Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden
Abstract
1. Introduction, Background Literature, and Motivation
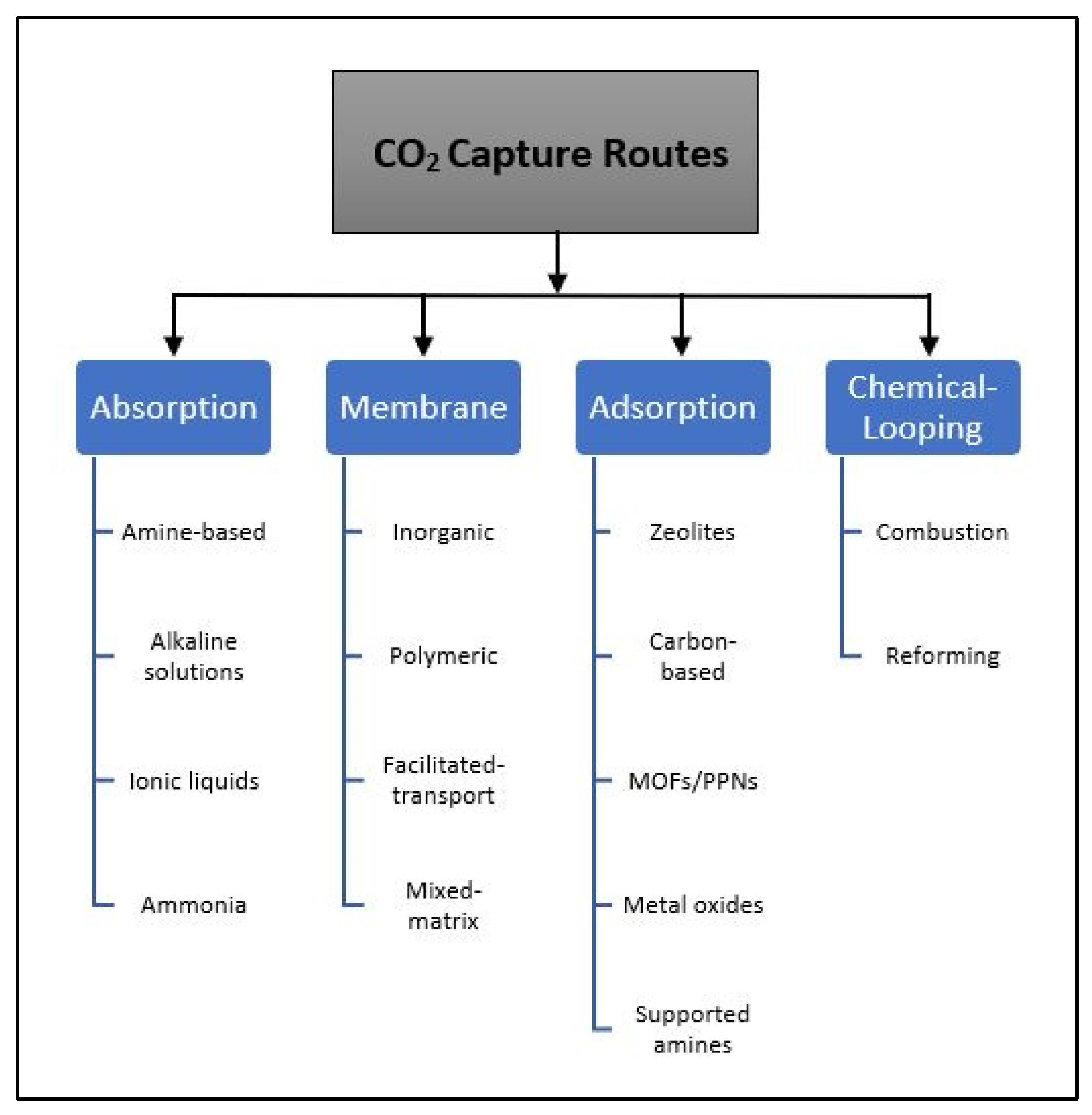
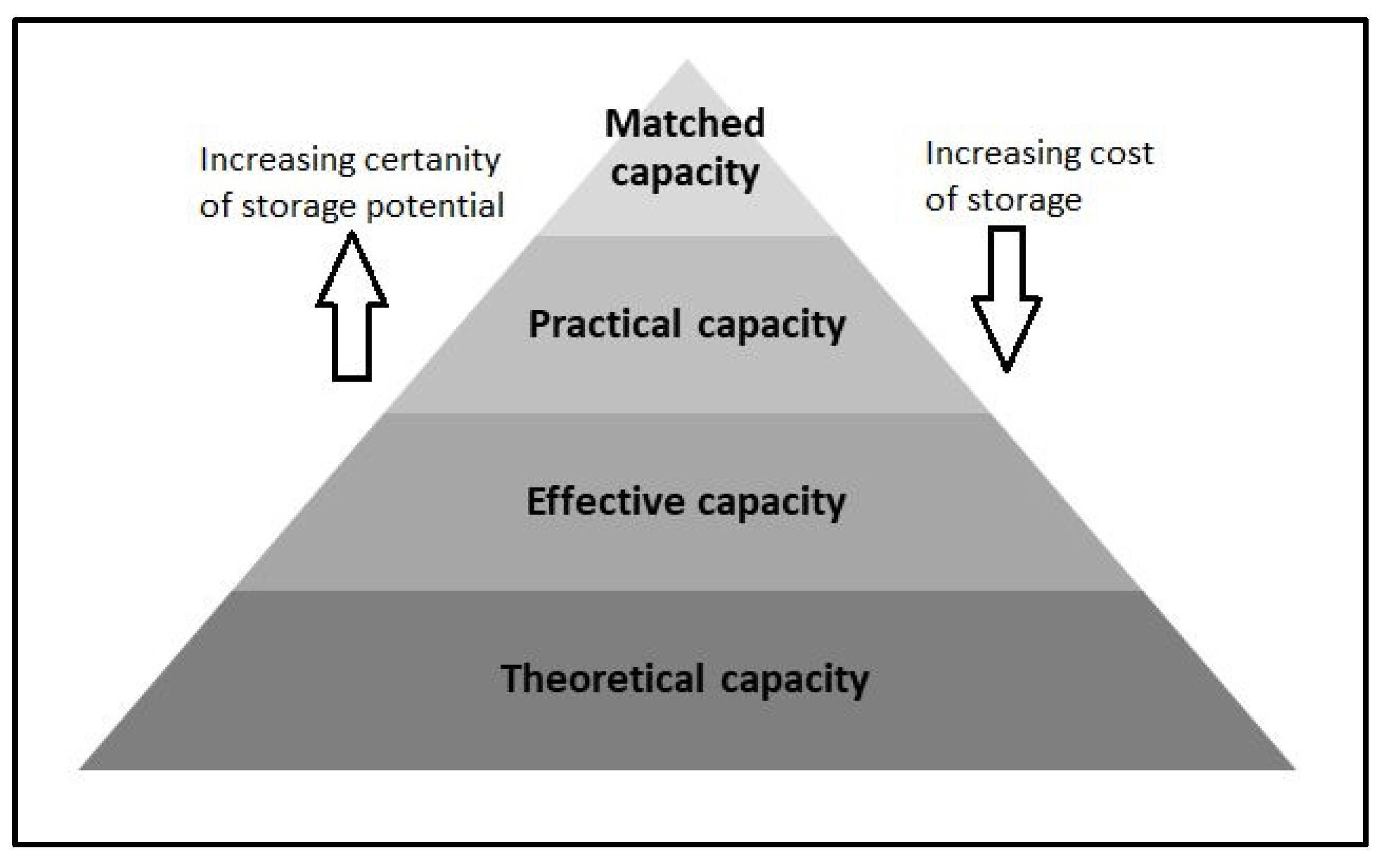
2. Focused Literature Review
3. Aim and Goals of this Study
4. Methodology
- Literature review (already presented in an earlier section);
- Data gathering for the case study;
- Techno-economic analysis (TEA); and
- Partial environmental life-cycle analysis (E-LCA) with a sensitivity analysis.
