Responses of Anammox Granular Sludge to Long-Term Rare Earth Element Feeding: Lanthanum as a Case
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Setup and Operation
2.2. EPS Extraction and Characterization
2.3. La Distribution
2.4. Other Analytical Procedures
2.5. Data Processing
3. Results
3.1. NRE in Response to La (III) Feeding
3.2. Variations in the Amount and Composition of EPS
3.3. Fate and Behavior of La in Anammox Reactor
3.4. ROS Generation, Extracellular LDH Release, and DHA Change
3.5. Microbial Community Analysis
3.5.1. Microbial Community Composition and Diversity
3.5.2. Potential Dominant Genera Driven by La (III)
3.5.3. Functional Genes Associated with EPS Production
3.5.4. Metal Transport Genes Predicted by PICRUSt
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclatures
| 2D-COS | two-dimensional correlation spectroscopy |
| 3D-EEM | three-dimensional excitation-emission matrix |
| ANAMMOX | Anaerobic ammonium oxidation |
| CNDP | conventional nitrification-denitrification process |
| DHA | dehydrogenase activity |
| EPS | extracellular polymeric substance |
| FTIR | Fourier transform infrared |
| La | lanthanum |
| LDH | lactate dehydrogenase |
| NRE | nitrogen removal efficiency |
| OR | odds ratio |
| PCA | principal component analysis |
| PN | protein |
| PS | Polysaccharide |
| REE | rare earth element |
| RDA | redundancy analysis |
| ROS | reactive oxygen species |
| SAA | specific anammox activity |
| SBR | sequencing batch reactor |
| SS | suspended solids |
| TN | total nitrogen |
| VSS | volatile suspended solids |
References
