Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry
Abstract
1. Introduction
2. Materials and Methods
2.1. Pre-Sowing Soil Analysis
2.2. Experimental Setup
2.3. Preparation of Rhizobacterial Inocula and Seed Inoculation and Biogas Slurry
2.4. Characteristics of the PGPR Strains
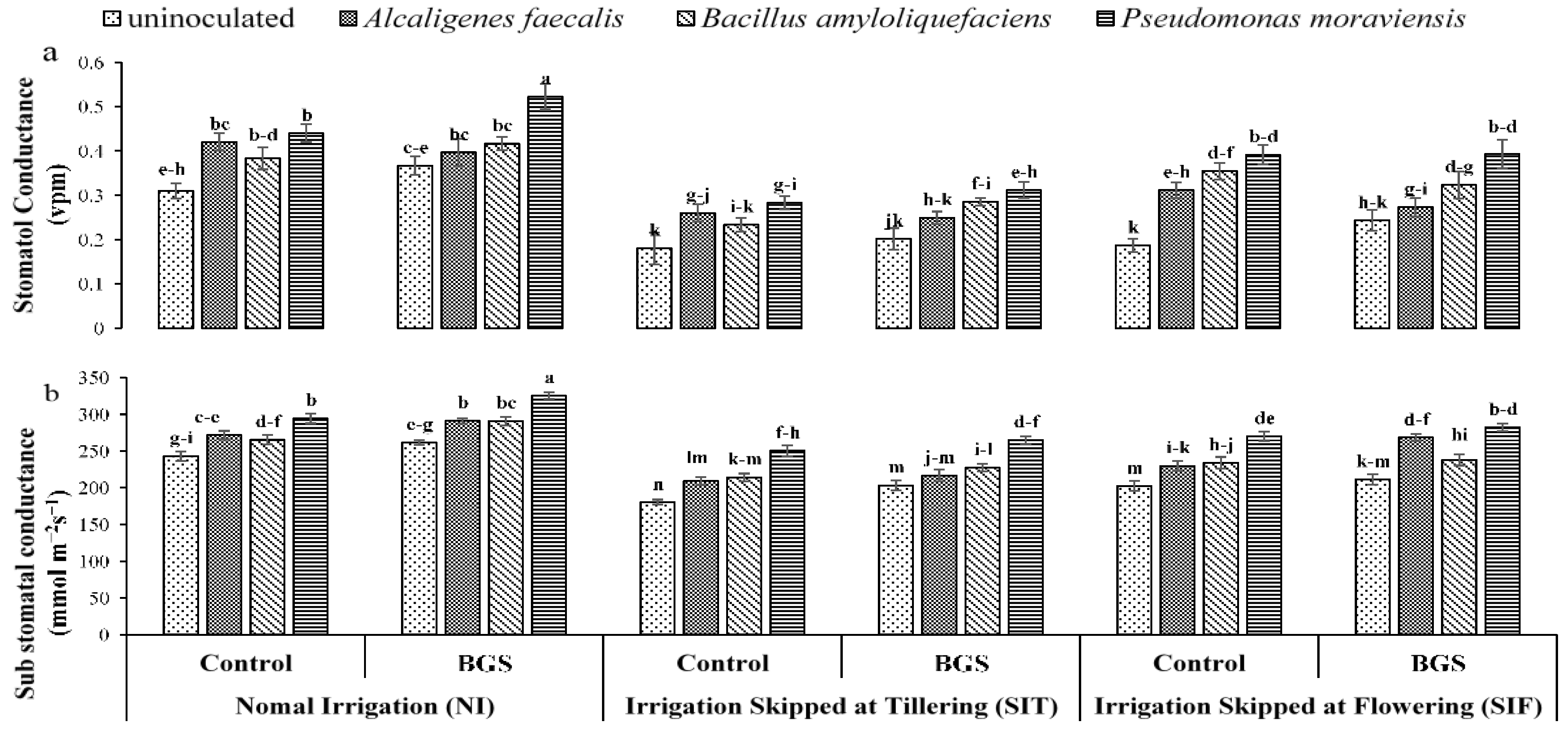
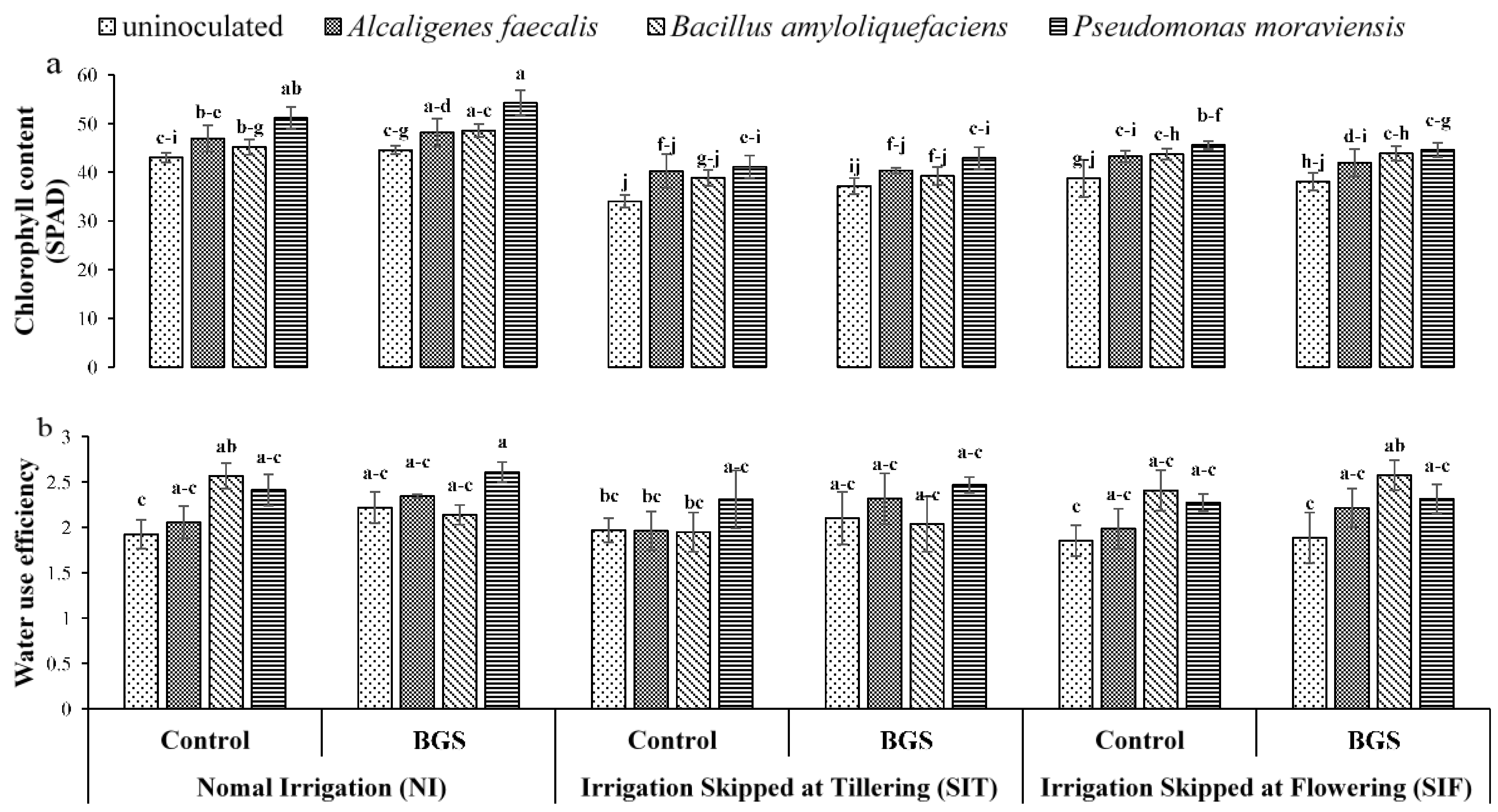
2.5. Plant Physiological Parameters
2.6. Relative Water Content, Electrolyte Leakage, Total Phenolics, and Proline in Plant Leaves
2.7. Enzymatic Antioxidant Activity Assay
2.8. Measurement of Growth and Yield Parameters and Mineral Nutrients of Plant
2.9. Statistical Analysis
3. Results
3.1. Growth Physiology
3.2. Relative Water Content, Electrolyte Leakage, Proline Content, and Total Phenolic Content
3.3. The Enzymatic Antioxidant Activity
3.4. Growth and Agronomic Yield
3.5. Mineral Nutrition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | 1-Aminocyclopropane-1-carboxylate |
| ACCD | ACC-deaminase |
| ANOVA | Analysis of variance |
| BGS | Biogas slurry |
| CAT | Catalase |
| CFU | Colony forming unit |
| EC | Electrical conductivity |
| EL | Electrolyte leakage |
| HSD | Honest Significant Difference |
| IAA | Indole acetic acid |
| NI | Normal irrigation |
| PGPR | Plant growth promoting rhizobacteria |
| ppm | Parts per million |
| RCBD | Randomized complete block design |
| rpm | Resolution per minute |
| RWC | Relative water content |
| SIF | Skipped irrigation flowering stage |
| SIT | Skipped irrigation tillering stage |
| WUE | Water-use efficiency |
References
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A potential candidate for phytoremediation of metals—A review. Plants 2020, 9, 258. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the correlations between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Rivas, R.; Falcão, H.; Ribeiro, R.; Machado, E.; Pimentel, C.; Santos, M. Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S. Afr. J. Bot. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 136675. [Google Scholar] [CrossRef] [PubMed]
- Canavar, Ö.; Kaptan, M.A. Changes In macro and micro plant nutrients of sunflower (Helianthus annuus L.) under drought stress. Sci. Pap. Ser. A. Agron. 2014, 57, 1. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Kulkarni, M.; Phalke, S. Evaluating variability of root size system and its constitutive traits in hot pepper (Capsicum annum L.) under water stress. Sci. Hortic. 2009, 120, 159–166. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; El-Daim, I.A.