Solid Waste Incineration Modelling for Advanced Moving Grate Incinerators
Abstract
1. Introduction
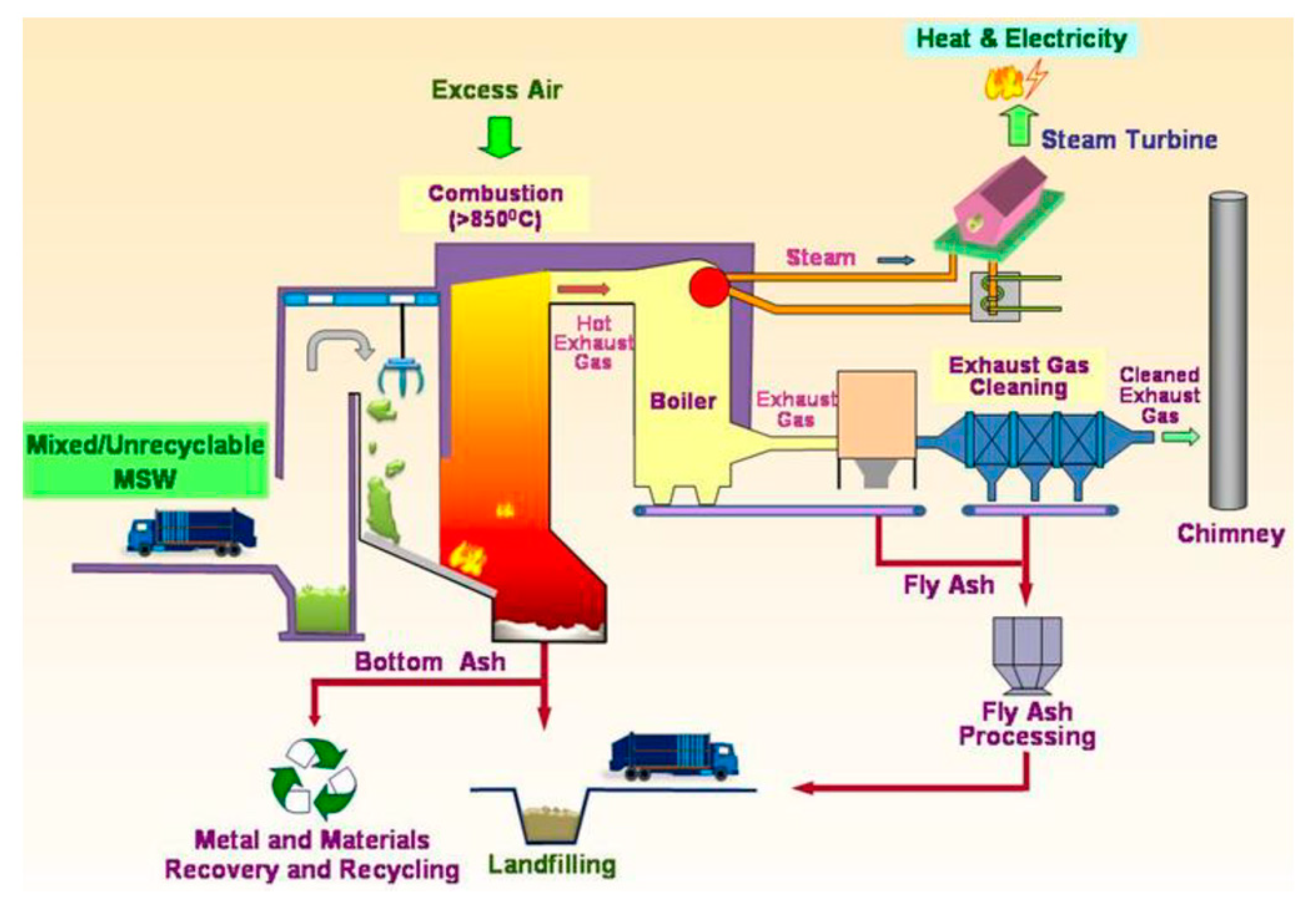
2. Simulation Models
2.1. Freeboard Model
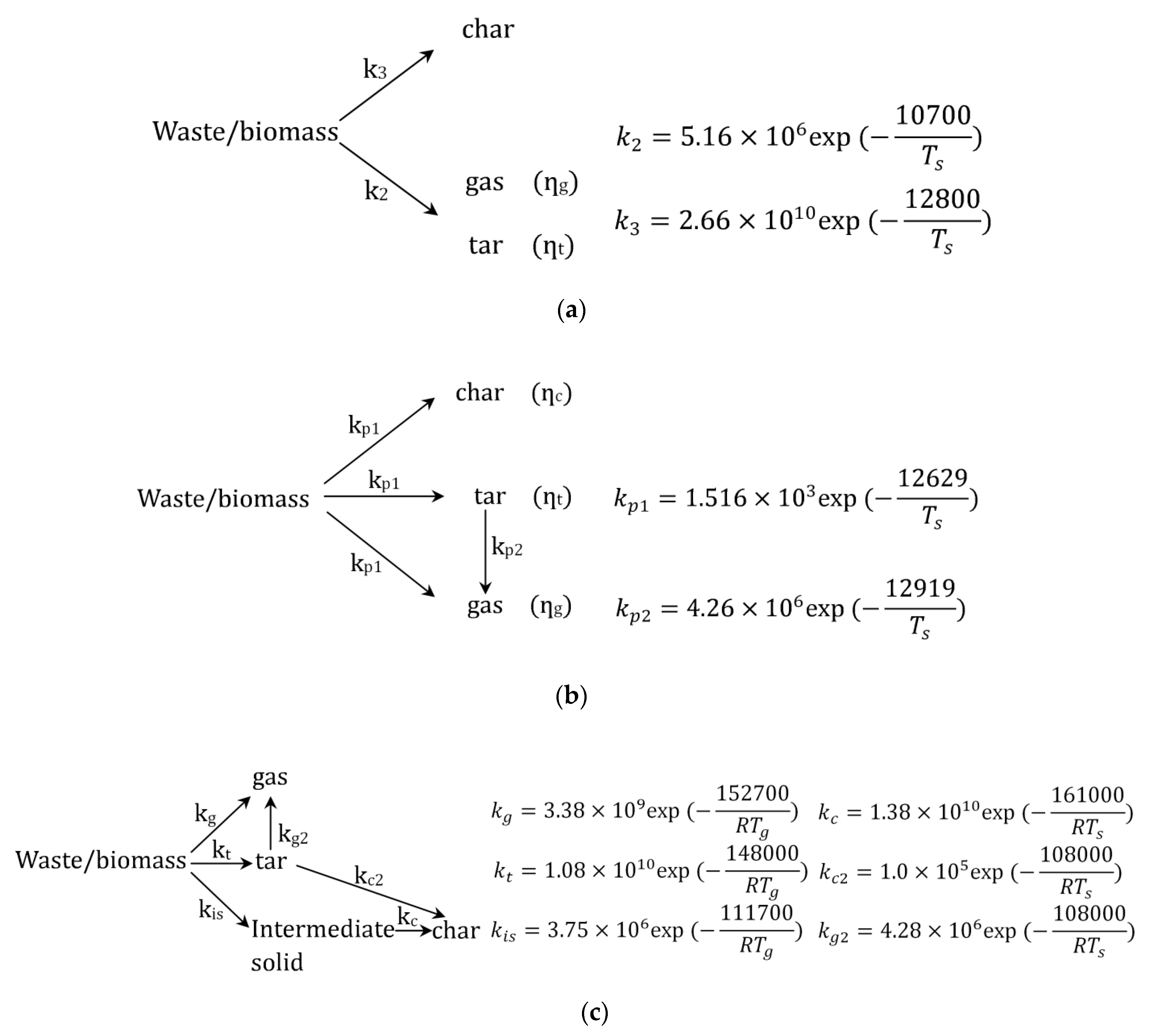
2.2. Pyrolysis Mechanisms
3. A New Coupling Approach
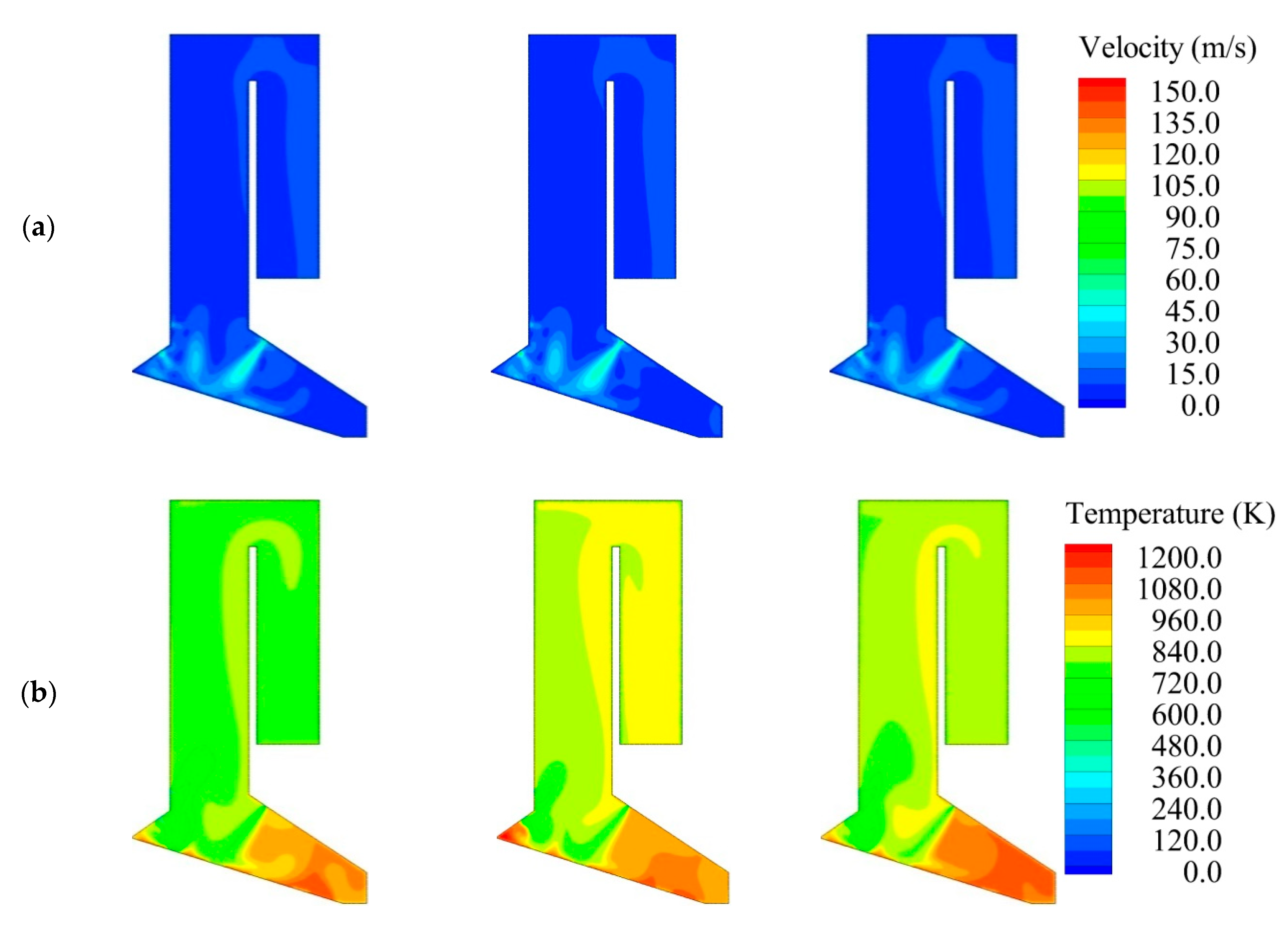
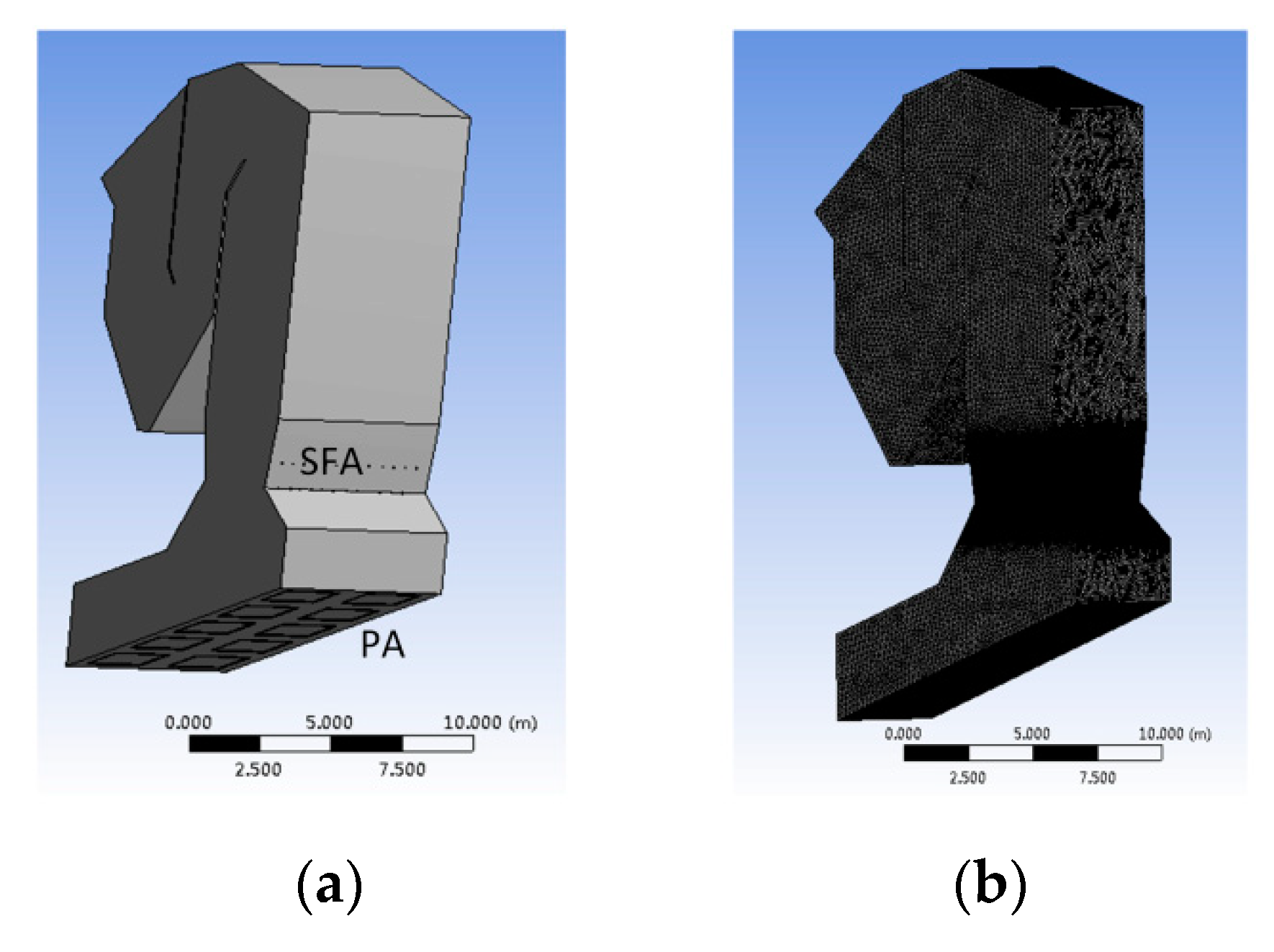
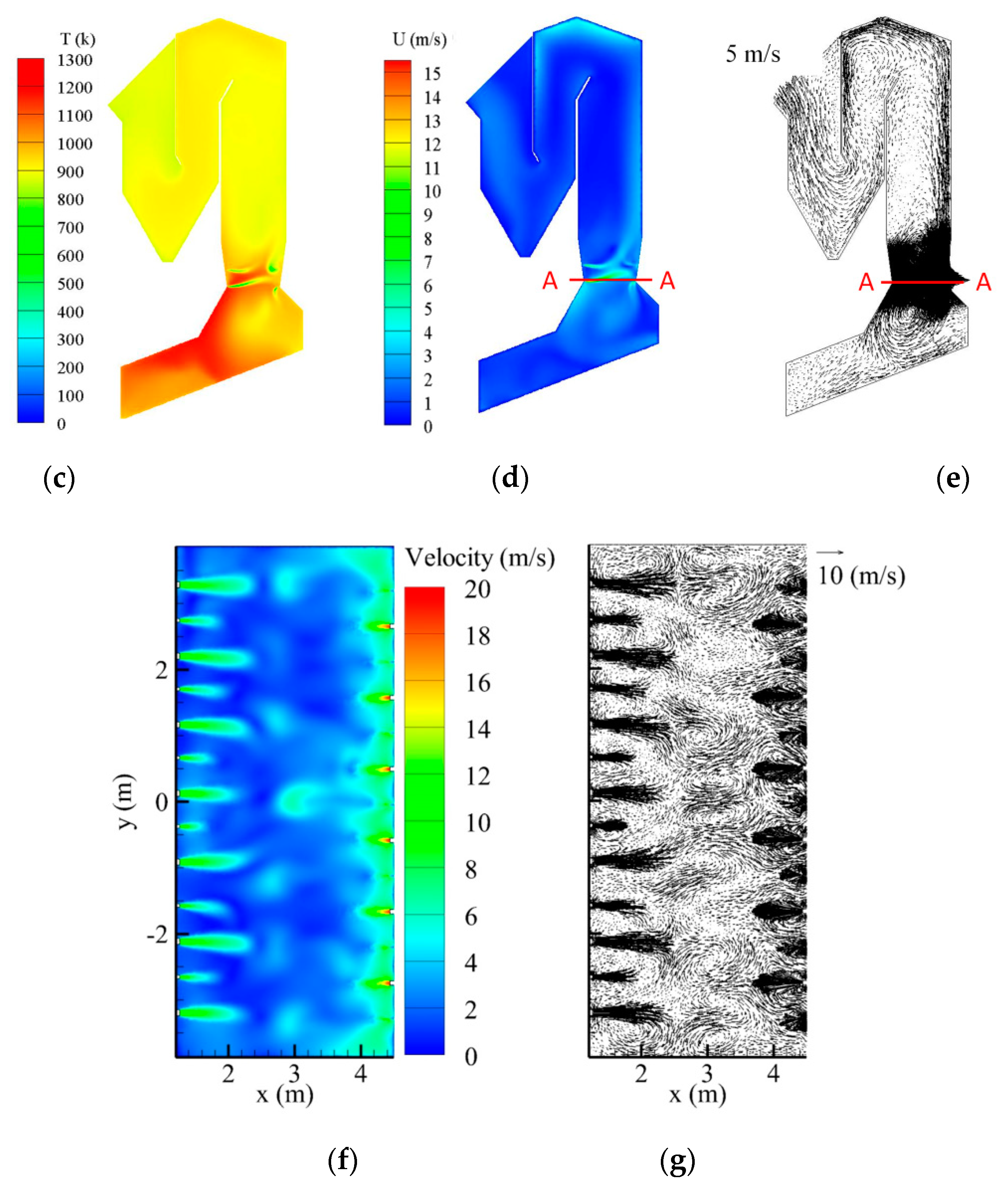
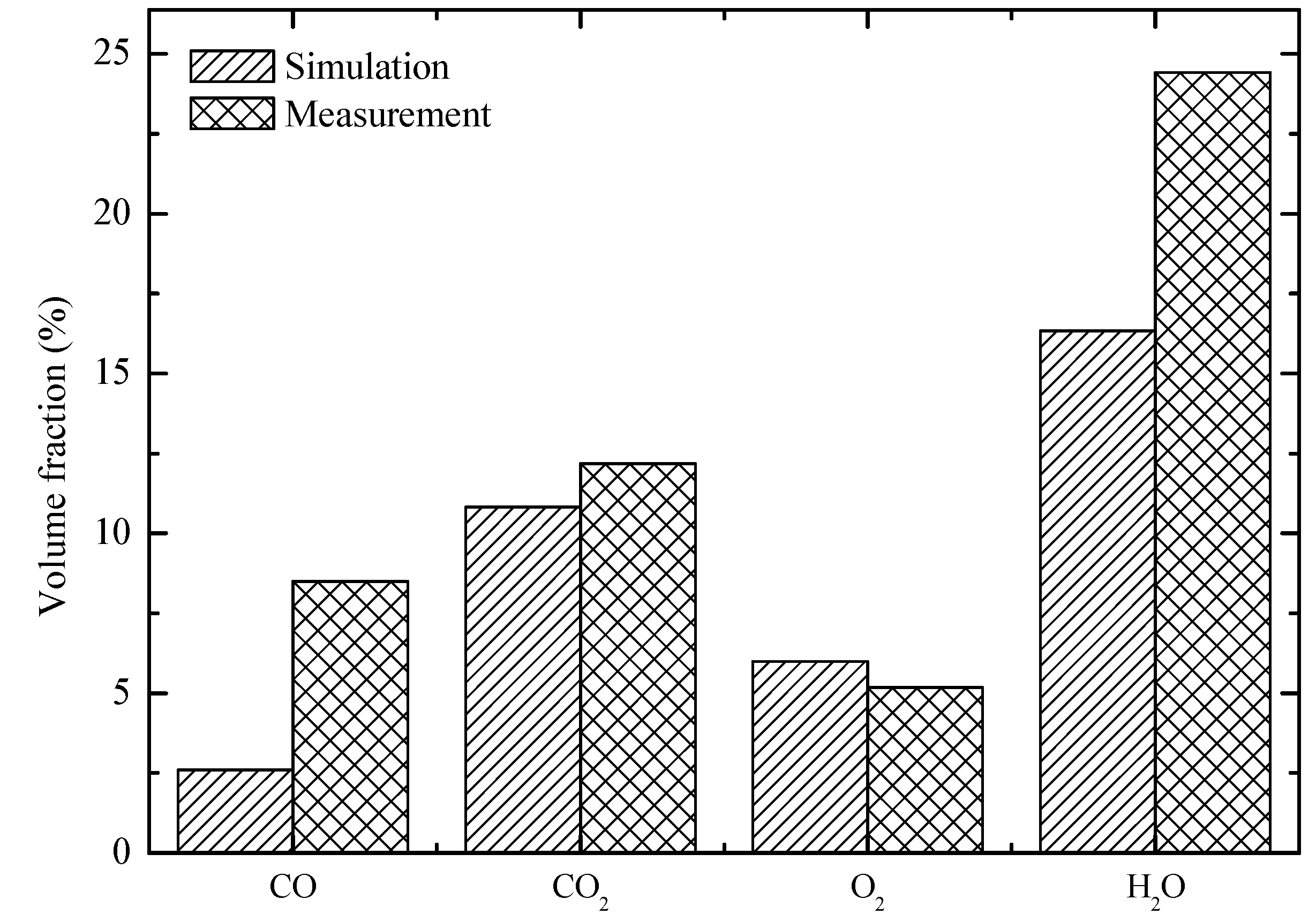
4. Predictions for a Large Scale WTE Plant
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hettiarachchi, H.; Ryu, S.; Caucci, S.; Silva, R. Municipal Solid Waste Management in Latin America and the Caribbean: Issues and Potential Solutions from the Governance Perspective. Recycling 2018, 3, 19. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, S. Economic and Environmental Cost Analysis of Incineration and Recovery Alternatives for Flammable Industrial Waste: The Case of South Korea. Sustainability 2017, 9, 1638. [Google Scholar]
- Schneider, P.; Anh, L.H.; Wagner, J.; Reichenbach, J.; Hebner, J. Solid Waste Management in Ho Chi Minh City, Vietnam: Moving towards a Circular Economy? Sustainability 2017, 9, 286. [Google Scholar] [CrossRef]
