Rainfall and Human Impacts on Weathering Rates and Carbon-Nutrient Yields in the Watershed of a Small Mountainous River (Kaoping) in Southwestern Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Background
2.2. Sampling and Analytical Methods
- (i)
- Dissolved elemental flux = Σ([dissolved elemental concentration (corrected from atmospheric contribution)] × [discharge]);
- (ii)
- Particulate elemental flux = Σ([particulate elemental concentration] × [sediment load]);
- (iii)
- Sediment, carbon, and nutrient yields = (annual fluxes of sediment, carbon, and nutrients)/(watershed area, 3257 km2).
3. Results
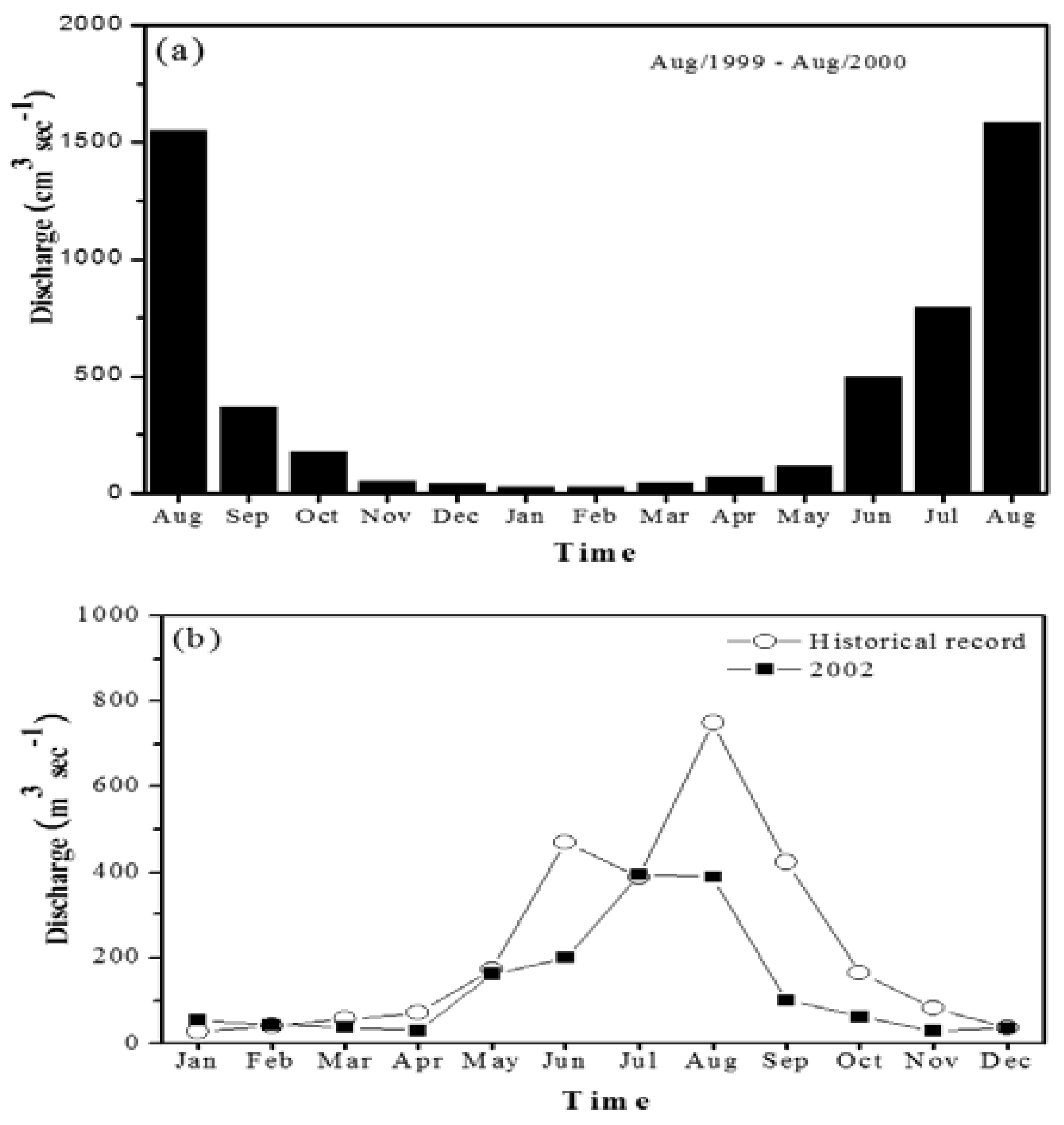
3.1. General Hydrological Characteristics and River Water Chemistry
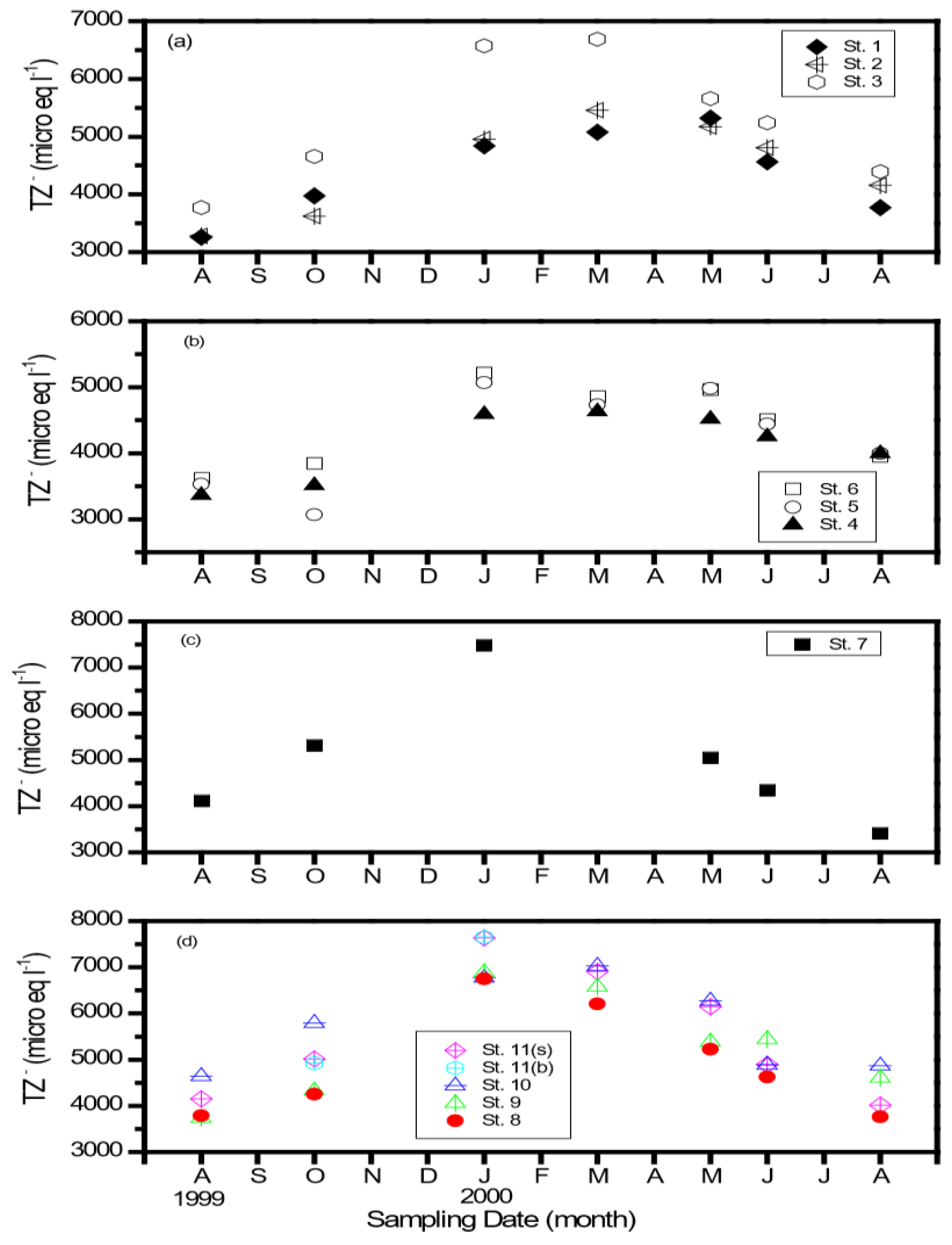
3.2. Distribution of Major Cations and Anions
3.3. Dissolved Inorganic and Organic Nutrients
3.4. Dissolved Inorganic Carbon (DIC) and Organic Carbon (DOC)
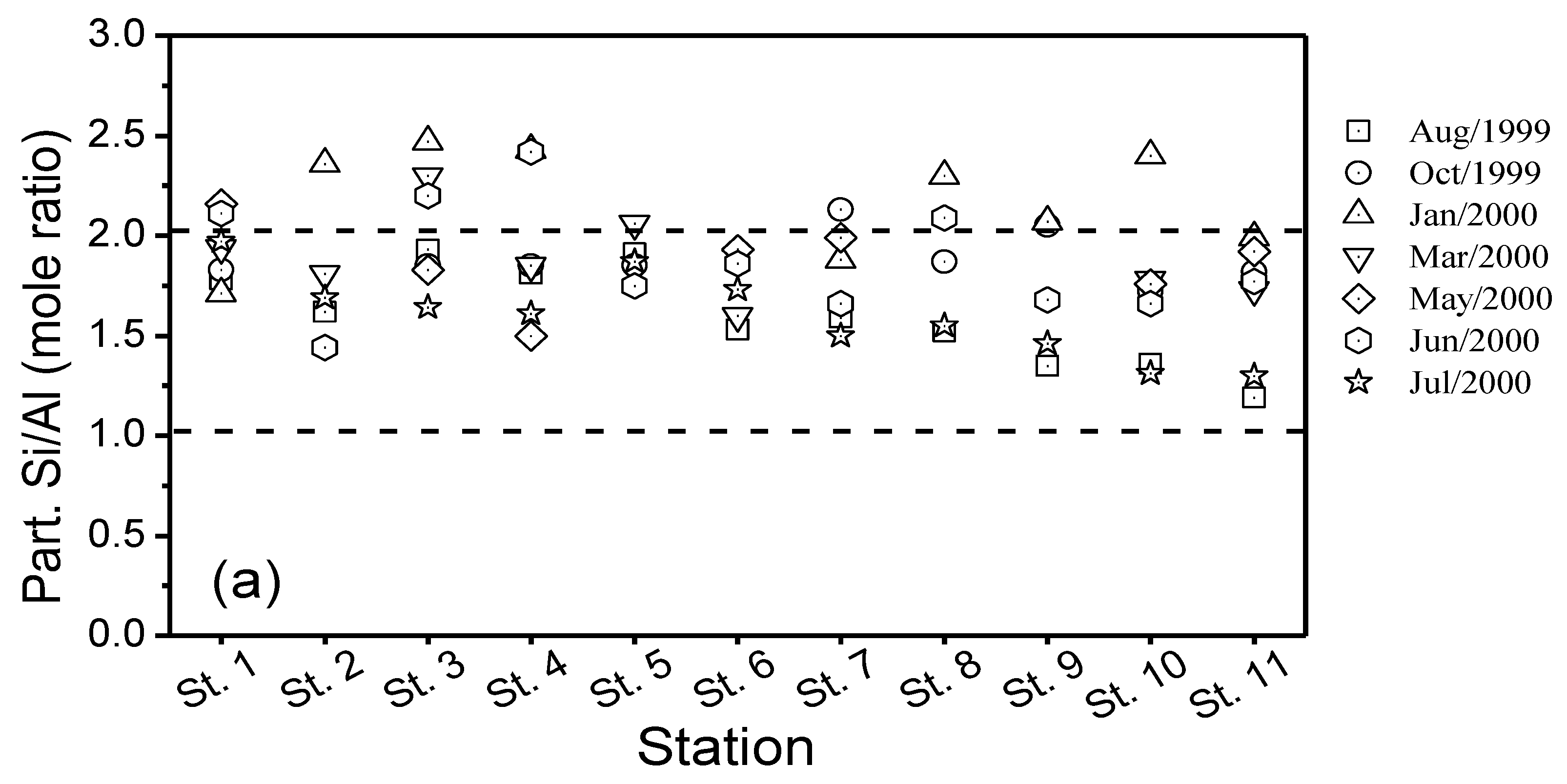
3.5. Total Suspended Matter (TSM), Particulate Organic and Inorganic Carbon (POC, PIC) and Particulate Nitrogen (PN)
4. Discussion
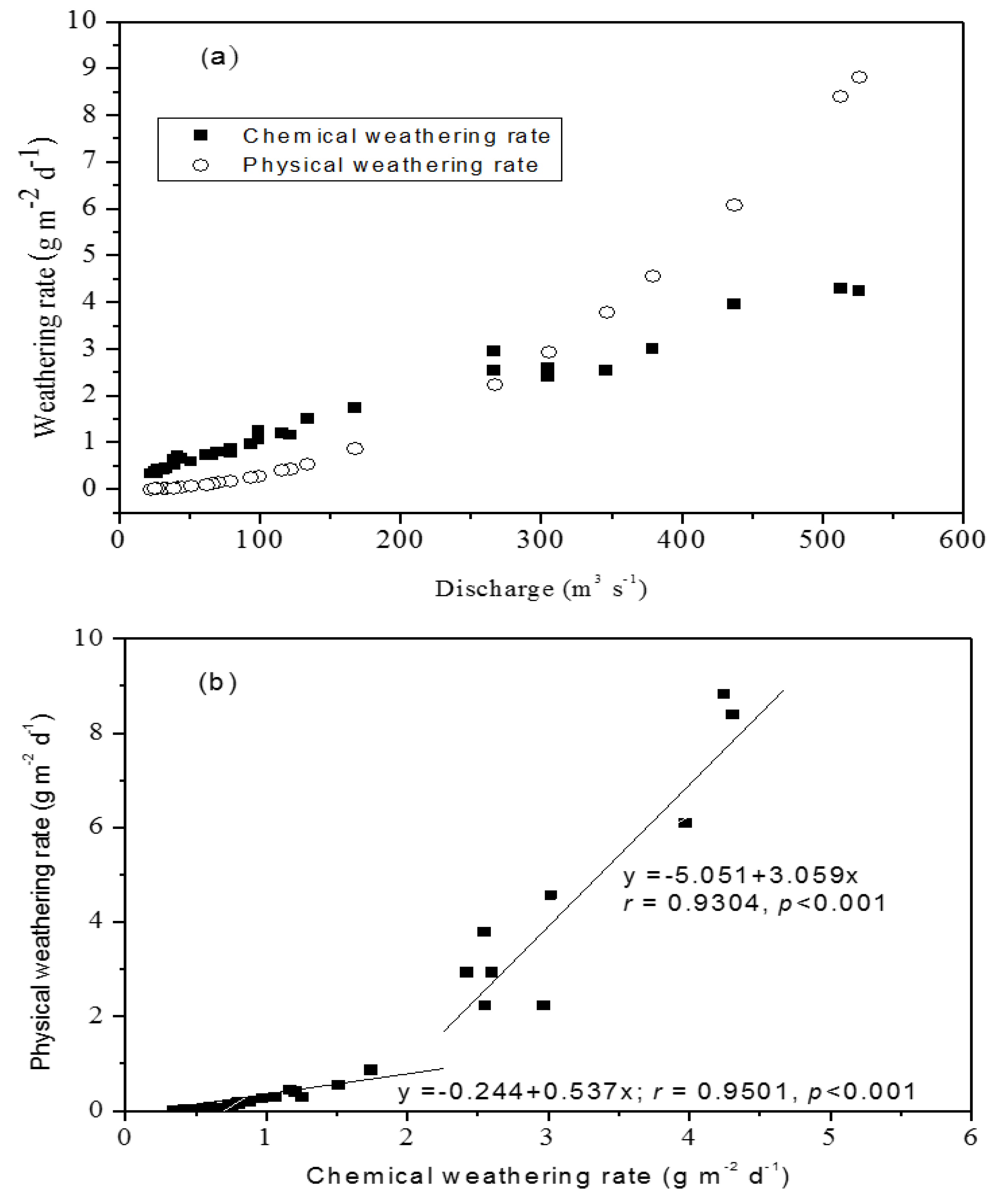
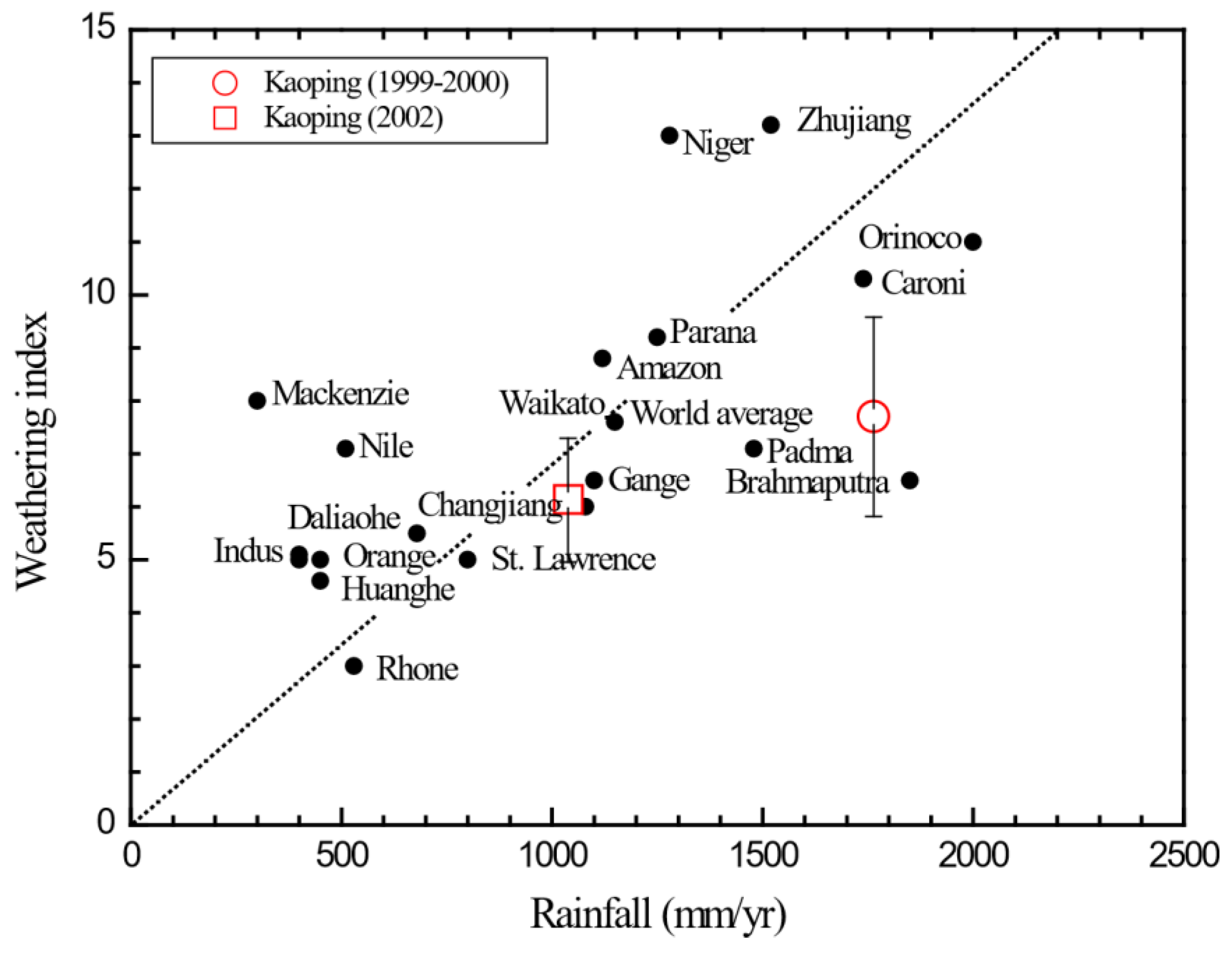
4.1. Physical Weathering Rates in the Kaoping Watershed
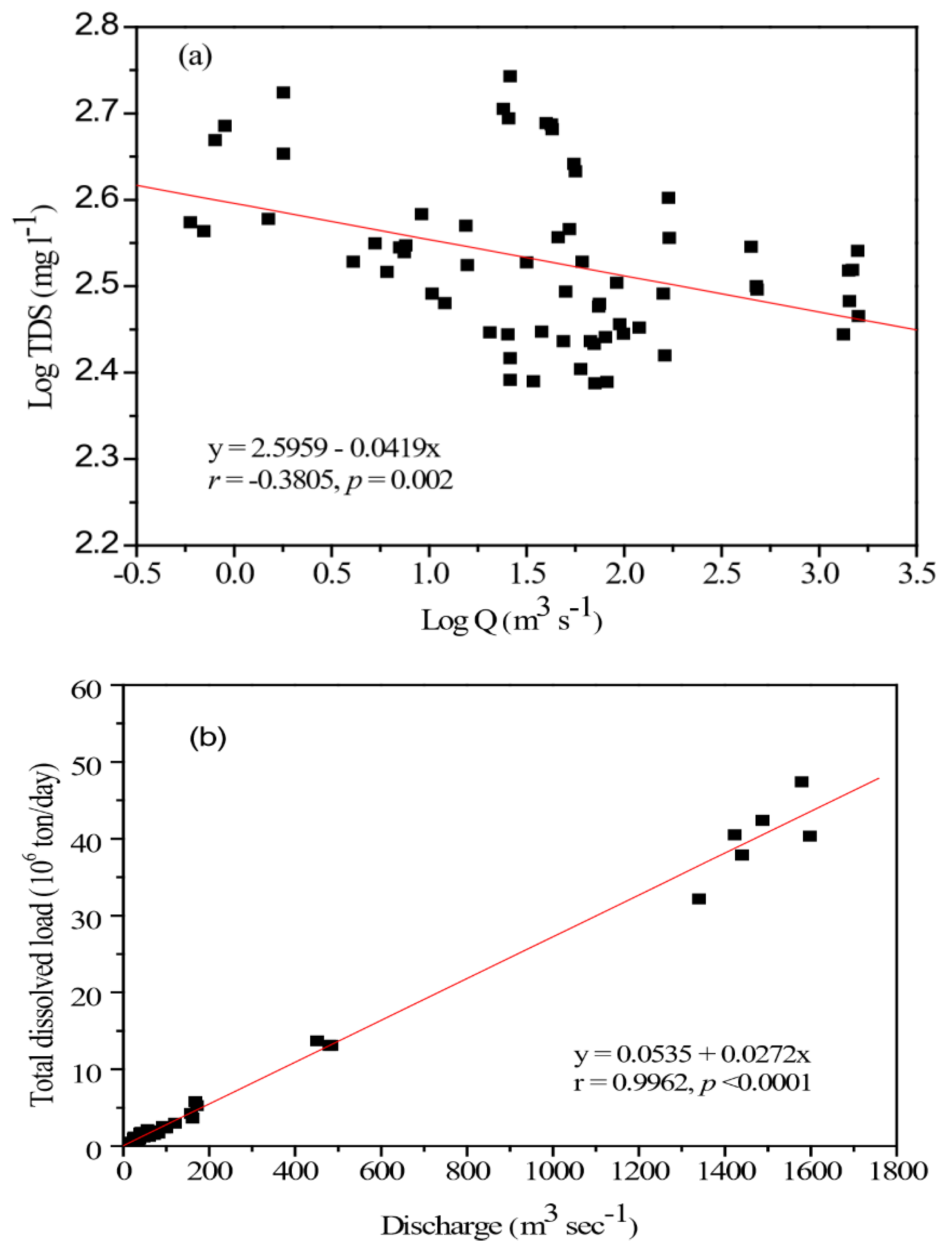
4.2. Chemical Weathering Rates in the Kaoping Watershed
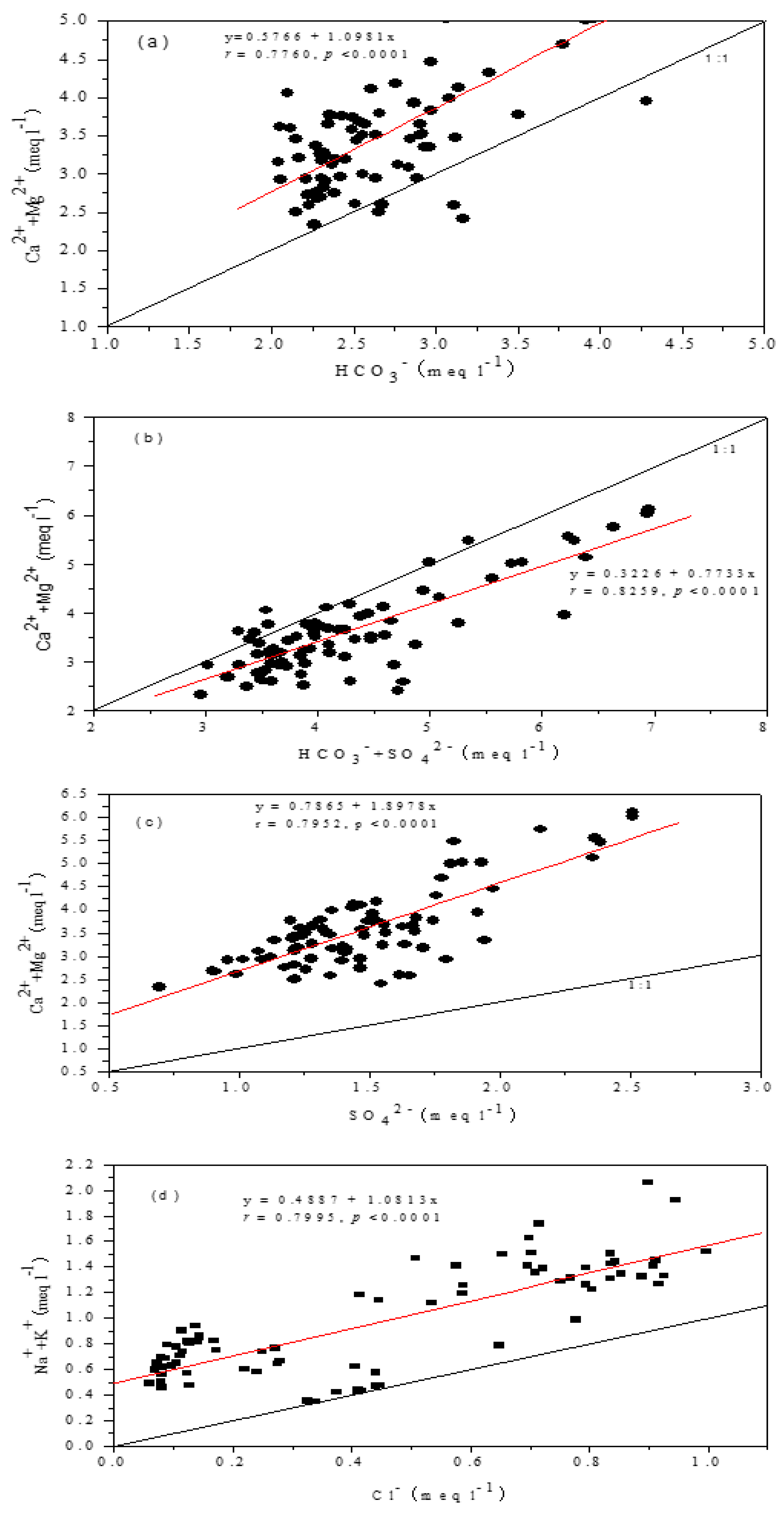
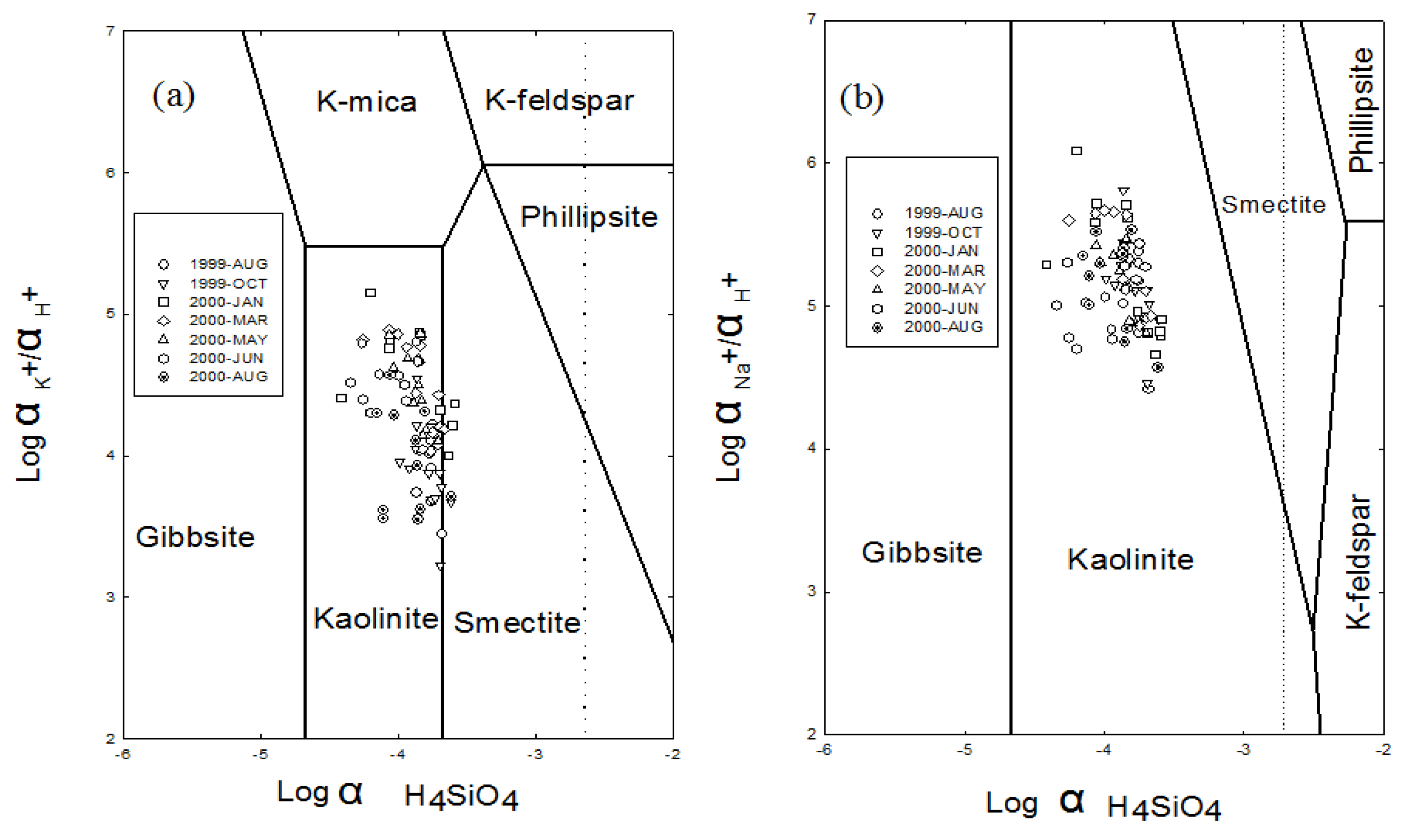
4.3. Mechanisms of Solutes Acquisition
4.4. Relative Contributions of Carbonate and Silicate Weathering
4.5. Carbon Yields in the Watershed
4.6. Nutrient Yields and Human Influence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.-H. Denudation of Taiwan Island since the Pliocene Epoch. Geology 1976, 4, 105–107. [Google Scholar] [CrossRef]
- Milliman, J.D.; Meade, R.H. World-Wide Delivery of River Sediment to the Oceans. J. Geol. 1983, 91, 1–21. [Google Scholar] [CrossRef]
- Chest, R. Marine Geochemistry; Chapman & Hall: London, UK, 2000; pp. 11–82. [Google Scholar]
- White, A.F.; Blum, A.E. Effects of climate on chemical_ weathering in watersheds. Geochim. Cosmochim. Acta 1995, 59, 1729–1747. [Google Scholar] [CrossRef]
- Stumm, W.; Wollast, R. Coordination chemistry of weathering: Kinetics of the surface-controlled dissolution of oxide minerals. Rev. Geophys. 1990, 28, 53–69. [Google Scholar] [CrossRef]
