Mineralization Patterns of Maize Straw in Fluvio-Aquatic Soil as Determined by Isotopic Traces
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Maize Straw
2.2. Incubation Experiment
2.3. Measurement of Gas Emissions and Soil C and N
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Carbon and Nitrogen Losses in CO2 and N2O
3.2. Isotopic Characteristics of CO2 and N2O
3.3. Changes in δ13C-SOC and δ15N-TN During Incubation
4. Discussion
4.1. Effect of Maize Straw on C Mineralization
4.2. Effect of Maize Straw on N Loss
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Desjardins, T.; Folgarait, P.J.; Pando-Bahuon, A.; Girardin, C.; Lavelle, P. Soil organic matter dynamics along a rice chronosequence in north-eastern Argentina: Evidence from natural 13C abundance and particle size fractionation. Soil Biol. Biochem. 2006, 38, 2753–2761. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Badalucco, L.; Palazzolo, E. Effects of compost input and tillage intensity on soil microbial biomass and activity under Mediterranean conditions. Biol. Fertil. Soils 2011, 47, 63–70. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sinicco, T.; Mondini, C. Mineralization dynamics and biochemical properties during initial decomposition of plant and animal residues in soil. Appl. Soil Ecol. 2009, 41, 118–127. [Google Scholar] [CrossRef]
- Vinther, F.P.; Hansen, E.M.; Olesen, J.E. Effects of plant residues on crop performance, N mineralisation and microbial activity including field CO2 and N2O fluxes in unfertilised crop rotations. Nutr. Cycl. Agroecosyst. 2004, 70, 189–199. [Google Scholar] [CrossRef]
- Kaewpradit, W.; Toomsan, B.; Cadisch, G.; Vityakon, P.; Limpinuntana, V.; Saenjan, P.; Jogloy, S.; Patanothai, A. Mixing groundnut residues and rice straw to improve rice yield and N use efficiency. Field Crops Res. 2009, 110, 130–138. [Google Scholar] [CrossRef]
- Andresen, L.C.; Jonasson, S.; Ström, L.; Michelsen, A. Uptake of pulse injected nitrogen by soil microbes and mycorrhizal and non-mycorrhizal plants in a species-diverse subarctic heath ecosystem. Plant Soil 2008, 313, 283–295. [Google Scholar] [CrossRef]
