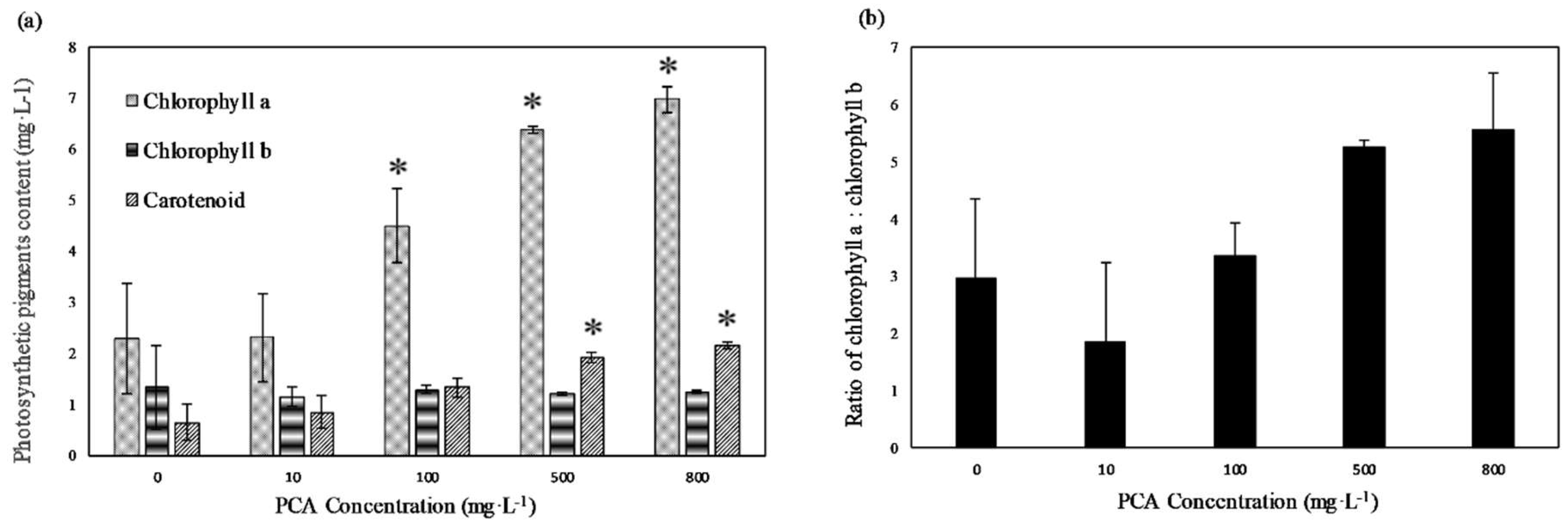
As shown in Figure 3a, both chlorophyll a and carotenoid content increased concentration-dependently, but no significant change in chlorophyll b content was observed. In the 800 mg·L−1 treatment group, chlorophyll a was the highest, at 6.97 mg·L−1, followed by carotenoids, with a content of 2.15 mg·L−1, and chlorophyll b, with a content of 1.25 mg·L−1, which were 3.1, 3.3, and 0.9 times greater than those in the control group, respectively. There was no significant difference in pigments’ contents for the 100, 500, and 800 mg·L−1 concentrations. Figure 3b shows the ratio of chlorophyll a/b (Chl a/b) per alga cell. The Chl a/b ratio of the control group was approximately 2.9. At PCA concentrations of 500 and 800 mg·L−1, the Chl a/b ratios were 5.3 and 5.5, respectively.
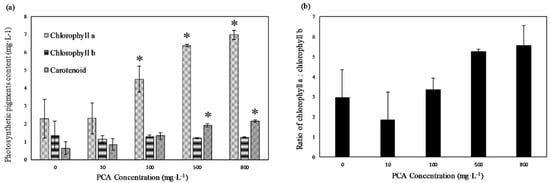
Figure 3.
(a) Chlorophyll a, chlorophyll b, and carotenoids content of E. gracilis at different concentrations of PCA. (b) Ratio of chlorophyll a to chlorophyll b at different concentrations of PCA. Values represent the mean of three independent measurements (n = 3), and error bars indicate the standard deviation; * in each column indicates a significant difference, with p < 0.05, according to Student’s t-test.
