Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review
Abstract
1. Introduction
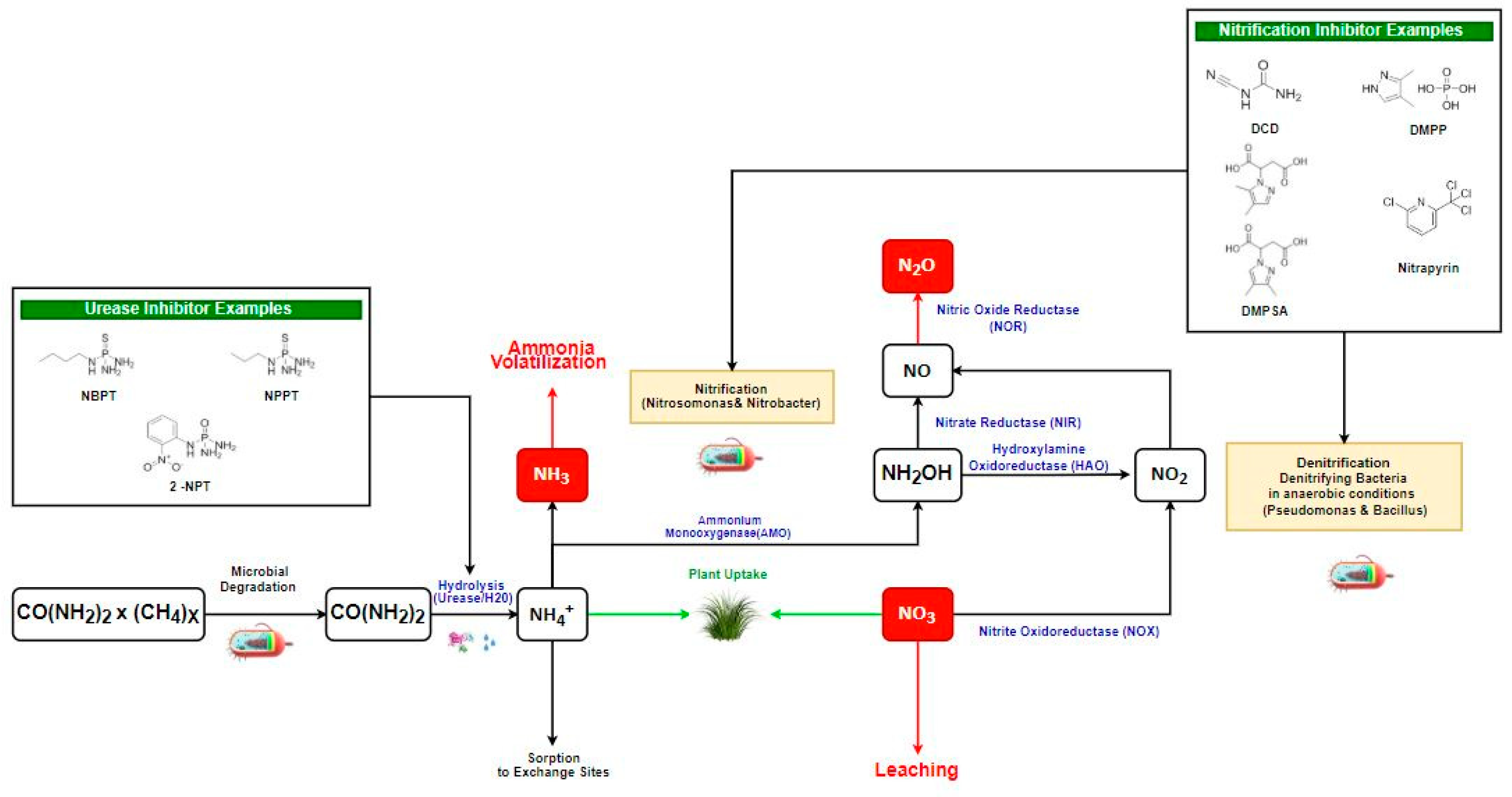
2. Nitrogen-Based Gas Emissions and Their Formation
3. Urease and Nitrification Inhibitor EU Regulation
4. Urease Inhibitors Types, Mode of Action, and Their Role in GHG Reduction
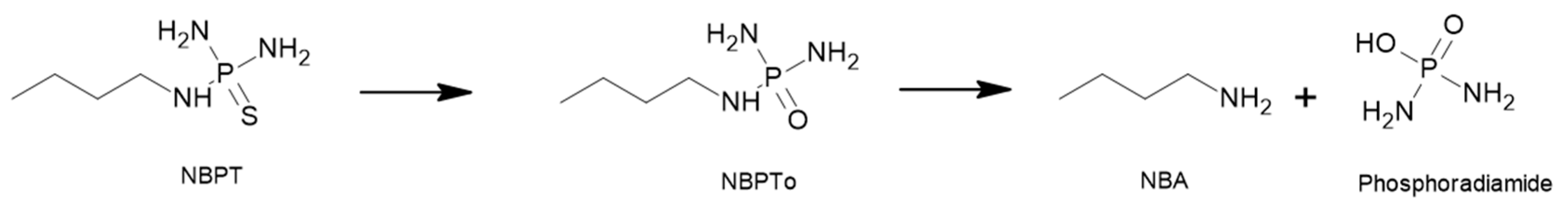
4.1. N-(n-butyl) Thiophosphoric Triamide (NBPT)
4.2. N-(n-propyl) Thiophosphoric Triamide (NPPT)
4.3. N-(2-nitrophenyl) Phosphoric Triamide (2-NPT)
5. Nitrification Inhibitors Types, Mode of Action, and Their Role in GHG Reduction
5.1. Dicyandiamide (DCD)
5.2. Nitrapyrin
5.3. 3,4-Dimethylpyrazole Phosphate (DMPP)
5.4. 3,4-Dimethylpyrazole Succinic (DMPSA)
6. Inhibitor Plant Uptake Potential
6.1. Urease Inhibitor Plant Uptake
6.2. Nitrification Inhibitor Plant Uptake
7. Survival of Inhibitor Compounds in Rumen
7.1. Survival of Urease Inhibitors in the Rumen
7.2. Survival of Nitrification Inhibitors in the Rumen
8. Passage of Contaminants into Milk via the Blood-Milk Barrier
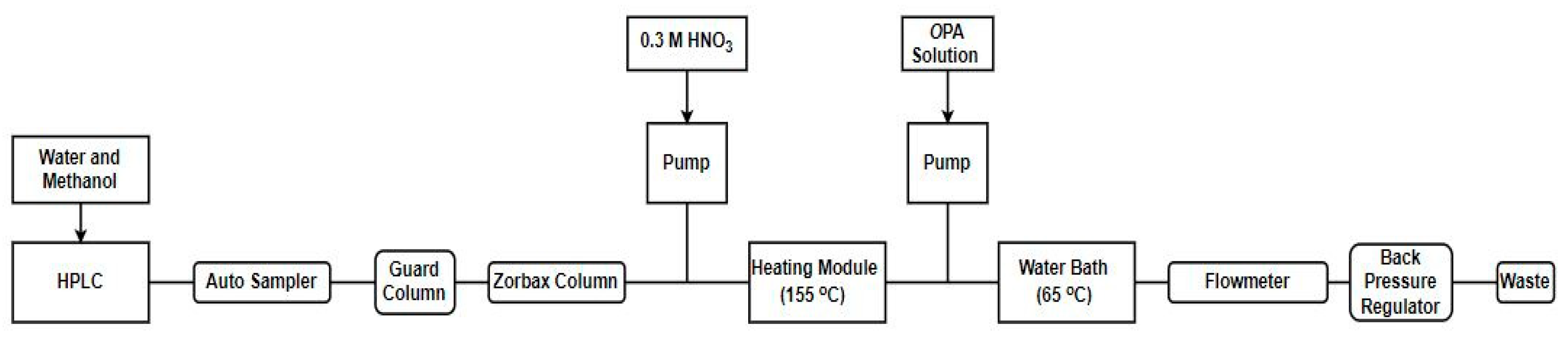
9. Analytical Methods for the Detection of Inhibitors in Complex Milk and Environmental Matrices
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hertel, T.W. The global supply and demand for agricultural land in 2050: A perfect storm in the making? Am. J. Agric. Econ. 2011, 93, 259–275. [Google Scholar] [CrossRef]
- Irish Department of Communications, Climate and Environoment—National Mitigation Plan July 2017 Ireland. Available online: https://www.dccae.gov.ie/en-ie/climate-action/publications/Documents/7/National%20Mitigation%20Plan%202017.pdf (accessed on 9 June 2020).