- Denef, K.; Bubenheim, H.; Lenhart, K.; Vermeulen, J.; Cleemput, O.V.; Boeckx, P.; Müller, C. Community shifts and carbon translocation within metabolically-active rhizosphere microorganisms in grasslands under elevated CO2. Biogeosci. Discuss. 2007, 4, 769–779. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, W. 13C isotope fractionation during rhizosphere respiration of C3 and C4 plants. Plant Soil 2011, 342, 277–287. [Google Scholar] [CrossRef]
- Rubino, M.; Dungait, J.A.J.; Evershed, R.P.; Bertolini, T.; Angelis, P.D.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F. Carbon input belowground is the major C flux contributing to leaf litter mass loss: Evidences from a 13 C labelled-leaf litter experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- Mambelli, S.; Bird, J.A.; Gleixner, G.; Dawson, T.E.; Torn, M.S. Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Organ. Geochem. 2011, 42, 1099–1108. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Blagodatsky, S.; Kuzyakov, Y.; Yuyukina, T. Turnover of soil organic matter and of microbial biomass under C3–C4 vegetation change: Consideration of 13C fractionation and preferential substrate utilization. Soil Biol. Biochem. 2011, 43, 159–166. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. Root-derived carbon in soil respiration and microbial biomass determined by 14C and 13C. Soil Biol. Biochem. 2008, 40, 625–637. [Google Scholar] [CrossRef]
- Ekblad, A.; Nyberg, G.; Högberg, P. 13C-discrimination during microbial respiration of added C3-, C4- and 13C-labelled sugars to a C3-forest soil. Oecologia 2002, 131, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wu, L.; Ouyang, Z.; Li, B.; Xu, Y.; Wu, S.; Gregorich, E.G. Priming effect of maize residue and urea N on soil organic matter changes with time. Appl. Soil Ecol. 2016, 100, 65–74. [Google Scholar] [CrossRef]
- Li, L.; Han, X.; You, M.; Yuan, Y.; Ding, X.; Qiao, Y. Carbon and nitrogen mineralization patterns of two contrasting crop residues in a Mollisol: Effects of residue type and placement in soils. Eur. J. Soil Biol. 2013, 54, 1–6. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L.; Goek, M.; Kara, E.E. Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turnover. Soil Biol. Biochem. 2004, 36, 255–266. [Google Scholar] [CrossRef]