- Meybeck, M. Global chemical weathering of surfacial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allègre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Milliman, J.D.; Syvistski, J.P.M. Geomorphic/tectonic control of sediment to oceans. J. Geol. 1992, 100, 525–544. [Google Scholar] [CrossRef]
- Milliman, J.D. Sediment discharge to the ocean from small mountainous rivers: The New Guinea example. Geomar. Lett. 1995, 15, 127–133. [Google Scholar] [CrossRef]
- Carey, A.E.; Goldsmith, S.T.; Lyons, W.B.; Gardner, C.B.; Hicks, D.M. Organic carbon yields from small, mountainous rivers, New Zealand. Geophys. Res. Lett. 2005, 32, L15404. [Google Scholar] [CrossRef]
- Milliman, J.D.; Farnsworth, K.L.; Albertin, C.S. Flux and fate of fluvial sediments leaving large islands in the East Indies. J. Sea Res. 1999, 41, 97–107. [Google Scholar] [CrossRef]
- Liu, J.T.; Hsu, R.T.; Hung, J.-J.; Chang, Y.-P.; Wang, Y.-H.; Rendle-Bühring, R.H.; Lee, C.-L.; Huh, C.-A.; Yang, R.J. From the highest to the deepest: The Gaoping River-Gaoping Submarine Canyon dispersal system. Earth Sci. Rev. 2016, 153, 274–300. [Google Scholar] [CrossRef]
- Degens, E.T.; Kempe, S.; Richey, J.E. Biogeochemistry of Major World Rivers; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Walling, D.E.; Webb, D.W. Material transport by world’s rivers: Evolving perpectives. In Water for Future: Hydrology in Perspective; IAHS Publication: Wallingford, UK, 1987; Volume 164. [Google Scholar]
- Hamid, A.; Bhat, S.U.; Jehangir, A. Local determinants influencing stream water quality. Appl. Water Sci. 2020, 10, 24. [Google Scholar] [CrossRef]
- Meybeck, M.; Helmer, R. The quality of rivers: From pristine stage to global pollution. Paleogeog. Paleoclim. Paleoecol. 1989, 75, 283–309. [Google Scholar] [CrossRef]
- Chen, X.; Zong, Y.; Zhang, E.; Xu, J.; Li, S. Human impacts on the Changjiang (Yangtze) River basin, China, with special reference to the impacts on the dry season water discharges into the sea. Geomorphology 2001, 41, 111–123. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Meybeck, M.; He, D.; Xia, X.; Zhang, L. Spatial and temporal analysis of water chemistry records (1958–2000) in the Huanghe (Yellow River) basin. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Hung, J.-J.; Yeh, Y.-T.; Huh, C.-A. Efficient transport of terrestrial particulate carbon in a tectonically-active marginal sea off southwestern Taiwan. Mar. Geol. 2012, 315, 29–43. [Google Scholar] [CrossRef]