5. Data Gathering and Calculations
5.1. Techno-Economic Analysis
5.2. Partial Environmental-Life Cycle Analysis
5.3. Sensitivity Analysis
6. Results and Discussion
7. Conclusions, Limitations, and Recommendations
- Stockholm Exergi must look into other possibilities for transport and storage to optimize the CCS process chain. For instance, if the favourable sites in southwestern Sweden can be harnessed, it would reduce the GHG emissions from the transport stage.
- If the firm acquires its own infrastructure such as pipelines and/or cargo ships and/or storage sites, the cost profile would be very different from the one in which it pays for the use of infrastructure it does not own.
- Optimization of the transport stage in the process chain is also likely to yield benefits, both environmental and economic. Due to the location of the incineration plant at Lövsta, there is a lock-in when it comes to the allowable sizes/volumes of the cargo ships that Stockholm Exergi can avail of. There may be other ports in the Stockholm region which may allow the use of larger ships, and greater flexibility in the choice of sea routes.
- In order to justify the employment of larger cargo ships, it may be a good idea to think in terms of creating a ’CO2-cluster’ of all the incineration plants owned and operated by Stockholm Exergi, and if possible, other point sources that may be beyond the firm’s remit. Alternately, a centralized hub can be created to which smaller carriers can ferry CO2 from different point sources in the area, and a larger cargo ship can thereafter travel from the hub to the storage site.
- CO2 is a raw material input in many processes in the industry both as gas and solid (dry ice). Stockholm Exergi can even consider finding markets for a part of the CO2 captured.
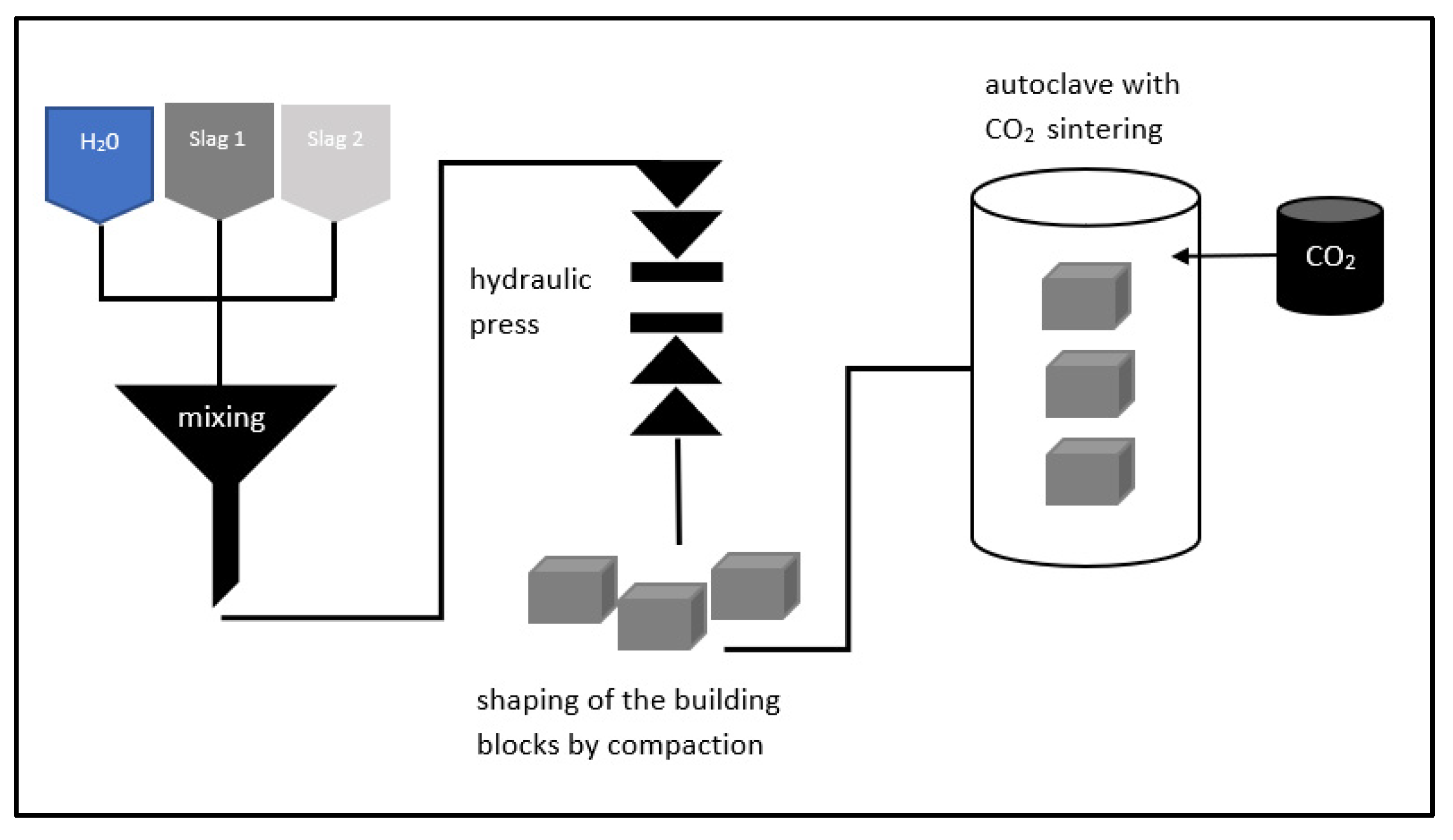
- CCU/S, as has been mentioned earlier, is a nascent technology. It is imperative to scout for potential buyers and investors in technologies like the one described in this article. Furthermore, studies to test different slag-types to identify the most suitable ones for the purpose of producing building blocks infused with CO2, are called for. The firm must also make sure that the slag-types they select are REACH–registered.
