Preliminary Studies on Fungal Contamination of Two Rupestrian Churches from Matera (Southern Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sampling
2.2. Fungi Isolation and Morphological Analyses
2.3. Molecular Analyses
2.3.1. Genomic DNA Isolation
2.3.2. PCR Amplification, Sequencing and Sequences Analyses
2.4. Phylogenetic Investigations
3. Results
3.1. Fungi Isolation and Morphological Characterization
3.2. Molecular Identification of the Fungal Species
3.3. Phylogenetic Identification of the Fungal Species
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Scheerer, S.; Ortega-Morales, B.O.; Gaylarde, C.C. Chapter 5 Microbial Deterioration of Stone Monuments—An Updated Overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Karbowska-Berent, J. Microbiodeterioration of mural paintings: A review. In Art, Biology and Conservation: Biodeterioration of Works of Art; Koestler, R.J., Koestler, V.H., Charola, A.E., Nieto-Fernanadez, F.E., Eds.; Metropolitan Museum of Art: New York, NY, USA, 2003; pp. 266–302. [Google Scholar]
- Saarela, M.; Alakomi, H.-L.; Suihko, M.-L.; Maunuksela, L.; Raaska, L.; Mattila-Sandholm, T. Heterotrophic microorganisms in air and biofilm smaples from Roman catacombs, with special emphasis on actinobacteria and fungi. Int. Biodeterior. Biodegrad. 2004, 54, 27–37. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. Microbiological and environmental issues in show caves. World J. Microbiol. Biotechnol. 2012, 28, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.E.C.; Ramos, A.P.; Sánchez, J.I.C.; Serrano, M.E.D. Origin and Control Strategies of Biofilms in the Cultural Heritage. In Antimicrobials, Antibiotric Resistance, Antibiofilm Strategies and Activity Methods; Kirmusaoğlu, S., Ed.; Intech Open Science: London, UK, 2018; Available online: https://www.intehopen.com/books/antimicrobials-antibiotic-resistance-antibiofilm-strategies-and-activity-methods/origin-and-control-strategies-of-biofilms-in-the-cultural-heritage (accessed on 16 May 2020). [CrossRef]
- Ponizovskaya, V.B.; Rebrikova, N.L.; Kachalkin, A.V.; Antropova, A.B.; Bilanenko, E.N.; Mokeeva, V.L. Micromycetes as colonizers of mineral building materials in historic monuments and museums. Fungal Biol. 2019, 123, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, J. Building Mycology: Management of Decay and Health in Buildings; Chapman & Hall: London, UK, 1994; pp. 1–269. [Google Scholar]
- Bornehag, C.G.; Blomquist, G.; Gyntelberg, F.; Järvholm, B.; Malmberg, P.; Nordvall, L.; Nielsen, A.; Pershagen, G.; Sundell, J. Dampness in Buildings and Health. Nordic Interdisciplinary Review of the Scientific Evidence on Associations between Exposure to “Dampness” in Buildings and Health Effects (NORDDAMP). Indoor Air 2001, 11, 72–86. [Google Scholar] [CrossRef]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W.; Barnes, C.; Grimes, C.; Horner, W.E.; Kennedy, K.; Levetin, E.; Miller, J.D.; et al. Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef]
- Pettigrew, H.D.; Selmi, C.; Teuber, S.S.; Gershwin, M.E. Mold and Human Health: Separating the Wheat from the Chaff. Clin. Rev. Allergy Immunol. 2009, 38, 148–155. [Google Scholar] [CrossRef]
