Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review
Abstract
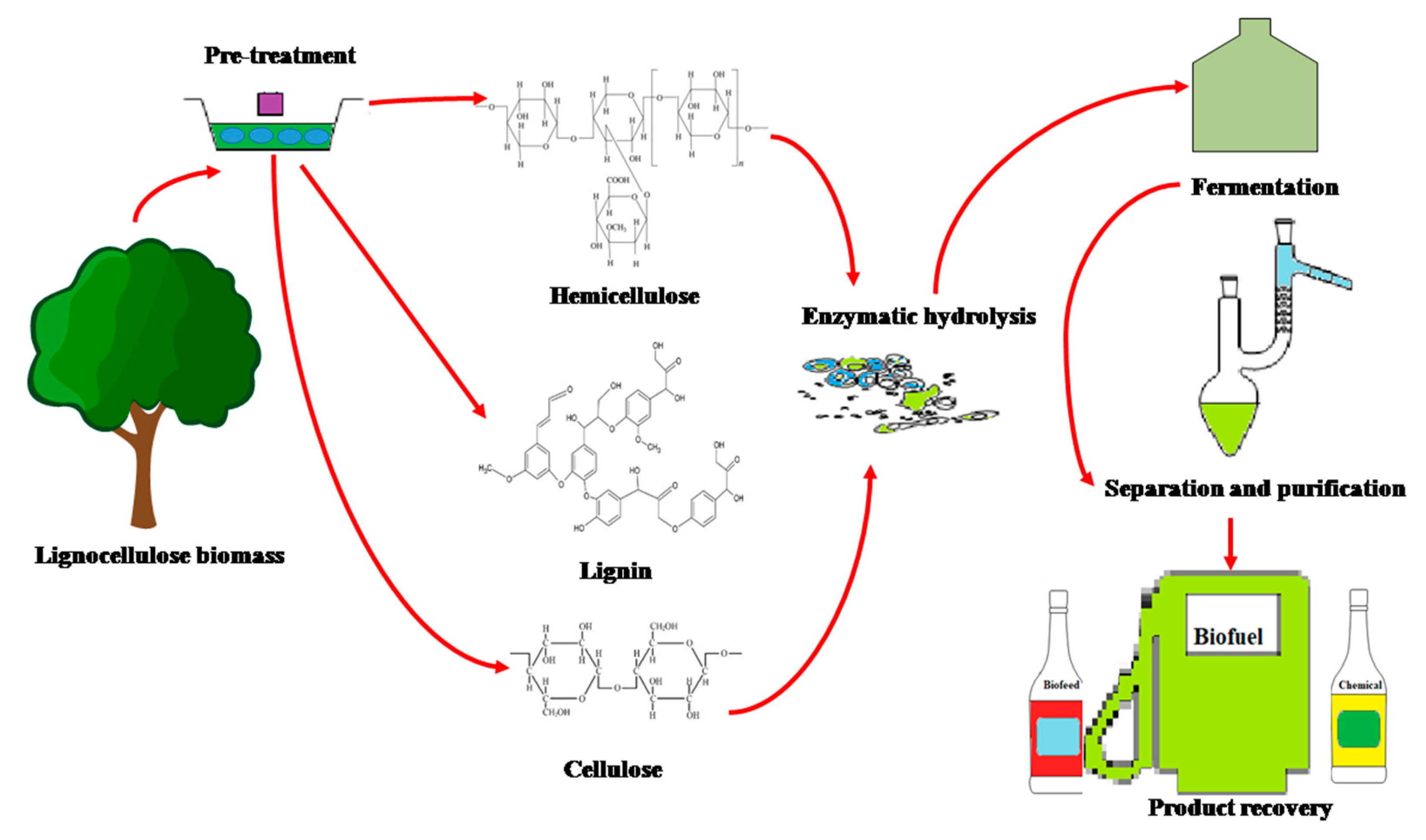
1. Introduction
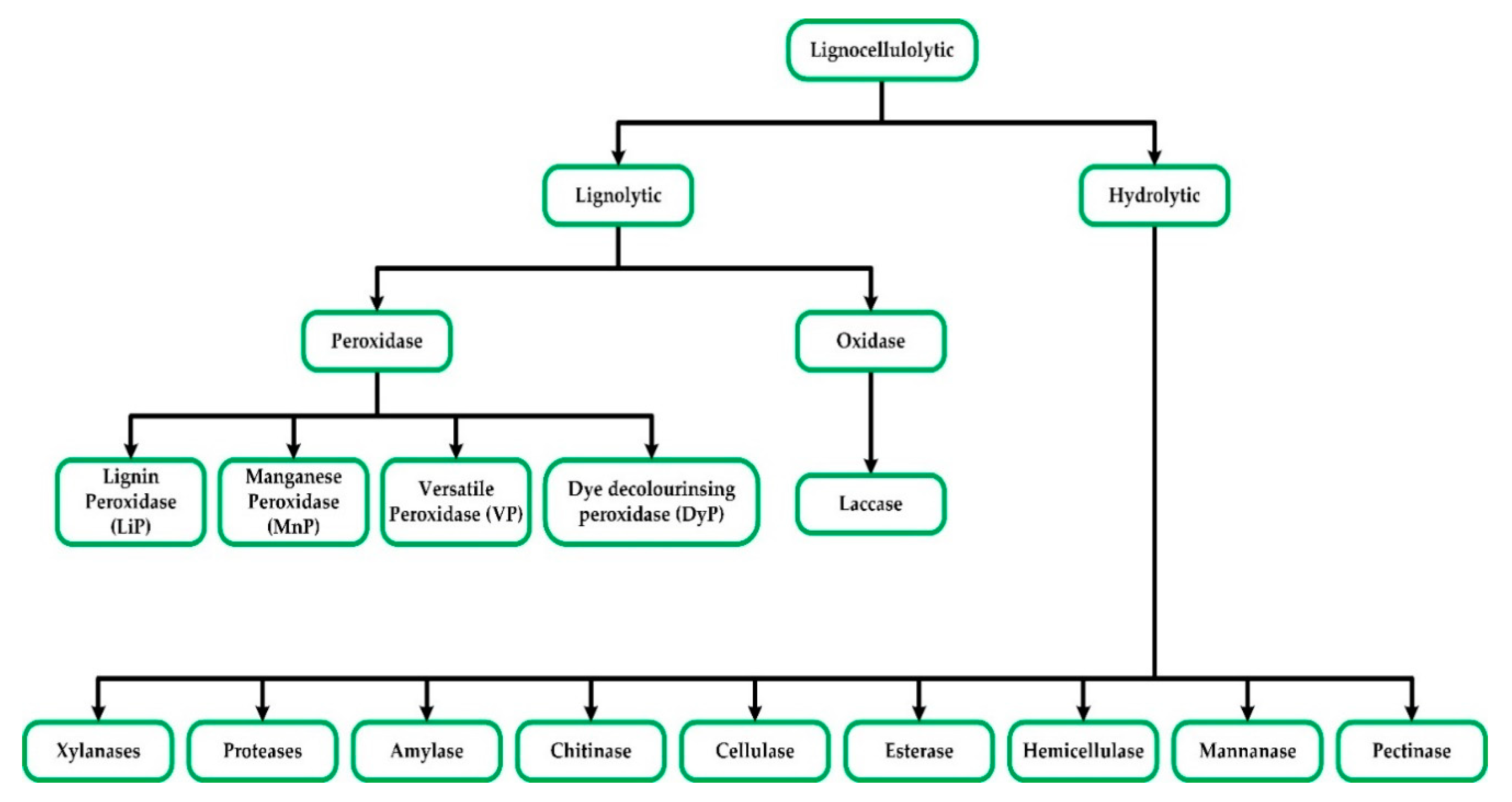
2. Lignocellulolytic Enzymes
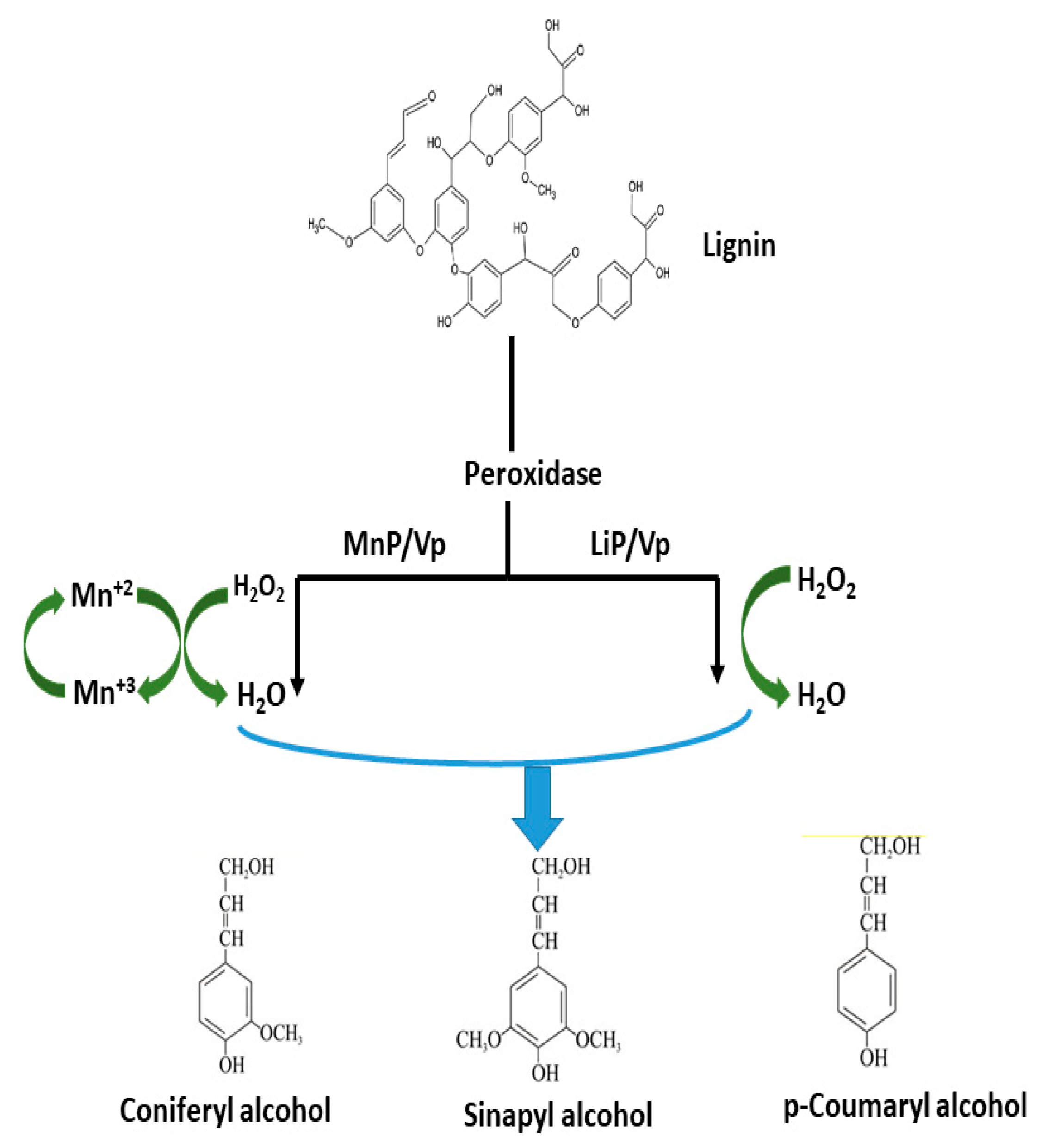
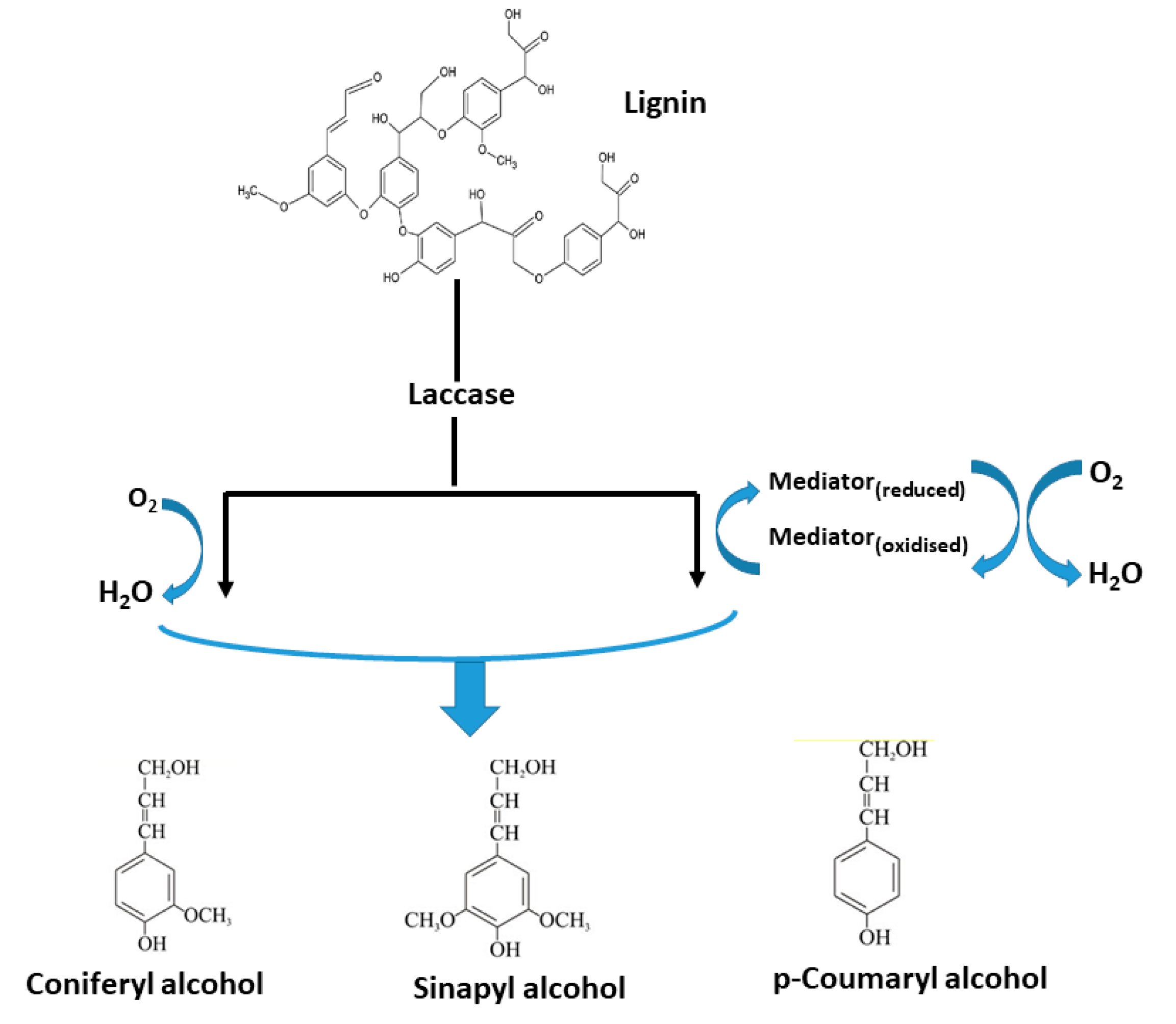
2.1. Lignin-Degraders (Ligninolytic Enzymes)
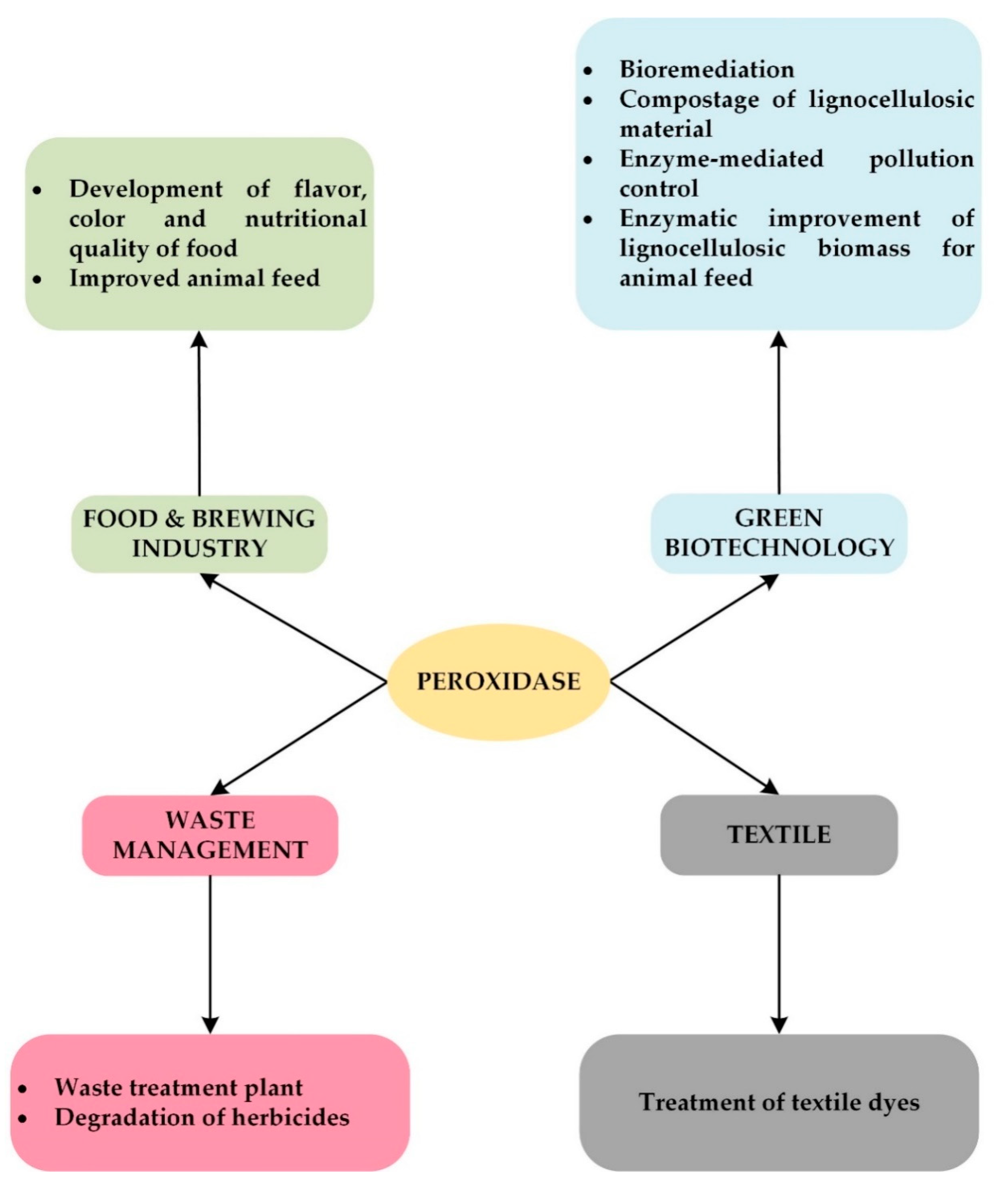
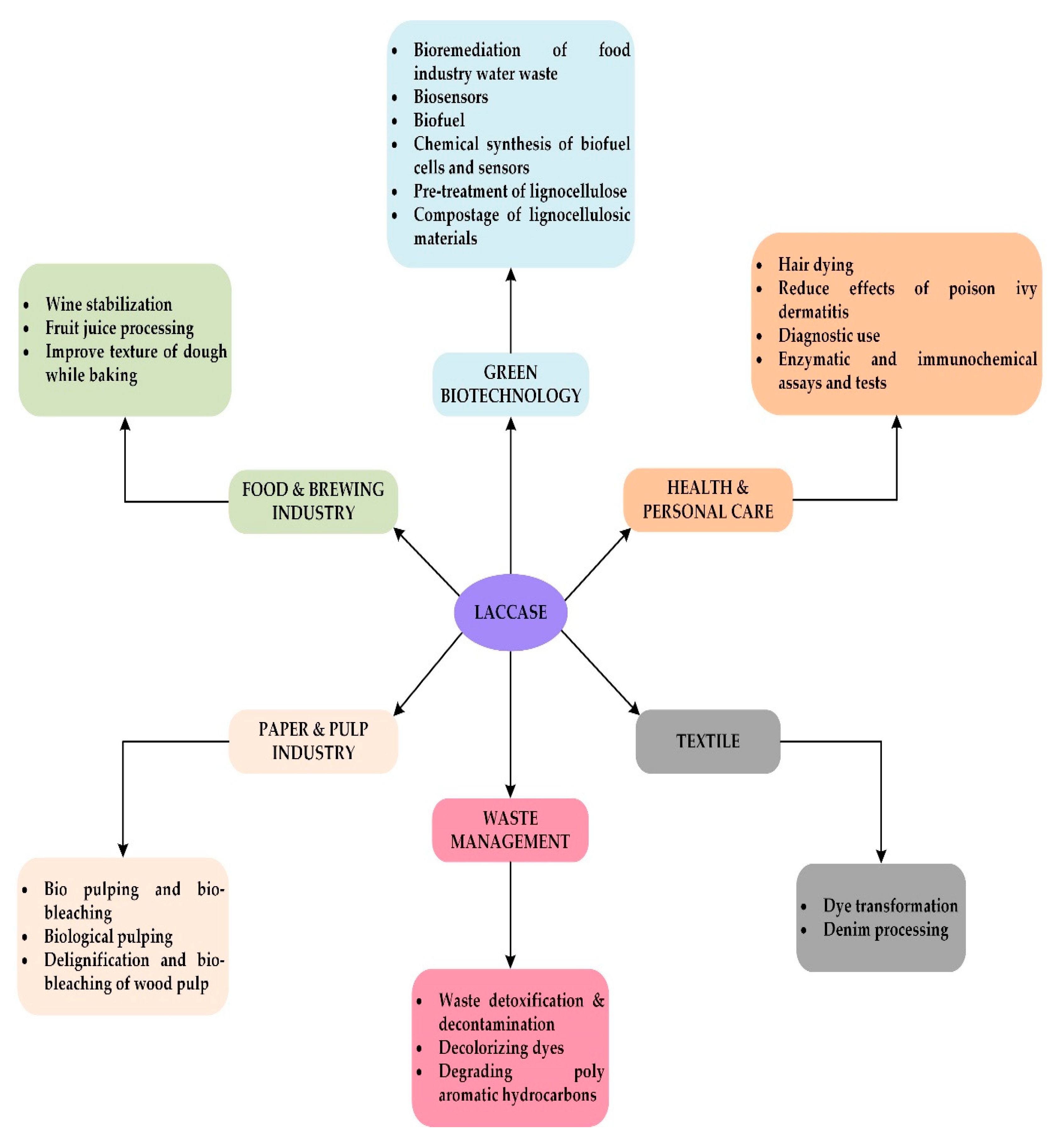
Applications of Ligninolytic Enzymes
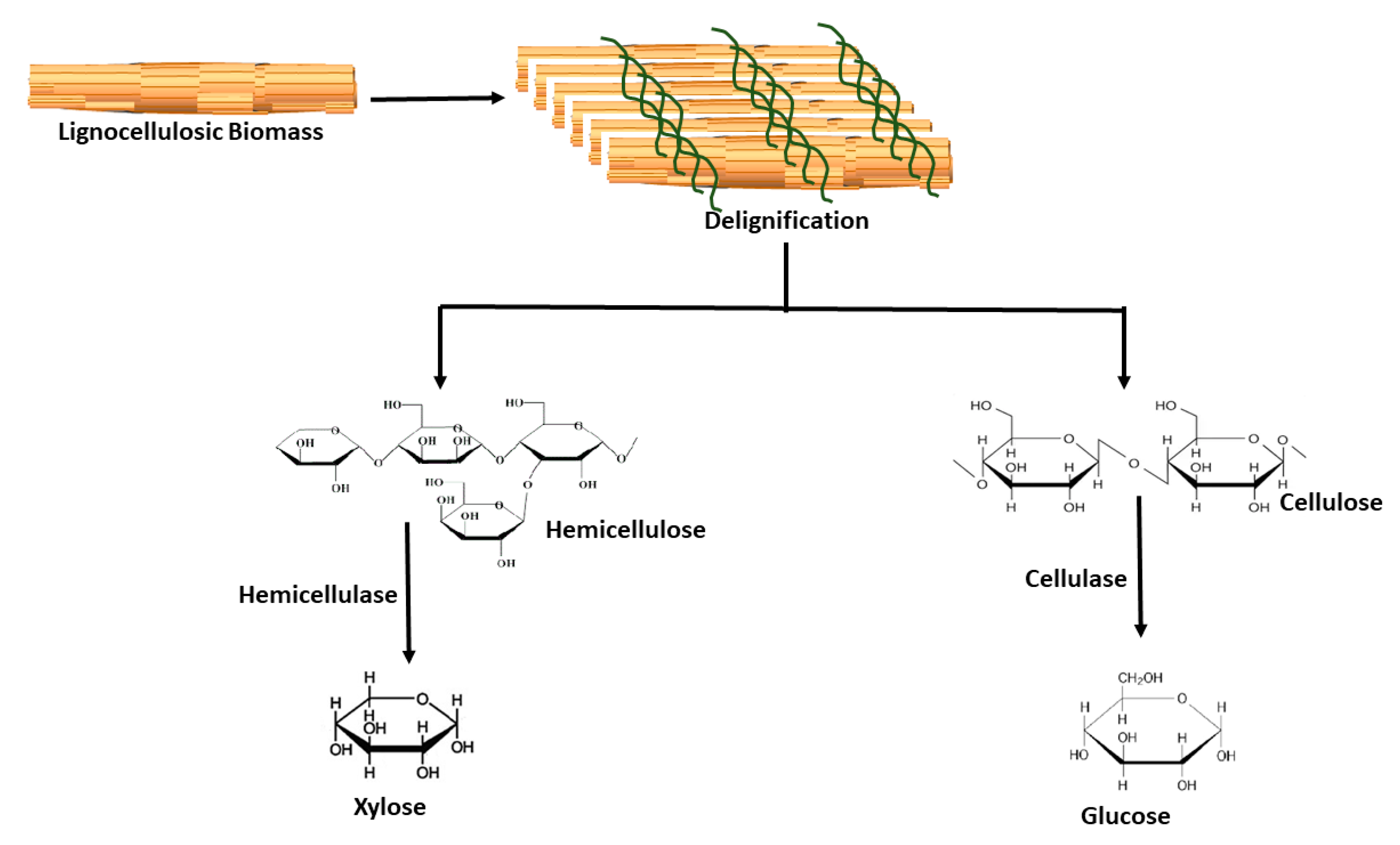
2.2. Hydrolytic Enzymes
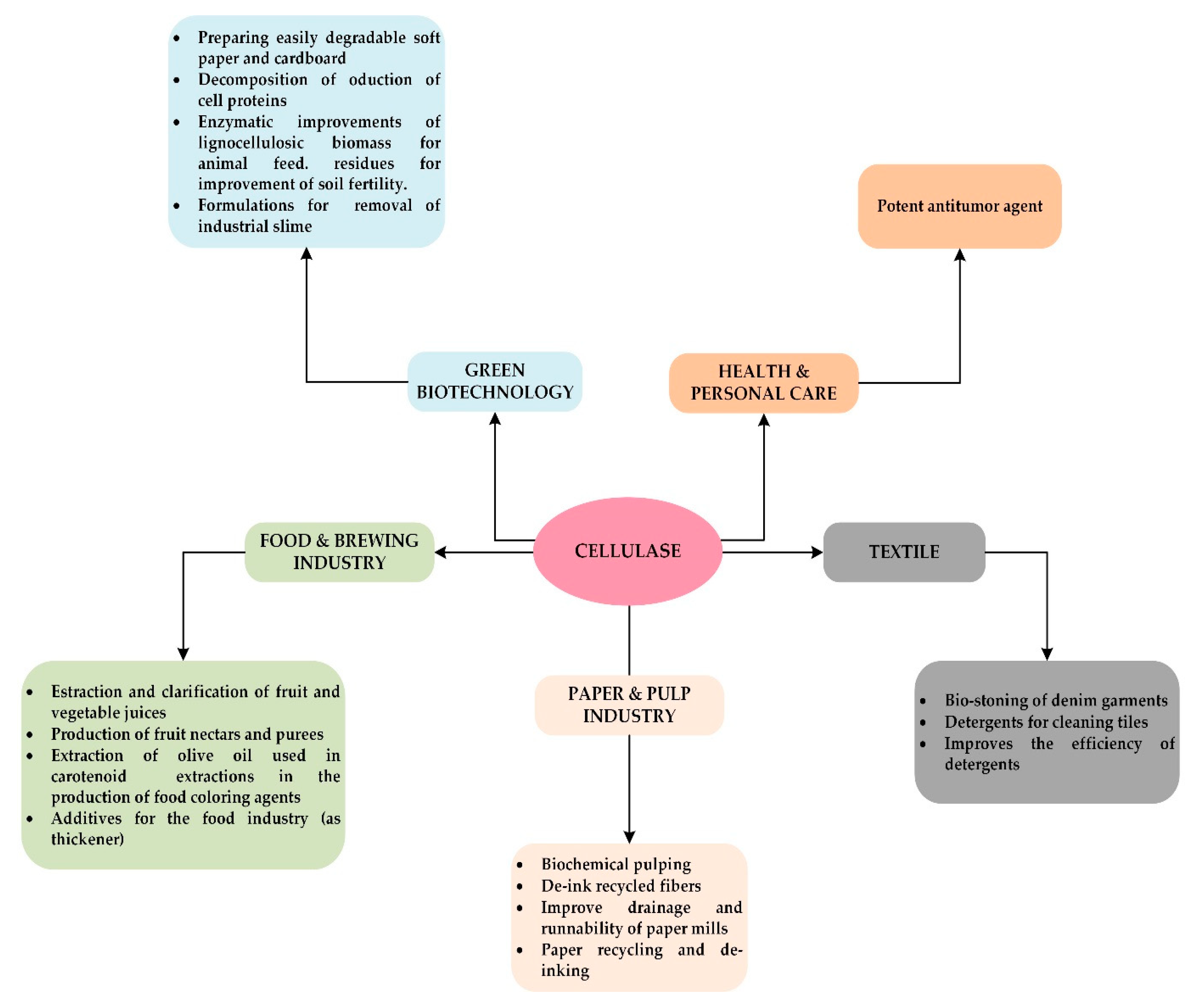
Applications of Cellulolytic Enzymes
3. Challenges Impeding the Use of Lignocellulolytic Enzymes
4. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsegaye, B.; Balomajumder, C.; Roy, P. Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: An overview and future prospect. Bull. Natl. Res. Cent. 2019, 43, 51. [Google Scholar] [CrossRef]
- Koupaie, E.H.; Dahadha, S.; Lakeh, A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Microbial cellulases Production, applications and challenges. J. Sci. Ind. Res. 2005, 64, 832–844. [Google Scholar]
- Singhania, R.R. Production of Celluloytic Enzymes for the Hydrolysis of Lignocellulosic Biomass. Biofuels 2011, 177–201. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Rahman, N.A. Food waste and food processing waste for biohydrogen production: A review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef]
- Brémond, U.; de Buyer, R.; Steyer, J.P.; Bernet, N.; Carrere, H. Biological pretreatments of biomass for improving biogas production: An overview from lab scale to full-scale. Renew. Sustain. Energy Rev. 2018, 90, 583–604. [Google Scholar] [CrossRef]
- Sokan-Adeaga, A.A.; Ana Godson, R.E.E.; Sokan-Adeaga, M.A.; Sokan-Adeaga, E.D. Lignocelluloses: An economical and ecological resource for bio-ethanol production—A review. Int. J. Nat. Res. Ecol. Manag. 2016, 1, 128–144. [Google Scholar] [CrossRef]
- Jaramillo, P.M.D.; Gomes, H.A.R.; Monclaro, A.; Silva, C.O.G.; Filho, E.X.F. Lignocellulose-Degrading Enzymes; Wiley: Hoboken, NJ, USA, 2015; pp. 73–85. [Google Scholar]
- Plácido, J.; Capareda, S. Ligninolytic enzymes: A biotechnological alternative for bioethanol production. Bioresour. Bioprocess. 2015, 2, 23. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Viikari, L. Lignocellulose Modifying Enzymes for Sustaniable Technologies. ACS Symp. Ser. 2003, 855, 30–44. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A. Microbial Enzyme Production Using Lignocellulosic Food Industry Wastes as Feedstock: A Review. Bioengineering 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Newcomb, M.; Wu, J.H.D. Cellulase, Clostridia, and Ethanol. Microbiol. Mol. Boil. Rev. 2005, 69, 124–154. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Yadav, A. Actinomycetes: A Source of Lignocellulolytic Enzymes. Enzym. Res. 2015, 2015, 279381. [Google Scholar] [CrossRef] [PubMed]
- Ezeilo, U.R.; Zakaria, I.I.; Huyop, F.; Wahab, R.A. Enzymatic breakdown of lignocellulosic biomass: The role of glycosyl hydrolases and lytic polysaccharide monooxygenases. Biotechnol. Biotechnol. Equip. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Woo, H.L.; Hazen, T.C.; Simmons, B.A.; DeAngelis, K.M. Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst. Appl. Microbiol. 2014, 37, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Tajarudin, H.A. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Sci. Total. Environ. 2020, 707, 134533. [Google Scholar] [CrossRef]
- Malherbe, S.; Cloete, T. Lignocellulose biodegradation: Fundamentals and applications. Rev. Environ. Sci. Bio/Technol. 2002, 1, 105–114. [Google Scholar] [CrossRef]
- Saadeddin, A. The complexities of hydrolytic enzymes from the termite digestive system. Crit. Rev. Biotechnol. 2012, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mtui, G.Y.S. Lignocellulolytic enzymes from tropical fungi: Types, substrates and applications. Sci. Res. Essays 2012, 7, 1544–1555. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.C. Cell Biochemistry and Function. Lehninger: Principles of Biochemistry, 4th ed.; Nelson, D.L., Cox, M.C., Eds.; W. H. Freeman & Co.: New York, NY, USA, 2004; Volume 23, p. 1119. [Google Scholar] [CrossRef]