- European Commission. 2020 Climate & Energy Package; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- EuropeanCommission. 2030 Climate & Energy Framework; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- EuroStat. Agri-Environmental Indicator-Greenhouse Gas Emission; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- EPA. Ireland’s Greenhouse Gas Emission Projections 2012-2030; Envionmental Protection Agency: Washington, DC, USA, 2013. [Google Scholar]
- Roche, L.; Forrestal, P.; Lanigan, G.; Richards, K.; Shaw, L.; Wall, D. Impact of fertiliser nitrogen formulation, and N stabilisers on nitrous oxide emissions in spring barley. Agric. Ecosyst. Environ. 2016, 233, 229–237. [Google Scholar] [CrossRef]
- Minet, E.; Jahangir, M.; Krol, D.; Rochford, N.; Fenton, O.; Rooney, D.; Lanigan, G.; Forrestal, P.; Breslin, C.; Richards, K.; et al. Amendment of cattle slurry with the nitrification inhibitor dicyandiamide during storage: A new effective and practical N2O mitigation measure for landspreading. Agric. Ecosyst. Environ. 2016, 215, 68–75. [Google Scholar] [CrossRef][Green Version]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.; de Brito, S. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Danaher, M.; Jordan, K. Identification of existing and emerging chemical residue contamination concerns in milk. Ir. J. Agric. Food Res. 2013, 52, 173–183. [Google Scholar]
- Singh, S.N.; Verma, A. The potential of nitrification inhibitors to manage the pollution effect of nitrogen fertilizers in agricultural and other soils: A review. Environ. Rev. 2007, 9, 266–279. [Google Scholar] [CrossRef]
- Change, C. Synthesis Report of the IPCC Fifth Assessment Report; IPCC: Geneva, Switzerlad, 2014. [Google Scholar]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in atmospheric constituents and in radiative forcing. Chapter 2. In Climate Change 2007; The Physical Science Basis; IPCC: Geneva, Switzerlad, 2007. [Google Scholar]
- Hyde, B.; Forrestal, P.J.; Jahangir, M.M.; Ryan, M.; Fanning, A.; Carton, O.T.; Lanigan, G.; Richards, K.G. The interactive effects of fertiliser nitrogen with dung and urine on nitrous oxide emissions in grassland. Ir. J. Agric. Food Res. 2016, 55, 1–9. [Google Scholar] [CrossRef][Green Version]
- Burger, M.; Venterea, R.T. Understanding Greenhouse Gas Emissions from Agricultural Management; Guo, L., Gunasekara, A.S., McConnell, L.L., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1072, pp. 179–202. [Google Scholar]
- Bøckman, O.C.; Olfs, H.W. Fertilizers, agronomy and N2O. Nutr. Cycl. Agroecosyst. 1998, 52, 165–170. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecuária Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Minick, K.; Pandey, C.; Fox, T.; Subedi, S. Dissimilatory nitrate reduction to ammonium and N2O flux: Effect of soil redox potential and N fertilization in loblolly pine forests. Biol. Fertil. Soils 2016, 52, 601–614. [Google Scholar] [CrossRef]
- Florio, A.; Bréfort, C.; Gervaix, J.; Bérard, A.; Le Roux, X. The responses of NO2-and N2O-reducing bacteria to maize inoculation by the PGPR Azospirillum lipoferum CRT1 depend on carbon availability and determine soil gross and net N2O production. Soil Biol. Biochem. 2019, 136, 107524. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Loubet, B.; Decuq, C.; Palumbo, A.D.; Di Tommasi, P.; Magliulo, V.; Masson, S.; Personne, E.; Cellier, P.; Rana, G.; et al. Ammonia volatilisation following urea fertilisation in an irrigated sorghum crop in Italy. Agric. For. Meteorol 2014, 195–196, 179–191. [Google Scholar] [CrossRef]
- Hyde, B.P.; Carton, O.T.; O’Toole, P.; Misselbrook, T.H. A new inventory of ammonia emissions from Irish agriculture. Atmos. Environ. 2003, 37, 55–62. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.; Blennerhassett, J.; Quin, B. Reducing NH3, N2O and NO3–N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Harty, M.A.; Forrestal, P.J.; Watson, C.J.; McGeough, K.L.; Carolan, R.; Elliot, C.; Krol, D.; Laughlin, R.J.; Richards, K.G.; Lanigan, G.J.; et al. Reducing nitrous oxide emissions by changing June d N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016, 563–564, 576–586. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdenas, L.M. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Shibata, H.; Branquinho, C.; McDowell, W.H.; Mitchell, M.J.; Monteith, D.T.; Tang, J.; Arvola, L.; Cruz, C.; Cusack, D.F.; Halada, L.; et al. Consequence of altered nitrogen cycles in the coupled human and ecological system under changing climate: The need for long-term and site-based research. Ambio 2015, 44, 178–193. [Google Scholar] [CrossRef]
- Kafarski, P.; Talma, M. Recent advances in design of new urease inhibitors: A review. J. Adv. Res. 2018, 13, 101–112. [Google Scholar] [CrossRef]
- Overrein, L.N.; Moe, P.G. Factors affecting urea hydrolysis and ammonia volatilization in soil1. Soil Sci. Soc. Am. J. 1967, 31, 57–61. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EC) No 1107/2008 of Amending Regulation (EC) No 2003/2003 of the European Parliament and of the Council Relating to Fertilisers; European Parliament: Brussel, Belgium, 7 November 2008.
- The European Food Safty Authority. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related toCreatine monohydrate for use in foods for particular nutritional uses Question number EFSA-Q-2003-12. EFSA J. 2004, 36, 1–6. [Google Scholar] [CrossRef]
- REGULATION (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers; Europian Parliament: Brussel, Belgium, 2003.
- European Commission. COMMISSION REGULATION (EU) No 1257/2014 Amending Regulation (EC) No 2003/2003; Eurpoean Parliament: Brussel, Belgium, 2014. [Google Scholar]
- The European Chemicals Agency ECHA. Nitrapyrin. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.016.076 (accessed on 9 June 2020).