- López-Martín, M.; Nowak, K.M.; Milter, A.; Knicker, H. Incorporation of N from burnt and unburnt 15N grass residues into the peptidic fraction of fire affected and unaffected soils. J. Soils Sediments 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Wei, X.; Huang, L.; Xiang, Y.; Shao, M.; Zhang, X.; Gale, W. The dynamics of soil OC and N after conversion of forest to cropland. Agric. For. Meteorol. 2014, 194, 188–196. [Google Scholar] [CrossRef]
- Bird, J.A.; Kessel, C.V.; Horwath, W.R. Stabilization of C-carbon and immobilization of N-nitrogen from rice straw in humic fractions. Soil Sci. Soc. Am. J. 2003, 67, 806–816. [Google Scholar] [CrossRef]
- Ismaili, K.; Ismaili, M.; Ibijbijen, J. The use of 13C and 15N based isotopic techniques for assessing soil C and N changes under conservation agriculture. Eur. J. Agron. 2015, 64, 1–7. [Google Scholar] [CrossRef]
- Thomsen, I.K.; Jensen, E.S. Recovery of nitrogen by spring barley following incorporation of 15N-labelled straw and catch crop material. Agric. Ecosyst. Environ. 1994, 49, 115–122. [Google Scholar] [CrossRef]
- Butterly, C.R.; Wang, X.; Armstrong, R.D.; Chen, D.; Tang, C. Elevated CO2 induced rhizosphere effects on the decomposition and N recovery from crop residues. Plant Soil 2016, 408, 1–7. [Google Scholar] [CrossRef]
- Moran, K. Role of mineral-nitrogen in residue decomposition and stable soil organic matter formation. Soil Sci. Soc. Am. J. 2005, 69, 1730–1736. [Google Scholar] [CrossRef]
- Mebius, L.J. A rapid method for the determination of organic carbon in soil. Anal. Chim. Acta 1960, 22, 120–124. [Google Scholar] [CrossRef]
- Bremner, J.M.; Tabatabai, M.A. Use of an ammonia electrode for determination of ammonium in Kjeldahl analysis of soils. Commun. Soil Sci. Plant Anal. 1972, 3, 159–165. [Google Scholar] [CrossRef]
- Zhang, W.L.; Wang, S.L. Effects of NH4+ and NO3− on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China. Soil Biol. Biochem. 2012, 47, 116–122. [Google Scholar] [CrossRef]
- Liang, B.C.; Gregorich, E.G.; MacKenzie, A.F. Short-term mineralization of maize residues in soils as determined by carbon-13 natural abundance. Plant Soil 1999, 208, 227–232. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Bol, R.; Bull, I.D.; Evershed, R.P. Tracking the fate of dung-derived carbohydrates in a temperate grassland soil using compound-specific stable isotope analysis. Organ. Geochem. 2009, 40, 1210–1218. [Google Scholar] [CrossRef]