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Yaseen, R.; Zafar-ul-Hye, M.; Hussain, M. Integrated application of ACC-deaminase containing plant growth promoting rhizobacteria and biogas slurry improves the growth and productivity of wheat under drought stress. Int. J. Agric. Biol. 2019, 21, 869–878. [Google Scholar]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Raghuwanshi, R.; Prasad, J.K. Perspectives of rhizobacteria with ACC deaminase activity in plant growth under abiotic stress. In Root Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 303–321. [Google Scholar]
- Vanderlinde, E.M.; Harrison, J.J.; Muszyński, A.; Carlson, R.W.; Turner, R.J.; Yost, C.K. Identification of a novel ABC transporter required for desiccation tolerance, and biofilm formation in Rhizobium leguminosarum bv. viciae 3841. FEMS Microbiol. Ecol. 2010, 71, 327–340. [Google Scholar] [CrossRef]
- Maqbool, S.; Ul Hassan, A.; JavedAkhtar, M.; Tahir, M. Integrated use of biogas slurry and chemical fertilizer to improve growth and yield of okra. Sci. Lett. 2014, 2, 56–59. [Google Scholar]
- Chen, R.; Blagodatskaya, E.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Kuzyakov, Y. Decomposition of biogas residues in soil and their effects on microbial growth kinetics and enzyme activities. Biomass Bioenergy 2012, 45, 221–229. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant. Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.-C.; Du, L. Soilless cultivation for high-quality vegetables with biogas manure in China: Feasibility and benefit analysis. Renew. Agric. Food Syst. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, S.M.E.; Rahman, M.M.; Oh, D.H.; Ra, C.S. The effects of biogas slurry on the production and quality of maize fodder. Turk. J. Agric. For. 2010, 34, 91–99. [Google Scholar]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T. Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis L.) seedlings exposed to copper stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef]
- Ahamd, M.; Zeshan, M.S.H.; Nasim, M.; Zahir, Z.A.; Nadeem, S.M.; Nazli, F.; Jamil, M. Improving the productivity of cucumber through combined application of organic fertilizers and pseudomonas fluorescens. Pak. J. Agric. Sci. 2015, 52, 1011–1016. [Google Scholar]
- Iqbal, M.A.; Khalid, M.; Zahir, Z.A.; Ahmad, R. Auxin producing plant growth promoting rhizobacteria improve growth, physiology and yield of maize under saline field conditions. Int. J. Agric. Biol. 2016, 18, 37–45. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. Growth response and nitrogen metabolism of sunflower (Helianthus annuus L.) to vermicompost and biogas slurry under salinity stress. J. Plant Nutr. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Tan, F.; Wang, Z.; Zhouyang, S.; Li, H.; Xie, Y.; Wang, Y.; Zheng, Y.; Li, Q. Nitrogen and phosphorus removal coupled with carbohydrate production by five microalgae cultures cultivated in biogas slurry. Bioresour. Technol. 2016, 221, 385–393. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, G.; Zhang, H.; Shen, Y.; Fei, H.; Cheng, W. Biogas slurry use as N fertilizer for two-season Zizania aquatica Turcz. Nutr. Cycl. Agroecosyst. 2017, 107, 303–320. (In Chinese) [Google Scholar] [CrossRef]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of biochar on composting of organic wastes and remediation of contaminated soils—A review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Ayers, R.; Westcot, D. Water Quality for Agriculture; FAO: Roma, Italy, 1985; Available online: http://www.fao.org/DOCReP/003/T0234e/T0234E00.htm (accessed on 23 July 2020).
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Iqbal, N.; Noman, A.; Ali, M. Influence of melatonin on antioxidant defense system and yield of wheat (Triticum aestivum L.) genotypes under saline condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef]
- Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Saleem, M.H.; Moussa, M.G.; Bhantana, P.; Afzal, J. Molybdenum-induced effects on nitrogen metabolism enzymes and elemental profile of winter wheat (Triticum aestivum L.) under different nitrogen sources. Int. J. Mol. Sci. 2019, 20, 3009. [Google Scholar] [CrossRef] [PubMed]
- Laboratory, R.S. Diagnosis and Improvement of Saline and Alkali Soils; US Department of Agriculture: Washington, DC, USA, 1954; pp. 6–7.
- Walkley, A. An examination of methods for determining organic carbon and nitrogen in soils 1.(with one text-figure.). J. Agric. Sci. 1935, 25, 598–609. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L.; Page, A. Methods of soil analysis. Part 1982, 2, 403–430. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants, and Waters; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1961; pp. 7–8. [Google Scholar]
- Moodie, C.; Smith, H.; McCreery, R. Laboratory Manual for Soil Fertility, (Mimeographed); Washington State College: Washington, DC, USA, 1959. [Google Scholar]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant. Analysis Laboratory Manual; ICARDA: Cairo, India, 2001; pp. 15–17. [Google Scholar]
- El-Tarabily, K.A. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil 2008, 308, 161–174. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Goldstein, A.H. Bacterial solubilization of mineral phosphates: Historical perspective and future prospects. Am. J. Altern. Agric. 1986, 1, 51–57. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- MacFaddin, J. Enterobacteriaceae and Other Intestinal Bacteria. In Biochemical Tests for Identification of Medical Bacteria; Williams & Wilkins: Baltimore, MD, USA, 1980; pp. 439–464. [Google Scholar]