Earlier studies have shown that chlorophyll plays an essential role in phototrophic organisms [39]. Chlorophyll a is a critical component of the light-harvesting pigment protein complex, and photosynthetic capacity is enhanced when the chlorophyll a content is increased [40], possibly leading to algal growth promotion. The Chl a/b ratio is closely related to the light-harvesting efficiency [41]. A higher ratio of Chl a/b indicates higher photosynthetic activity and faster growth of the cells. In this study, the higher Chl a/b ratio demonstrated that the PSII complex and photosynthetic efficiency were enhanced after treatment with different PCA concentrations. A similar effect was also observed when Cyclotella caspia was treated with gallic acid, which stimulated the algal cells to produce more chloroplasts to cope with the applied stress [42]. Carotenoids, as auxiliary pigments, also play an important role in plants and algae in absorbing light energy for photosynthesis. Increases in the content of carotenoids, scavengers of free radicals, might also be related to the stress induced by ROS produced by PCA [43]. Polyphenol-autoxidized products are known to induce the production of radicals [21]. Some carotenoids have been reported not only to scavenge free radicals directly but also to modulate the expression of genes involved in endogenous antioxidant pathways, thereby regulating physiological activities and protecting cells [44,45]. The more vigorous growth and the greater cell length reflected changes in the physiological activities of E. gracilis.
3.4. Paramylon Analysis
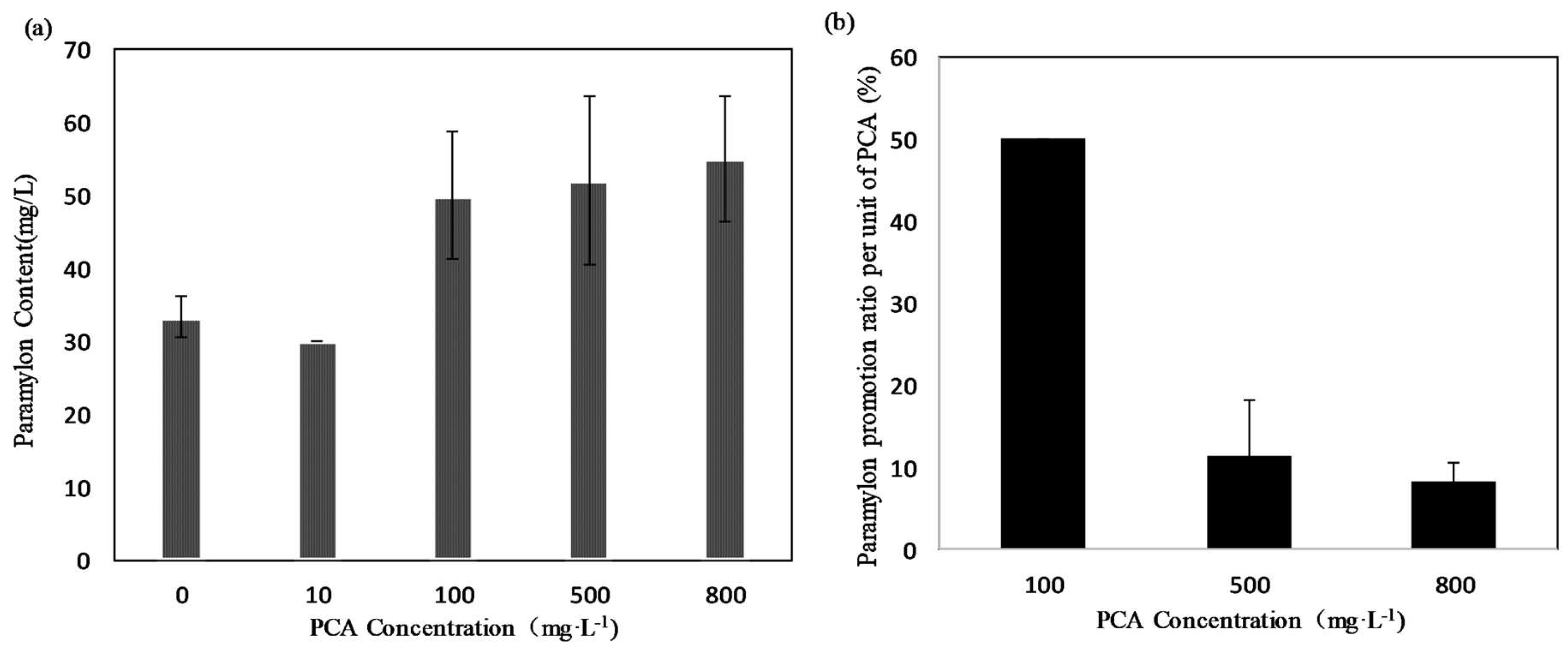
The total paramylon yield was increased by adding PCA to the medium. As shown in Figure 4a, the highest paramylon yield (55 mg·L−1) was obtained at 800 mg·L−1 of PCA, followed by the yields of 50 mg·L−1 at 100 mg·L−1 and 47 mg·L−1 at 500 mg·L−1 of PCA. Paramylon yield decreased to 30 mg·L−1 at 10 mg·L−1 of PCA, as low as that of the control group, which was 33 mg·L−1. Figure 4b shows the paramylon promotion ratio per unit of PCA (%). The highest paramylon promotion ratio per unit of PCA (5%) was obtained for the 100 mg·L−1 treatment group, followed by 1.2% and 0.8% at 500 and 800 mg·L−1 treatment, respectively. In this study, the total paramylon yield increased by approximately 65% with 800 mg·L−1 of PCA, due to the promotion of the growth of microalga biomass. However, if we compare the promotion effect per unit dosage of PCA, a 50% increment was obtained at 100 mg·L−1, whereas only 11.2% and 8.1% increments were observed at the higher dosages of 500 and 800 mg·L−1, respectively. Therefore, 100 mg·L−1 of PCA appeared to be the optimal dosage for producing paramylon economically.