- Siddiqi, M.M.; Naseer, M.N.; Abdul Wahab, Y.; Hamizi, N.A.; Badruddin, I.A.; Chowdhury, Z.Z.; Akbarzadeh, O.; Johan, M.R.; Khan, T.M.Y.; Kamangar, S. Evaluation of Municipal Solid Wastes Based Energy Potential in Urban Pakistan. Processes 2019, 7, 848. [Google Scholar] [CrossRef]
- Sikalidis, A.; Emmanouil, C. Description and Economic Evaluation of a Zero-Waste Mortar-Producing Process for Municipal Solid Waste Management in Greece. J. Open Innov. Technol. Mark. Complex. 2019, 5, 46. [Google Scholar] [CrossRef]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Hunsinger, H.; Jay, K.; Vehlow, J.; Seifert, H. Investigations on the combustion of various waste fractions inside a grate furnace. In Proceedings of the 7th Annual North American Waste-to-Energy Conference, Tampa, FL, USA, 17–19 May 1999; pp. 199–212. [Google Scholar]
- Frey, H.H.; Peters, B.; Hunsinger, H.; Vehlow, J. Characterization of municipal solid waste combustion in a grate furnace. Waste Manag. 2003, 23, 689–701. [Google Scholar] [CrossRef]
- Yang, Y.B.; Goodfellow, J.; Nasserzadeh, V.; Swithenbank, J. Study on the transient process of waste fuel incineration in a full-scale moving-bed furnace. Combust. Sci. Technol. 2004, 177, 127–150. [Google Scholar] [CrossRef]
- Essenhigh, R.H.; Kuo, T.J. Combustion and emission phenomena in incinerators: Development of physical and mathematical models of incinerators, Part I: Statement of the problem. In Proceedings of the National Incinerator Conference; ASME: New York, NY, USA, 1970; pp. 261–271. [Google Scholar]
- Klason, T.; Bai, X.S. Combustion process in a biomass grate fired industry furnace: A CFD study. Prog. Comput. Fluid Dyn. Int. J. 2006, 6, 278–286. [Google Scholar] [CrossRef]
- Brosch, B.; Scherer, V.; Wirtz, S. Simulation of municipal solid waste incineration in grate firing systems with a particle based novel discrete element method. VGB Powertech 2014, 1, 75. [Google Scholar]
- Wissing, F.; Wirtz, S.; Scherer, V. Simulating municipal solid waste incineration with a DEM/CFD method–influences of waste properties, grate and furnace design. Fuel 2017, 206, 638–656. [Google Scholar] [CrossRef]