- There is no dataset in SimaPro for marine transport powered by biogas, which is what Stockholm Exergi wishes to incorporate in its operations. The dataset used in this analysis was one in which liquefied natural gas was used (this is a fossil fuel, while biogas is not). The exact route followed by the cargo ship needs to be known for a more precise estimation of GHG emissions during the transport stage. Once the location of the plant in which the building blocks would be produced has been determined, a new LCA can be carried out, knowing the distance travelled and considering an electric vehicle.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Q1. How much carbon dioxide is incorporated/used per block and how much slag do you need for this purpose?
- A1. It depends on the material we use, for most of them, the carbon dioxide would account for 30% of the total mass.
- Q2. How much energy does the process require?
- A2. It is an exothermic reaction and so there is a lot of ‘free energy’ available. One needs a little energy to introduce the gas at atmospheric pressure into the blocks.
- Q3. How much time does it take to create these blocks? Do you produce these blocks piecemeal—one at a time—in the autoclave, or can several blocks be produced simultaneously?
- A3. We have a pilot plant with big autoclaves, and we can put around 1–2 tons into it at one time, per batch, that is, and fill it with CO2 thereafter. I am not very sure about the exact amount of CO2.
- Q4. Can all slag from the stainless steel industry be used for this purpose or are certain types of slag better suited for this?
- A4. Yes, all types of slags may not be suitable for the purpose. It depends on the content of magnesium and calcium in the slag.
- Q5. Is there an existing market for such blocks in Belgium? Your comments on the future market?
- A5. At the time of answering, we are collaborating with a partner firm which is doing the necessary research. Time will tell us if there is a market for this technology.