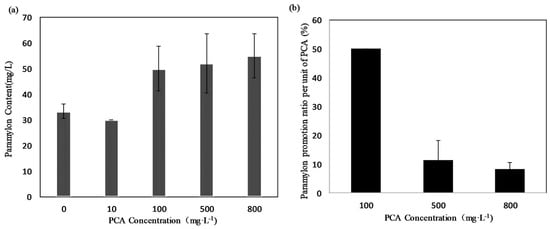
Figure 4.
(a) Paramylon yield (mg·L−1) of E. gracilis culture under different concentrations of PCA. (b) Paramylon promotion ratio per unit of PCA (%·mg−1). Values represent the mean of three independent measurements (n = 3), and error bars indicate the standard deviation.
Paramylon accumulation was enhanced when carbon and nitrogen sources were added to the medium as supplements or when E. gracilis was exposed to unfavorable conditions. As presented in Table 2, a paramylon content nearly 1.7 times higher than that of the control group was reported under treatment with 500 mg·L−1 of ferulic acid, but the effect was due to the dimethyl sulfoxide (DMSO) used as a co-solvent, which showed some inhibitory effect on the growth of E. gracilis [14]. It was also confirmed that the paramylon content was increased when adding other substances to the culture medium. A combination of 8.16 g·L−1 malate, 10.6 g·L−1 glucose acids, and 1.8 g·L−1 NH4Cl increased the paramylon content 2.0-fold; when the medium contained 17.7 g·L−1 glucose, 10 g·L−1 yeast extract, and some inorganic salt, the production of paramylon was increased by 1.65 folds in a heterotrophic culture [46,47]. Paramylon production required supplemental organic carbon sources, amino acids, and vitamins, which are expensive and to compete with humans for foods. In addition, these organics would increase the risk of media contamination as they are easily used by bacteria. In our studies, the high biomass and high levels of paramylon were obtained by simply adding PCA into the medium. This strategy provides a straightforward way to overcome the current difficulty in the production of both paramylon and biomass.

Table 2.
Effect of different supplemental sources and impact of paramylon on the growth of E. gracilis under different culture modes. DMSO, dimethyl sulfoxide.
4. Conclusions
In this study, the growth promotion effect of the phenolic compound PCA was confirmed on E. gracilis for the first time. The highest biomass yield, 3.1-fold greater than that of the control, was obtained at a PCA concentration of 800 mg·L−1. The yields of metabolites such as chlorophyll a, carotenoids, and paramylon at 800 mg·L−1 of PCA were increased by 3.3, 2.1, and 1.7 times with respect to those of the control group, respectively. The strongest effect of paramylon on growth per unit was obtained with 100 mg·L−1 of PCA, which therefore appears to be the optimal dosage for producing paramylon economically. As this study is preliminary, further investigation using intermediates or derivatives of PCA metabolism is essential to understand the effects of PCA on the promotion of the growth of E. gracilis.