- Karakasidou, K.; Nikolouli, K.; Amoutzias, G.D.; Pournou, A.; Manassis, C.; Tsiamis, G.; Mossialos, D. Microbial diversity in biodeteriorated Greek historical documents dating back to the 19th and 20th century: A case study. MicrobiologyOpen 2018, 7, e00596. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Mesquita, N.; Trovão, J.; Soares, F.; Tiago, I.; Coelho, C.; De Carvalho, H.P.; Gil, F.; Catarino, L.; Piñar, G.; et al. Limestone biodeterioration: A review on the Portuguese cultural heritage scenario. J. Cult. Herit. 2019, 36, 275–285. [Google Scholar] [CrossRef]
- Gonzales, J.M. Overview on existing molecular techniques of potential interest in cultural heritage. In Molecular Biology and Cultural Heritage; Gonzales-Jimenez, C., Ed.; Swets & Zeitlinger B.V. Publishers: Lisse, The Netherlands, 2003. [Google Scholar]
- Adamiak, J.; Otlewska, A.; Tafer, H.; Lopandic’, K.; Gutarowska, B.; Sterflinger, K.; Piñar, G. First evaluation of the microbiome of built cultural heritage by using the Ion Torrent next generation sequencing platform. Int. Biodeterior. Biodegrad. 2018, 131, 11–18. [Google Scholar] [CrossRef]
- Grottoli, A.; Beccaccioli, M.; Zoppis, E.; Fratini, R.S.; Schifano, E.; Santarelli, M.L.; Uccelletti, D.; Reverberi, M. Nanopore Sequencing and Bioinformatics for Rapidly Identifying Cultural Heritage Spoilage Microorganisms. Front. Mater. 2020, 7, 14. [Google Scholar] [CrossRef]
- Gutarowska, B.; Celikkol-Aydin, S.; Bonifay, V.; Otlewska, A.; Aydin, E.; Oldham, A.L.; Brauer, J.I.; Duncan, K.E.; Adamiak, J.; Sunner, J.A.; et al. Metabolomic and high-throughput sequencing analysis—Modern approach for the assessment of biodeterioration of materials from historic buildings. Front. Microbiol. 2015, 6, 979. [Google Scholar] [CrossRef] [PubMed]
- Trovão, J.; Gil, F.; Catarino, L.; Soares, F.; Tiago, I.; Portugal, A. Analysis of fungal deterioration phenomena in the first Portuguese King tomb using a multi-analytical approach. Int. Biodeterior. Biodegrad. 2020, 149, 104933. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, Q.; Zhu, Z.; Deng, Y.; Gu, J.-D. Microbiological community of the Royal Palace in Angkor Thom and Beng Mealea of Cambodia by Illumina sequencing based on 16S rRNA gene. Int. Biodeterior. Biodegrad. 2018, 134, 127–135. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, F.; Wang, W.; Gu, J.-D.; Li, Y.; Feng, H.; Chen, T.; Liu, G.; An, L.-Z. Differences of Microbial Community on the wall paintings preserved in situ and ex situ of the Tiantishan Grottoes, China. Int. Biodeterior. Biodegrad. 2018, 132, 102–113. [Google Scholar] [CrossRef]
- Caneva, G.; Bartoli, F.; Fontani, M.; Mazzeschi, D.; Visca, P. Changes in biodeterioration patterns of mural paintings: Multi-temporal mapping for a preventive conservation strategy in the Crypt of the Original Sin (Matera, Italy). J. Cult. Herit. 2019, 40, 59–68. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protoc.; Innis, M.A., White, T.J., Sninsky, J.J., Gelfand, D.H., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, J.D. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- CLUSTAL. Available online: http://www.ebi.ac.uk/clustalw (accessed on 23 April 2020).