Appendix B
| Material Type | Steel Slag | Stainless Steel (SS) Slag | Steel Slag | SS Slag (EAF) | SS Slag (EAF) | SS Slag (AOD) |
|---|---|---|---|---|---|---|
| Comp. (wt.%) | CaO: 56.8% MgO: 3.7% | CaO: 41% MgO: 7.6% | CaO: 44% MgO: 6.8% | CaO: 45% MgO: 9.3% | CaO: 55% MgO: 8.0% | |
| Precursor particle size [μm] | Median diameter 610 | <125 | 5–24 | <500 | 5–300 D50: ~100 | 10–200 D50: ~60 |
| Compact size and compaction pressure | 100 mm dia. × 200 mm height 25 × 25 × 25 cm 1 × 1 × 1 m bulk density: 2.30 g/cm3 | 90 × 40 × 10 mm 7.75 MPa | 61 × 61 × 40 mm 17.85 MPa | 300 × 100 × 50 mm 29.42 MPa | 40 × 40 × 40 mm Fresh bulk density: 2.25 g/cm3 | |
| Pressure/CO2 conc. | 1.005 atm 1 L/min 1.030 atm | 0.3 MPa 100% CO2 | 0.536 MPa 100% CO2 | 2.0 MPa 100% CO2 | 2.0 MPa 100% CO2 | Atm. Pressure 5 vol.% CO2 |
| 0.8 MPa 100% CO2 | ||||||
| Temp [°C] | 140 | 140 | 22 | |||
| 80 | ||||||
| Moisture content/ RH | L/S = 0.053-0.063 | L/S = 0.125 | L/S = 0.125 RH: 60–80% | L/S = 0.12 | L/S = 0.10 | L/S = 0.15 RH: 80% |
| L/S = 0.15 | ||||||
| Duration | 120 min | 16 h | 16 h | 3 weeks | ||
| 15 h | ||||||
| CO2-uptake | 6 ± 1 weight % | 18 | 108 g CO2/kg slag | 177–188 g CO2/kg slag | 150–200 g CO2/kg slag | 4.3 weight % |
| 8.1 weight % | ||||||
| Compressive strength [MPa] | 18.3 19 | 9 | 45 | 55 (tensile splitting strength: 2.7MPa) | 134 | 43 |
| 60 |
References
- Poura, N.; Webley, P.A.; Cook, P.J. A sustainability framework for bioenergy with carbon capture and storage (BECCS) technologies. Energy Procedia 2017, 114, 6044–6056. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Summary for Policymakers of IPCC Special Report on Global Warming of 1.5 °C Approved by Governments. 2018. Available online: http://www.ipcc.ch/2018/10/08/summary-for-policymakers-ofipcc-special-report-on-global-warming-of-1-5c-approved-by-governments/ (accessed on 30 January 2019).
- Dahlin, E. Hållbar Utveckling: En Introduktion för Ingenjörer (Sustainable Development: An Introduction for Engineers), 1st ed.; Studentlitteratur AB: Lund, Sweden, 2014. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA; Cambridge, MA, USA, 2013. [Google Scholar]
- Fridahl, M.; Lehtveer, M. Bioenergy with carbon capture and storage (BECCS): Global potential, investment preferences, and deployment barriers. Energy Res. Soc. Sci. 2018, 42, 155–165. [Google Scholar] [CrossRef]
- Kjärstad, J.; Skagestad, R.; Eldrup, N.H.; Johnsson, F. Ship transport—A low cost and low risk CO2 transport option in the Nordic countries. Int. J. Greenh. Gas Control 2016, 54, 168–184. [Google Scholar] [CrossRef]
- Nyström, J. Så ska Koldioxiden Sugas Tillbaka. (Thus Can CO2 Be Sucked Back) Forskning & Framsteg. 18 April 2016. Available online: http://fof.se/tidning/2016/5/artikel/sa-ska-koldioxiden-sugas-tillbaka (accessed on 29 January 2019).
- Regeringskansliet. Regeringen Tillsätter Utredning om Negativa Utsläpp av Växthusgaser. The Government Stresses on Negative Emissions of GHGs. 2018. Available online: http://www.regeringen.se/pressmeddelanden/2018/07/regeringen-tillsatterutredning-om-negativa-utslapp-av-vaxthusgaser/ (accessed on 29 January 2019).
- Naturvårdsverket. Fossila bränslen (Fossil Fuels). 2018. Available online: http://www.naturvardsverket.se/Miljoarbete-i-samhallet/Miljoarbete-iSverige/Uppdelat-efter-omrade/Energi/Fossila-branslen/ (accessed on 24 April 2019).
- International Energy Agency (IEA). Utilisation. Available online: http://www.iea.org/topics/carbon-capture-and-storage/utilisation/ (accessed on 2 February 2019).