Author Contributions
Conceptualization, Methodology, Software, Data curation, Visualization, Writing—original draft: X.T.; Methodology, Software, Validation, Writing editing: J.Z.; Investigation, Supervision, Software, Validation, Writing—review & editing: M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Xiaomiao Tan would like to thank the Yangzhou University International Academic Exchange Fund, China (YZUIAEF201901027).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Thankamony, R.L.; Agusti, S. Euglena as a potential natural source of value-added metabolites. A review. Algal Res. 2019, 37, 154–159. [Google Scholar] [CrossRef]
- Watanabe, T.; Shimada, R.; Matsuyama, A.; Yuasa, M.; Sawamura, H.; Yoshida, E.; Suzuki, K. Antitumor activity of the β-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in mice. Food Funct. 2013, 4, 1685. [Google Scholar] [CrossRef]
- Ivušić, F.; Šantek, B. Optimization of complex medium composition for heterotrophic cultivation of Euglena gracilis and paramylon production. Bioprocess Biosyst. Eng. 2015, 38, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Ho, M.Y.; Kanehara, K.; Nakamura, Y. Functional study of diacylglycerol acyltransferase type 2 family in Chlamydomonas reinhardtii. FEBS Lett. 2013, 587, 2364–2370. [Google Scholar] [CrossRef]
- Banerjee, A.; Banerjee, C.; Negi, S.; Chang, J.S.; Shukla, P. Improvements in algal lipid production: A systems biology and gene editing approach. Crit. Rev. Biotechnol. 2018, 38, 369–385. [Google Scholar] [CrossRef]
- Soares, J.; Kriiger Loterio, R.; Rosa, R.M.; Santos, M.O.; Nascimento, A.G.; Santos, N.T.; Williams, T.C.R.; Nunes-Nesi, A.; Martins, M.A. Scenedesmus sp. cultivation using commercial-grade ammonium sources. Ann. Microbiol. 2018, 68, 35–45. [Google Scholar] [CrossRef]
- Zheng, Y.; Chi, Z.; Lucker, B.; Chen, S. Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. Bioresour. Technol. 2012, 103, 484–488. [Google Scholar] [CrossRef]
- Yee, W. Feasibility of various carbon sources and plant materials in enhancing the growth and biomass productivity of the freshwater microalgae Monoraphidium griffithii NS16. Bioresour. Technol. 2015, 196, 1–8. [Google Scholar] [CrossRef]
- Bajguz, A.; Czerpak, R.; Piotrowska, A.; Polecka, M. Effect of isomers of hydroxybenzoic acid on the growth and metabolism of Chlorella vulgaris Beijerinck (Chlorophyceae). Acta Soc. Bot. Pol. 2014, 70, 253–259. [Google Scholar] [CrossRef]
- Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Biodegradation of phenols by microalgae. Biotechnol. Lett. 2002, 24, 2047–2051. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M. Growth promotion of Euglena gracilis by ferulic acid from rice bran. AMB Express 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.E.K. The Metabolism of Aromatic Compounds Related to Lignin by some Hyphomycetes and Yeast-like Fungi of Soil. J. Gen. Microbiol. 1961, 26, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tillett, R.; Walker, J.R.L. Metabolism of ferulic acid by a Penicillium sp. Arch. Microbiol. 1990, 154, 206–208. [Google Scholar] [CrossRef]
- Owumi, S.; Ajijola, I.; Agbeti, O. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum. Exp. Toxicol. 2019, 38, 1254–1265. [Google Scholar] [CrossRef]
- Choi, J.R.; Kim, J.H.; Lee, S.; Cho, E.J.; Kim, H.Y. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer’s disease mouse model. Food Chem. Toxicol. 2020, 144, 111571. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Olaveson, M.M.; Nalewajko, C. Effects of acidity on the growth of two Euglena species. Hydrobiologia 2000, 433, 39–56. [Google Scholar] [CrossRef]
- Nakai, S. Algal growth inhibition effects and inducement modes by plant-producing phenols. Water Res. 2001, 35, 1855–1859. [Google Scholar] [CrossRef]
- Zuo, S.; Fang, Z.; Yang, S.; Wan, K.; Han, Y. Effect of allelopathic potential from selected aquatic macrophytes on algal interaction in the polluted water. Biochem. Syst. Ecol. 2015, 61, 133–138. [Google Scholar] [CrossRef]