- Larkin, M.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.; McWilliam, H.; Valentin, F.; Wallace, I.; Wilm, A.; López, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Morton, L.H.G. Deteriogenic biofilms on buildings and their control: A review. Biofouling 1999, 14, 59–74. [Google Scholar] [CrossRef]
- Dakal, T.C.; Cameotra, S.S. Microbially induced deterioration of architectural heritages: Routes and mechanisms involved. Environ. Sci. Eur. 2012, 24, 36. [Google Scholar] [CrossRef]
- Jain, A.; Bhadauria, S.; Kumar, V.; Chauhan, R.S. Biodeterioration of sandstone under the influence of different humidity levels in laboratory conditions. Build. Environ. 2009, 44, 1276–1284. [Google Scholar] [CrossRef]
- Braams, J. Ecological Studies on the Fungal Microflora Inhabiting Historical Sandstone Monuments. Ph.D. Thesis, University of Oldenburg, Oldenbury, Germany, 1992. [Google Scholar]
- Grbić, M.L.; Vukojevic, J. Role of fungi in biodeterioration process of stone in historic buildings. Zb. Matice Srp. Prir. Nauk. 2009, 245–251. [Google Scholar] [CrossRef]
- Salvadori, O.; Municchia, A.C. The Role of Fungi and Lichens in the Biodeterioration of Stone Monuments. Open Conf. Proc. J. 2016, 7, 39–54. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the past: “Find the guilty bug—microorganisms involved in the biodeterioration of archeological and historical artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, E.; Chisesi, R.; Barresi, G.; Barbaro, S.; Lombardo, G.; Rotolo, V.; Sebastianelli, M.; Travagliato, G.; Palla, F. Fungi and Bacteria in Indoor Cultural Heritage Environments: Microbial-related Risks for Artworks and Human Health. Environ. Ecol. Res. 2016, 4, 257–264. [Google Scholar] [CrossRef]
- Abdel-Ghany, T.M.; Omar, A.; Elwkeel, F.M.; Al Abboud, M.A.; AlAwlaqi, M. Fungal deterioration of limestone false-door monument. Heliyon 2019, 5, e02673. [Google Scholar] [CrossRef] [PubMed]
- Jurado, V.; Sanchez-Moral, S.; Saiz-Jimenez, C. Entomogenous fungi and the conservation of the cultural heritage: A review. Int. Biodeterior. Biodegrad. 2008, 62, 325–330. [Google Scholar] [CrossRef]