- Li, L.J.; Zeng, D.H.; Yu, Z.Y.; Fan, Z.P.; Yang, D.; Liu, Y.X. Impact of litter quality and soil nutrient availability on leaf decomposition rate in a semi-arid grassland of Northeast China. J. Arid Environ. 2011, 75, 787–792. [Google Scholar] [CrossRef]
- Kristiansen, S.M.; Brandt, M.; Hansen, E.M.; Magid, J.; Christensen, B.T. 13C signature of CO2 evolved from incubated maize residues and maize-derived sheep faeces. Soil Biol. Biochem. 2004, 36, 99–105. [Google Scholar] [CrossRef][Green Version]
- Henn, M.R.; Chapela, I.H. Differential C isotope discrimination by fungi during decomposition of C3- and C4-derived sucrose. Appl. Environ. Microbiol. 2000, 66, 4180–4186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngao, J.; Cotrufo, M.F. Carbon isotope discrimination during litter decomposition can be explained by selective use of substrate with differing δ13C. Biogeosci. Discuss. 2011, 8, 51–82. [Google Scholar] [CrossRef]
- Yang, W.J.; Bruun, S.; Rønn, R.; Ekelund, F.; Magid, J. Turnover and isotopic fractionation of carbon from maize leaves as influenced by clay type and content in constructed soil media. Biol. Fertil. Soils 2016, 52, 447–454. [Google Scholar] [CrossRef]
- Coyle, J.S.; Dijkstra, P.; Doucett, R.R.; Schwartz, E.; Hart, S.C.; Hungate, B.A. Relationships between C and N availability, substrate age, and natural abundance 13C and 15N signatures of soil microbial biomass in a semiarid climate. Soil Biol. Biochem. 2009, 41, 1605–1611. [Google Scholar] [CrossRef]
- Wedin, D.A.; Tieszen, L.L.; Dewey, B.; Pastor, J. Carbon isotope dynamics during grass decomposition and soil organic matter formation. Ecology 1995, 76, 1383–1392. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Soil organic carbon decomposition from recently added and older sources estimated by δ13C values of CO2 and organic matter. Soil Biol. Biochem. 2012, 55, 40–47. [Google Scholar] [CrossRef]
- Flessa, H.; Ludwig, B.; Heil, B.; Merbach, W. The origin of soil organic C, dissolved organic C and respiration in a long-term maize experiment in Halle, Germany, determined by 13C natural abundance. J. Plant Nutr. Soil Sci. 2000, 163, 157–163. [Google Scholar] [CrossRef]