- Hung, J.-J.; Hsu, C.-L. Present state and historical changes of trace metal pollution in Kaoping coastal sediments, southwestern Taiwan. Mar. Pollut. Bull. 2004, 49, 986–998. [Google Scholar] [CrossRef]
- Hung, J.-J.; Lu, C.-C.; Huh, C.-A.; Liu, J.T. Geochemical controls on distributions and speciation of As and Hg in sediments along the Gaoping (Kaoping) Estuary–Canyon system off southwestern Taiwan. J. Mar. Syst. 2009, 76, 479–495. [Google Scholar] [CrossRef]
- Water Resources Bureau. Hydrological Year Book of Taiwan; Ministry Economic Affairs: Taiwan, 2004. [Google Scholar]
- Ho, C.-S. An Introduction to the Geology of Taiwan: Explanatory Text of the Geologic Map of Taiwan, 2nd ed.; Central Geological Survey, Ministry of Economic Affairs: Taiwan, 1988. [Google Scholar]
- Blattmann, T.M.; Wang, S.-L.; Lupker, M.; Märki, L.; Haghipour, N.; Wacker, L.; Chung, L.-H.; Bernasconi, S.M.; Plötze, M.; Eglinton, T.I. Sulphuric acid-mediated weathering on Taiwan buffers geological atmospheric carbon sinks. Sci. Rep. 2019, 9, 2945. [Google Scholar] [CrossRef]
- Harrison, J.F.; Chang, C.-H.; Liu, C.-C. Identification of inventory-based susceptibility models for assessing landslide probability: A case study of the Gaoping River Basin, Taiwan. Geomat. Nat. Hazards Risk 2017, 8, 1730–1751. [Google Scholar] [CrossRef]
- Sparkes, R.; Lin, I.-T.; Hovius, N.; Galy, A.; Liu, J.T.; Xu, X.; Yang, R. Redistribution of multi-phase particulate organic carbon in a marine shelf and canyon system during an exceptional river flood: Effects of Typhoon Morakot on the Gaoping River–Canyon system. Mar. Geol. 2015, 363, 191–201. [Google Scholar] [CrossRef]
- Welschmeyer, N.A. Fluorometric analysis of chlorophyII aon the presence of chlorophyII b and phenopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Hung, J.-J.; Hung, P.-Y. Carbon and nutrient dynamics in a hypertrophic lagoon in southwestern Taiwan. J. Mar. Syst. 2003, 42, 97–114. [Google Scholar] [CrossRef]