- Karigar, C.; Rao, S.S. Role of Microbial Enzymes in the Bioremediation of Pollutants: A Review. Enzym. Res. 2011, 2011, 805187. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial Enzymes—An Overview. In Advances in Enzyme Technology; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Nawani, N.N.; Prakash, M.; Bodas, M.; Mandal, A.; Khetmalas, M.; Kapadnis, B. Actinomycetes: A Repertory of Green Catalysts with a Potential Revenue Resource. BioMed Res. Int. 2013, 2013, 264020. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M.R. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2011, 32, 172–186. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pandey, A. Production, Purification, and Application of Microbial Enzymes. Biotechnol. Microb. Enzym. 2017, 13–41. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.; Solbiati, J.O.; Cann, I.K. Insights into Lignin Degradation and its Potential Industrial Applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.R. Microbial Degradation of Lignocellulosic Biomass. Sustain. Degrad. Lignocellul. Biomass—Technol. Appl. Commer. 2013, 9, 1–13. [Google Scholar] [CrossRef]
- Vandenberghe, L.D.S.; De Carvalho, J.C.; Libardi, N.; Rodrigues, C.; Soccol, C.R. Microbial Enzyme Factories. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Vicuña, R. Ligninolysis: A Very Peculiar Microbial Process. Mol. Biotechnol. 2000, 14, 173–176. [Google Scholar] [CrossRef]
- Underkofler, L.A.; Barton, R.R.; Rennert, S.S. Microbiological process report Production of microbial enzymes and their applications. Appl. Microbiol. 1957, 6, 212–221. [Google Scholar] [CrossRef]
- Yoshida, T.; Sugano, Y. A structural and functional perspective of DyP-type peroxidase family. Arch. Biochem. Biophys. 2015, 574, 49–55. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Rahmanpour, R.; Bugg, T.D. Characterisation of Dyp-type peroxidases from Pseudomonas fluorescens Pf-5: Oxidation of Mn(II) and polymeric lignin by Dyp1B. Arch. Biochem. Biophys. 2015, 574, 93–98. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef]
- Su, C.; Lei, L.; Duan, Y.; Zhang, K.-Q.; Yang, J. Culture-independent methods for studying environmental microorganisms: Methods, application, and perspective. Appl. Microbiol. Biotechnol. 2011, 93, 993–1003. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R. New Trends in Fungal Biooxidation. In Industrial Applications; Springer: Berlin, Germany, 2010; Volume 10, pp. 425–449. [Google Scholar]
- Asina, F.; Brzonova, I.; Kozliak, E.; Kubátová, A.; Ji, Y. Microbial treatment of industrial lignin: Successes, problems and challenges. Renew. Sustain. Energy Rev. 2017, 77, 1179–1205. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Filho, E.F.; Moreira, L.R.D.S. An update on enzymatic cocktails for lignocellulose breakdown. J. Appl. Microbiol. 2018, 125, 632–645. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Techology prospecting on enzymes: Application, marketing and engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formánek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Wongwilaiwalin, S.; Rattanachomsri, U.; Laothanachareon, T.; Eurwilaichitr, L.; Igarashi, Y.; Champreda, V. Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzym. Microb. Technol. 2010, 47, 283–290. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, N. Microbial Laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Couto, S.R.; Toca-Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Nunes, C.S.; Kunamneni, A. Laccases—Properties and applications. Enzym. Hum. Anim. Nutr. 2018, 4, 133–161. [Google Scholar] [CrossRef]
- Parisutham, V.; Kim, T.H.; Lee, S.K. Feasibilities of consolidated bioprocessing microbes: From pretreatment to biofuel production. Bioresour. Technol. 2014, 161, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Wahab, A.; Bilal, M.; Iqbal, H.M. Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal. Agric. Biotechnol. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Rohr, C.O.; Levin, L.N.; Mentaberry, A.N.; Wirth, S.A. A First Insight into Pycnoporus sanguineus BAFC 2126 Transcriptome. PLoS ONE 2013, 8, e81033. [Google Scholar] [CrossRef] [PubMed]