- Pulimood, T.B.; Corden, J.M.; Bryden, C.; Sharples, L.; Nasser, S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J. Allergy Clin. Immunol. 2007, 120, 610–617. [Google Scholar] [CrossRef]
- Peat, J.K.; Dickerson, J.; Li, J. Effects of damp and mould in the home on respiratory health: A review of the literature. Allergy 1998, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Crameri, R.; Garbani, M.; Rhyner, C.; Huitema, C. Fungi: The neglected allergenic sources. Allergy 2013, 69, 176–185. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Pöll, V.; Rid, R.; Breitenbach, M. The Spectrum of Fungal Allergy. Int. Arch. Allergy Immunol. 2007, 145, 58–86. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Du, Y.; Tian, T.; Xiang, T.; Liu, X.; Wu, F.; An, L.; Wang, W.; Gu, J.-D.; et al. The community distribution of bacteria and fungi on ancient wall paintings of the Mogao Grottoes. Sci. Rep. 2015, 5, 7752. [Google Scholar] [CrossRef]
- Mohammadi, P.; Maghboli-Balasjin, N. Isolation and molecular identification of deteriorating fungi from Cyrus the Great tomb stones. Iran. J. Microbiol. 2014, 6, 361–370. [Google Scholar]
- Kustrzeba-Wojcicka, I.; Siwak, E.; Terlecki, G.; Wolanczyk-Medrala, A.; Medrala, W. Alternaria alternata and Its Allergens: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2014, 47, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Safaiefarahani, B. Indoor damp surfaces harbor molds with clinical significance. Curr. Med. Mycol. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Ana, S.G.; Torres-Rodríguez, J.M.; Ramírez, E.A.; García, S.M.; Belmonte-Soler, J. Seasonal distribution of Alternaria, Aspergillus, Cladosporium and Penicillium species isolated in homes of fungal allergic patients. J. Investig. Allergol. Clin. Immunol. 2006, 16, 357–363. [Google Scholar] [PubMed]
- Gabriel, M.; Postigo, I.; Tomaz, C.; Martinez, J. Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016, 89, 71–80. [Google Scholar] [CrossRef]
- Berner, M.; Wanner, G.; Lubitz, W. A comparative study of the fungal flora present in medieval wall paintings in the chapel of the castle Herberstein and in the parish church of St Georgen in Styria, Austria. Int. Biodeterior. Biodegrad. 1997, 40, 53–61. [Google Scholar] [CrossRef]