- European Commission. COMMISSION REGULATION (EU) 2019/1102; European Parliament: Brussel, Belgium, 2019. [Google Scholar]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol. Biochem. 1999, 31, 789–796. [Google Scholar] [CrossRef]
- Kumari, D.; Qian, X.Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced carbonate precipitation for immobilization of toxic metals. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 94, pp. 79–108. [Google Scholar]
- Hadjipavlou-Litina, D.; Gupta, S.P. Design and development of inhibitors of herpes viral proteases and their SAR and QSAR. In Viral Proteases and Their Inhibitors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 441–467. [Google Scholar]
- Shah, M.R.; Soomro, Z.H. Urease inhibition. In Enzyme Inhibition and Bioapplications; InTech: London, UK, 2012. [Google Scholar]
- Upadhyay, L.S.B. Urease Inhibitors: A Review; NISCAIR-CSIR: New Delhi, India, 2012. [Google Scholar]
- Engelking, L.R. Textbook of Veterinary Physiological Chemistry, Updated 2/e; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Krajewska, B.; Ureases, I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B. Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Benini, S.; Ciurli, S. The impact of pH on catalytically critical protein conformational changes: The case of the urease, a nickel enzyme. Chem. Eur. J. 2019, 25, 12145–12158. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, L.; Cianci, M.; Contaldo, U.; Musiani, F.; Ciurli, S. Urease inhibition in the presence of N-(n-Butyl) thiophosphoric triamide, a suicide substrate: Structure and kinetics. Biochemistry 2017, 56, 5391–5404. [Google Scholar] [PubMed]
- Karamanos, R.; Holzapfel, C.; Nybo, B.; Puurveen, D.; Shirtliffe, S. The impact of DCD and NBPT concentration on nitrification and volatilization. In Proceedings of the Soils and Crops Workshop, Saskatoon, SK, Canada, 9–10 March 2019. [Google Scholar]
- Ning, J.; Ai, S.; Cui, L. Dicyandiamide has more inhibitory activities on nitrification than thiosulfate. PLoS ONE 2018, 13, e0200598. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Torralbo, F.; Menéndez, S.; Barrena, I.; Estavillo, J.M.; Marino, D.; González-Murua, C. Dimethyl pyrazol-based nitrification inhibitors effect on nitrifying and denitrifying bacteria to mitigate N2O emission. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Forrestal, P.J.; Harty, M.; Carolan, R.; Lanigan, G.J.; Watson, C.J.; Laughlin, R.J.; McNeill, G.; Chambers, B.J.; Richards, K.G. Ammonia emissions from urea, stabilized urea and calcium ammonium nitrate: Insights into loss abatement in temperate grassland. Soil Use Manag. 2016, 32, 92–100. [Google Scholar] [CrossRef]
- Watson, C.J.; Miller, H. Short-term effects of urea amended with the urease inhibitor N-(n-butyl) thiophosphoric triamide on perennial ryegrass. Plant Soil 1996, 184, 33–45. [Google Scholar] [CrossRef]
- Forrestal, P.J.; Harty, M.A.; Carolan, R.; Watson, C.J.; Lanigan, G.J.; Wall, D.P.; Hennessy, D.; Richards, K.G. Can the agronomic performance of urea equal calcium ammonium nitrate across nitrogen rates in temperate grassland? Soil Use Manag. 2017, 33, 243–251. [Google Scholar] [CrossRef]
- Domínguez, M.J.; Sanmartín, C.; Font, M.; Palop, J.A.; San Francisco, S.; Urrutia, O.; Houdusse, F.; García-Mina, J.M. Design, synthesis, and biological evaluation of phosphoramide derivatives as urease inhibitors. J. Agric. Food Chem. 2008, 56, 3721–3731. [Google Scholar] [CrossRef] [PubMed]
- Rochette, P.; MacDonald, J.D.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; Bertrand, N. Banding of urea increased ammonia volatilization in a dry acidic soil. J. Environ. Qual. 2009, 38, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.; Williams, E.; Wallander, R.; Hilmer, J. Apparent persistence of N-(n-butyl) thiophosphoric triamide is greater in alkaline soils. Soil Sci. Soc. Am. J. 2013, 77, 1424–1429. [Google Scholar] [CrossRef]
- The European Chemicals Agency ECHA. [diamino(sulfanylidene)-lambda5-phosphanyl](propyl)amine. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.127.866 (accessed on 9 June 2020).
- Pro, D.; Huguet, S.; Arkoun, M.; Nugier-Chauvin, C.; Garcia-Mina, J.M.; Ourry, A.; Wolbert, D.; Yvin, J.C.; Ferrières, V. From algal polysaccharides to cyclodextrins to stabilize a urease inhibitor. Carbohydr. Polym. 2014, 112, 145–151. [Google Scholar] [CrossRef] [PubMed]
- The Australian Governoment Department of Health and Aging; National Industrial Chemicals Notification and Assessment Scheme NICNAS. Existing Chemical Secondary Notification Assessment NA/467S N-(n-butyl) Thiophosphoric Triamide (NBPT) Aging; The Australian Governoment Department of Health and Aging: Sydney, Australia, 2011. [Google Scholar]
- Engel, R.E.; Towey, B.D.; Gravens, E. Degradation of the urease inhibitor NBPT as affected by soil pH. Soil Sci. Soc. Am. J. 2015, 79, 1674–1683. [Google Scholar] [CrossRef]
- The European Chemicals Agency. NPPT; ECHA: Helsinki, Finland, 2019. [Google Scholar]
- The European Chemicals Agency ECHA. N-(2-nitrophenyl)phosphoric Triamide. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.105.163 (accessed on 9 June 2020).
- The European Chemicals Agency ECHA. Cyanoguanidine. Available online: https://echa-term.echa.europa.eu/da/web/guest/substance-information/-/substanceinfo/100.006.649 (accessed on 9 June 2020).
- The European Chemicals Agency ECHA. 3,4-Dimethyl-1H-pyrazol-1-ium Dihydrogen Phosphate. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.102.315 (accessed on 9 June 2020).
- Marsden, K.A.; Marín-Martínez, A.J.; Vallejo, A.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: A comparison between DCD and DMPP. Biol. Fertility Soils 2016, 52, 491–503. [Google Scholar] [CrossRef]
- Tao, L.; Yuanliang, S.; Xuewen, L.; Guolin, L. Degradation and its affecting factors of NBPT in soil. Chin. J. Ecol. 2006, 25, 1082–1086. [Google Scholar]
- ECHA, E.C.A. Pre-registered Reach Substances. Available online: https://echa.europa.eu/information-on-chemicals/pre-registered-substances/-/dislist/substance/100.112.861 (accessed on 5 June 2019).