- Prabha, M.; Ravi, V.; Swamy, N.R. Activity of Hydrolytic Enzymes in Various Regions of Normal Human Brain Tissue. Indian J. Clin. Biochem. 2012, 28, 283–291. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kaminski, M.A. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Van Dyk, J.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Arnold, W.N. Hydrolytic Enzymes. Arnold, W.N., Ed.; CRC Press: Boca Raton, FL, USA, 1987; Volume II, Chapter 12; pp. 369–399. [Google Scholar]
- Mishra, B.K.; Lata, A.K.P. Lignocellulolytic enzyme production from submerged fermentation of paddy straw. Indian J. Microbiol. 2007, 47, 176–179. [Google Scholar] [CrossRef]
- Marques, N.P.; Pereira, J.D.C.; Gomes, E.; Da Silva, R.; Araujo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.; Bocchini, D.A. Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind. Crop. Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Tiwari, R.; Nain, L.; Labrou, N.E.; Shukla, P. Bioprospecting of functional cellulases from metagenome for second generation biofuel production: A review. Crit. Rev. Microbiol. 2017, 44, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, S.; Wei, Z.; Shen, Q.; Xu, Y. Insights into high-efficiency lignocellulolytic enzyme production by Penicillium oxalicum GZ-2 induced by a complex substrate. Biotechnol. Biofuels 2014, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.T.; Cherry, J. Progress and Challenges in Enzyme Development for Biomass Utilization. Biomed. Inorg. Polym. 2007, 108, 95–120. [Google Scholar] [CrossRef]
- Nevoigt, E. Progress in Metabolic Engineering of Saccharomyces cerevisiae. Microbiol. Mol. Boil. Rev. 2008, 72, 379–412. [Google Scholar] [CrossRef]
- García-Aparicio, M.P.; Ballesteros, M.; Manzanares, P.; Ballesteros, I.; Gonzalez, A.; Negro, M.J. Xylanase contribution to the efficiency of cellulose enzymatic hydrolysis of barley straw. Appl. Biochem. Biotechnol. 2007, 137, 353–365. [Google Scholar] [CrossRef]
- Chadha, B.S.; Kaur, B.; Basotra, N.; Tsang, A.; Pandey, A. Thermostable xylanases from thermophilic fungi and bacteria: Current perspective. Bioresour. Technol. 2019, 277, 195–203. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- De Souza, P.M.; Magalhães, P.O. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.; Distel, D.L.; DuPree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Boil. 2015, 29, 108–119. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenerg. 2020, 134, 105481. [Google Scholar] [CrossRef]
- Hasunuma, T.; Okazaki, F.; Okai, N.; Hara, K.Y.; Ishii, J.; Kondo, A. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresour. Technol. 2013, 135, 513–522. [Google Scholar] [CrossRef]
- Tuncer, M.; Rob, A.; Ball, A.S.; Eady, R.R.; Henderson, N.; Wilson, M.T. Optimisation of production of extracellular nonhaem peroxidases by Thermomonospora fusca BD25 in aerobic bio-reactor conditions. Biochem. Soc. Trans. 1997, 25, 65S. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M. Biomass Availability, Potential and Characteristics. In Lecture Notes in Energy; 57; Rabaçal, M., Ferreira, A., Silva, C., Costa, M., Eds.; Springer: Berlin, Germany, 2017; Chapter 2. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- Zhang, K.; Pei., Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of Cellulose-Degrading Bacteria and Determination of Their Cellulolytic Potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.D.S.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenerg. 2020, 132, 105419. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Rosero-Henao, J.C.; Bueno, B.E.; De Souza, R.; Ribeiro, R.; De Oliveira, A.L.; Gomide, C.A.; Gomes, T.M.; Tommaso, G. Potential benefits of near critical and supercritical pre-treatment of lignocellulosic biomass towards anaerobic digestion. Waste Manag. Res. 2018, 37, 74–82. [Google Scholar] [CrossRef]
- Galante, Y.; Formantici, C. Enzyme Applications in Detergency and in Manufacturing Industries. Curr. Org. Chem. 2003, 7, 1399–1422. [Google Scholar] [CrossRef]