- Yang, X.J.; Lin, A.; Li, X.L.; Wu, Y.; Zhou, W.; Chen, Z. China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Hao, X.; Wang, D.; Wang, P.; Wang, Y.; Zhou, D. Evaluation of water quality in surface water and shallow groundwater: A case study of a rare earth mining area in southern Jiangxi Province, China. Environ. Monit. Assess. 2016, 188, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, G.; Pan, A.; Chen, F.; Zheng, C. Protecting the environment and public health from rare earth mining. Earths Future 2016, 4, 532–535. [Google Scholar] [CrossRef]
- Liu, Y. Present Situation and Countermeasures of Soil and Water Conservation for mining areas using advanced rare earth mining technology. Water Resour. Dev. Res. 2002, 2, 30–32. [Google Scholar]
- State Environmental Protection Administration of China and General Administration of Quality Supervision. Standards for Underground Water Quality (GB/T14848-2017); Inspection and Quarantine of China: Beijing, China, 2017.
- Liu, W.S.; Guo, M.N.; Liu, C.; Yuan, M.; Chen, X.T.; Huot, H.; Zhao, C.M.; Tang, Y.T.; Morel, J.L.; Qiu, R.L. Water, sediment and agricultural soil contamination from an ion-adsorption rare earth mining area. Chemosphere 2019, 216, 75–83. [Google Scholar] [CrossRef]
- Luo, Z.J. MBR Process for Treatment of Rare Earth High-Salt and Ammonia-Nitrogen Wastewater; Jiangxi University of Science and Technology: Nanchang, China, 2018. [Google Scholar]
- Gao, L.Q.; Han, J.H.; Fan, B.H. The pretreatment of rare earth wastewater by UASB reactor. J. Inn. Mong. Univ. Sci. Technol. 2010, 29, 69–72. [Google Scholar]
- Zhang, Z.Z.; Cheng, Y.F.; Xu, L.Z.J.; Bai, Y.H.; Xu, J.J.; Shi, Z.J.; Zhang, Q.Q.; Jin, R.C. Transient disturbance of engineered ZnO nanoparticles enhances the resistance and resilience of anammox process in wastewater treatment. Sci. Total Environ. 2018, 622, 402–409. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Hu, Z.; Lotti, T.; Kreuk, M.; Kleerebezem, R.; Loosdrecht, M.; Kruit, J.; Jetten, M.S.M.; Kartal, B. Nitrogen Removal by a Nitritation-Anammox Bioreactor at Low Temperature. Appl. Environ. Microbiol. 2013, 79, 2807–2812. [Google Scholar] [CrossRef]