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- The Australian Governoment Department of Health and Aging; National Industrial Chemicals Notification and Assessment Scheme NICNAS. N-(n-butyl) Thiophosphoric Triamide (NBPT) Aging; The Australian Governoment Department of Health and Aging: Sydney, Australia, 1997. [Google Scholar]
- Silva, A.G.; Sequeira, C.H.; Sermarini, R.A.; Otto, R. Urease inhibitor NBPT on ammonia volatilization and crop productivity: A meta-analysis. Agron. J. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Ramaty, E.; Maor, E.; Peltz-Sinvani, N.; Brom, A.; Grinfeld, A.; Kivity, S.; Segev, S.; Sidi, Y.; Kessler, T.; Sela, B.; et al. Low ALT blood levels predict long-term all-cause mortality among adults. A historical prospective cohort study. Eur. J. Intern. Med. 2014, 25, 919–921. [Google Scholar]
- Van de Ligt, J.; Borghoff, S.J.; Yoon, M.; Ferguson, L.J.; DeMaio, W.; McClanahan, R.H. Nondetectable or minimal detectable residue levels of N-(n-butyl) thiophosphoric triamide in bovine tissues and milk from a 28-d NBPT dosing study. Transl. Anim. Sci. 2019, 3. [Google Scholar] [CrossRef]
- Li, Q.; Yang, A.; Wang, Z.; Roelcke, M.; Chen, X.; Zhang, F.; Pasda, G.; Zerulla, W.; Wissemeier, A.H.; Liu, X.; et al. Effect of a new urease inhibitor on ammonia volatilization and nitrogen utilization in wheat in north and northwest China. Field Crops Res. 2015, 175, 96–105. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Lu, J.; Wang, Z. Effect of mixed urease inhibitors on N losses from surface-applied urea. Intern. J. Agric. Sci. Technol. 2015, 3, 23–27. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Cui, X.; Liu, X.; Roelcke, M.; Pasda, G.; Zerulla, W.; Wissemeier, A.H.; Chen, X.; Goulding, K.; Zhang, F.; et al. A new urease-inhibiting formulation decreases ammonia volatilization and improves maize nitrogen utilization in North China Plain. Sci. Rep. 2017, 7, 43853. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Kage, H.; Pacholski, A. Effects of novel nitrification and urease inhibitors (DCD/TZ and 2-NPT) on N2O emissions from surface applied urea: An incubation study. Atmos. Environ. 2018, 175, 75–82. [Google Scholar] [CrossRef]
- Ni, K.; Pacholski, A.; Kage, H. Ammonia volatilization after application of urea to winter wheat over 3 years affected by novel urease and nitrification inhibitors. Agric. Ecosyst. Environ. 2014, 197, 184–194. [Google Scholar] [CrossRef]
- Schraml, M.; Gutser, R.; Schmidhalter, U. Abatement of NH3 emissions following application of urea to grassland by means of the new urease inhibitor 2-NPT. In Proceedings of the 18th Symposium of the International Scientific Centre of Fertilizers, Rome, Italy, 8–12 November 2010. [Google Scholar]
- Kirschke, T.; Spott, O.; Vetterlein, D. Impact of urease and nitrification inhibitor on NH4+ and NO3− dynamic in soil after urea spring application under field conditions evaluated by soil extraction and soil solution sampling. J. Plant Nutr. Soil Sci. 2019, 182, 441–450. [Google Scholar] [CrossRef]
- Lan, T.; Han, Y.; Roelcke, M.; Nieder, R.; Cai, Z. Effects of the nitrification inhibitor dicyandiamide (DCD) on gross N transformation rates and mitigating N2O emission in paddy soils. Soil Biol. Biochem. 2013, 67, 174–182. [Google Scholar] [CrossRef]
- Skiba, U.; Smith, K.A.; Fowler, D. Nitrification and denitrification as sources of nitric oxide and nitrous oxide in a sandy loam soil. Soil Biol. Biochem. 1993, 25, 1527–1536. [Google Scholar] [CrossRef]
- McTaggart, I.; Clayton, H.; Parker, J.; Swan, L.; Smith, K.A. Nitrous oxide emissions from grassland and spring barley, following N fertiliser application with and without nitrification inhibitors. Biol. Fertil. Soils 1997, 25, 261–268. [Google Scholar] [CrossRef]
- McGeough, K.L.; Watson, C.J.; Müller, C.; Laughlin, R.J.; Chadwick, D.R. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol. Biochem. 2016, 94, 222–232. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)–A new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Lasa, B.; Aparicio-Tejo, P.M.; González-Murua, C.; Marino, D. 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: Quantification in plant tissues and toxicity assays. Sci. Total Environ. 2018, 624, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, E.; Minet, E.; Ernfors, M.; Müller, C.; Devaney, D.; Forrestal, P.J.; Richards, K.G. The effect of precipitation and application rate on dicyandiamide persistence and efficiency in two Irish grassland soils. Soil Use Manag. 2015, 31, 367–374. [Google Scholar] [CrossRef]
- Shepherd, M.; Wyatt, J.; Welten, B. Effect of soil type and rainfall on dicyandiamide concentrations in drainage from lysimeters. Soil Res. 2012, 50, 67–75. [Google Scholar] [CrossRef]
- Zacherl, B.; Amberger, A. Effect of the nitrification inhibitors dicyandiamide, nitrapyrin and thiourea onNitrosomonas europaea. Fertil. Res. 1990, 22, 37–44. [Google Scholar] [CrossRef]
- Klotz, M.G.; Stein, L.Y. Research on Nitrification and Related Processes; Academic Press: Cambridge, MA, USA, 2011; Volume 496. [Google Scholar]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use Manag. 2002, 18, 395–403. [Google Scholar] [CrossRef]
- Subbarao, G.; Ito, O.; Sahrawat, K.; Berry, W.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I.M.; et al. Scope and strategies for regulation of nitrification in agricultural systems—Challenges and opportunities. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Kuo, J. Air Pollution Control: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Hauser, M.; Haselwandter, K. Degradation of dicyandiamide by soil bacteria. Soil Biol. Biochem. 1990, 22, 113–114. [Google Scholar] [CrossRef]
- Wilcock, R.; Elliott, S.; Hudson, N.; Parkyn, S.; Quinn, J. Climate change mitigation for agriculture: Water quality benefits and costs. Water Sci. Technol. 2008, 58, 2093–2099. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Espín, S.; García-Fernández, A. Nitrapyrin. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 519–522. [Google Scholar]
- Keiblinger, K.M.; Zehetner, F.; Mentler, A.; Zechmeister-Boltenstern, S. Biochar application increases sorption of nitrification inhibitor 3,4-dimethylpyrazole phosphate in soil. Environ. Sci. Pollut. Res. 2018, 25, 11173–11177. [Google Scholar] [CrossRef]
- Huérfano, X.; Fuertes-Mendizábal, T.; Fernández-Diez, K.; Estavillo, J.M.; González-Murua, C.; Menéndez, S. The new nitrification inhibitor 3, 4-dimethylpyrazole succinic (DMPSA) as an alternative to DMPP for reducing N2O emissions from wheat crops under humid Mediterranean conditions. Eur. J. Agron. 2016, 80, 78–87. [Google Scholar] [CrossRef]
- Pacholski, A.; Berger, N.; Bustamante, I.; Ruser, R.; Guardia, G.; Mannheim, T. Effects of the novel nitrification inhibitor DMPSA on yield, mineral N dynamics and N2O emissions. In Proceedings of the 2016 International Nitrogen Initiative Conference, Solutions to Improve Nitrogen Use Efficiency for the World, Melbourne, Australia, 4–8 December 2016. [Google Scholar]
- The European Chemicals Agency ECHA. Reaction Mass of 2-(3,4-Dimethyl-1H-pyrazol-1-yl)succinic Acid and 2-(4,5-Dimethyl-1H-pyrazol-1-yl)succinic Acid. Available online: https://echa.europa.eu/da/registration-dossier/-/registered-dossier/11890/2/3 (accessed on 9 June 2020).