- Goh, Y.R.; Siddall, R.G.; Nasserzadeh, V.; Zakaria, R.; Swithenbank, J.; Lawrence, D.; Garrod, N.; Jones, B. Mathematical modelling of the burning bed of a waste incinerator. J. Inst. Energy 1998, 71, 110–118. [Google Scholar]
- Goh, Y.R.; Yang, Y.B.; Zakaria, R.; Siddall, R.G.; Nasserzadeh, V.; Swithenbank, J. Development of an incinerator bed model for municipal solid waste incineration. Combust. Sci. Technol. 2001, 162, 37–58. [Google Scholar] [CrossRef]
- Shin, D.; Choi, S. The combustion of simulated waste particles in a fixed bed. Combust. Flame 2000, 121, 167–180. [Google Scholar] [CrossRef]
- Kær, S.K. Straw combustion on slow-moving grates—a comparison of model predictions with experimental data. Biomass Bioenergy 2005, 28, 307–320. [Google Scholar] [CrossRef]
- Miljković, B.; Pešenjanski, I.; Vićević, M. Mathematical modelling of straw combustion in a moving bed combustor: A two dimensional approach. Fuel 2013, 104, 351–364. [Google Scholar] [CrossRef]
- Zhou, H.; Jensen, A.D.; Glarborg, P.; Jensen, P.A.; Kavaliauskas, A. Numerical modeling of straw combustion in a fixed bed. Fuel 2005, 84, 389–403. [Google Scholar] [CrossRef]