- Chambers, J. Hydrolytic Enzymes. Biochem. Labfax 1993, 93, 145–165. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.; Deswal, D.; Sharma, S.; Bhattacharya, A.; Jain, K.K.; Kaur, A.; Pletschke, B.; Singh, A.; Karp, M. Revisiting cellulase production and redefining current strategies based on major challenges. Renew. Sustain. Energy Rev. 2016, 55, 249–272. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Euverink, G.J.W. Consolidated briefing of biochemical ethanol production from lignocellulosic biomass. Electron. J. Biotechnol. 2016, 23, 44–53. [Google Scholar] [CrossRef]
- Hüttner, S.; Nguyen, T.T.; Granchi, Z.; Chin-A-Woeng, T.; Ahrén, D.; Larsbrink, J.; Thanh, V.N.; Olsson, L. Combined genome and transcriptome sequencing to investigate the plant cell wall degrading enzyme system in the thermophilic fungus Malbranchea cinnamomea. Biotechnol. Biofuels 2017, 10, 265. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.-X.; Tu, R. Unexpectedly high bacterial diversity in decaying wood of a conifer as revealed by a molecular method. Int. Biodeterior. Biodegrad. 2008, 62, 471–474. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Algora, C.; Baldrian, P. Lignocellulolytic systems of soil bacteria: A vast and diverse toolbox for biotechnological conversion processes. Biotechnol. Adv. 2019, 37, 107374. [Google Scholar] [CrossRef]
- Maeda, K.; Hanajima, D.; Toyoda, S.; Yoshida, N.; Morioka, R.; Osada, T. Microbiology of nitrogen cycle in animal manure compost. Microb. Biotechnol. 2011, 4, 700–709. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Rinta-Kanto, J.M.; Sun, S.; Sharma, S.; Poretsky, R.; Moran, M.A. Transcriptomic analysis of a marine bacterial community enriched with dimethylsulfoniopropionate. ISME J. 2010, 4, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Naz, S. Study of Ligninolytic Bacteria Isolation and Characterization from Kuthrel Agro Field of Bhilai-Durg Region. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 141–150. [Google Scholar] [CrossRef]
- Khan, P.A.; Wuyep, U.; Nok, J. Production and regulation of lignin degrading enzymes from Lentinus squarrosulus (mont.) Singer and Psathyrella atroumbonata Pegler. Afr. J. Biotechnol. 2003, 2, 444–447. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating biowaste into circular bioeconomy: A critical review of current trend and scaling up feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.-S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; Milessi, T.S.S.; Antunes, F.A.F.; Freitas, W.L.D.C.; Felipe, M.D.G.A.; Da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Production of hydrogen and methane from lignocellulose waste by fermentation. A review of chemical pretreatment for enhancing the efficiency of the digestion process. J. Clean. Prod. 2020, 267, 267. [Google Scholar] [CrossRef]
- Moodley, P.; Sewsynker-Sukai, Y.; Kana, E.G. Progress in the development of alkali and metal salt catalysed lignocellulosic pretreatment regimes: Potential for bioethanol production. Bioresour. Technol. 2020, 310, 123372. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.U.; Taherzadeh, M.J. Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour. Technol. 2020, 304, 122998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-Y.; Li, M.-C.; Zhu, X.-L.; Fan, F.; Wang, L.-L.; Ma, J. Microbial synthesized biodegradable PHBHHxPEG hybrid copolymer as an efficient intracellular delivery nanocarrier for kinase inhibitor. BMC Biotechnol. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Ping, Y.; Bai, Y.; Luo, H.; Huang, H.; Yao, B. Engineering of a Bacillus amyloliquefaciens Strain with High Neutral Protease Producing Capacity and Optimization of Its Fermentation Conditions. PLoS ONE 2016, 11, e0146373. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lin, A.; Zhang, G.; Zhang, J.; Chen, Y.; Shen, T.; Zhao, J.; Wei, D.; Wang, W. Enhancement of cellulase production in Trichoderma reesei RUT-C30 by comparative genomic screening. Microb. Cell Factories 2019, 18, 81. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, H.; Li, D.; Zhang, H.; Wang, H.; Lu, F. Optimized expression and enhanced production of alkaline protease by genetically modified Bacillus licheniformis 2709. Microb. Cell Factories 2020, 19, 1–13. [Google Scholar] [CrossRef]
| Enzyme | pH | Optimum Temperature | Active Sites | Mode of Action | Fungi | Bacteria | Remarks | References |