- HussainF, B. Use of chlorophyll meter sufficiency indices for nitrogen management of irrigated rice in Asia. Agron. J. 2000, 92, 875–879. [Google Scholar]
- Teulat, B.; Zoumarou-Wallis, N.; Rotter, B.; Salem, M.B.; Bahri, H.; This, D. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor. Appl. Genet. 2003, 108, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–297. [Google Scholar]
- Giannakoula, A.E.; Ilias, I.F.; Maksimović, J.J.D.; Maksimović, V.M.; Živanović, B.D. The effects of plant growth regulators on growth, yield, and phenolic profile of lentil plants. J. Food Compos. Anal. 2012, 28, 46–53. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Y.; Zhang, X.-S.; Huang, Z.-Y. Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J. Plant Ecol. 2010, 3, 79–87. [Google Scholar] [CrossRef]
- Steel, R.G. Pinciples and Procedures of Statistics a Biometrical Approach; McGraw-Hill: New York, NY, USA, 1997; p. 666. [Google Scholar]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana L. Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Nagargade, M.; Tyagi, V.; Singh, M. Plant growth-promoting rhizobacteria: A biological approach toward the production of sustainable agriculture. In Role of Rhizospheric Microbes in Soil; Springer: Berlin/Heidelberg, Germany, 2018; pp. 205–223. [Google Scholar]
- Rana, M.S.; Hu, C.X.; Shaaban, M.; Imran, M.; Afzal, J.; Moussa, M.G.; Elyamine, A.M.; Bhantana, P.; Saleem, M.H.; Syaifudin, M. Soil phosphorus transformation characteristics in response to molybdenum supply in leguminous crops. J. Environ. Manag. 2020, 268, 110610. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, S.; Rehman, M.; Rana, M.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Hussein, M.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Kachare, S.; Tiwari, S. Molecular diversity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing PGPR from wheat (Triticum aestivum L.) rhizosphere. Plant Soil 2017, 414, 213–227. [Google Scholar] [CrossRef]
- Hussain, M.B.; Zahir, Z.A.; Asghar, H.N.; Mahmood, S. Scrutinizing rhizobia to rescue maize growth under reduced water conditions. Soil Sci. Soc. Am. J. 2014, 78, 538–545. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, C.B.; Rouina, B.B.; Boukhris, M. Effects of water deficit on olive trees cv. Chemlali under field conditions in arid region in Tunisia. Sci. Hortic. 2007, 113, 267–277. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Khalid, M.; Nazli, F.; Arshad, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.; Escalante, M.; Gallardo, M.; Alemano, S.; Abdala, G. Effects of bacterial single inoculation and co-inoculation on growth and phytohormone production of sunflower seedlings under water stress. Acta Physiol. Plant. 2013, 35, 2299–2309. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Rehman, M.; Saud, S.; Jamal, Y.; Khan, S.; Liu, L. Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. PeerJournal 2020, 8, e8321. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Irshad, S.; Hussaan, M.; Rizwan, M.; Rana, M.S.; Hashem, A.; Abd_Allah, E.F.; Ahmad, P. Copper uptake and accumulation, ultra-structural alteration, and bast fibre yield and quality of fibrous jute (Corchorus capsularis L.) plants grown under two different soils of China. Plants 2020, 9, 404. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Mohamed, I.A.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R.; Liu, L. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 2020, 19, 100895. [Google Scholar] [CrossRef]
- Pereyra, M.; Zalazar, C.; Barassi, C. Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress. Plant Physiol. Biochem. 2006, 44, 873–879. [Google Scholar] [CrossRef]
- Barbosa, R.H.; Tabaldi, L.A.; Miyazaki, F.R.; Pilecco, M.; Kassab, S.O.; Bigaton, D. Foliar copper uptake by maize plants: Effects on growth and yield. Ciência Rural 2013, 43, 1561–1568. [Google Scholar] [CrossRef]
- Garg, R.N.; Pathak, H.; Das, D.; Tomar, R. Use of flyash and biogas slurry for improving wheat yield and physical properties of soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef]
- Du, H.; Gao, W.; Li, J.; Shen, S.; Wang, F.; Fu, L.; Zhang, K. Effects of digested biogas slurry applicationmixed with irrigation water on nitrate leaching during wheat-maize rotation in the North China Plain. Agric. Water Manag. 2019, 213, 882–893. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.L.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Khan, M.H.U.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Rehman, M.; Fahad, S.; Tung, S.A.; Iqbal, N.; Hassan, A.; Ayub, A.; Wahid, M.A.; Shaukat, S.; Liu, L.; et al. Leaf gas exchange, oxidative stress, and physiological attributes of rapeseed (Brassica napus L.) grown under different light-emitting diodes. Photosynthetica 2020, 58, 836–845. [Google Scholar] [CrossRef]
- Rana, M.; Bhantana, P.; Sun, X.-C.; Imran, M.; Shaaban, M.; Moussa, M.; Hamzah Saleem, M.; Elyamine, A.; Binyamin, R.; Alam, M.; et al. Molybdenum as an Essential Element for Crops: An Overview. Int. J. Sci. Res. Growth 2020, 24, 18535. [Google Scholar]