- Yang, Y.B.; Ryu, C.; Goodfellow, J.; Sharifi, V.N.; Swithenbank, J. Modelling waste combustion in grate furnaces. Process Saf. Environ. Prot. 2004, 82, 208–222. [Google Scholar] [CrossRef]
- Ryu, C.; Shin, D.; Choi, S. Combined simulation of combustion and gas flow in a grate-type incinerator. J. Air Waste Manag. Assoc. 2002, 52, 189–197. [Google Scholar] [CrossRef][Green Version]
- Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biomass furnace study via 3D numerical modeling. Int. J. Numer. Methods Heat Fluid Flow 2016, 26, 515–533. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.; Clausen, S.; Hvid, S.L. Characterizing and modeling of an 88 MW grate-fired boiler burning wheat straw: Experience and lessons. Energy 2012, 41, 473–482. [Google Scholar] [CrossRef]
- Goerner, K.; Klasen, T. Modelling, simulation and validation of the solid biomass combustion in different plants. Progress Comput. Fluid Dyn. Int. J. 2006, 6, 225–234. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling wood gasification in a countercurrent fixed-bed reactor. AIChE J. 2004, 50, 2306–2319. [Google Scholar] [CrossRef]
- Park, W.C.; Atreya, A.; Baum, H.R. Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis. Combust. Flame 2010, 157, 481–494. [Google Scholar] [CrossRef]
- Lai, A.C.H.; Law, A.W.K. Numerical modeling of municipal waste bed incineration. Int. J. Numer. Methods Heat Fluid Flow 2019, 29, 504–522. [Google Scholar] [CrossRef]
- Yakhot, V.; Orszag, S.A. Renormalization group analysis of turbulence. I. Basic theory. J. Sci. Comput. 1986, 1, 3–51. [Google Scholar] [CrossRef]
- Magnussen, B.F.; Hjertager, B.H. On mathematical modeling of turbulent combustion with special emphasis on soot formation and combustion. Symp. (Int.) Combust. 1977, 16, 719–729. [Google Scholar] [CrossRef]
- Cheng, P. Two-dimensional radiating gas flow by a moment method. AIAA J. 1964, 2, 1662–1664. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, A.; Xu, M.; Yang, W.; Siah, K.B.; Prabakaran, S. Experimental and numerical study on the combustion of a 32 MW wood-chip grate boiler with internal flue gas recirculation technology. Energy Procedia 2017, 143, 591–598. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.; Kær, S.K.; Clausen, S.; Hvid, S.L.; Hille, T. Mathematical modeling and experimental study of biomass combustion in a thermal 108 MW grate-fired boiler. Energy Fuels 2008, 22, 1380–1390. [Google Scholar] [CrossRef]


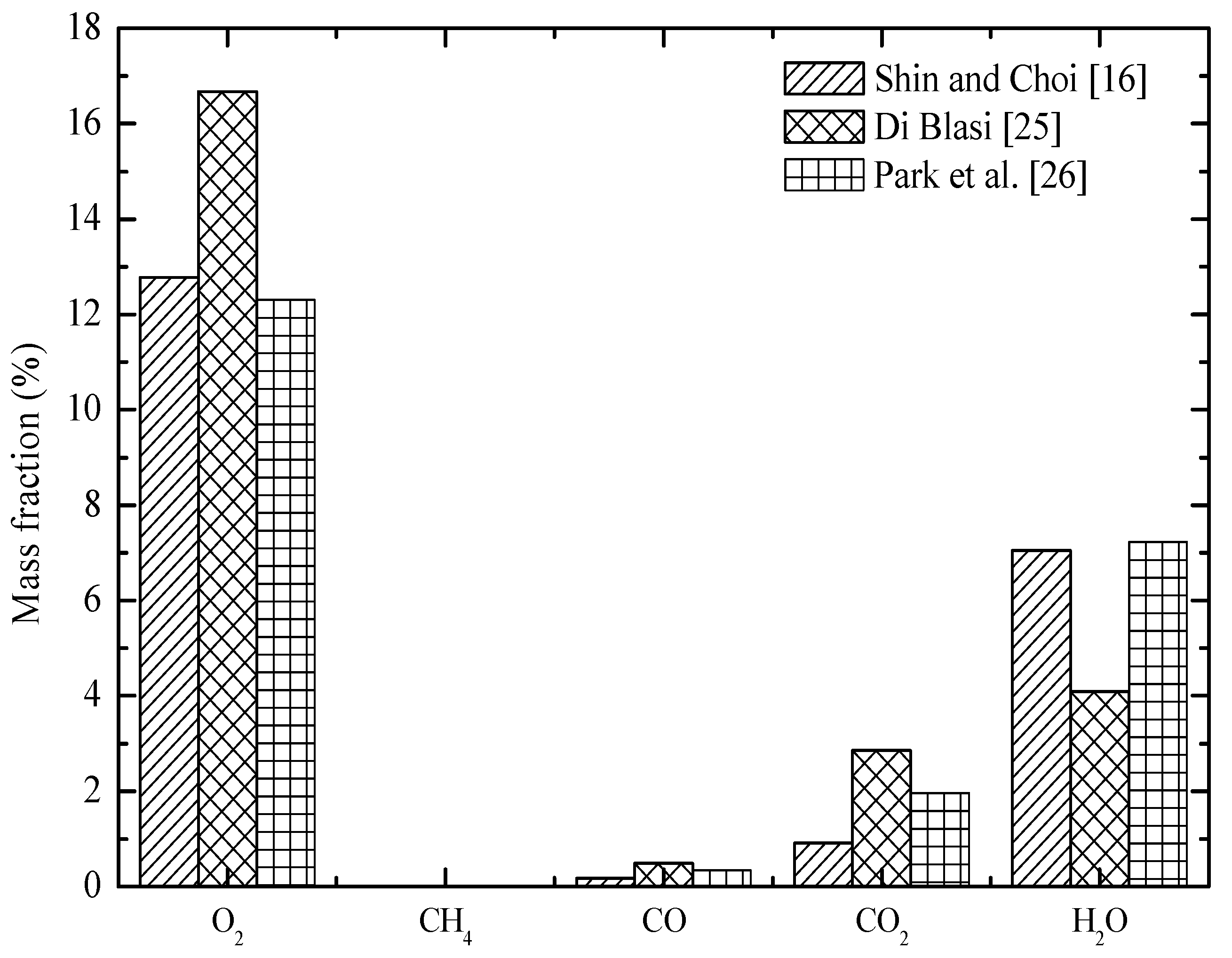
| Area (m2) | Grate Length | Flow Rate (kg/s) | Temp (K) | O2 (%) | H2O (%) | CO2 (%) | CO (%) | CH4 (%) | H2 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| E1L | 3.94 | 0–2 | 1.31 | 1100 | 4.9 | 23.8 | 9.6 | 13.3 | 3.8 | 0.1 |
| E1R | 3.94 | 0–2 | 1.31 | 1100 | 4.9 | 23.8 | 9.6 | 13.3 | 3.8 | 0.1 |
| E2L | 3.85 | 2–4 | 1.87 | 1100 | 0.2 | 18.3 | 32.6 | 16.8 | 4.0 | 0.2 |
| E2R | 3.85 | 2–4 | 1.87 | 1100 | 0.2 | 18.3 | 32.6 | 16.8 | 4.0 | 0.2 |
| E3L | 3.85 | 4–6 | 1.89 | 1100 | 3.2 | 8.2 | 24.0 | 2.6 | 1.0 | 0.0 |
| E3R | 3.85 | 4–6 | 1.89 | 1100 | 3.2 | 8.2 | 24.0 | 2.6 | 1.0 | 0.0 |
| E4L | 3.85 | 6–8 | 0.73 | 1100 | 11.5 | 0.0 | 16.8 | 0.2 | 0.1 | 0.0 |
| E4R | 3.85 | 6–8 | 0.73 | 1100 | 11.5 | 0.0 | 16.8 | 0.2 | 0.1 | 0.0 |