|---|---|---|---|---|---|---|---|---|
| Peroxidase (EC number 1.11.1.7) (Molecular weight 55 kDA) (CAS number 90663-99-0) | --- | --- | --- | --- | Trametes versicolor, Coniochaeta ligniaria | --- | Review on using lignocellulosic waste to produce lignocellulose-degrading enzymes | [15] |
| --- | --- | --- | --- | Thanatephorus sp., Auricularia sp., Pleurotus ostreatus, Umbelopsis isabellina, Penicillium geastrovirus | Escherichia coli, Bacillus sp., Pseudomonas sp. | Overview of some important microbial enzymes, their mode of action, and producing organisms | [26] | |
| --- | --- | Heme cofactor | Oxidizes substrates in a hydrogen peroxide dependent manner | Basidiomycetes | Thermobifida sp., Rhodococcus sp., Streptomyces sp., Shewanella sp., Bacillus sp., Staphylococcus | Review on DyP-type peroxidases | [39] | |
| --- | --- | --- | --- | --- | Saccharomonospora sp., Thermomonospora sp., Amycolatopsis sp., Pseudomonas putida, Bacillus sp. | Bacterial enzymes involved in delignification | [40] | |
| --- | --- | --- | --- | --- | Pseudomonas fluorescens | Use of Psedomonas to successfully produce peroxide which was able to breakdown lignin | [41] | |
| 4 | 24–55 °C | Heme-containing; need hydrogen peroxide as oxidant | --- | Phanerochaete chrysosporium, Ceriporiopsis subvermispora, Ceriporia lacerata, Cyathus stercolerus, Pycnoporus cinnarbarinus, Pleurotus ostreaus, Phlebia subserialis, Pleurotus streatus, Postia placenta, Gloeophyllum trabeum, and Echindodontium taxodi | --- | Current trends in pre-treating lignocellose to derive useful products | [42] | |
| --- | --- | --- | --- | --- | Pseudomonads sp., Rhodococci sp., Bacillus subtilis, Amycolatopsis sp. | Positives and downsides of biological methods of breaking down lignin into monomers | [43] | |
| 7 | --- | --- | --- | --- | --- | Best environment for culturing and studying lignocellulose degraders from the environment | [44] | |
| Laccase (EC number 1.10.3.2) (Molecular weight 60–100 kDA) (CAS number 80498-15-3) | 6 | 30 °C | --- | --- | Trametes versicolor, Coniochaeta ligniaria Cerrena unicolor | --- | Review on using lignocellulosic waste to produce lignocellulose-degrading enzyme | [15] |
| --- | --- | --- | Oxidize phenolic compounds/reduce molecular oxygen to water | Pleurotus florida, P. ostreatus, P. pulmonarius, P. tailandia, Trametes sp., Coriolopsis sp., Grifola sp., Lentinula sp. | --- | Overview of some important microbial enzymes, their mode of action, and producing organisms | [26] | |
| 7.4 | --- | Multi- copper centers | --- | T. versicolor, Trametes hirsuta, Trametes ochracea, Trametes villosa, Trametes gallica, Cerrena maxima, P. radiata, C. subvermispora, and P. eryngii | Azospirillum lipoferum, Bacillus subtilis, Streptomyces cyaneus, Streptomyces lavendulae, Streptomyces griseus, Streptomyces coelicolor, and Thermus thermophilus | Analysis on lignin breakdown and the potential applications in industry | [34] | |
| --- | --- | Multi-copper oxidases | Use a cluster of four copper ions for oxidation; generate water as byproduct | --- | Streptomyces sp., Bacillus sp., Thermus thermophilius | Bacterial enzymes involved in delignification | [40] | |
| --- | --- | --- | --- | Trametes versicolor, Tremetes trogii, Phlebia floridensis | Citrobacter spp., Straphylococcus saprophlticus, Bacillus subtilis | Current trends in pre-treating lignocellose to derive useful products | [42] | |
| --- | --- | Blue copper oxidases; four copper ions in the active site | Use a cluster of four copper ions for oxidation; generate water as byproduct | Trametes trogii, T. ochracea, T. villosa, Cerrena maxima, Pycnoporus cinnabarinus, P. sanguineus, T. versicolor, T. villosa, Myceliophthora thermo- phila | NA | Recent processes in fungal biooxidation | [45] | |
| 5 | 30–35 °C | --- | --- | Phanerochaete chrysosporium, Chrysonilia sitophila, and Botryosphaeria sp. | Sphingomonas paucimobilis | Successes and challenges with using microbial methods for biodegradation of lignin | [46] | |
| --- | --- | Blue multicopper oxidases | --- | Basidiomycota species | A. lipoferum, Bacillus subtilis, S. lavendulae, Sinorhizobium meliloti | [47] |