- Jasso-Chávez, R.; Pacheco-Rosales, A.; Lira-Silva, E.; Gallardo-Pérez, J.C.; García, N.; Moreno-Sánchez, R. Toxic effects of Cr(VI) and Cr(III) on energy metabolism of heterotrophic Euglena gracilis. Aquat. Toxicol. 2010, 100, 329–338. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic Acid Composition, Antiatherogenic and Anticancer Potential of Honeys Derived from Various Regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phyther. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zeng, X.B.; Guo, S.Y.; Li, Z.T. Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagnetics 2008, 29, 39–46. [Google Scholar] [CrossRef]
- Cho, K.; Lee, C.-H.; Ko, K.; Lee, Y.J.; Kim, K.N.; Kim, M.K.; Chung, Y.H.; Kim, D.; Yeo, I.K.; Oda, T. Use of phenol-induced oxidative stress acclimation to stimulate cell growth and biodiesel production by the oceanic microalga Dunaliella salina. Algal Res. 2016, 17, 61–66. [Google Scholar] [CrossRef]
- Kim, J.Y.; Oh, J.J.; Jeon, M.S.; Kim, G.H.; Choi, Y.E. Improvement of Euglena gracilis Paramylon Production through a Cocultivation Strategy with the Indole-3-Acetic Acid-Producing Bacterium Vibrio natriegens. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.; Thimann, K.V. Interactions of Phenolic Acids, Metallic Ions and Chelating Agents on Auxin-Induced Growth. Plant Physiol. 1966, 41, 1443–1454. [Google Scholar] [CrossRef]
- Vendrig, J.C.; Buffel, K. Growth-stimulating Activity of Trans-Caffeic Acid isolated from Coleus rhenaltianus. Nature 1961, 192, 276–277. [Google Scholar] [CrossRef]
- Bird, J.A.; Cain, R.B. Microbial degradation of alkylbenzenesulphonates. Metabolism of homologues of short alkyl-chain length by an Alcaligenes sp. Biochem. J. 1974, 140, 121–134. [Google Scholar] [CrossRef]
- Walker, J.D.; Colwell, R.R.; Petrakis, L. Degradation of petroleum by an alga, Prototheca zopfii. Appl. Microbiol. 1975, 30, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Cain, R.B. The metabolism of protocatechuic acid by a vibrio. Biochem. J. 1961, 79, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Danilov, R.A.; Ekelund, N.G.A. Responses of Photosynthetic Efficiency, Cell Shape and Motility in Euglena gracilis (Euglenophyceae) to Short-Term Exposure to Heavy Metals and Pentachlorophenol. Water Ai Soil Pollut. 2001, 132, 61–73. [Google Scholar] [CrossRef]
- Peng, C.; Lee, J.W.; Sichani, H.T.; Ng, J.C. Toxic effects of individual and combined effects of BTEX on Euglena gracilis. J. Hazard. Mater. 2015, 284, 10–18. [Google Scholar] [CrossRef]
- Lonergan, T.A. Regulation of cell shape in Euglena gracilis. II. The effects of altered extra- and intracellular Ca2+ concentrations and the effect of calmodulin antagonists. J. Cell Sci. 1984, 71, 37–50. [Google Scholar]
- Zhu, J.; Wakisaka, M. Effect of two lignocellulose related sugar alcohols on the growth and metabolites biosynthesis of Euglena gracilis. Bioresour. Technol. 2020, 303, 122950. [Google Scholar] [CrossRef]
- Mostafa, S.S.M.; Shalaby, E.A.; Mahmmoud, G.I. Cultivating Microalgae in Domestic Wastewater for Biodiesel Production. Not. Sci. Biol. 2012, 4, 56–65. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, J.; Gong, D.; Zhao, X.; Qi, Y.; Su, Y.; Ma, W. The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci. Total Environ. 2018, 633, 593–599. [Google Scholar] [CrossRef]
- Paulsen, H.; Rümler, U.; Rüdiger, W. Reconstitution of pigment-containing complexes from light-harvesting chlorophyll a/b-binding protein overexpressed in Escherichia coli. Planta 1990, 181, 204–211. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Huang, Q. Allelopathic effects of gallic acid from Aegiceras corniculatum on Cyclotella caspia. J. Environ. Sci. 2013, 25, 776–784. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Minireview. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Ye, Z.W.; Jiang, J.G.; Wu, G.H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, L.; Luo, W.; Li, H.; Zhou, Y.; Yu, X. Effect of Inoculation Process on Lycopene Production by Blakeslea trispora in a Stirred-Tank Reactor. Appl. Biochem. Biotechnol. 2015, 175, 770–779. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.S.; Ortiz-Cruz, M.A.; Mendoza-Hernández, G.; Moreno-Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Hao, W.; Zou, Y.; Shi, M.; Jiang, Y.; Xiao, P.; Lei, A.; Hu, Z.; Zhang, W.; Zhao, L.; et al. Fatty acid and metabolomic profiling approaches differentiate heterotrophic and mixotrophic culture conditions in a microalgal food supplement “Euglena”. BMC Biotechnol. 2016, 16, 49. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).