- Hung, J.-J.; Wang, S.-M.; Chen, Y.-L. Biogeochemical controls on distributions and fluxes of dissolved and particulate organic carbon in the Northern South China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1486–1503. [Google Scholar] [CrossRef]
- Hung, J.-J.; Lin, C.; Hung, G.; Chung, Y. Lateral Transport of Lithogenic Particles from the Continental Margin of the Southern East China Sea. Estuar. Coast. Shelf Sci. 1999, 49, 483–499. [Google Scholar] [CrossRef]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis; Verlag Chimie: Weinheim, Germany, 1983; pp. 143–187. [Google Scholar]
- Pai, S.C.; Yang, C.C. Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylbdum blue complex. Anal. Chim. Acta 1990, 229, 115–120. [Google Scholar]
- Ridal, J.; Moore, R. A re-examination of the measurement of dissolved organic phosphorus in seawater. Mar. Chem. 1990, 29, 19–31. [Google Scholar] [CrossRef]
- David, L.T. Calculation of River Discharge. Loicz Rep. Stud. 2000, 14, 141–147. [Google Scholar]
- McLennan, S.M. Weathering and Global Denudation. J. Geol. 1993, 101, 295–303. [Google Scholar] [CrossRef]
- Wu, T.-T.; Lee, C.-P. The effects of El Niño Southern Oscillation on temperature and precipitation in Taiwan. In Proceedings of the 36th Ocean Engineering Conference in Taiwan, Taiwan, 4–5 December 2014; pp. 471–476, (in Chinese with Abstract in English). [Google Scholar]
- Summerfield, M.A.; Hulton, N.J. Natural controls of fluvial denudation rates in major world drainage basins. J. Geophys. Res. Space Phys. 1994, 99, 13871–13883. [Google Scholar] [CrossRef]
- Chakrapani, G.J. Factors controlling variations in river sediment loads. Curr. Sci. 2005, 88, 569–575. [Google Scholar]
- Hovius, N.; Stark, C.P.; Hao-Tsu, C.; Jiun-Chuan, L. Supply and Removal of Sediment in a Landslide-Dominated Mountain Belt: Central Range, Taiwan. J. Geol. 2000, 108, 73–89. [Google Scholar] [CrossRef]
- Dadson, S.J.; Hovius, N.; Chen, H.; Dade, W.B.; Hsieh, M.L.; Willett, S.D.; Hu, J.-C.; Horng, M.-J.; Chen, M.-C.; Stark, C.P.; et al. Links between erosion, runoff variability and seismicity in the Taiwan orogeny. Nature 2003, 426, 648–651. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, K.-T.; Chiu, Y.-J.; Lau, S.-M.; Lee, H.-Y. Quantifying rainfall controls on catchment-scale land-slide erosion in Taiwan. Earth Surf. Proc. Landf. 2012, 38, 372–382. [Google Scholar] [CrossRef]