- Luo, J.; Huo, Y.; Shen, Y.; Hu, J.; Ji, H. Effects of colloidal particle size on the geochemical characteristics of REE in the water in southern Jiangxi province, China. Environ. Earth Sci. 2016, 75, 81. [Google Scholar] [CrossRef]
- Gonzalez, V.; Vignati, D.A.L.; Leyval, C.; Giamberini, L. Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int. 2014, 71, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Zhou, Q.; Yang, G.; Ding, X.L.; Li, X.; Cai, C.X.; Zhang, Z.; Wei, H.Y.; Lu, T.H.; et al. Rare earth elements activate endocytosis in plant cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12936–12941. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, L.; Morgana, M.; Carboni, M.A.; Staiano, M.; Antisari, M.V.; Re, M.; Lorito, M.; Vinale, F.; Abadi, K.M.; Woo, S.L. Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol. Biochem. 2009, 41, 2406–2413. [Google Scholar] [CrossRef]
- Li, W.; Zhao, R.; Xie, Z.; Chen, X.; Shen, P. Effects of La3+ on Growth, Transformation, and Gene Expression of Escherichia coli. Biol. Trace Elem. Res. 2003, 94, 167–178. [Google Scholar]
- Tanaka, Y.; Hosaka, T.; Ochi, K. Rare earth elements activate the secondary metabolite–biosynthetic gene clusters in Streptomyces coelicolor A3(2). J. Antibiot. 2010, 63, 477–481. [Google Scholar] [CrossRef]
- Li, J.; Verweij, R.A.; van Gestel, C.A.M. Lanthanum toxicity to five different species of soil invertebrates in relation to availability in soil. Chemosphere 2018, 193, 412–420. [Google Scholar] [CrossRef]