- Saleem, M.H.; Rehman, M.; Kamran, M.; Afzal, J.; Noushahi, H.A.; Liu, L. Investigating the potential of different jute varieties for phytoremediation of copper-contaminated soil. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rehman, M.; Zahid, M.; Imran, M.; Xiang, W.; Liu, L. Morphological changes and antioxidative capacity of jute (Corchorus capsularis, Malvaceae) under different color light-emitting diodes. Braz. J. Bot. 2019, 42, 581–590. [Google Scholar] [CrossRef]


| Characteristics | Unit | Value |
|---|---|---|
| Textural Class | sandy clay loam | |
| Sand | % | 54 |
| Silt | % | 26 |
| Clay | % | 20 |
| pHs | - | 7.9 |
| ECe | dS m−1 | 2.42 |
| Organic Matter | % | 0.58 |
| CaCO3 | % | 0.59 |
| Total Nitrogen | % | 0.04 |
| Available Phosphorus | mg kg−1 | 7.1 |
| Extractable Potassium | mg kg−1 | 107 |
| Characteristics | Alcaligenes faecalis | Bacillus amyloliquefaciens | Pseudomonas moraviensis | |
|---|---|---|---|---|
| ACC-deaminase activity (nmol α-ketobutyrateg−1 protein h−1) | 384 | 435 | 532 | |
| IAA production (mg L−1) | Without L–Tryptophan | 2.21 | 6.12 | 5.63 |
| With L–Tryptophan (1 g L−1) | 15.33 | 14.32 | 22.23 | |
| Phosphate solubilization | - | - | - | |
| Exopolysaccharides production ability | - | - | - | |
| Catalase activity | - | - | - | |
| Treatments | Electrolyte Leakage (%) | Relative Water Content (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | SIT | SIF | NI | SIT | SIF | |||||||
| No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | |
| No PGPR | 64 c–f | 57 f–k | 80 a | 73 ab | 69 bc | 68 b–d | 60 e–j | 67 b–g | 45 k | 52 i–k | 52 i–k | 57 g–j |
| A. faecalis | 50 k–n | 55 h–m | 73 ab | 61 c–h | 63 c–f | 58 f–k | 69 a–f | 71 a–c | 50 jk | 59 f–j | 61 d–j | 59 f–j |
| B. amyloliquefaciens | 55 g–m | 49 l–n | 62 c–g | 66 b–e | 61 d–i | 59 e–j | 63 c–h | 76 ab | 57 g–j | 56 h–j | 60 d–j | 70 a–e |
| P. moraviensis | 47 mn | 45 n | 56 f–l | 53 i–m | 51 k–n | 52 j–n | 71 a–d | 78 a | 59 f–j | 61 c–i | 65 c–h | 71 a–d |
| Proline Content (µg g−1) | Total Phenolic (µg g−1) | |||||||||||
| No PGPR | 0.47 c–f | 0.45 c–g | 0.63 ab | 0.71 a | 0.52 cd | 0.63 a | 99 g–j | 90 h–j | 165 b | 192 a | 183 a | 160 b |
| A. faecalis | 0.43 c–h | 0.37 f–i | 0.50 c–e | 0.65 a | 0.41 e–i | 0.42 d–i | 72 kl | 88 jk | 132 c | 112 fg | 122 c–f | 113 e–g |
| B. amyloliquefaciens | 0.36 g–i | 0.41 e–i | 0.53 bc | 0.48 c–e | 0.44 c–g | 0.44 c–h | 90 ij | 70 l | 135 c | 132 c | 131 cd | 130 c–e |
| P. moraviensis | 0.33 i | 0.39 e–i | 0.40 e–i | 0.38 f–i | 0.33 hi | 0.41 e–i | 67 l | 58 l | 106 f–i | 114 d–g | 107 f–h | 91 h–j |