- Soliman, M.S.; Monem, M.A.A. Effect of method of N-application and modified urea on N-15 recovery by rice. Fertil. Res. 1995, 43, 143–148. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, L.; Van Cleemput, O.; Wang, Z. Fate of urea-15N in a soil-wheat system as influenced by urease inhibitor hydroquinone and nitrification inhibitor dicyandiamide. Plant Soil 2000, 220, 261–270. [Google Scholar] [CrossRef]
- Gioacchini, P.; Nastri, A.; Marzadori, C.; Giovannini, C.; Antisari, L.V.; Gessa, C. Influence of urease and nitrification inhibitors on N losses from soils fertilized with urea. Biol. Fertil. Soils 2002, 36, 129–135. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Zamboni, A.; Varanini, Z.; Pinton, R. The urease inhibitor NBPT negatively affects DUR3-mediated uptake and assimilation of urea in maize roots. Front. Plant Sci. 2015, 6, 1007. [Google Scholar] [CrossRef]
- Cruchaga, S.; Artola, E.; Lasa, B.; Ariz, I.; Ignacio, I.; Moran, J.; Aparicio-Tejo, P. Short term physiological implications of NBPT application on the N metabolism of Pisum sativum and Spinacea oleracea. J. Plant Physiol. 2010, 168, 329–336. [Google Scholar] [CrossRef]
- Krogmeier, M.J.; McCarty, G.W.; Bremner, J.M. Potential phytotoxicity associated with the use of soil urease inhibitors. Proc. Natl. Acad. Sci. USA 1989, 86, 1110–1112. [Google Scholar] [CrossRef]
- Ariz, I.; Cruchaga, S.; Lasa, B.; Moran, J.F.; Jauregui, I.; Aparicio-Tejo, P.M. The physiological implications of urease inhibitors on N metabolism during germination of Pisum sativum and Spinacea oleracea seeds. J. Plant Physiol. 2012, 169, 673–681. [Google Scholar] [CrossRef][Green Version]
- Zacharia, J.T. Identity, physical and chemical properties of pesticides. In Pesticides in the Modern World-Trends in Pesticides Analysis; BoD–Books on Demand: Norderstedt, Germany, 2011; pp. 1–18. [Google Scholar]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine-e-Book: A Textbook of the Diseases of Cattle, Horses, Hheep, Pigs and Goats; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- O’Connor, P.J.; Hennessy, D.; Lynch, M.B.; Slattery, H.; Lewis, E. The effect of dicyandiamide on rumen and blood metabolites, diet digestibility and urinary excretion. Livest. Sci. 2013, 155, 30–37. [Google Scholar] [CrossRef]
- Welten, B.G.; Ledgard, S.F.; Schipper, L.A.; Waller, J.E.; Kear, M.J.; Dexter, M.M. Effects of prolonged oral administration of dicyandiamide to dairy heifers on excretion in urine and efficacy in soil. Agric. Ecosyst. Environ. 2013, 173, 28–36. [Google Scholar] [CrossRef]
- Welten, B.; Ledgard, S.; Balvert, S.; Kear, M.; Dexter, M. Effects of oral administration of dicyandiamide to lactating dairy cows on residues in milk and the efficacy of delivery via a supplementary feed source. Agric. Ecosyst. Environ. 2016, 217, 111–118. [Google Scholar] [CrossRef]
- Minet, E.; Ledgard, S.; Lanigan, G.; Murphy, J.; Grant, J.; Hennessy, D.; Lewis, E.; Forrestal, P.; Richards, K. Mixing dicyandiamide (DCD) with supplementary feeds for cattle: An effective method to deliver a nitrification inhibitor in urine patches. Agric. Ecosyst. Environ. 2016, 231, 114–121. [Google Scholar] [CrossRef]
- Luo, J.; Ledgard, S.; Wise, B.; Welten, B.; Lindsey, S.; Judge, A.; Sprosen, M. Effect of dicyandiamide (DCD) delivery method, application rate, and season on pasture urine patch nitrous oxide emissions. Biol. Fertil. Soils 2015, 51, 453–464. [Google Scholar] [CrossRef]
- Kelsey, J.J. Drug Principles in Lactation. Available online: https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf (accessed on 20 June 2020).
- Peck, T.; Hill, S.; Williams, M. Drug passage across the cell membrane. In Pharmacology for Anaesthesia and Intensive Care, 2nd ed.; Greenwich Medical Media: London, UK, 2003. [Google Scholar]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. 2008, 25, 172–180. [Google Scholar] [CrossRef]
- Bhal, S.K. LogP—Making Sense of the Value; Advanced Chemistry Development: Toronto, ON, Canada, 2007; pp. 1–4. [Google Scholar]
- Rainard, P. Consequences of interference of milk with chemoattractants for enzyme-linked immunosorbent assay quantifications. Clin. Vaccine Immunol. CVI 2010, 17, 848–852. [Google Scholar] [CrossRef]
- Douglass, E.A.; Hendrickson, L.L. HPLC Method for the Analysis of the Urease Inhibitor N-(n-butyl) thiophosphoric Triamide and Its Metabolites. J. Agric. Food Chem. 1991, 39, 2318–2321. [Google Scholar] [CrossRef]
- Fertilizers, E. Determination of Urease Inhibitor N-(nbutyl) Thiophosphoric Triamide (NBPT) Using High-Performance Liquid Chromatography (HPLC); European Commission: Brussel, Belgium, 2007. [Google Scholar]
- Yogesha, S.; Arun, K.; Shetty, S.G.; Ravi, H.; Ramesh, N. Simultaneous quantification of N-butylthiophosphoric triamide and dicyandiamide in urea formulation by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2018, 42, 484–490. [Google Scholar] [CrossRef]
- Scheurer, M.; Brauch, H.J.; Schmidt, C.K.; Sacher, F. Occurrence and fate of nitrification and urease inhibitors in the aquatic environment. Environ. Sci. Proces. Impacts 2016, 18, 999–1010. [Google Scholar] [CrossRef]
- Schwarzer, C.; Haselwandter, K. Rapid quantification of the nitrification inhibitor dicyandiamide in soil samples, nutrient media and bacterial cell-free extracts. J. Chromatogr. A 1996, 732, 390–393. [Google Scholar] [CrossRef]
- Shen, Y.; Han, C.; Zhou, X.; Chen, X.; Huang, F.; Zhu, Z. Microwave-assisted extraction and determination of dicyandiamide residue in infant formula samples by liquid chromatography-tandem mass spectrometry. J. Dairy Sci. 2013, 96, 6877–6882. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, S.; Begley, T.H.; Diachenko, G.W.; Stromgren, S.A. A liquid chromatography–tandem mass spectrometry method for the detection of economically motivated adulteration in protein-containing foods. J. Chromatogr. A 2012, 1220, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Zhou, L.X.; Zhao, Y.G.; Pan, S.D.; Jin, M.C. Application of nanoring amino-functionalized magnetic polymer dispersive micro-solid-phase extraction and ultra fast liquid chromatography–tandem mass spectrometry in dicyandiamide residue analysis of powdered milk. Talanta 2014, 119, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Sakamoto, T.; Min, J.Z.; Todoroki, K.; Toyo’oka, T. Determination of dicyandiamide in infant formula by stable isotope dilution hydrophilic interaction liquid chromatography with tandem mass spectrometry. Food Chem. 2014, 156, 390–393. [Google Scholar] [CrossRef]