- Lee, T.Y.; Huang, J.-C.; Lee, J.-Y.; Jien, S.-H.; Zehetner, F.; Kao, S.-J. Magnified Sediment Export of Small Mountainous Rivers in Taiwan: Chain Reactions from Increased Rainfall Intensity under Global Warming. PLoS ONE 2015, 10, e0138283. [Google Scholar] [CrossRef]
- Shou, K.J.; Wu, C.C.; Lin, J.F. Predictive analysis of landslide susceptibility in the Kao-Ping watershed, Taiwan under climate change conditions. Nat. Hazards Earth Syst. Sci. Discuss. 2015, 3, 575–606. [Google Scholar] [CrossRef]
- Kao, S.J.; Milliman, J.D. Water and Sediment Discharge from Small Mountainous Rivers, Taiwan: The Roles of Lithology, Episodic Events, and Human Activities. J. Geol. 2008, 116, 431–448. [Google Scholar] [CrossRef]
- Hudson, N. Soil Conservation, 3rd ed.; B.T. Batsford Ltd.: London, UK, 1995. [Google Scholar]
- Chiang, S.-H. Climate and soil erodibility (in Chinese). Sci. Mon. 1989, 20, 132–134. [Google Scholar]
- Martin, J.-M.; Whitfield, M. The Significance of the River Input of Chemical Elements to the Ocean. In Trace Metals in Sea Water; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1983; pp. 265–296. [Google Scholar]
- Meybeck, M. How to establish and use world budgets of riverine materials. In Physical and Chemical Weathering in Geochemical Cycles; Lerman, A., Meybeck, M., Eds.; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Garrels, R.M.; Mackenzie, F.T. Evolution of Sedimentary Rocks; W.W. Norton & Co.: New York, NY, USA, 1971. [Google Scholar]
- Zhang, S.-R.; Lu, X.; Higgitt, D.L.; Chen, C.-T.A.; Sun, H.-G.; Han, J.-T. Water chemistry of the Zhujiang (Pearl River): Natural processes and anthropogenic influences. J. Geophys. Res. Space Phys. 2007, 112, F01011. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Xia, X.; Zhang, L. Major element chemistry of the Changjiang (Yangtze River). Chem. Geol. 2002, 187, 231–255. [Google Scholar] [CrossRef]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon: The influence of geology and weathering environment on the dissolved load. J. Geophys. Res. Space Phys. 1983, 88, 9671–9688. [Google Scholar] [CrossRef]