| Treatments | Plant Height (cm) | Number of Tillers (m−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | SIT | SIF | NI | SIT | SIF | |||||||
| No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | |
| No PGPR | 82 de | 82 c–e | 63 ij | 62 j | 76 d–h | 75 e–h | 214 jk | 284 b–e | 215 jk | 216 i–k | 213 k | 222 h–k |
| A. faecalis | 90 a–c | 92 a | 70 g–i | 70 g–i | 83 b–e | 84 b–d | 302 bc | 353 a | 232 g–k | 243 f–k | 280 b–f | 295 bc |
| B. amyloliquefaciens | 91 ab | 93 a | 69 h–j | 74 f–h | 79 d–f | 84 b–d | 317 ab | 345 a | 251 e–j | 265 c–g | 253 e–i | 269 c–g |
| P. moraviensis | 93 a | 93 a | 72 f–h | 78 d–g | 84 b–d | 84b–d | 307 b | 347 a | 243 f–k | 256 d–h | 268 c–g | 294 b–d |
| 1000-Grain Weight (g) | Grain Yield (Mg ha−1) | |||||||||||
| No PGPR | 36 c–g | 37 c–g | 29 i | 31 i | 31 hi | 36 e–g | 3.16 d–g | 3.34 b–f | 2.29 j | 2.40 ij | 3.05 e–h | 2.91 gh |
| A. faecalis | 40 a–c | 41 ab | 30 i | 32 hi | 35 f–h | 38 b–f | 3.47 b–d | 3.72 ab | 2.84 gh | 2.82 gh | 3.34 b–f | 3.32 c–f |
| B. amyloliquefaciens | 40 a–d | 39 a–d | 31 hi | 32 hi | 36 c–g | 36 d–g | 3.39 b–e | 3.63 a–c | 2.74 hi | 2.98 f–h | 3.30 c–f | 3.31 c–f |
| P. moraviensis | 41 ab | 42 a | 32 hi | 34 gh | 38 b–g | 39 a–e | 3.62 a–c | 3.90 a | 2.98 f–h | 3.05 e–h | 3.19 dg | 3.19 d–g |
| Straw Yield (Mg ha−1) | Biological Yield (Mg ha−1) | |||||||||||
| No PGPR | 5.05 b–f | 5.28 a–e | 4.05 i | 4.12 i | 4.80 c–h | 4.80 c–h | 10.9 d–f | 11.7 c–f | 8.4 g | 10.0 fg | 9.9 fg | 11.0 d–f |
| A. faecalis | 5.27 a–e | 5.54 ab | 4.35 g–i | 4.71 d–i | 4.92 b–h | 5.29 a–e | 12.7 b–d | 14.1 ab | 10.5 ef | 11.3 c–f | 11.3 d–f | 11.6 c–f |
| B. amyloliquefaciens | 5.18 a–e | 5.46 a–c | 4.26 hi | 4.64 e–i | 5.08 b–f | 5.00 b–g | 12.1 c–e | 12.8 a–d | 10.6 ef | 10.8 d–f | 11.2 d–f | 11.6 c–f |
| P. moraviensis | 5.36 a–d | 5.75 a | 4.49 f–i | 5.03 b–f | 5.33 a–d | 5.36 a–d | 13.2 a–c | 14.7 a | 10.6 ef | 11.4 c–f | 11.7 c–f | 12.2 b–e |
| Treatments | Grain Nitrogen (%) | Shoot Nitrogen (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | SIT | SIF | NI | SIT | SIF | |||||||
| No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | No BGS | BGS | |
| No PGPR | 1.56 h–k | 1.83 c–f | 1.15 q | 1.27 o–q | 1.25 pq | 1.30 n–q | 1.21 d–f | 1.44 bc | 0.79 j | 1.02 g–i | 1.01 g–i | 1.17 e–g |
| A. faecalis | 1.88 b–e | 2.02 a–c | 1.32 m–q | 1.46 k–o | 1.49 j–n | 1.53 h–l | 1.36 cd | 1.51 a–c | 0.93 ij | 1.03 g–i | 1.22 d–f | 1.24 de |
| B. amyloliquefaciens | 1.79 d–g | 1.95 a–d | 1.35 l–p | 1.50 i–m | 1.69 e–i | 1.70 e–h | 1.44 bc | 1.53 ab | 1.05 f–i | 0.95 h–j | 1.17 e–g | 1.26 de |