- Draher, J.; Pound, V.; Reddy, T.M. Validation of a rapid method of analysis using ultrahigh-performance liquid chromatography-tandem mass spectrometry for nitrogen-rich adulterants in nutritional food ingredients. J. Chromatogr. A 2014, 1373, 106–113. [Google Scholar] [CrossRef]
- Zou, H.; Xu, K.; Feng, Y.; Liang, B. Application of first order derivative UV spectrophotometry coupled with H-point standard addition to the simultaneous determination of melamine and dicyandiamide in milk. Food Anal. Methods 2015, 8, 740–748. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, W.; Feng, Y.; Liang, B. Simultaneous determination of melamine and dicyandiamide in milk by UV spectroscopy coupled with chemometrics. Anal. Methods 2014, 6, 5865–5871. [Google Scholar] [CrossRef]
- Deng, H.H.; Hong, G.L.; Lin, F.L.; Liu, A.L.; Xia, X.H.; Chen, W. Colorimetric detection of urea, urease, and urease inhibitor based on the peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 915, 74–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; He, N.; Long, M.; Huang, J.; Zhang, G.; Wang, Q.; Han, X. Increase in ammonia volatilization from soil in response to N deposition in Inner Mongolia grasslands. Atmos. Environ. 2014, 84, 156–162. [Google Scholar] [CrossRef]
| Inhibitor Augmentation Based on % (w/w) by Mass of the total N Present as AN and Urea | |||||||
|---|---|---|---|---|---|---|---|
| Compound Name | Inhibitor Class | Min % (w/w) | Max % (w/w) | EC No 1 | RR Status 2 | TTB 3 | References |
| NBPT | Urease Inhibitor | 0.09 | 0.2 | 435-740-7 | Full | 100–1000 tpa | [30] |
| Mixture NBPT and NPPT (ratio 3:1 (1)) | 0.02 | 0.3 | 700-457-2 | 10–100 tpa | [31] | ||
| 2-NPT | 0.04 | 0.15 | 477-690-9 | 10–100 tpa | [30] | ||
| DCD | Nitrification Inhibitor | 2.3 | 4.5 | 207-312-8 | Full | 10,000–100,000 tpa | [30] |
| Nitrapyrin | N/A | N/A | 217-682-2 | 100–1000 tpa | [32] | ||
| DMPP | 0.8 | 1.6 | 424-640-9 | 100–1000 tpa | [31] | ||
| Isomeric mixture of 2-(3,4-dimethylpyrazole-1-yl)-succinic acid and DMPSA | 0.8 | 1.6 | 940-877-5 | 100 + tpa | [33] | ||
| Compound Name | Inhibitor Class | Mode of Inhibition Action | Inhibitor Chemical Class | Inhibition Type | Enzyme | References |
|---|---|---|---|---|---|---|
| NBPT | Urease inhibitor (Urea Analogue) | Delays urea hydrolysis via urease inhibition | Organic | Competitive, irreversible (suicide substrate) | Urease | [9,39] |
| NPPT | [9,39] | |||||
| 2-NPT | Competitive Metal chelator | [9,39] | ||||
| DCD | Nitrification inhibitor | Inhibition of oxidation of NH3 to NO2− via inhibition of ammonia monooxygenase (AMO) | Organic | Competitive Metal chelator | Ammonia monooxygenase (AMO) | [43,44] |
| Nitrapyrin | Non-competitive, Metal chelator | [45] | ||||
| DMPP | Metal chelator | [45] | ||||
| DMPSA | Expected to be competitive | [46] |
| Compound | Class/Use | Molecular Formula | Molecular Weight | Melting Point | Relative Density | Vapor Pressure | Water Solubility | Log Pow | References |
|---|---|---|---|---|---|---|---|---|---|
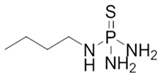 NBPT | C4H14N3PS | 167.2 | 59.1 °C at 101,300 Pa | 1.22 ca. at 20 °C | 0 Pa at 20 °C | 43.04 g/L at 25 °C pH = 7.26. | 0.444 at 20 °C | [53] | |
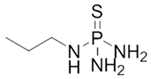 NPPT | Urease inhibition | C3H14N3PS | 153.186 | 91 °C at 101,325 Pa | 1.253 at 20 °C | 0 Pa at 20 °C | 51.5 g/L at 20 °C | 0.3 at 24 °C | [57] |
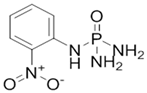 2-NPT | C6H9N4O3P | 216.13 | >260 °C | 1.558 at 20 °C | 0 Pa at 20 °C | 1394 mg/L at 20 °C | 0.51 at 25 °C | [58] | |
 DCD | Nitrification inhibition | C2H4N4 | 84.08 | 210−212 °C | 1.404 | 0.0045 Pa | 40 g/L | −1.0 at 20 +/− 0.5 °C | [59] |
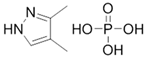 DMPP | C5H11N2O4P | 194.2 | 165 °C | 1.51 | <10−4 at 20 °C and 50 °C. | 132 g/L −1 at pH 3 and 25 °C | 0.92 | [60,61] | |
 DMPSA | C9H12N2O4 | 150–178 °C at ca. 1013 hPa | 1.402 at 20 °C | 0.002 Pa at 25 °C | 15.7 g/L at 20 °C, pH 1.9 | <−2.2 at 21 °C, pH 6 | [60] | ||
 Nitrapyrin | C6H3Cl4N | 230.9 | 62.5–63.6 °C | 1.55 at 20 °C | 0.37–0.64 Pa at 22.8–25 °C | 20.1–40.1 mg/L at 18.5–20 °C | 3.325–3.41 at 23.5–25 °C | [32] |
| Compound | Animal | Parameter | Exposure Type | Method of Administration | Dose (mg/kg/bw) | Sex | Toxicology Results and Clinical Observations | References |
|---|---|---|---|---|---|---|---|---|
| NBPT | Rat | LD50 | Acute exposure | Oral | 2000 | Male/Female | Low toxicity via oral and dermal exposure, LD50 in rats exceeded 2000 mg/kg/bw | [65] |
| Dermal | ||||||||
| Guinea Pig | Skin Sensitizer | Acute exposure | intradermal injections | - | Male/Female | Not a skin sensitizer. | [55] | |
| Rabbit | Ocular toxicity | Acute exposure | Ocular | 0.1 mL of test material (AGROTAIN, 25% NBPT) | Male/Female | Severe eye irritant with irritation observed on the conjunctiva, cornea, and iris. | [65] | |
| Rabbit | Ocular toxicity | Acute exposure | Ocular | 0.1 mL of NBPT (white powder) | Male/Female | Irreversible eye damage. Scattered or subtle corneal opacity and vascularization noted in the treated eye. NBPT considered an irritant with the potential to cause severe of eye damage. | [55] | |
| Rat | Neurotoxicity | Repeat exposure (13 weeks) | Oral | 200, 1000 and 5000 | Male | Decreased food utilization and lower body weights noted at 5000 mg/kg bw. neurotoxic study observed decreased gripping strength in 5000 mg/kg bw male. No effect observed during ophthalmoscopic examination. | [53] | |
| Female | Decreased food utilization and lower body weights noted at 5000 mg/kg bw. Decreased gripping strength and hunched posture observed in 5000 mg/kg bw females. No effect observed during ophthalmoscopic examination. | |||||||