- Macêdo, D.P.C.; Neves, R.P.; De Souza-Motta, C.M.; Magalhães, O.M.C. Engyodontium album fungaemia: The first reported case. Braz. J. Microbiol. 2007, 38, 110–112. [Google Scholar] [CrossRef]
- Augustinsky, J.; Kammeyer, P.; Husain, A.; DeHoog, G.S.; Libertin, C.R. Engyodontium album endocarditis. J. Clin. Microbiol. 1990, 28, 1479–1481. [Google Scholar] [CrossRef]
- Seeliger, H.P. Infections of man by opportunistic molds—their identification and nomenclature of their diseases. Mykosen 1983, 26, 587–598. [Google Scholar] [CrossRef]
- McDonnell, P.J.; Werblin, T.P.; Sigler, L.; Green, W.R. Mycotic keratitis due to Beauveria alba. Cornea 1984, 3, 213–216. [Google Scholar] [CrossRef]
- Hasnain, S.M.; Al-Frayh, A.S.; Al-Suwaine, A.; Gad-El-Rab, M.O.; Fatima, K.; Al-Sedairy, S. Cladosporium and respiratory allergy: Diagnostic implications in Saudi Arabia. Mycopathologia 2004, 157, 171–179. [Google Scholar] [CrossRef]
- Maciel, N.O.; Johann, S.; Brandão, L.R.; Kucharíková, S.; Morais, C.G.; Oliveira, A.P.; Freitas, G.J.; Borelli, B.M.; Pellizzari, F.M.; Santos, D.A.; et al. Occurrence, antifungal susceptibility, and virulence factors of opportunistic yeasts isolated from Brazilian beaches. Mem. Inst. Oswaldo Cruz 2019, 114, e180566. [Google Scholar] [CrossRef] [PubMed]
- Corte, L.; di Cagno, R.; Groenewald, M.; Roscini, L.; Colabella, C.; Gobbetti, M.; Cardinali, G. Phenotypic and molecular diversity of Myerozyma guilliermondii strains isolated from food and other environmental niches, hints for an incipient speciation. Food Microbiol. 2015, 48, 206–215. [Google Scholar] [CrossRef] [PubMed]
- De Marco, L.; Epis, S.; Capone, A.; Martín, E.; Bozic, J.; Crotti, E.; Ricci, I.; Sassera, D. The Genomes of Four Meyerozyma caribbica Isolates and Novel Insights into the Meyerozyma guilliermondii Species Complex. G3 Genes Genomes Genet. 2018, 8, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Houbraken, J.; Groenewald, J.; Meijer, M.; Andersen, B.; Nielsen, K.; Crous, P.; Samson, R.A. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud. Mycol. 2016, 84, 145–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; McMullin, D.R. Fungal secondary metabolites as harmful indoor air contaminants: 10 years on. Appl. Microbiol. Biotechnol. 2014, 98, 9953–9966. [Google Scholar] [CrossRef] [PubMed]