- Edmond, J.; Palmer, M.; Measures, C.; Brown, E.T.; Huh, Y. Fluvial geochemistry of the eastern slope of the northeastern Andes and its foredeep in the drainage of the Orinoco in Colombia and Venezuela. Geochim. Cosmochim. Acta 1996, 60, 2949–2974. [Google Scholar] [CrossRef]
- Lyons, W.B.; Carey, A.E.; Hicks, D.M.; Nezat, C.A. Chemical weathering in high-sediment-yielding watersheds, New Zealand. J. Geophys. Res. Space Phys. 2005, 110, F01008. [Google Scholar] [CrossRef]
- Walling, D.E.; Webb, D.W. Solutes in River Systems, in Solute Processes; Trudgill, S.D., Ed.; John Wiley: Hoboken, NJ, USA, 1986; pp. 251–327. [Google Scholar]
- Gaillardet, J.; Dupré, B.; Allègre, C.J.; Negrel, P. Chemical and physical denudation in the Amazon River Basin. Chem. Geol. 1997, 142, 141–173. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Strong, P.J.; Li, Z.; Jiang, P. Plant impact on the coupled terrestrial biogeochemical cycles of silicon and carbon: Implications for biogeochemical carbon sequestration. Earth Sci. Rev. 2012, 115, 319–331. [Google Scholar] [CrossRef]
- Zhang, J. Geochemistry of Trace Metals from Chinese River/Estuary Systems: An Overview. Estuar. Coast. Shelf Sci. 1995, 41, 631–658. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Selvaraj, K.; Chen, C.-T.A. Moderate Chemical Weathering of Subtropical Taiwan: Constraints from Solid-Phase Geochemistry of Sediments and Sedimentary Rocks. J. Geol. 2006, 114, 101–116. [Google Scholar] [CrossRef]
- Hung, J.-J.; Yeh, Y.-C. Quantifying Phosphorus Sources and Sinks in the Gaoping River-Sea System in Southwestern Taiwan. Estuar. Coast. 2018, 42, 425–438. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, C.-T.; Hung, J.-J. Aquatic Chemistry of Lakes and Reservoirs in Taiwan. Terr. Atmos. Ocean. Sci. 1997, 8, 405. [Google Scholar] [CrossRef]
- Das, A.; Chung, C.-H.; You, C.-F. Disproportionately high rates of sulfide oxidation from mountainous river basins of Taiwan orogeny: Sulfur isotope evidence. Geophys. Res. Lett. 2012, 39, 39. [Google Scholar] [CrossRef]
- Huh, Y.; Edmond, J.M. The fluvial geochemistry of the rivers of Eastern Siberia: III. Tributaries of the Lena and Anabar draining the basement terrain of the Siberian Craton and the Trans-Baikal Highlands. Geochim. Cosmochim. Acta 1999, 63, 967–987. [Google Scholar] [CrossRef]