| Hemotology | Repeat exposure (13 weeks) | Oral | 200, 1000 and 5000 | Male | At 5000 mg/kg bw increase lymphocyte counts were observed which resulted in significantly lower white blood cell counts. Alanine transaminase (ALT) levels and aspartate aminotransferase (AST) at 500 and 1000 mg/kg bw decreased | |||
| Female | At 5000 mg/kg bw platelets, were significantly increased. Alanine transaminase (ALT) levels and Aspartate Aminotransferase (AST) at 500 and 1000 mg/kg bw decreased. Phosphorus levels significantly decreased at 200 and 1000 mg/kg bw. | |||||||
| Organ toxcicity | Repeat exposure (13 weeks) | Oral | 200, 1000 and 5000 | Male | Increase in liver weights at 5000 mg/kg bw. Minimal centrilobular hepatocyte hypertrophy at 5000 mg/kg bw. | |||
| Female | Increase in uterine weights at 5000 mg/kg bw. Increased in uterine fluid and luminal distension at all dosages. Minimal centrilobular hepatocyte hypertrophy at 5000 mg/kg bw. Increased incidence of foci mineralization in kidney at 5000 mg/kg bw | |||||||
| Rat | Neurotoxicity | Repeat exposure (15 d) | Oral | 0, 250, 500, 1000 and 2000 | Male/Female | Decreases in cholinesterase levels observed in brain tissue and erythrocytes the at 500, 1000 and 2000 mg/kg bw | [65] | |
| Hematology | Repeat exposure (15 d) | Oral | 0, 250, 500, 1000 and 2000 | Male | Liver damage observed with no chlorosis at 500 mg/kg bw, no change in liver weight. At 500–2000 mg/kg bw decreased triglycerides observed. | |||
| Female | Liver damage observed with no chorosis at 500 mg/kg/bw no change in liver weight. At 500 mg/kg/bw decreased blood urea & total cholesterol observed. | |||||||
| Organ toxcicity | Repeat exposure (15 d) | Oral | 0, 250, 500, 1000 and 2000 | Male/Female | Spleen: body weight ratio affected at 500 mg/kg/bw and above. | |||
| Rat | Reproductive toxicity | Repeat exposure (over two generations) | Oral | 0, 200, 800 and 3200 | Male | Abnormalities in sperm counts at 84 mg/kg bw/day(d), male rats exhibited epididymal lesions. The No-observed-adverse-effect level (NOAEL) is 21 mg/kg bw/d. | [55] | |
| Female | Increases in uterine weight at 88 mg/kg bw/d. NOAEL females 17 mg/kg bw/d. NBPT meets the requirements to be classified as a category three reproductive toxin. | |||||||
| Neurotoxicity | Repeat exposure (over two generations) | Oral | 0, 200, 800 and 3200 | Male | Decreased erythrocyte cholinesterase levels at 800 and 3200 mg/kg/bw. NOAEL males 74 mg/kg bw/d. based on neurobehavioral changes. | |||
| Female | Decreased erythrocyte cholinesterase levels at 800 and 3200 mg/kg/bw. | |||||||
| Hemotology | Repeat exposure (over two generations) | Oral | 0, 200, 800 and 3200 | Male | Effects on the liver and changes in hematology indicate a lowest-observed-adverse-effect level LOAEL of 377mg/kg bw/d. | |||
| Female | LOAEL for females 17 mg/kg bw/d due to uterine changes. |
| Compound | Animal | Parameter | Exposure Type | Method of Administration | Dose (mg/kg/bw) | Sex | Toxicology Results and Clinical Observations | References |
|---|---|---|---|---|---|---|---|---|
| NPPT | Rat | LD50 | Acute exposure | Oral | 2000 | Male/Female | 2000 mg/kg bw. | [57] |
| LD50 | Repeat exposure (28 d) | Oral | 200 | Male | 200 mg/kg bw. NOAEL 18.1 mg/kg bw | |||
| Female | 200 mg/kg bw. NOAEL 19.8 mg/kg bw. | |||||||
| LD50 | Acute exposure | Dermal | 2000 | Male/Female | 2000 mg/kg bw. | |||
| Developmental toxicity | Repeat Exposure | Oral | 100 | Female | NOAEL 100 mg/kg bw. |
| Compound | Animal | Parameter | Exposure Type | Method of Administration | Dose (mg/kg bw) | Sex | Toxicology Results and CLINICAL OBSERVATIONS | References |
|---|---|---|---|---|---|---|---|---|
| 2-NPT | Rat | LD50 | Acute exposure | Oral | >2000 | Male/Female | >2000 mg/kg bw. | [58] |
| Dermal | ||||||||
| Rat | Reproductive toxicity | Repeat Exposure | Oral | 0, 45, 135, 450 | Male/Female | NOAEL for parental animals is 45 mg/kg bw/d. NOAEL for reproductive toxicity and toxicity to first-generation pups is 135 mg/kg bw. | ||
| Rat | Reproductive toxicity | Repeat Exposure (28 d) | Oral | 0, 30, 100 and 300 | Male | NOAEL 30 mg/kg bw. At 300 mg/kg/bw increased leucocyte count and decreased organ weights for testes and epididymides. Irreversible effects to the micro-orchidia and epididymides. Damage to the testes across all dose groups and high dose groups completely void of spermatozoa. | ||
| Rat | Hemotology | Repeat Exposure (28 d) | Oral | 0, 30, 100 and 300 | Male/Female | Decreased levels of alkaline phosphatase and aspartate aminotransferase (AST) at high doses. | ||
| Rat | Organ toxicity | Repeat Exposure (28 d) | Oral | 0, 30, 100 and 300 | Male | Increased spleen weights. Dose-dependent kidney damage with degenerated tubular epithelium in the medulla of the kidneys observed at high doses. Dose dependent urine glucose increase. Discoloration of urine on day of administration. | ||
| Female | Dose-dependent kidney damage with degenerated tubular epithelium in the medulla of the kidneys at high doses. Dose-dependent urine glucose increase. Discoloration of urine on day of administration. |
| Compound | Animal | Parameter | Exposure Type | Method of Administration | Dose (mg/kg bw) | Sex | Toxicology Results and Clinical Observations | References |
|---|---|---|---|---|---|---|---|---|
| DCD | Rat | LD50 | Acute exposure | Oral | 10,000 | Male/Female | 10,000 mg/kg bw. | [59] |
| Rat | LD50 | Acute exposure | Inhalation | 259 mg/m³ | Male/Female | 259 mg/m³ | ||
| Rat | LD50 | Acute exposure | Dermal | 2000 | Male/Female | 2000 mg/kg bw. | ||
| Rat | Reproductive toxicity | Repeat exposure | Oral | 0, 30, 100 and 300 | Male | NOEAL for Reproductive toxicity 15,000 mg/kg bw | ||
| Female | NOEAL for Reproductive toxicity 15,000 mg/kg bw. Significantly lower body weights in the high dose females exposed to 50,000 mg/kg and slightly lower fertility and pregnancy rates in females in this group, therefore, the LOAEL for reproductive toxicity is 50,000 mg/kg. | |||||||