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). IPCC Special Report on Carbon Dioxide Capture and Storage. In Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA; Cambridge, MA, USA, 2005. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate change 2014 Mitigation of climate change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA; Cambridge, MA, USA, 2014. [Google Scholar]
- Bataille, C.; Åhman, M.; Neuhoff, K.; Nilsson, L.J.; Fischedick, M.; Lechtenböhmer, S.; Solano-Rodriquez, B.; Denis-Ryan, A.; Stiebert, S.; Waisman, H.; et al. A review of technology and policy deep decarbonization pathway options for making energy-intensive industry production consistent with the Paris Agreement. J. Clean. Prod. 2018, 187, 960–973. [Google Scholar] [CrossRef]
- Viebahn, P.; Vallentin, D.; Höller, S. Prospects of carbon capture and storage (CCS) in China’s power sector—An integrated assessment. Appl. Energy 2015, 157, 229–244. [Google Scholar] [CrossRef]
- Tan, R.R.; Tapia, J.F.D.; Lee, J.-Y.; Ooi, R.E.H.; Foo, D.C. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCU/S) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar]
- Quaghebeur, M.; Nielsen, P.; Horckmans, L.; Van Mechelen, D. Accelerated Carbonation of Steel Slag Compacts: Development of High Strength Construction Materials. Front. Energy Res. 2015, 3, 52. [Google Scholar] [CrossRef]
- Bergmo, P.E.S.; Emmel, B.U.; Anthonsen, K.L.; Aagaard, P.; Mortensen, G.M.; Sundal, A. Quality ranking of the best CO2 storage aquifers in the Nordic countries. Energy Procedia 2017, 114, 4374–4381. [Google Scholar] [CrossRef]
- Mortensen, G.M. Koldioxidlagring i Sverige—Sammanställning och Resultat från NORDICCS (Carbon Dioxide Storage in Sweden—Summary and Results from NORDICCS); SGU-rapport 2016:20; Sveriges Geologiska Undersökning (Swedish Geological Survey): Uppsala, Sweden, 2016. [Google Scholar]
- Mortensen, G.M.; Erlström, M.; Nordström, S.; Nyberg, J. Geologisk Lagring av Koldioxid i Sverige—Lägesbeskrivning Avseende Förutsättningar, Lagstiftning och Forskning Samt Olje-och Gasverksamhet i Östersjöregionen (Geological Storage of Carbon Dioxide in Sweden—Status quo vis-a-vis Requisites, Legislation and Research in the Oil and Gas Sector in the Baltic Sea Region: Reports and Communication 142); Rapporter och meddelanden 142; Sveriges Geologiska Undersökning (Swedish Geological Survey): Uppsala, Sweden, 2017. [Google Scholar]
- Karlsson, H.; Byström, L.; Wiklund, J. BECCS som Klimatåtgärd, en Rapport om Koldioxidlagring från Biomassa i ett Svensk-Norskt Perspektiv (BECCS as a Climate Strategy, a Report on Carbon Dioxide Storage from Biomass from a Swedish-Norwegian Perspective); Biorecro AB: Stockholm, Sweden, 2010. [Google Scholar]
- Bellona. An Industry’s Guide to Climate Action. Brussel: Bellona Europa. Bellona (inter alia). Transport of CO2. 2018. Available online: http://bellona.org/about-ccs/howccs/transport-of-co2 (accessed on 19 March 2019).
- Ringrose, P.S. The CCS hub in Norway: Some insights from 22 years of saline aquifer storage. Energy Procedia 2018, 146, 166–172. [Google Scholar] [CrossRef]
- Bastian, P.; Kraus, J.; Scheichl, R.; Wheeler, M. Simulation of Flow in Porous Media Applications in Energy and Environment (Electronic); De Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Sundal, A.; Nystuen, J.P.; Rørvik, K.-L.; Dypvik, H.; Aagaard, P. The Lower Jurassic Johansen Formation, northern North Sea Depositional model and reservoir characterization for CO2 storage. Mar. Pet. Geol. 2016, 77, 1376–1401. [Google Scholar] [CrossRef]
- Zahid, U.; Han, C.; Choi, S.C.; An, J.; Lee, U. Techno-economic assessment of CO2 liquefaction for ship transportation. Greenh. Gas Sci. Technol. 2014, 4, 734–749. [Google Scholar] [CrossRef]
- Chang, D.; Seo, Y.; Huh, C.; Lee, S. Comparison of CO2 liquefaction pressures for ship-based carbon capture and storage (CCS) chain. Int. J. Greenh. Gas Control 2016, 52, 1–12. [Google Scholar]