- Trinsoutrot, I.; Nicolardot, B.; Justes, E.; Recous, S. Decomposition in the field of residues of oilseed rape grown at two levels of nitrogen fertilisation. Effects on the dynamics of soil mineral nitrogen between successive crops. Nutr. Cycl. Agroecosyst. 2000, 56, 125–137. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S. Addition of external organic carbon and native soil organic carbon decomposition: A meta-analysis. PLoS ONE 2013, 8, e54779. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, S.; Mao, J.; Olk, D.C.; Cao, X.; Zhang, B. Chemical composition of organic matter in a deep soil changed with a positive priming effect due to glucose addition as investigated by 13C NMR spectroscopy. Soil Biol. Biochem. 2015, 85, 137–144. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Wang, J.Y.; Jia, J.X.; Xiong, Z.Q.; Mak, K.; Xing, G.X. Water regime-nitrogen fertilizer-straw incorporation interaction: Field study on nitrous oxide emissions from a rice agroecosystem in Nanjing, China. Agric. Ecosyst. Environ. 2011, 141, 437–446. [Google Scholar] [CrossRef]
- Khalil, M.I.; Rosenani, A.B.; Ovan, C.; Boeckx, P.; Shamshuddin, J.; Fauziah, C.I. Nitrous oxide production from an ultisol of the humid tropics treated with different nitrogen sources and moisture regimes. Biol. Fertil. Soils 2002, 36, 59–65. [Google Scholar]
- Toma, Y.; Hatano, R. Effect of crop residue C:N ratio on N2O emissions from Gray Lowland soil in Mikasa, Hokkaido, Japan. Soil Sci. Plant Nutr. 2010, 53, 198–205. [Google Scholar] [CrossRef]
- Al-Sheikh, A.; Delgado, J.A.; Barbarick, K.; Sparks, R.; Dillon, M.; Qian, Y.; Cardon, G. Effects of potato–grain rotations on soil erosion, carbon dynamics and properties of rangeland sandy soils. Soil Tillage Res. 2005, 81, 227–238. [Google Scholar] [CrossRef]
- Zhu, L.X.; Xiao, Q.; Shen, Y.F.; Li, S.Q. Effects of biochar and maize straw on the short-term carbon and nitrogen dynamics in a cultivated silty loam in China. Environ. Sci. Pollut. Res. 2017, 24, 1019–1029. [Google Scholar] [CrossRef]