- Connan, R.; Dabert, P.; Khalil, H.; Bridoux, G.; Béline, F.; Magrí, A. Batch enrichment of anammox bacteria and study of the underlying microbial community dynamics. Chem. Eng. J. 2016, 297, 217–228. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Xu, J.J.; Shi, Z.J.; Cheng, Y.F.; Ji, Z.Q.; Deng, R.; Jin, R.C. Short-term impacts of Cu, CuO, ZnO and Ag nanoparticles (NPs) on anammox sludge: CuNPs make a difference. Bioresour. Technol. 2017, 235, 281–291. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Cheng, Y.F.; Xu, L.Z.J.; Bai, Y.H.; Jin, R.C. Anammox granules show strong resistance to engineered silver nanoparticles during long-term exposure. Bioresour. Technol. 2018, 259, 10–17. [Google Scholar] [CrossRef]
- Helmeczi, E.; Wang, Y.; Brindle, I.D. A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry. Talanta 2016, 160, 521–527. [Google Scholar] [CrossRef]
- APHA; AWWA; AEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Wang, H.; Li, H.X.; Fang, F.; Guo, J.; Chen, Y.P.; Yan, P.; Yang, J.X. Underlying mechanisms of ANAMMOX bacteria adaptation to salinity stress. J. Ind. Microbiol. Biotechnol. 2019, 46, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Liang, R.; Hong, Y.; Ding, L.; Ren, H.; Mao, Y.; Zhao, M. Effects of La, Ce on nitrogen removal in sequencing batch reactor. Front. Environ. Sci. Eng. 2009, 3, 369–374. [Google Scholar] [CrossRef]
- Balboul, B.; El Roudi, A.M.; Samir, E.; Othman, A.G. Non-isothermal studies of the decomposition course of lanthanum oxalate decahydrate. Thermochim. Acta. 2002, 387, 109–114. [Google Scholar] [CrossRef]
- Moriwaki, H.; Koide, R.; Yoshikawa, R.; Warabino, Y.; Yamamoto, H. Adsorption of rare earth ions onto the cell walls of wild-type and lipoteichoic acid-defective strains of Bacillus subtilis. Appl. Microbiol. Biotechnol. 2013, 97, 3721–3728. [Google Scholar] [CrossRef] [PubMed]