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; Imperial Collage Press: London, UK, 2014. [Google Scholar]
- Selosse, S.; Ricci, O. Carbon capture and storage: Lessons from a storage potential and localization analysis. Appl. Energy 2016, 188, 32–44. [Google Scholar] [CrossRef]
- Bruhn, T.; Naims, H.; Olfe-Kräutlein, B. Separating the debate on CO2 utilisation from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D. Carbon Capture and Utilisation in the Green Economy; Report No. 501; The Center for Low Carbon Futures: New York, NY, USA, 2011. [Google Scholar]
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Versteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. [Google Scholar] [CrossRef]
- Rosell, E. Carbon Capture and Storage—Hjälper Eller Stjälper Klimatet? (CCS—Does it Help or Upend the Climate?); FORES bakgrundsrapport 2016:1; Fores—Forum för Reformer och Entreprenörskap (Fores—Forum for Reforms and Entrepreneurship): Stockholm, Sweden, 2016. [Google Scholar]
- Grafström, J.; Hvalgren, N.; Korpi, M. Förutsättningar för Storskaligt Infångande av Koldioxid (Pre-Requisites for Large-Scale Capture of Carbon Dioxide); Ratio Working Paper No. 309; Ratio—Näringslivets forskningsinstitut: Stockholm, Sweden, 2018. [Google Scholar]
- International Energy Agency (IEA). Overview. Available online: http://www.iea.org/topics/carbon-capture-and-storage/ (accessed on 5 April 2019).
- Erlström, M.; Fredriksson, D.; Juhojuntti, N.; Sivhed, U.; Wickström, L. Lagring av Koldioxid i Berggrunden– Krav, Förutsättningar och Möjligheter (Carbon Storage in the Lithosphere—Pre-Requisites and Possibilities: Reports and Communications 131); Rapporter och Meddelanden 131; Sveriges Geologiska Undersökning (Swedish Geological Survey): Uppsala, Sweden, 2011. [Google Scholar]
- Serpa, J.; Morbee, J.; Tzimas, E. Technical and Economic Characteristics of a CO2 Transmission Pipeline Infrastructure; Report No.: JRC62502; European Union, Joint Research Centre, Institute for Energy: Maastricht, The Netherlands, 2011. [Google Scholar]
- Neele, F.; de Kler, R.; Nienoord, M.; Brownsort, P.; Koornneef, J.; Belfroid, S.; Peters, L.; van Wijhe, A.; Loeve, D. CO2 transport by ship: The way forward in Europe. Energy Procedia 2017, 114, 6824–6834. [Google Scholar] [CrossRef]
- de Kler, R.; Neele, F.; Nienoord, M.; Brownsort, P.; Koornneef, J.; Belfroid, S.; Peters, L.; van Wijhe, A.; Loeve, D. Transportation and Unloading of CO2 by Ship—A Comparative Assessment; Report number: CCU/S-T2013-09-D08; NordForsk: Oslo, Norway, 2016. [Google Scholar]
- Global CCS Institute. Transport. Available online: http://www.globalccsinstitute.com/why-ccs/what-is-ccs/transport/ (accessed on 18 April 2019).
- Skagestad, R.; Eldrup, N.; Hansen, H.R.; Belfroid, S.; Anette Mathisen, A.; Lach, A.; Haugen, H.A. Ship Transport of CO2 Status and Technology Gaps; Tel-Tek Report no. (2214090); Tel-Tek: Porsgrunn, Norway, 2014. [Google Scholar]
- Kjärstad, J.; Skagestad, R.; Eldrup, N.H.; Johnsson, F. Linking the effect of reservoir injectivity and CO2 transport logistics in the Nordic Region. Energy Procedia 2017, 114, 6860–6869. [Google Scholar] [CrossRef]
- Leung, D.; Caramanna, G.; Maroto-Valer, M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Global CCS Institute. Storage. Available online: http://www.globalccsinstitute.com/why-ccs/what-is-ccs/storage/ (accessed on 6 March 2019).
- International Energy Agency (IEA). Storage. Available online: http://www.iea.org/topics/carbon-capture-and-storage/storage/ (accessed on 4 February 2019).
- Havercroft, I.; Macrory, R. Legal Liability and Carbon Capture and Storage. Available online: http://decarboni.se/sites/default/files/publications/179798/legal-liabilitycarbon-capture-storage-comparative-perspective.pdf (accessed on 29 April 2019).