| Enzyme | pH | Optimum Temperature | Mode of Fermentation | Fungi | Bacteria | Remarks | References |
|---|---|---|---|---|---|---|---|
| Cellulase (EC number 3.2.1.4) (Molecular weight 23.5–52 kDA) (CAS number 9012-54-8) (Iso electric point 4.5–7.2) | --- | 30 °C | SMF, SSF | Aspergillus niger Trichoderma viride | --- | Review on using lignocellulosic waste to produce lignocellulose-degrading enzyme | [15] |
| 6–7 | 40–55 °C | --- | --- | Cellulomonas fimi, Microbispora bispora, and Thermobifida fusca | Actinomycetes as a source of lignocelluloses degraders | [17] | |
| --- | 50 °C | --- | Trichoderma reesei and Trichoderma viride | --- | Use of enzymes to improve yield of biogas | [33] | |
| 3.2–11.2 | 30–50 °C | SSF, SHF | Actinomucor, A. niger, Trichoderma viride | Agaricus arvensis | Synergy between hemicellulases leading to increased effectiveness of cellulase in cellulose degradation | [62] | |
| --- | --- | --- | Aspergillus Protuberus, A. ellipticus, A. fumigatus, A. niger, Trichoderma viride, T. asperellum, Penicillium echinulatum, Rhizopus oryzae, Myceliophthora thermophila Trichoderma reesei, Humicola, | --- | Characteristics of hydrolytic enzymes | [64] | |
| --- | --- | SMF | Phanerochaete chrysosporium | Cellulomonas spp., Cytophaga spp. | Synergistic action of cellulolytic and lignolytic enzymes | [65] | |
| 5–9 | 30–80 °C | SSF | Botryosphaeria sp., Saccharicola sp. | --- | Endophytic fungi are potential producers of cellulases and xylanases by SSF when using lignocellulosic materials | [66] | |
| --- | 25–90 °C | --- | Aspergillus Protuberus, A. ellipticus, A. fumigatus, A. niger, Trichoderma viride, T. asperellum, Penicillium echinulatum, Rhizopus oryzae, Myceliophthora thermophila Trichoderma reesei | Vibrio, Cellvibrio, Plesiocystis, Prevotella, Clostridium, Sorangium, Micromonospora, Klebsiella, Rhodothermus, Dysgonomonas, Fibrobacter, Bacillus, Rhizobium, Enterobactercance, Cellulomonas | Using metagenomics to bioprospect for cellulases | [67] | |
| Chitinase (EC number 3.2.1.14) (Molecular weight 20–90 kDA) (CAS number 9001-06-3) (Iso electric point 4.5–8.5) | 3–7 | 30–60 °C | SSF | Trichoderma, Penicillium, Neurospora, Mucor, Lycoperdon, Aspergillus, Conidiobolous, Stachybotrys | Serratia, Chromobacterium, Bacillus, Pseudomonas, Clostridium, Arthobacter, and Aeromonas | Producing microbial enzymes | [38] |
| --- | --- | --- | Aspergillus Protuberus, A. ellipticus, A. fumigatus, A. niger, Trichoderma viride, T. asperellum, Penicillium echinulatum, Rhizopus oryzae, Myceliophthora thermophila Trichoderma reesei, Humicola, | --- | Characteristics of hydrolytic enzymes | [64] | |
| --- | --- | --- | Penicillium oxalicum | --- | Production and characterization of cellulolytic enzymes from Penicillium oxalicum | [68] | |
| --- | --- | --- | T. reesei, Thielavia terrestris | --- | Progress in enzyme development | [69] | |
| --- | --- | --- | Saccharomyces cerevisiae | --- | Use of Saccharomyces cerevisiae in metabolic engineering for enzyme production | [70] | |
| Xylanase (EC number 3.2.1.8) (Molecular weight 29.8 kDA) (CAS number 9025-57-4) (Iso electric point 3–10) | 4–11 | 30–60 °C | SSF | Aspergillus, Fusarium, Penicillium, Geotrichum, Paecilomyces, Cephalosporium, and Trichoderma | Bacillus sp. (B. circulans, B. stearothermophilus, B. polymyxa, B. subtilis, and B. amyloliquifaciens), Arthrobacter, Cellulomonas, Micrococcus, Paenibcillus, Staphylococcus, Thermotoga, Microbacterium, Pseudoxanthomonas, and Rhodococcus | [61] | |
| --- | 90 °C | --- | Aspergillus sp. Phanerochaete chrysosporium, Phlebia radiate, Dichmitus squalens, Rigidosporus lignosus, and Jungua separabilima | Tetraselmis suecica, Cellulomonas, Thermobifida, Clostridium, Ruminococus | Xylanase inclusion to enzymatic mixture increases the efficiency of cellulose hydrolysis | [61] | |
| 4–11 | 30–60 °C | SSF | Aspergillus, Fusarium, Penicillium, Geotrichum, Paecilomyces, Cephalosporium, and Trichoderma | Bacillus sp. Arthrobacter, Cellulomonas, Micrococcus, Paenibcillus, Staphylococcus, Thermotoga, Microbacterium, Pseudoxanthomonas, and Rhodococcus | Synergistic interactions between lignocellulolytic enzymes | [62] | |