- Noda, I.; Dowrey, A.E.; Marcott, C.; Story, G.M.; Ozaki, Y. Generalized two-dimensional correlation spectroscopy. Appl. Spectrosc. 2000, 54, 236A–248A. [Google Scholar] [CrossRef]
- Wei, D.; Li, M.; Wang, X.; Han, F.; Li, L.; Guo, J.; Ai, L.; Fang, L.; Liu, L.; Du, B.; et al. Extracellular polymeric substances for Zn (II) binding during its sorption process onto aerobic granular sludge. J. Hazard. Mater. 2016, 301, 407–415. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef]
- Pagano, G.; Aliberti, F.; Guida, M.; Oral, R.; Siciliano, A.; Trifuoggi, M.; Tommasi, F. Rare earth elements in human and animal health: State of art and research priorities. Environ. Res. 2015, 142, 215–220. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Cheng, Y.F.; Liu, Y.Y.; Zhang, Q.; Zhu, B.Q.; Jin, R.C. Deciphering the evolution characteristics of extracellular microbial products from autotrophic and mixotrophic anammox consortia in response to nitrogen loading variations. Environ. Int. 2019, 124, 501–510. [Google Scholar] [CrossRef]
- Li, J.; Wang, N. The gpsX gene encoding a glycosyltransferase is important for polysaccharide production and required for full virulence in Xanthomonas citri subsp. citri. BMC Microbiol. 2012, 12, 31. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, J.; Huo, T.; Zhao, Y.; Liu, S. Enhanced microbial metabolism in one stage partial nitritation-anammox system treating low strength wastewater by novel composite carrier. Water Res. 2019, 163, 114872. [Google Scholar] [CrossRef]
- Nagatani, H.; Shimizu, M.; Valentine, R.C. The mechanism of ammonia assimilation in nitrogen fixing bacteria. Arch. Für Mikrobiol. 1971, 79, 164–175. [Google Scholar] [CrossRef]