| E5L | 3.50 | 8–10 | 0.15 | 1100 | 18.2 | 0.4 | 6.3 | 0.0 | 0.0 | 0.0 |
| E5R | 3.50 | 8–10 | 0.13 | 1100 | 18.2 | 0.4 | 6.3 | 0.0 | 0.0 | 0.0 |
| SAF | 0.21 | -- | 1.42 | 1100 | 23.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| SAR | 0.21 | -- | 2.18 | 1100 | 23.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Lai, A.C.H.; Law, A.W.-K. Solid Waste Incineration Modelling for Advanced Moving Grate Incinerators. Sustainability 2020, 12, 8007. https://doi.org/10.3390/su12198007
Jiang M, Lai ACH, Law AW-K. Solid Waste Incineration Modelling for Advanced Moving Grate Incinerators. Sustainability. 2020; 12(19):8007. https://doi.org/10.3390/su12198007
Chicago/Turabian StyleJiang, Mingtao, Adrian C. H. Lai, and Adrian Wing-Keung Law. 2020. "Solid Waste Incineration Modelling for Advanced Moving Grate Incinerators" Sustainability 12, no. 19: 8007. https://doi.org/10.3390/su12198007
APA StyleJiang, M., Lai, A. C. H., & Law, A. W.-K. (2020). Solid Waste Incineration Modelling for Advanced Moving Grate Incinerators. Sustainability, 12(19), 8007. https://doi.org/10.3390/su12198007