- Došen, I.; Nielsen, K.F.; Clausen, G.; Andersen, B. Potentially harmful secondary metabolites produced by indoor Chaetomium species on artificially and naturally contaminated building materials. Indoor Air 2016, 27, 34–46. [Google Scholar] [CrossRef]
- Lin, W.-R.; Chen, Y.-H.; Lee, M.-F.; Hsu, L.-Y.; Tien, C.-J.; Shih, F.-M.; Hsiao, S.-C.; Wang, P.-H. Does Spore Count Matter in Fungal Allergy? The Role of Allergenic Fungal Species. Allergy Asthma Immunol. Res. 2016, 8, 404–411. [Google Scholar] [CrossRef]
- Medrela-Kuder, E. Seasonal variations in the occurrence of culturable airborne fungi in outdoor and in indoor air in Craćow. Int. Biodeterior. Biodegrad. 2003, 52, 203–205. [Google Scholar] [CrossRef]
- Sharma, K. Seasonal variation and ecological study on fungi in relation to biodeterioration. Recent Res. Sci. Technol. 2012, 4, 6–8. [Google Scholar]
- Caneva, G.; Tescari, M. Stone biodeterioration: Treatments and preventive conservation. In Proceedings of the International Symposium on Stone Conservation, Conservation Technologies fort Stone Cultural Heritages: Status and Future Prospects, Seoul, Korea, 1 September 2017; pp. 95–114. [Google Scholar]
- Caneva, G.; Fidanza, M.R.; Tonon, C.; Favero-Longo, S.E. Biodeterioration Patterns and Their Interpretation for Potential Applications to Stone Conservation: A Hypothesis from Allelopathic Inhibitory Effects of Lichens on the Caestia Pyramid (Rome). Sustainability 2020, 12, 1132. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed]

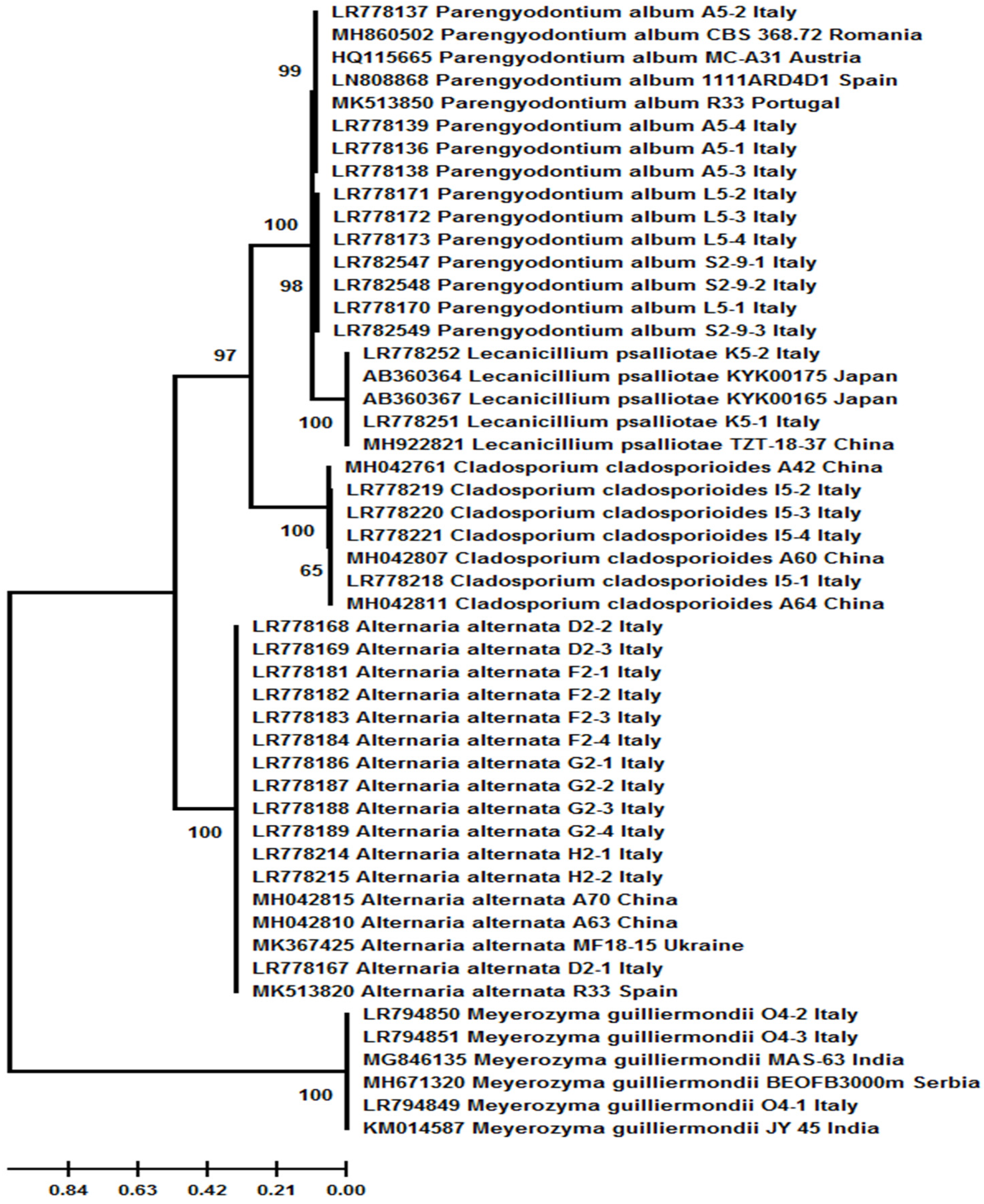
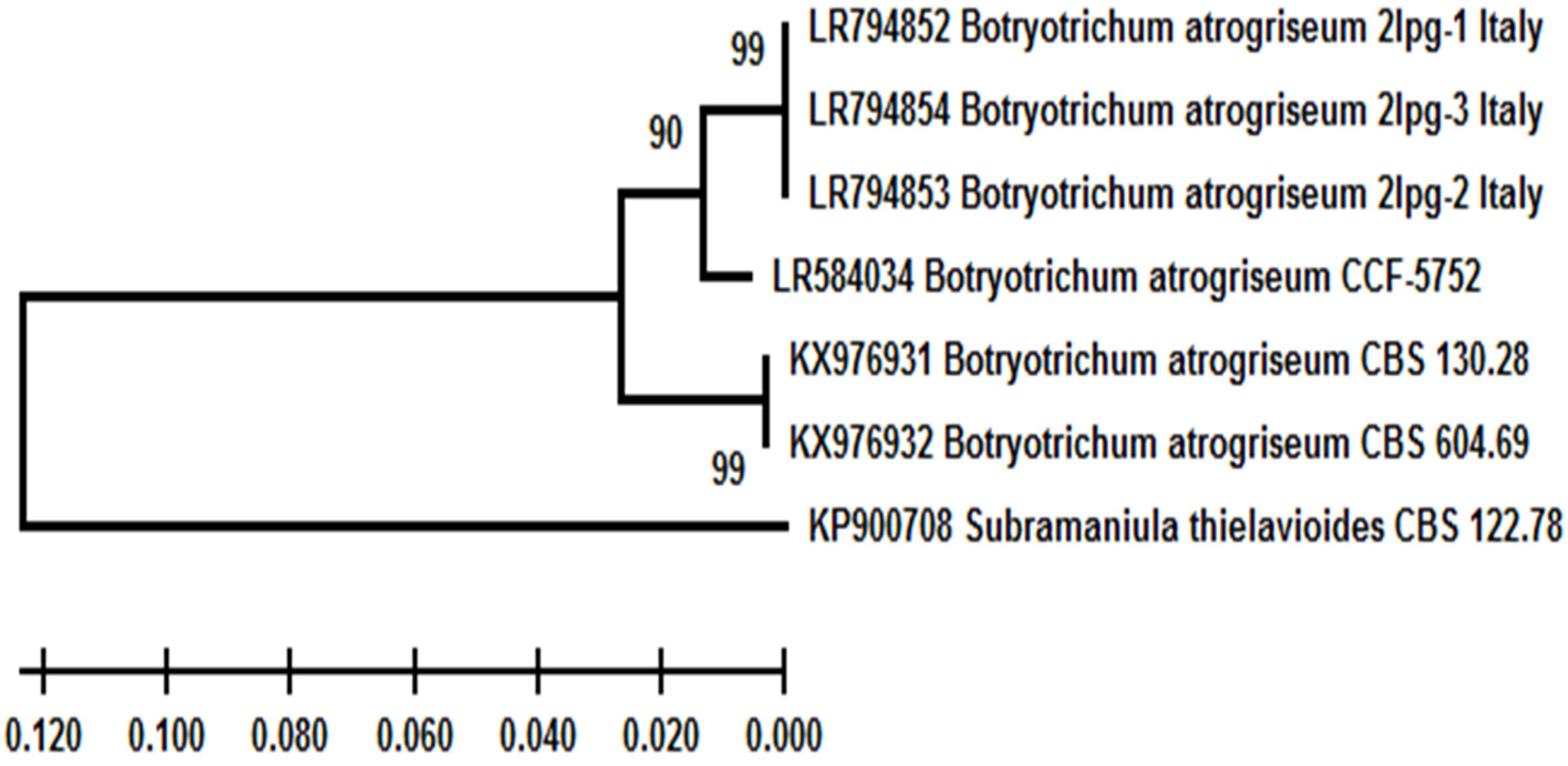
| Taxon | Isolate/Strain | Acc. No. (ITS/TUB2 *) | Isolation Source as Described in GenBank | Country | Year a |
|---|---|---|---|---|---|
| Parengyondontium album | R33 | MK513850 | limestone walls of old Cathedral Coimbra UNESCO World Heritage Site | Portugal | 2019 |