- Yao, Z.; Zhou, Z.; Zheng, X.; Xie, B.; Mei, B.; Wang, R.; Butterbach-Bahl, K.; Zhu, J. Effects of organic matter incorporation on nitrous oxide emissions from rice-wheat rotation ecosystems in China. Plant Soil 2010, 327, 315–330. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Beusichem, M.L.V.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Xie, B.; Mei, B.; Wang, R.; Klaus, B.B.; Zhu, J.; Yin, R. Tillage and crop residue management significantly affects N-trace gas emissions during the non-rice season of a subtropical rice-wheat rotation. Soil Biol. Biochem. 2009, 41, 2131–2140. [Google Scholar] [CrossRef]
- Say, E.W.; Dines, A.J. Dissolved organic carbon and nitrogen relationships in forest litter as affected by nitrogen deposition. Soil Biol. Biochem. 2000, 32, 603–613. [Google Scholar]
- Pérez, T.; Trumbore, S.E.; Tyler, S.C.; Matson, P.A.; Ortiz-Monasterio, I.; Rahn, T.; Griffith, D.W.T. Identifying the agricultural imprint on the global N2O budget using stable isotopes. J. Geophys. Res. Atmos. 2001, 106, 9869–9878. [Google Scholar] [CrossRef]
- Xia, Z.; Xu, H.; Chen, G.; Dong, D.; Bai, E.; Luo, L. Soil N2O production and the δ15N–N2O value: Their relationship with nitrifying/denitrifying bacteria and archaea during a growing season of soybean in northeast China. Eur. J. Soil Biol. 2013, 58, 73–80. [Google Scholar] [CrossRef]
- Aita, C.; Recous, S.; Angers, D.A. Short-term kinetics of residual wheat straw C and N under field conditions: Characterization by 13C15N tracing and soil particle size fractionation. Eur. J. Soil Sci. 1997, 48, 283–294. [Google Scholar] [CrossRef]
- Barrow, N.J.; Jenkinson, D.S. The effect of water-logging on fixation of nitrogen by soil incubated with straw. Plant Soil 1962, 16, 258–262. [Google Scholar] [CrossRef]
- Katsuki, A.; Iwao, W.; Michiharu, K.; Eiichi, T. Effect of application of glucose, cellulose, and rice straw on nitrogen fixation (acetylene reduction and soil-nitrogen components) in anaerobic soil. Soil Sci. Plant Nutr. 1989, 35, 235–243. [Google Scholar]
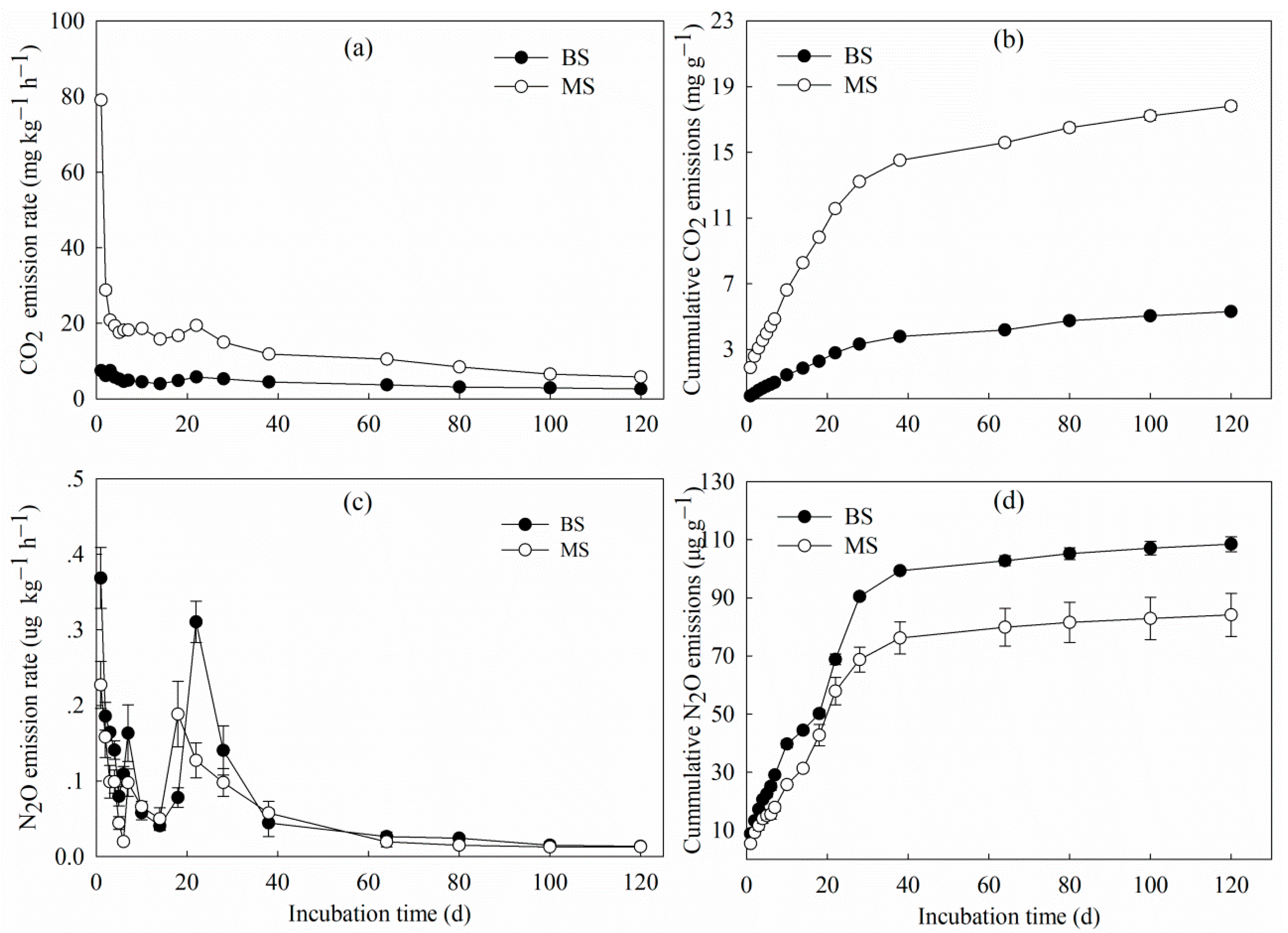
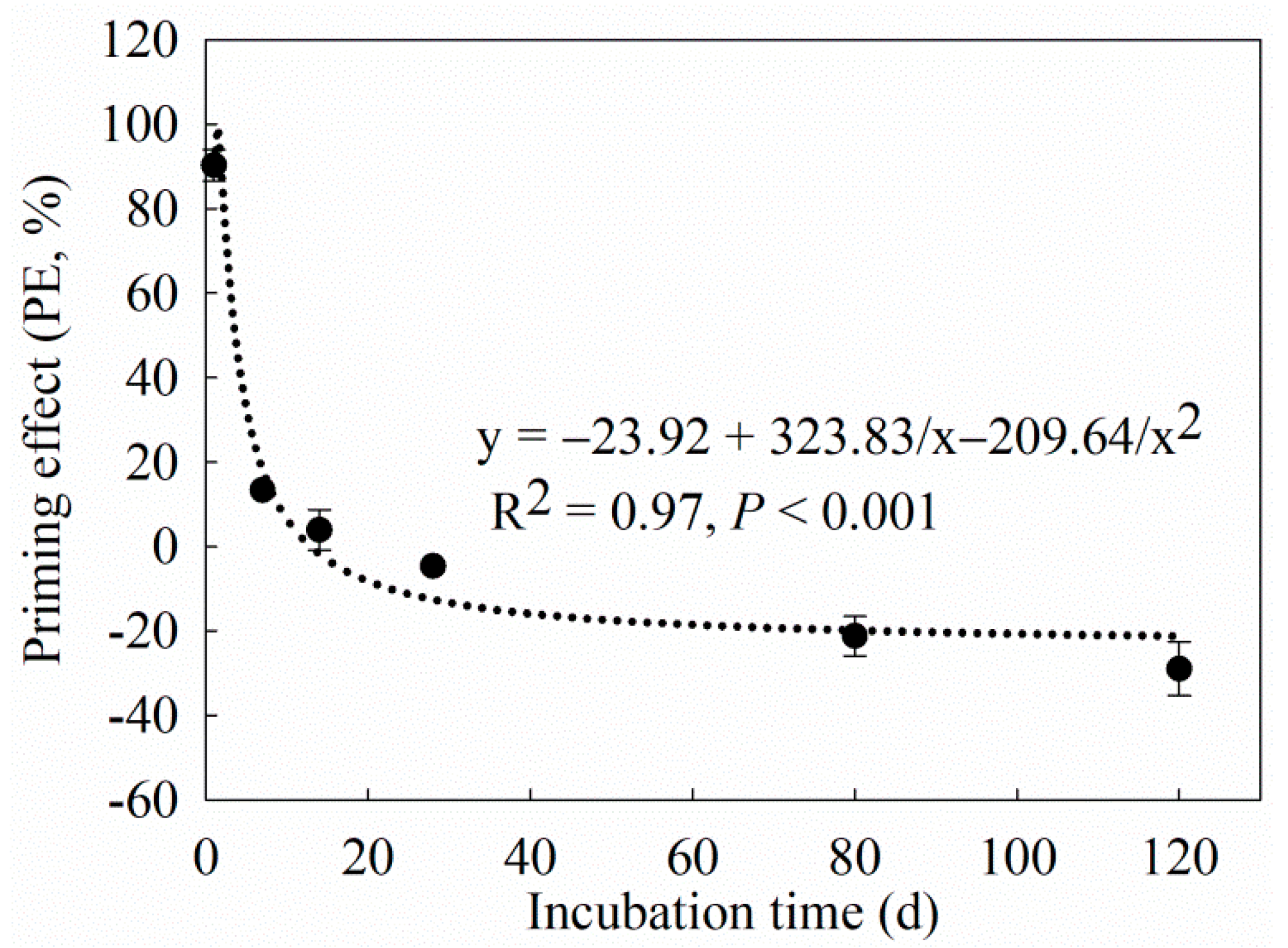
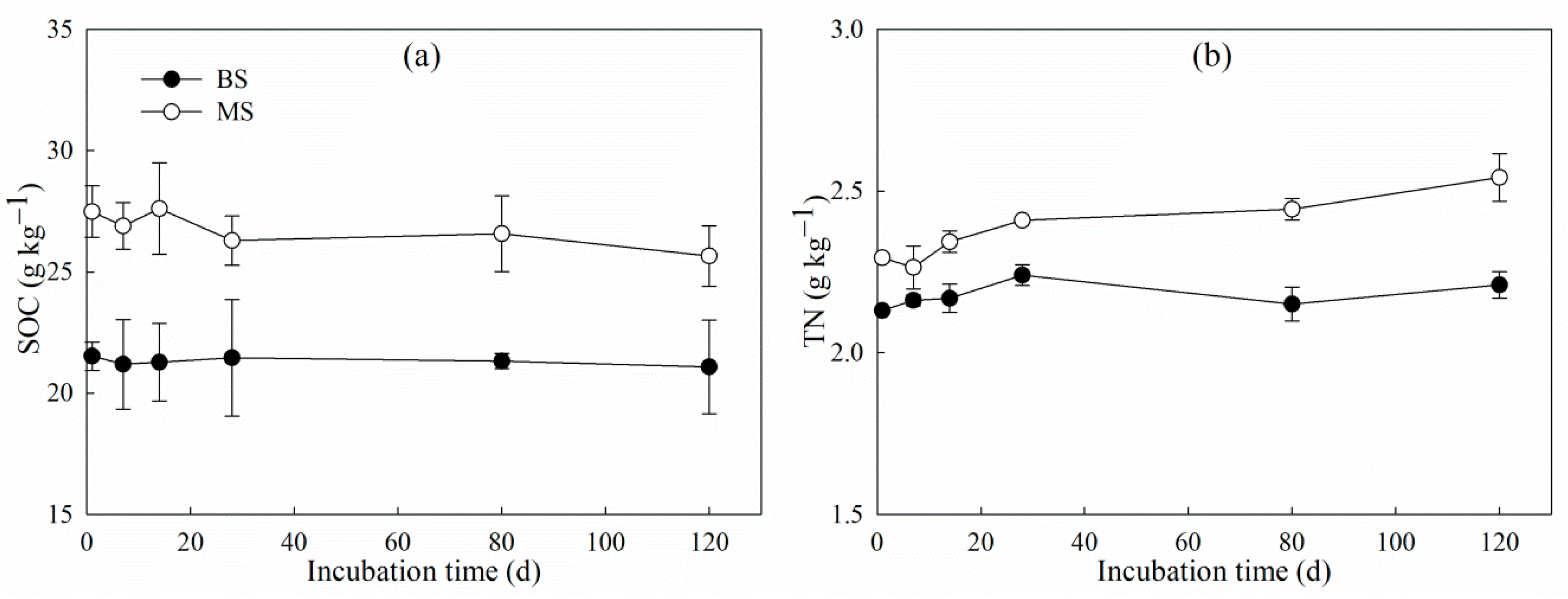
| Treatment | Cm (mg g−1) | Cp (mg g−1) | K (d−1) | T1/2 (d) | R2 |
|---|---|---|---|---|---|
| BS | 1.385 ± 0.008 b | 1.388 ± 0.012 b | 0.034 ± 0.001 b | 20.39 b | 0.99 |