- Lawson, C.E.; Wu, S.; Bhattacharjee, A.S.; Hamilton, J.J.; McMahon, K.D.; Goel, R.; Noguera, D.R. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 2017, 8, 15416. [Google Scholar] [CrossRef]
- Lansman, J.B. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J. Gen. Physiol. 1990, 95, 679–696. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, H.; Li, X.; Zhang, C.; Liu, Y. Study on the toxic mechanism of La3+ to Escherichia coli. Biol. Trace Elem. Res. 2006, 114, 293–299. [Google Scholar]
- Lewis, B.D.; Spalding, E.P. Nonselective Block by La3+ of Arabidopsis Ion Channels Involved in Signal Transduction. J. Membr. Biol. 1998, 162, 81–90. [Google Scholar] [CrossRef]
- Merroun, M.L.; Ben Chekroun, K.; Arias, J.M.; González-Muñoz, M.T. Lanthanum fixation by Myxococcus xanthus: Cellular location and extracellular polysaccharide observation. Chemosphere 2003, 52, 113–120. [Google Scholar] [CrossRef]
- Evans, C.H. Interesting and useful biochemical properties of lanthanides. Trends Biochem. Sci. 1983, 8, 445–449. [Google Scholar] [CrossRef]
- Das, T.; Sharma, A.; Talukder, G. Effects of lanthanum in cellular systems. Biol. Trace Elem. Res. 1988, 18, 201–228. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size Dependent and Reactive Oxygen Species Related Nanosilver Toxicity to Nitrifying Bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Zuo, J. Response of anammox granules to ZnO nanoparticles at ambient temperature. Environ. Technol. Innov. 2019, 13, 146–152. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, R.; Chen, Y. Effects of ZnO Nanoparticles on Wastewater Biological Nitrogen and Phosphorus Removal. Environ. Sci. Technol. 2011, 45, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zheng, X.; Mu, H. The impacts of silver nanoparticles and silver ions on wastewater biological phosphorous removal and the mechanisms. J. Hazard. Mater. 2012, 15, 88–94. [Google Scholar]