| --- | --- | SMF | Phanerochaete chrysosporium | Cellulomonas spp., Cytophaga spp. | Synergistic action of cellulolytic and lignolytic enzymes | [65] | |
| 5–9 | 30–80 °C | SSF | Botryosphaeria sp., Saccharicola sp. | --- | Endophytic fungi are potential producers of cellulases and xylanases by SSF when using lignocellulosic materials | [66] | |
| 4.8 | 50 °C | SMF | --- | --- | Xylanase improving the efficiency of enzymatic hydrolysis | [71] | |
| --- | 50 °C | ---- | --- | Geobacillus spp. Thermopolyspora flexuosa, Caldicellulosiruptor | Reviews diverse thermophilic fungi and bacteria that produce xylanases and the recent methods used in discovering them. | [72] | |
| Amylase (EC number 3.2.1.1) (Molecular weight 51.0–54.0 KDA) (CAS number 9000-90-2) (Iso electric point 3.25 to 10.1) | --- | --- | SMF | --- | Bacillus subtilis, | Review on using lignocellulosic waste to produce lignocellulose-degrading enzyme | [15] |
| 4.8 | 50 °C | SMF | --- | --- | Improving the efficiency of enzymatic hydrolysis | [73] | |
| --- | 28–50 °C | SSF, SHF, SMF | Fusarium oxysporum, Trichoderma reesei, Paecilomyces variotii, Aspergillus oryzae | Clostridium thermocellum, C. phytofermentans, C. cellulolyticum, Thermoanaerobacterium saccharolyticum, Caldicellulosiruptor bescii, Escherichia coli, Zymomonas mobilis | Process of second-generation bioethanol production | [74] | |
| 5.0–7.0 | 45–50 °C | SMF, SSF | Aspergillus oryzae, A. niger, A. awamori, A. fumigates, A. kawachii, and A. flavus and Penicillium sp.; Penicillium brunneum, P. fellutanum, P. expansum, P. chrysogenum, P. roqueforti, P. janthinellum, P. camemberti, and P. olsonii | B. amyloliquefaciens, B. cereus, B. coagulans, B. licheniformis, B. polymyxa, B. subtilis, B. stearothermophilus, B. mesentericus, B. vulgaris, B. megaterium, B. halodurans, Caldimonas taiwanensis, Chromohalobacter sp., Corynebacterium gigantean, Geobacillus thermoleovorans, Lactobacillus ferment, and Lactobacillus manihotivorans; Bacillus dipsosauri, Halobacillus sp., Haloarcula hispanica, Chromohalobacter sp., and Halomonas meridian | A review on microbial amylases | [75] | |
| Esterase (EC number 3.1.1.73) (Molecular weight 40 kDA) (CAS number 9026-00-0) (Iso electric point 5) | --- | --- | --- | Trichoderma reesei, Dictyostelium discoideum, Chaetomium thermophilum, Coniophora puteana, Daphnia pulex, and Limnoria quadripunctata | Bacteriodetes, Firmicutes, Spirochaetes, Proteobacteria, and Elusibacteria | Microbial family tree showing enzyme ability | [76] |
| 3–9 | 30 °C | SSF | A. niger, A. oryzae, A. awamori, Neurospora crassa | --- | Insights to enzymatic degradation of lignocellulose | [77] | |
| Hemicellulase (EC number 3.2.1.8) (Molecular weight 132kDA) (CAS number 9025-56-3) (Iso electric point 8) | 5.5 | 55 °C | SSF | Aspergillus terreus | Bacillus amyloliquefaciens, B. subtilis; Dictyoglomus thermophilum | Review on using lignocellulosic waste to produce lignocellulose-degrading enzyme | [15] |
| --- | --- | --- | Trichoderma and Humicola | Bacillus sp. | Role of enzymes in bioremediation | [25] | |
| --- | 5–35 °C | --- | Aspergillus and Trichoderma | Cellulomonas sp., Cellvibrio sp., Microbispora sp., Thermomonospora sp., Clostridium sp., and Ruminococcus sp. | Overview of some important microbial enzymes, their mode of action, and producing organisms | [26] | |
| --- | --- | SMF | Phanerochaete chrysosporium | Cellulomonas spp., Cytophaga spp. | Synergistic action of cellulolytic and lignolytic enzymes | [65] | |
| 5–9 | 30–80 °C | SSF | Botryosphaeria sp., Saccharicola sp. | --- | Endophytic fungi are potential producers of cellulases and xylanases by SSF when using lignocellulosic materials | [66] | |
| 5.5 | 55 °C | SSF | Aspergillus terreus | Bacillus amyloliquefaciens, B. subtilis. Dictyoglomus thermophilum | Review on using lignocellulosic waste to produce lignocellulose-degrading enzyme | [77] | |
| Mannase (EC number 3.2.1.78) (Molecular weight 49 kDA) (CAS number 37288-54-3) (Iso electric point 2.5–5) | --- | --- | --- | --- | Cellulomonas fimi, Clostridium thermocellum | Enzymatic hydrolysis of lignocellulose is extensively reviewed | [18] |