- Meybeck, M. Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar] [CrossRef]
- EPA (Taiwan) Report. The Planning of Pollution Control in the Kaoping River; EPA (Taiwan): Taiwan, 1993; p. 179. (in Chinese) [Google Scholar]
- Billen, G.; Lancelot, C.; Meybeck, M. N, P and Si Retention along the Aquatic Continuum from Land to Ocean. In Ocean Margin Processes in Global Change; Mantoura, R.F.C., Martin, J.-M., Wollast, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]

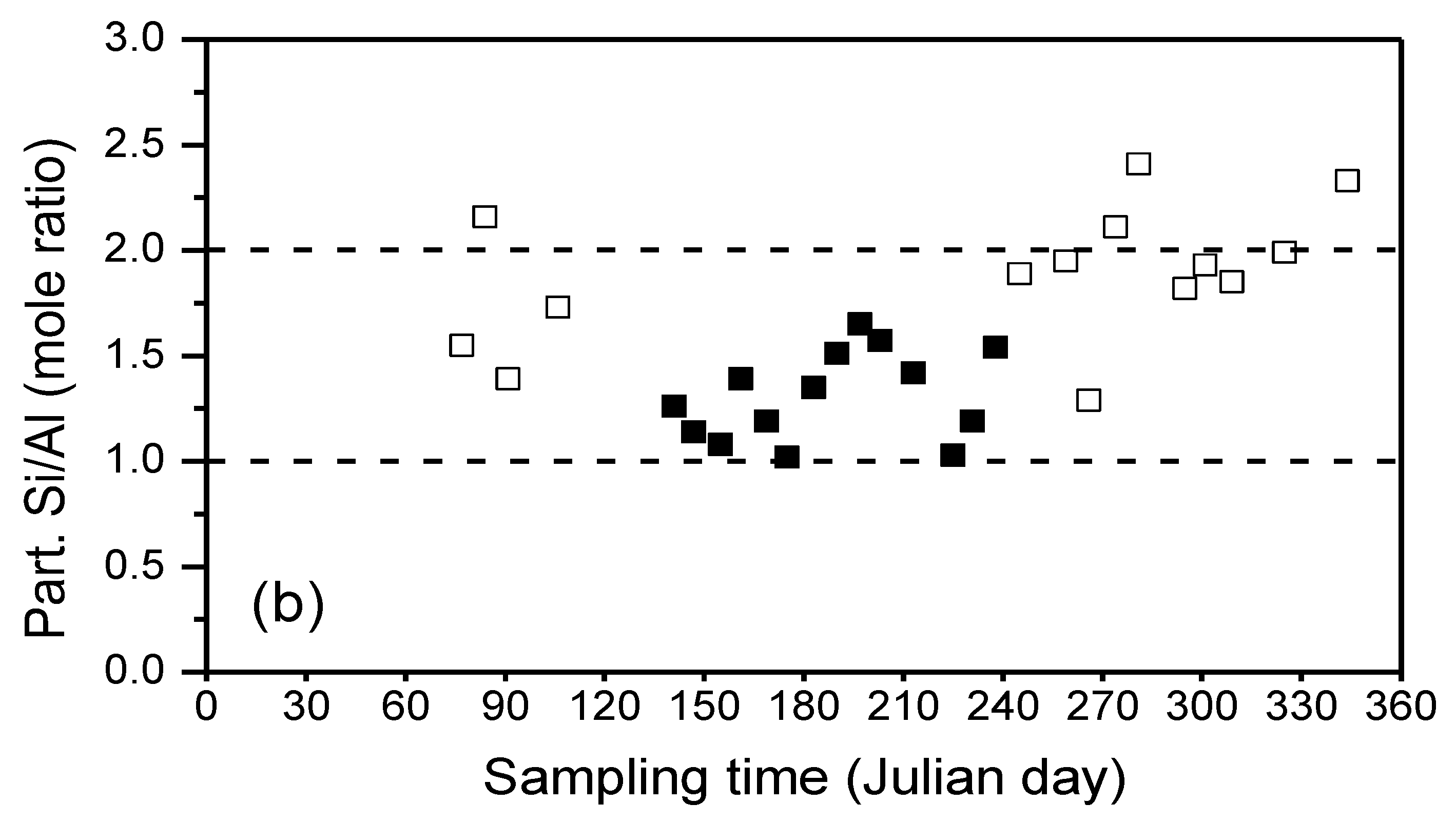
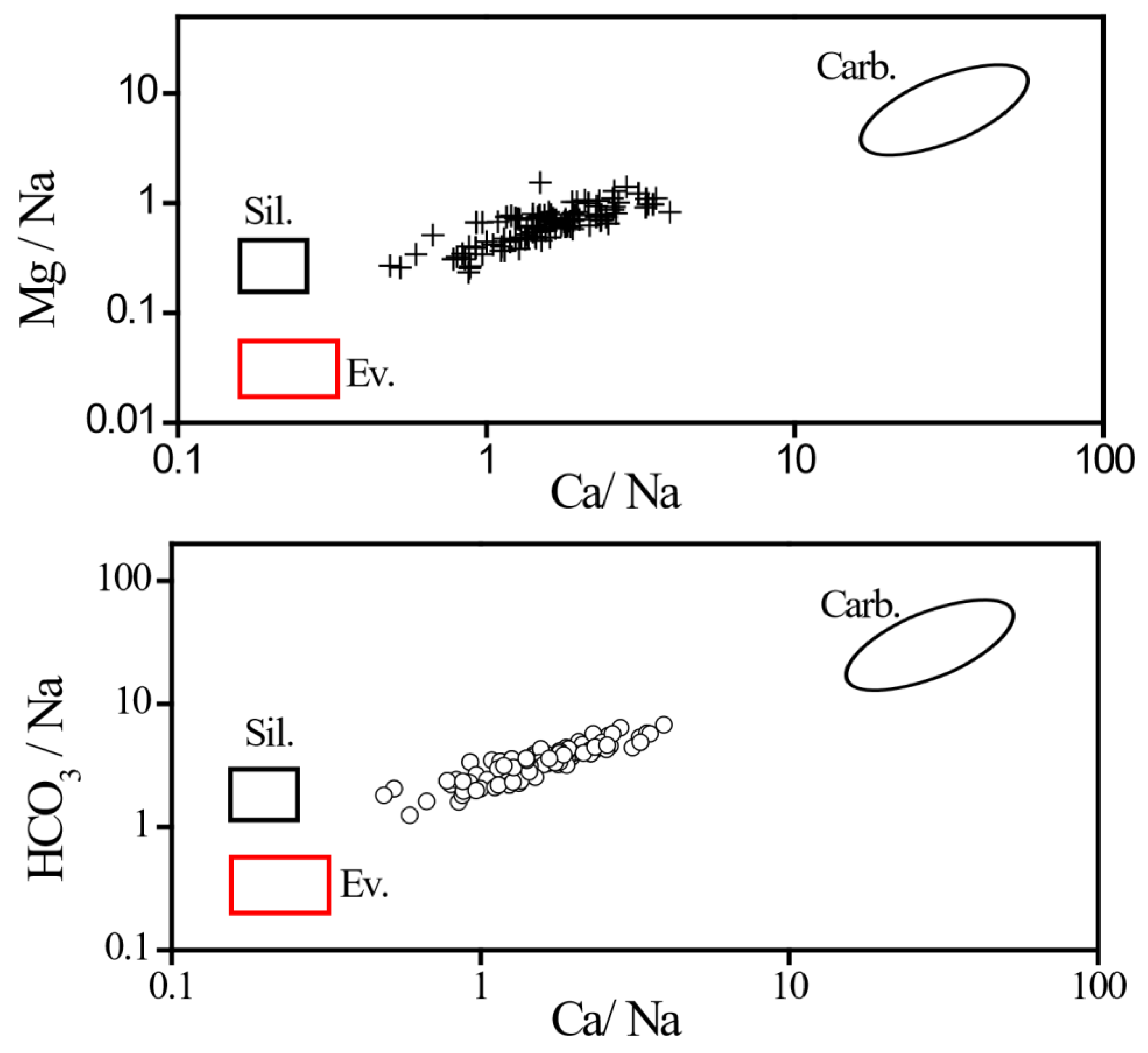
| River branch | Ca2+ | Mg2+ | K+ | Na+ | HCO3− | Cl− | SO42v |
|---|---|---|---|---|---|---|---|
| (Stream) | μeq L−1 | ||||||
| Lao-Nung (mean) | 1096 | 412 | 117 | 778 | 2341 | 350 | 682 |
| Lao-Nung (range) | 823–1331 | 291–570 | 26–270 | 221–1309 | 2040–2924 | 20–794 | 348–897 |
| Chi-San (mean) | 1150 | 497 | 143 | 957 | 2809 | 346 | 664 |
| Chi-San (range) | 735–1761 | 353–625 | 35–295 | 397–1818 | 2221–4279 | 29–907 | 448–956 |
| Yai-Liao (mean) | 1219 | 458 | 113 | 664 | 2775 | 362 | 712 |
| Yai-Liao (range) | 1035–2281 | 383–599 | 15–200 | 180–1114 | 2054–4471 | 81–768 | 477–1077 |
| Kaoping (mean) | 1553~ | 541 | 137 | 792 | 2994 | 429 | 842 |
| Kaoping (range) | 1023–2313 | 393–705 | 16–251 | 245–1278 | 2094–4433 | 17–997 | 602–1254 |
| Sampling Station | Empirical Relationship | Regression Coefficient | Significant Level |
|---|---|---|---|
| Station 10 | Log[TSM] = 0.0318 + 1.0095Log[Q] | r = 0.8770 | p < 0.0001 |
| Log[sed load] = −1.0649 + 2.0233Log[Q] | r = 0.9659 | p < 0.0001 | |
| Station 9 | Log[TSM] = 0.4995 + 0.8755Log[Q] | r = 0.8386 | p < 0.0001 |
| Log[sed load] = −0.6061 + 1.8878Log[Q] | r = 0.9565 | p < 0.0001 | |
| Station 6 | Log[TSM] = 0.9652 + 0.8681Log[Q] | r = 0.8559 | p < 0.0001 |
| Log[sed load] = −0.062 + 1.851Log[Q] | r = 0.9616 | p < 0.0001 | |
| Station 5 | Log[TSM] = 0.0668 + 1.2804Log[Q] | r = 0.8375 | p < 0.0001 |
| Log[sed load] = −1.0451 + 2.3036Log[Q] | r = 0.9395 | p < 0.0001 | |
| Station 1 | Log[TSM] = 0.2526 + 1.2755Log[Q] | r = 0.7120 | p < 0.0001 |
| Log[sed load] = −0.8285 + 2.279Log[Q] | r = 0.8712 | p < 0.0001 | |
| Station 7 | Log[TSM] = 1.0218 + 0.8263Log[Q] | r = 0.7618 | p < 0.0001 |