| -″- | 1111ARD4D1 | LN808868 | wall of tourist grottoes “Cueva de Ardales” | Spain | 2015 |
| -″- | MC_A31 | HQ115665 | wall after water damage | Austria | 2011 |
| -″- | CBS 368. 72 | MH860502 | not mentioned | Romania | 2020 |
| Alternaria alternata | A63 | MH042810 | wall paintings in cave temples in grottoes | China | 2018 |
| -″- | A70 | MH042815 | wall paintings in cave temples in grottoes | China | 2018 |
| -″- | MF18_15 | MK367425 | deteriorated walls from the 11th c. St. Sophia Cathedral | Ukraine | 2019 |
| -″- | R33 | MK513820 | limestone walls of old Cathedral Coimbra UNESCO World Heritage Site | Portugal | 2019 |
| Cladosporium cladosporioides | A42 | MH042761 | wall paintings in cave temples in grottoes | China | 2018 |
| -″- | A60 | MH042807 | wall paintings in cave temples in grottoes | China | 2018 |
| -″- | A64 | MH042811 | wall paintings in cave temples in grottoes | China | 2018 |
| Lecanicillium psalliotae | KYK00165 | AB360367 | soil | Japan | 2014 |
| -″- | KYK00175 | AB360364 | not mentioned | Japan | 2014 |
| -″- | TZT-18-37 | MH922821 | not mentioned | China | 2018 |
| Meyerozyma guilliermondii | BOEFB3000m | MH671320 | cultural heritage conservation facility | Serbia | 2018 |
| -″- | JY 45 | KM014587 | continental shelf sediments | India | 2014 |
| -″- | MAS-63 | MG846135 | soil | India | 2018 |
| Botryotrichum atrogriseum * | CBS 130.28 | KX976931 | dung of rabbit | Netherlands | 2017 |
| -″- | CBS 604.69 | KX976932 | corn field soil | Canada | 2017 |
| -″- | CCF 5752 | LR584034 | air in the restroom | USA | 2019 |
| Subramaniula thielavioides ** | CBS 122.78 | KP900708 | not mentioned | Netherlands | 2015 |
| Isolate | Closest Species | Length (bp) a | Sequence Similarity (% Identity) | GenBank Acc. No. |
|---|---|---|---|---|
| A5_1 | Parengyondontium album | 575 | 99.83 | LR778136 |
| A5_2 | -″- | 575 | 99.83 | LR778137 |
| A5_3 | -″- | 575 | 99.83 | LR778138 |
| A5_4 | -″- | 575 | 99.83 | LR778139 |
| S2_9_1 | -″- | 589 | 100 | LR782547 |
| S2_9_2 | -″- | 589 | 100 | LR782548 |
| S2_9_3 | -″- | 589 | 100 | LR782549 |
| L5_1 | -″- | 578 | 100 | LR778170 |