| P. moraviensis | 2.08 ab | 2.13 a | 1.47 k–n | 1.60 g–k | 1.68 f–j | 1.80 d–g | 1.59 ab | 1.61 a | 1.14 e–g | 1.10 e–h | 1.23 de | 1.35 cd |
| Grain Phosphorus (%) | Shoot Phosphorus (%) | |||||||||||
| No PGPR | 0.54 e–i | 0.54 e–i | 0.44 k | 0.45 jk | 0.49 i–k | 0.51 g–j | 0.27 c–e | 0.27 c–e | 0.12 i | 0.14 hi | 0.15 g–i | 0.21 d–f |
| A. faecalis | 0.61 b–d | 0.65 ab | 0.50 g–k | 0.52 f–i | 0.57 d–g | 0.54 d–i | 0.31 a–c | 0.36 a | 0.13 i | 0.21 e–g | 0.19 f–h | 0.29 bc |
| B. amyloliquefaciens | 0.60 b–e | 0.57 c–g | 0.50 h–k | 0.52 f–i | 0.54 e–i | 0.56 d–h | 0.29 bc | 0.30 bc | 0.17 f–i | 0.20 fg | 0.14 hi | 0.22 d–f |
| P. moraviensis | 0.63 a–c | 0.68 a | 0.54 e–i | 0.55 d–i | 0.56 d–h | 0.58 c–f | 0.32 a–c | 0.35 ab | 0.22 d–f | 0.22 d–f | 0.22 d–f | 0.27 cd |
| Grain Potassium (%) | Shoot Potassium (%) | |||||||||||
| No PGPR | 0.47 f–i | 0.54 d–f | 0.36 jk | 0.31 k | 0.40 i–k | 0.39 i–k | 0.94 i–k | 1.15 c–g | 0.79 k | 0.98 h–j | 0.84 jk | 0.97 h–j |
| A. faecalis | 0.64 a–c | 0.70 a | 0.43 h–j | 0.45 g–j | 0.52 d–g | 0.50 e–h | 1.25 b–e | 1.31 bc | 1.16 c–g | 1.12 e–h | 1.03 g–i | 1.17 c–g |
| B. amyloliquefaciens | 0.54 d–f | 0.67 ab | 0.50 e–h | 0.48 e–h | 0.48 e–h | 0.51 e–h | 1.15 c–g | 1.39 ab | 1.06 g–i | 1.07 f–i | 1.09 e–i | 1.12 e–h |
| P. moraviensis | 0.65 ab | 0.71 a | 0.53 d–f | 0.54 d–f | 0.60 b–d | 0.56 c–e | 1.29 b–d | 1.51 a | 1.18 c–g | 1.13 d–h | 1.22 c–f | 1.24 b–e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, R.; Aziz, O.; Saleem, M.H.; Riaz, M.; Zafar-ul-Hye, M.; Rehman, M.; Ali, S.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A.; et al. Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability 2020, 12, 6022. https://doi.org/10.3390/su12156022
Yaseen R, Aziz O, Saleem MH, Riaz M, Zafar-ul-Hye M, Rehman M, Ali S, Rizwan M, Nasser Alyemeni M, El-Serehy HA, et al. Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability. 2020; 12(15):6022. https://doi.org/10.3390/su12156022
Chicago/Turabian StyleYaseen, Rizwan, Omar Aziz, Muhammad Hamzah Saleem, Muhammad Riaz, Muhammad Zafar-ul-Hye, Muzammal Rehman, Shafaqat Ali, Muhammad Rizwan, Mohammed Nasser Alyemeni, Hamed A. El-Serehy, and et al. 2020. "Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry" Sustainability 12, no. 15: 6022. https://doi.org/10.3390/su12156022
APA StyleYaseen, R., Aziz, O., Saleem, M. H., Riaz, M., Zafar-ul-Hye, M., Rehman, M., Ali, S., Rizwan, M., Nasser Alyemeni, M., El-Serehy, H. A., Al-Misned, F. A., & Ahmad, P. (2020). Ameliorating the Drought Stress for Wheat Growth through Application of ACC-Deaminase Containing Rhizobacteria along with Biogas Slurry. Sustainability, 12(15), 6022. https://doi.org/10.3390/su12156022