| Log[sed load] = −0.0494 + 1.830Log[Q] | r = 0.9323 | p < 0.0001 | |
| Station 2 | Log[TSM] = 0.3765 + 1.150Log[Q] | r = 0.8560 | p < 0.0001 |
| Log[sed load] = −0.6656 + 2.142Log[Q] | r = 0.9516 | p < 0.0001 | |
| Station 4 | Log[TSM] = 0.4279 + 1.035Log[Q] | r = 0.7771 | p < 0.0001 |
| Log[sed load] = −0.6981 + 2.0712log[Q] | r = 0.9322 | p < 0.0001 |
| Parameter | Aug. 1999–July 2000 (a) | Jan.–Dec. 2002 (b) | a/b |
|---|---|---|---|
| Physical weathering rate (g m−2 year−1) | 3.6 × 103 | 0.656 × 103 | 5.5 |
| Chemical weathering rate (g m−2 year−1) | 1.14 × 103 | 0.416 × 103 | 2.74 |
| POC yield (g m−2 year−1) | 37.0 | 2.92 | 12.6 |
| PIC yield (g m−2 year−1) | 15.2 | 1.27 | 11.9 |
| DOC yield (g m−2 year−1) | 1.87 | 1.8 | 1.04 |
| DIC yield (g m−2 year−1) | 91.5 | 47.7 | 1.92 |
| PN yield (g m−2 year−1) | 0.70 | 0.47 | 1.49 |
| DIN yield (mol m−2 year−1) | 0.59 | 0.13 | 4.54 |
| DIP yield (mmol m−2 year−1) | 6.29 | 5.43 | 1.16 |
| DSi yield (mol m−2 year−1) | 0.41 | 0.31 | 1.32 |
| River discharge (mean) (m3 s−1) | 389 | 128 | 3.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, J.-J.; Yang, C.-Y.; Lai, I.-J.; Li, Y.-H. Rainfall and Human Impacts on Weathering Rates and Carbon-Nutrient Yields in the Watershed of a Small Mountainous River (Kaoping) in Southwestern Taiwan. Sustainability 2020, 12, 7689. https://doi.org/10.3390/su12187689
Hung J-J, Yang C-Y, Lai I-J, Li Y-H. Rainfall and Human Impacts on Weathering Rates and Carbon-Nutrient Yields in the Watershed of a Small Mountainous River (Kaoping) in Southwestern Taiwan. Sustainability. 2020; 12(18):7689. https://doi.org/10.3390/su12187689
Chicago/Turabian StyleHung, Jia-Jang, Chun-Yi Yang, I-Jen Lai, and Yuan-Hui Li. 2020. "Rainfall and Human Impacts on Weathering Rates and Carbon-Nutrient Yields in the Watershed of a Small Mountainous River (Kaoping) in Southwestern Taiwan" Sustainability 12, no. 18: 7689. https://doi.org/10.3390/su12187689
APA StyleHung, J.-J., Yang, C.-Y., Lai, I.-J., & Li, Y.-H. (2020). Rainfall and Human Impacts on Weathering Rates and Carbon-Nutrient Yields in the Watershed of a Small Mountainous River (Kaoping) in Southwestern Taiwan. Sustainability, 12(18), 7689. https://doi.org/10.3390/su12187689