| MS | 4.858 ± 0.071 a | 4.664 ± 0.047 a | 0.050 ± 0.001 a | 13.86 a | 0.99 |
| Incubation Time (day) | δ13C-CO2 (‰) | fco2 (%) | δ15N-N2O (‰) | fN2O (%) | ||
|---|---|---|---|---|---|---|
| BS | MS | BS | MS | |||
| 1 | −17.84 ± 0.02a | −13.91 ± 0.04a | 81.24 ± 0.52 a | 2.03 ± 0.21 a | 5.48 ± 0.01 b | 0.041 ± 0.001 b |
| 7 | −18.38 ± 0.04 a | −14.70 ± 0.19 b | 71.40 ± 2.33 b | 1.90 ± 0.10 a | 5.42 ± 0.04 b | 0.037 ± 0.003 b |
| 14 | −18.57 ± 0.07 a | −14.63 ± 0.05 b | 72.22 ± 0.65 b | 1.88 ± 0.06 a | 5.38 ± 0.02 b | 0.034 ± 0.001 b |
| 28 | −18.26 ± 0.04 a | −15.08 ± 0.01 c | 66.55 ± 0.05 c | 1.90 ± 0.03 a | 5.53 ± 0.02 b | 0.044 ± 0.002 b |
| 80 | −18.21 ± 0.02 a | −14.71 ± 0.01 b | 71.20 ± 0.01 b | 1.98 ± 0.07 a | 5.81 ± 0.07 ab | 0.062 ± 0.005 ab |
| 120 | −18.91 ± 0.85 a | −14.81 ± 0.07 b | 69.96 ± 0.83 b | 1.85 ± 0.04 a | 6.05 ± 0.49 a | 0.078 ± 0.032 a |
| Incubation Time (day) | δ13C (‰) | fOC (%) | XOC (g kg−1) | δ15N (‰) | fN (%) | XN (g kg−1) |
|---|---|---|---|---|---|---|
| 1 | −18.02 | 29.67 | 10.43 | 159.13 | 10.01 | 0.23 |
| 7 | −18.54 | 23.17 | 7.94 | 158.60 | 9.97 | 0.23 |
| 14 | −18.65 | 21.70 | 7.67 | 170.44 | 10.74 | 0.26 |
| 28 | −18.70 | 21.09 | 7.34 | 177.81 | 11.22 | 0.27 |
| 80 | −19.29 | 13.98 | 4.81 | 209.78 | 13.04 | 0.32 |
| 120 | −19.47 | 11.45 | 3.66 | 248.07 | 15.75 | 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Chen, J.; Li, L.; Zhang, F.; Liu, T. Mineralization Patterns of Maize Straw in Fluvio-Aquatic Soil as Determined by Isotopic Traces. Sustainability 2020, 12, 621. https://doi.org/10.3390/su12020621
Zhu L, Chen J, Li L, Zhang F, Liu T. Mineralization Patterns of Maize Straw in Fluvio-Aquatic Soil as Determined by Isotopic Traces. Sustainability. 2020; 12(2):621. https://doi.org/10.3390/su12020621
Chicago/Turabian StyleZhu, Lixia, Jutian Chen, Lili Li, Fuli Zhang, and Tianxue Liu. 2020. "Mineralization Patterns of Maize Straw in Fluvio-Aquatic Soil as Determined by Isotopic Traces" Sustainability 12, no. 2: 621. https://doi.org/10.3390/su12020621
APA StyleZhu, L., Chen, J., Li, L., Zhang, F., & Liu, T. (2020). Mineralization Patterns of Maize Straw in Fluvio-Aquatic Soil as Determined by Isotopic Traces. Sustainability, 12(2), 621. https://doi.org/10.3390/su12020621