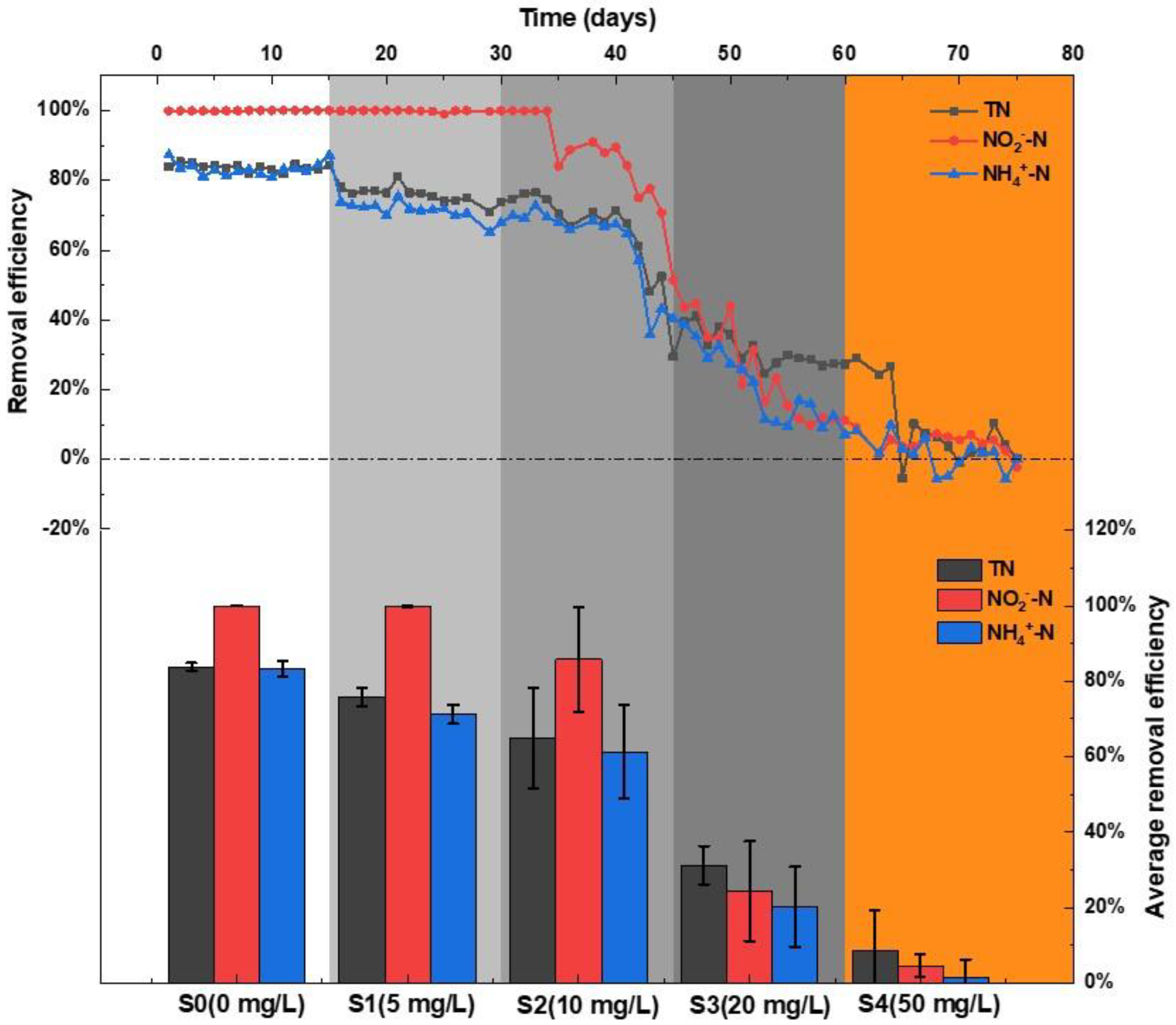

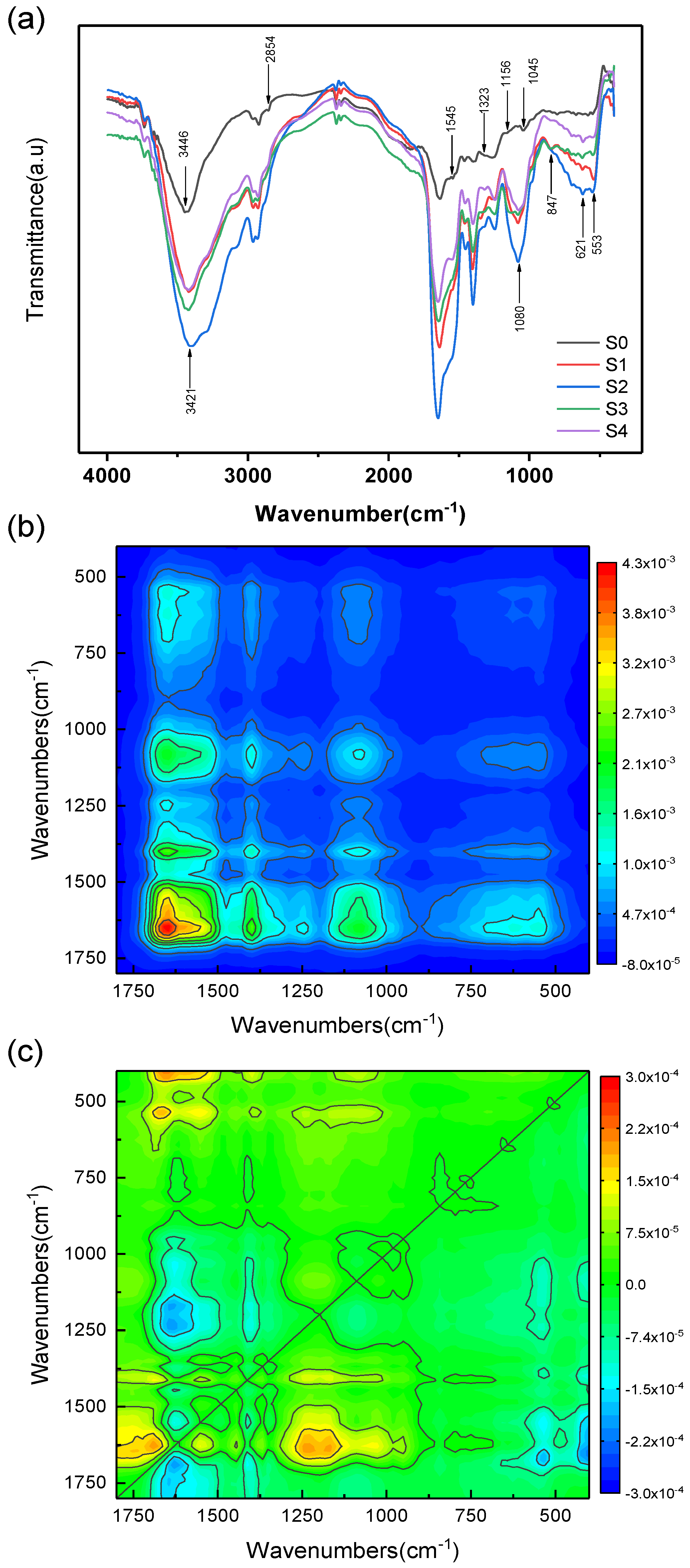
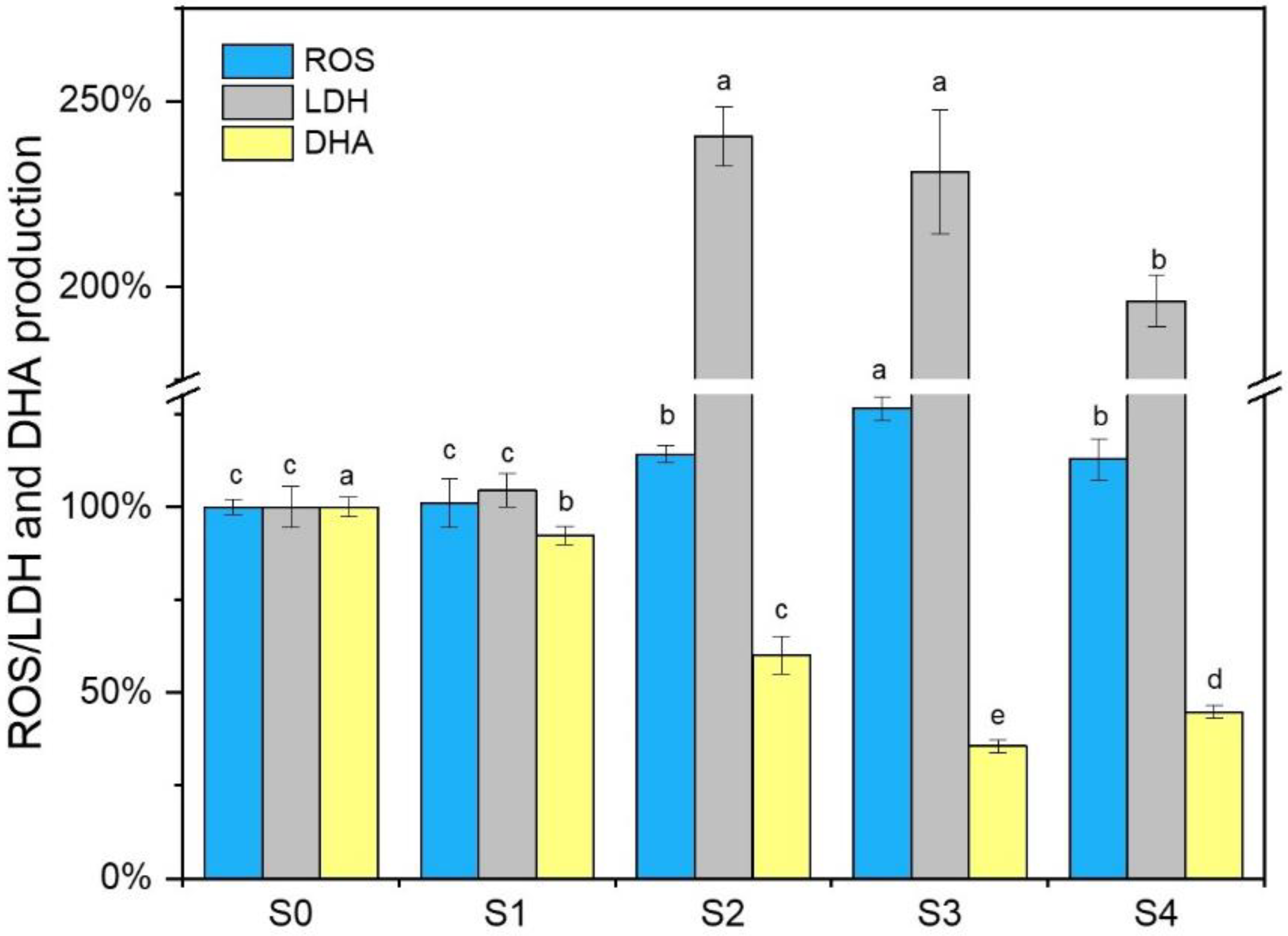
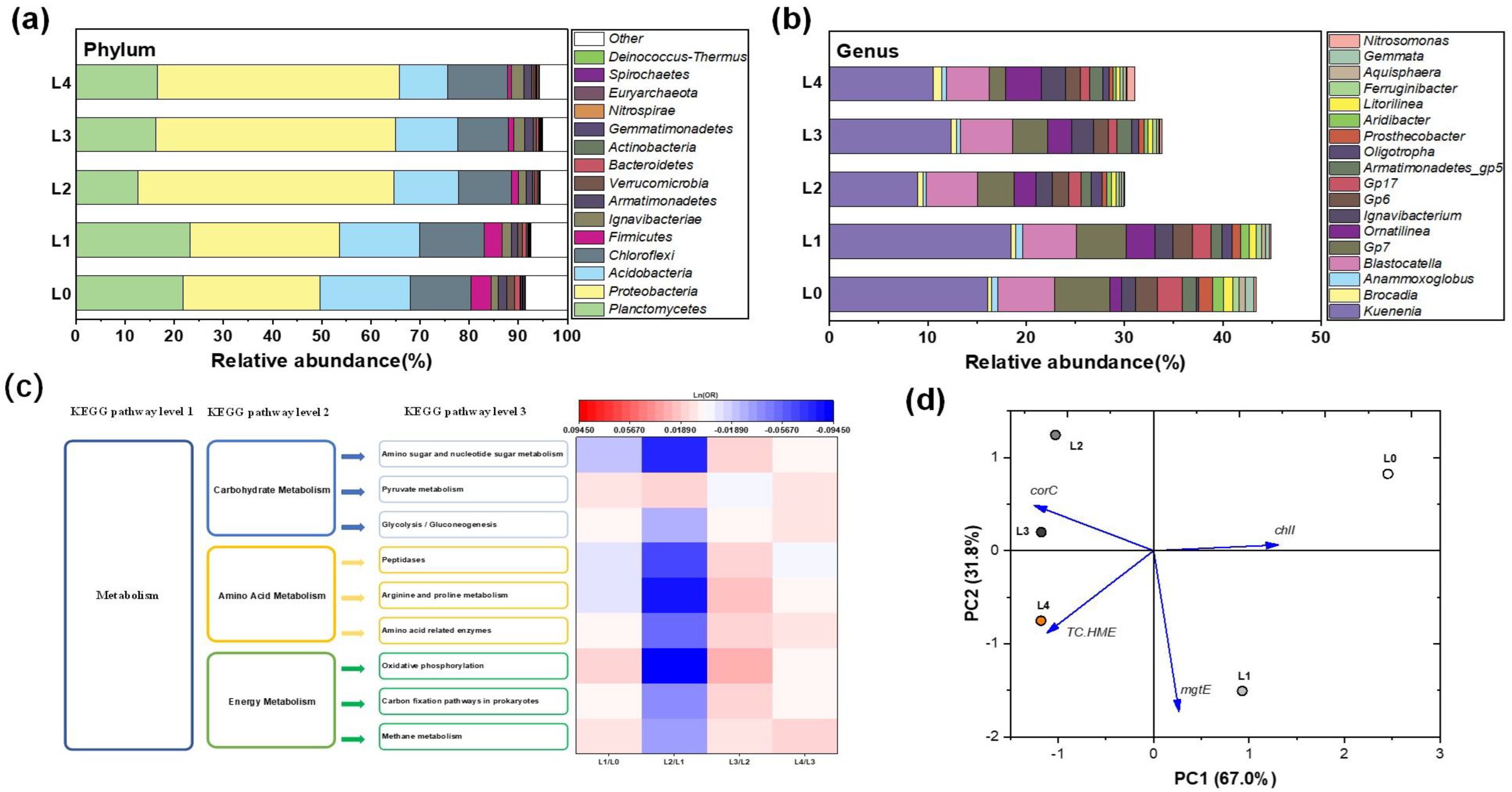
| Stage | Days | La Added | Dispersed La | PN | PS | Total EPS | PN/PS |
|---|---|---|---|---|---|---|---|
| mg L−1 | mg L−1 | mg·g−1 VSS | mg·g−1 VSS | mg·g−1 VSS | |||
| S0 | 0–15 | 0 | ND | 260.9 ± 20.4 (n = 3) b | 32.0 ± 2.9 (n = 3) b | 292.9 ± 17.5 (n = 3) b | 8.2 |
| S1 | 16–30 | 5 | ND | 363.0 ± 26.7 (n = 3) a | 35.3 ± 1.2 (n = 3) a | 398.3 ± 26.8 (n = 3) a | 10.3 |
| S2 | 31–45 | 10 | ND | 273.4 ± 37.8 (n = 3) b | 50.6 ± 4.5 (n = 3) b | 324.0 ± 40.4 (n = 3) b | 5.4 |
| S3 | 46–60 | 20 | ND | 192.8 ± 16.6 (n = 3) c | 39.4 ± 4.3 (n = 3) c | 232.2 ± 20.9 (n = 3) c | 4.9 |
| S4 | 61–75 | 50 | ND | 180.1 ± 18.3 (n = 3) c | 45.0 ± 3.0 (n = 3) c | 225.1 ± 17.5 (n = 3) c | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Wu, D. Responses of Anammox Granular Sludge to Long-Term Rare Earth Element Feeding: Lanthanum as a Case. Sustainability 2020, 12, 7887. https://doi.org/10.3390/su12197887
Huang S, Wu D. Responses of Anammox Granular Sludge to Long-Term Rare Earth Element Feeding: Lanthanum as a Case. Sustainability. 2020; 12(19):7887. https://doi.org/10.3390/su12197887
Chicago/Turabian StyleHuang, Shuanglei, and Daishe Wu. 2020. "Responses of Anammox Granular Sludge to Long-Term Rare Earth Element Feeding: Lanthanum as a Case" Sustainability 12, no. 19: 7887. https://doi.org/10.3390/su12197887
APA StyleHuang, S., & Wu, D. (2020). Responses of Anammox Granular Sludge to Long-Term Rare Earth Element Feeding: Lanthanum as a Case. Sustainability, 12(19), 7887. https://doi.org/10.3390/su12197887