| 6.5 | 30 °C | SSF | A. niger Trichoderma harzianum Polyporus versicolor, Penicillium purpurogenum, Thielavia terrestris, Aspergillus tamarii, and Aspergillus niger | --- | Fungal sources of lignocellulolytic enzymes | [23] | |
| --- | --- | --- | A. niger Trichoderma | Aeromonas hydrophila Bacillus sp., Enterococcus casseliflavus and Streptomyces | Green catalysts and their future potentials | [28] | |
| --- | --- | --- | A. niger Trichoderma | Bacillus sp., Streptomyces | Promising technologies for pre-treatment of lignocellulosic biomass | [29] | |
| Protease (EC number 3.4.21.112) (Molecular weight 15 to 30 kDA) (CAS number 9001-92-7) (Iso electric point 9–10) | 4.0–7.0 | 28–60 °C | SF, SMF | Aspergillus sp. Aspergillus flavus, A. oryzae, [A. sojae], and A. niger Entomophthora coronate, Cladosporium herbarum, F. solani, and P. chrysogenum | Bacillus sp., including B. subtilis, B. firmus, B. licheniformis B. subtilis [Lactobacillus acidophilus], Lactobacillus delbrueckii Microbacterium sp., Pseudomonas stutzeri, and Engyodontium album | Application of enzymes | [75] |
| Pectinase (EC number 3.2.1.15) (Molecular weight 66 kDA) (CAS number 9032-75-1) (Iso electric point 6) | --- | --- | --- | --- | Clostridium phytofermentans, E. coli | Consolidated bioprocessing technology | [78] |
| 4.0–7.0 | 30–45 °C | SSF, SF | --- | Thermomonospora fusca | Optimizing enzyme production using a thermophile | [79] |
| Pre-treatment Method | Limitation | Cost | Advantages | Products | Procedure | References |
|---|---|---|---|---|---|---|
| Physico-chemical | High cost of equipment, low yields | Low–high | Efficient lignin removal, high retention of hemicellulose and cellulose | Bioethanol | Steam explosion, microwave-chemical, liquid hot water, hydrothermal liquefaction, ammonia fiber explosion, and recycle percolation | [102] |
| Higher power consumption than inherent biomass energy | High | Effective in reducing cellulose crystallinity | Heat, power, alcohol, olefins, gasoline, diesel, methane, oils, specialty chemicals, hydrogen | Hydrothermal, combustion, gasification, liquefaction, pyrolysis | [103] | |
| Difficult to operate, with complex processes. | --- | Efficient lignin removal and reduced generation of inhibitors, environmentally friendly | Ethanol | Steam explosion or hydrothermal, ammonia fiber explosion, CO2 explosion, hot water | [104] | |
| Chemical | High cost, corrosive in nature | High | High delignification rate, good yields | Hydrogen, methane | Acidic, alkaline, oxidative, ionic-liquid, and carbon-dioxide | [105] |
| Toxic by-product, cost of process. | Medium | Least selective in terms of substrate, ease of adoption on an industrial scale, effective removal of lignin | Bioethanol | Alkali and metal salts | [106] | |
| Physical | High amounts of operation and energy costs, complexity of the system, inadequate delignification | Low–high | Easy to operate, can be combined or used with other methods to maximize results | Bioethanol and biogas | Milling, extrusion, freezing, microwave, grinding. | [107] |
| Biological | Time consuming and requires close monitoring, difficult to scale up for industrial processes, low efficiency, considerable loss of carbohydrates | Low–medium | Low energy input, no chemical application, low cost | Biofuels such as ethanol, hydrogen, and methane; biomaterials such as enzymes, lactates, acetates, and organic acids | Microorganisms, enzymes | [107] |
| Slow reaction rate, enzyme price | --- | Increased yields, environmentally friendly, mild operating conditions | Methane | Fungi, microbial consortium, and enzymes | [108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability 2020, 12, 7282. https://doi.org/10.3390/su12187282
Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability. 2020; 12(18):7282. https://doi.org/10.3390/su12187282
Chicago/Turabian StyleChukwuma, Ogechukwu Bose, Mohd Rafatullah, Husnul Azan Tajarudin, and Norli Ismail. 2020. "Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review" Sustainability 12, no. 18: 7282. https://doi.org/10.3390/su12187282
APA StyleChukwuma, O. B., Rafatullah, M., Tajarudin, H. A., & Ismail, N. (2020). Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability, 12(18), 7282. https://doi.org/10.3390/su12187282