| L5_2 | -″- | 578 | 100 | LR778171 |
| L5_3 | -″- | 578 | 100 | LR778172 |
| L5_4 | -″- | 578 | 100 | LR778173 |
| D2_1 | Alternaria alternata | 543 | 100 | LR778167 |
| D2_2 | -″- | 543 | 100 | LR778168 |
| D2_3 | -″- | 543 | 100 | LR778169 |
| F2_1 | -″- | 547 | 100 | LR778181 |
| F2_2 | -″- | 547 | 100 | LR778182 |
| F2_3 | -″- | 547 | 100 | LR778183 |
| F2_4 | -″- | 547 | 100 | LR778184 |
| G2_1 | -″- | 548 | 100 | LR778186 |
| G2_2 | -″- | 548 | 100 | LR778187 |
| G2_3 | -″- | 548 | 100 | LR778188 |
| G2_4 | -″- | 548 | 100 | LR778189 |
| H2_1 | -″- | 560 | 100 | LR778214 |
| H2_2 | -″- | 560 | 100 | LR778215 |
| I5_1 | Cladosporium cladosporioides | 531 | 100 | LR778218 |
| I5_2 | -″- | 531 | 100 | LR778219 |
| I5_3 | -″- | 531 | 100 | LR778220 |
| I5_4 | -″- | 531 | 100 | LR778221 |
| K5_1 | Lecanicillium psalliotae | 586 | 100 | LR778251 |
| K5_2 | -″- | 586 | 100 | LR778252 |
| O4_1 | Meyerozyma guilliermondii | 565 | 100 | LR794849 |
| O4_2 | -″- | 565 | 100 | LR794850 |
| O4_3 | -″- | 565 | 100 | LR794851 |
| 2Ipg_1 | Botryotrichum atrogriseum | 452 | 98.99 | LR794852 |
| 2Ipg_2 | -″- | 452 | 98.99 | LR794853 |
| 2Ipg_3 | -″- | 452 | 98.99 | LR794854 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mang, S.M.; Scrano, L.; Camele, I. Preliminary Studies on Fungal Contamination of Two Rupestrian Churches from Matera (Southern Italy). Sustainability 2020, 12, 6988. https://doi.org/10.3390/su12176988
Mang SM, Scrano L, Camele I. Preliminary Studies on Fungal Contamination of Two Rupestrian Churches from Matera (Southern Italy). Sustainability. 2020; 12(17):6988. https://doi.org/10.3390/su12176988
Chicago/Turabian StyleMang, Stefania Mirela, Laura Scrano, and Ippolito Camele. 2020. "Preliminary Studies on Fungal Contamination of Two Rupestrian Churches from Matera (Southern Italy)" Sustainability 12, no. 17: 6988. https://doi.org/10.3390/su12176988
APA StyleMang, S. M., Scrano, L., & Camele, I. (2020). Preliminary Studies on Fungal Contamination of Two Rupestrian Churches from Matera (Southern Italy). Sustainability, 12(17), 6988. https://doi.org/10.3390/su12176988