| Rat | Two-year carcinogenicity study | Chronic exposure | Oral | Up to 50,000 | Male/Female | No increase in tumours; non-carcinogenic |
| Compound | Animal | Parameter | Exposure Type | Method of Administration | Dose (mg/kg bw) | Sex | Toxicology Results and Clinical Observations | References |
|---|---|---|---|---|---|---|---|---|
| Nitrapyrin | Rat | LD50 | Acute exposure | Oral | 5000 | Male/Female | 5000 mg/kg bw | [32] |
| Rat | LD50 | Acute exposure | Inhalation | 630–3510 mg/m³ | Male/Female | 630–3510 mg/m³ | ||
| Rabbit | LD50 | Acute exposure | Dermal | 2000 | Male/Female | 2000 mg/kg bw. | ||
| Rat | NOEAL | Repeat exposure | Oral | 5 | Male/Female | NOAEL 5 mg/kg bw/day | ||
| Rabbit | NOEAL | Repeat exposure | Dermal | 2000, 2.2 mg/cm² | Male/Female | Dermal NOAEL for systemic effects 1000 mg/kg bw/day, Dermal NOAEL for Local effects 2.2 mg/cm² | ||
| Rat | NOEAL Reproductive toxicity | Repeat exposure | Oral | 75, 50 | Male/Female | NOEAL for Reproductive toxicity 75 mg/kg bw/day | ||
| NOEAL for Developmental toxicity 50 mg/kg bw/day | ||||||||
| Rat | NOEAL Neurotoxicity | Repeat exposure | Oral | 40 | Male/Female | 40 mg/kg bw/day | ||
| Mouse | NOAEL carcinogenicity | Chronic exposure | Oral | 125 | Male/Female | NOAEL 125 mg/kg bw/day |
| Compound | Animal | Parameter | Exposure Type | Method of Administration | Dose (mg/kg bw) | Sex | Toxicology Results and Clinical Observations | References |
|---|---|---|---|---|---|---|---|---|
| DMPSA | Rat | LD50 | Acute exposure | Oral | >2000 | Male/Female | >2000 mg/kg bw | [32,96] |
| Rat | LD50 | Acute exposure | Dermal | >2000 | Male/Female | >2000 mg/kg bw. | ||
| Rat | NOEAL | Repeat exposure | Oral | ≥1000 | Male/Female | NOAEL ≥ 1000 mg/kg bw/day | ||
| Rat | NOEAL Reproductive toxicity | Repeat exposure | Oral | 1000 | Male/Female | NOEAL for Reproductive toxicity 1000 mg/kg bw/day. No developmental effects were observed. |
| Detection Method | Target Analytes | Matrix | Sample Preparation | Column | Mobile Phase | Observed Ions | Limit of Quantification (LOQ) (μg/kg) | References |
|---|---|---|---|---|---|---|---|---|
| HPLC & post-column derivatisation with fluorescence detection | NBPT, NBPTo, NBA | Soil | Extracted with deionized water & filtered (0.22 µm) | Zorbax-Rx C8 column (4.6 × 250 mm) | 40% H2O 60% MeOH | N/A | 1000 | [117] |
| HPLC | NBPT and NPPT | Urea Fertilizer | Detailed in EN 1482-1 | C18 Nucleosil (250 mm × 4 mm, 5 μm) | MeCN + H2O 10 + 90 | N/A | N/A | [118] |
| Detailed in EN 1482-1 | LiChroSpher C18 (250 mm × 4 mm, 5 μm) | MeCN + H2O 15 + 85 | 168.2 m/z and 74 m/z | N/A | ||||
| LC-MS/MS (Positive mode) | DCD and NBPT | Urea Fertilizer | Dilution & filtration (0.45 μm) | Obelsc-R column (4.6 × 150 mm, 5 um) | (A) 0.05% formic acid + 10mM ammonium acetate + H20 (B) 0.05% formic acid + MeCN | m/z values of 85→68 for DCD and m/z 168.2 → 74 for NBPT | 25 & 5 respectively | [119] |
| LC-ESI-MS/MS (Positive mode) | DCD, NBPT, 2-NPT, 1H-1,2,4-triazole, and 3 MP, and 3,4-DMPP | Water | Dillution | Hypercarb column (150 × 2.1 mm, 5 µm) | (A) 0.01% formic acid in H2O & (B) MeOH and 0.01% formic acid | m/z vaules 85.0 → 68.0 42.9, 168.1 → 95.0 74.0, 217.0 → 199.9 121.0, 73.0 → 44.0, 83.0 → 42.0 56.1, 97.0 → 56.1 42.0 respectively | 20, 30, 50, 100 and 250 respectively | [120] |
| LC/MS/MS (Positive mode) | NBPT | Milk, Tissues (Liver, Kidney, Mussel, Fat) | Homogenization & extracted with acetonitrile | Luna C8 column (50 × 2 mm, 3μm) | (A) 0.2% formic acid and 0.012% NH4OH in H2O and (B) MeCN. | 168.1 m/z and product ion 74 m/z | 50 in milk samples and 40 in tissue samples | [68] |
| HPLC/ESI/QTOF (Positive mode) | NBPT, NBPTo, NBA | Soil | Extraction with deionised water and centrifugation | RRHD (50 × 2.1 mm 1.8 μm) C18 | (A) 20 mM ammonium formate (pH 6.5) & (B) MeCN | [M+H]+ (168.02 m/z), [M+Na]+ (190.063), decay ions: [NBPTo+Na]+ (174.084), [NBPTo+H]+ (152.090), 151.046, and 135.071. NBA derivativem/z = 252.064 | 0.3 nmole g-1 | [56] |
| HPLC | DCD | Soil extracts, bacterial cultures, culture filtrates | Dilution, Deprotaination and centrifugation | Prepacked cation H+-column (30×4.6 mm) | Isocratic elution with 0.025M HeSO4 | N/A | 500 | [121] |
| LC/MS/MS (Positive mode) | DCD & 15N4-DCD | Infant formula | Microwave-assisted extraction with solid-phase extraction | ZIC-HILIC HPLC column (150 × 2.1 mm i.d., 5-µm) | (A) 20 mM ammonium acetate (B) MeCN | 85.0, 68.02, 43.0 89.0, 71.02, 45.0 | 3 and 10 | [122] |
| LC/MS/MS (Positive mode) | DCD, Urea, Biuret,Cyromazine, Triuret, Amidinourea,Melamine | Wheat Flower & Skim Milk | Extraction with 0.2% formic acid and MeCN, Centrifugation, Filtration (0.20 μm) | ZIC-HILIC (150 mm × 2.1 mm, 5 μm) | (A) (95:5 ACN:0.1% formic acid/10 mM ammonium formate in H2O) (B) (50:50 ACN:0.1% formic acid/10 mM ammonium formate in H2O) | 85.0 → 68.0→ 43.1, 61.0 → 44.0, 104.1 → 61.0 → 44.0, 147.1 → 130.1 → 104.1 → 61.1, 167.1 → 85.1 → 125 → 68.0, 103.1 → 60.1 → 43.1, 127.0 → 85.0 → 68.0 | (18, 60), (4320, 28800), (54, 240), (18, 180), (18, 180), (54, 240), (162, 480) | [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’Callaghan, T.F. Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review. Sustainability 2020, 12, 6018. https://doi.org/10.3390/su12156018
Byrne MP, Tobin JT, Forrestal PJ, Danaher M, Nkwonta CG, Richards K, Cummins E, Hogan SA, O’Callaghan TF. Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review. Sustainability. 2020; 12(15):6018. https://doi.org/10.3390/su12156018
Chicago/Turabian StyleByrne, Maria P., John T. Tobin, Patrick J. Forrestal, Martin Danaher, Chikere G. Nkwonta, Karl Richards, Enda Cummins, Sean A. Hogan, and Tom F. O’Callaghan. 2020. "Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review" Sustainability 12, no. 15: 6018. https://doi.org/10.3390/su12156018
APA StyleByrne, M. P., Tobin, J. T., Forrestal, P. J., Danaher, M., Nkwonta, C. G., Richards, K., Cummins, E., Hogan, S. A., & O’Callaghan, T. F. (2020). Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review. Sustainability, 12(15), 6018. https://doi.org/10.3390/su12156018