- Det Norske Veritas (DNV). Report Activity 4: CO2 Storage; Report No./DNV Reg No./13REPT4-2; Det Norske Veritas: Høvik, Norway, 2012. [Google Scholar]
- Eiken, O.; Ringrose, P.; Hermanrud, C.; Nazarian, B.; Torp, T.A.; Høier, L. Lessons Learned from 14 years of CCS Operations: Sleipner, In Salah and Snøhvit. Energy Procedia 2011, 4, 5541–5548. [Google Scholar] [CrossRef]
- Furre, A.-K.; Eiken, O.; Alnes, H.; Nesland Vevatne, J.; Kiær, A.F. 20 years of monitoring CO2-injection at Sleipner. Energy Procedia 2017, 114, 3916–3926. [Google Scholar] [CrossRef]
- Global CCS Institute. The Global Status of CCS: 2017; Global CCS Institute: Docklands, Australia, 2017. [Google Scholar]
- Global CCS institute. Highlights for 2018. Available online: http://www.globalccsinstitute.com/resources/global-status-report/ (accessed on 14 March 2019).
- International Energy Agency (IEA). Technology Roadmap: Carbon Capture and Storage; IEA & OECD: Paris, France, 2009. [Google Scholar]
- Nagabhushan, D.; Thompson, J. Carbon Capture and Storage in the United States Power Sector, the Impact of 45Q Federal Tax Credits; Director at Clean Air Task Force (CATF): Boston, MA, USA, 2019. [Google Scholar]
- Zhao, X.; Liao, X.; Wang, W.; Chen, C.; Rui, Z.; Wang, H. The CO2 storage capacity evaluation: Methodology and determination of key factors. J. Energy Inst. 2014, 87, 297–305. [Google Scholar] [CrossRef]
- Global CCS Institute. Technology Readiness Level (TRL). Available online: http://hub.globalccsinstitute.com/publications/technology-options-co2-capture/technology-readiness-level-trl (accessed on 16 May 2019).
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- de Weireld, G.; Chauvy, R.; Meunier, N.; Thomas, D. Selecting emerging CO2 utilization products for short- to mid-term deployment. Appl. Energy 2019, 236, 662–680. [Google Scholar]
- Patricio, J.; Angelis-Dimakis, A.; Castillo-Castillo, A.; Kalmykova, Y.; Rosado, L. Region prioritization for the development of carbon capture and utilization technologies. J. CO2 Util. 2017, 17, 50–59. [Google Scholar] [CrossRef]
- Jernkontoret. Stålindustrin gör Mer än Stål, Handbok för Restprodukter 2018 (The Steel Sector Produces more than just Steel—Handbook for Waste Products 2018); Jernkontorets teknikområde 55, Restprodukter; Jernkontoret (Stockholm: Office of the Railways): Stockholm, Sweden, 2018. [Google Scholar]
- Wulfert, H.; Schiffers, A.; Jungmann, A. Processes for the Dry Processing of Steel Slags with Loesch Mills for Metal Recovery and Production of Silicate Composite Material for Use in Building Materials Industry; Loesche Innovative Engineering: Düsseldorf, Germany, 2017. [Google Scholar]
- Quaghebeur, M.; Nielsen, P.; Laenen, B.; ENguyen, E.; Van Mechelen, D. Carbstone: Sustainable Valorisation Technology for Fine Grained Steel Slags and CO2. Refract. Worldforum 2010, 2, 75–79. [Google Scholar]
- Vito. Carbstone. Available online: http://vito.be/en/carbstone (accessed on 20 May 2019).
- EARTO. VITO—CO2 Negative Construction Materials from Recycled Resources. Available online: http://www.earto.eu/rto-innovation/vito-co2-negative-constructionmaterials-from-recycled-resources/ (accessed on 20 May 2019).
- Zero Emission Platform (ZEP). Policy & Regulation; National and EU Policy Supporting CCS. Available online: http://www.zeroemissionsplatform.eu/policy-andregulation.html (accessed on 12 March 2019).
- Extavour, M.; Bunje, P. CCU/S: Utilizing CO2 to reduce emissions. Chem. Eng. Prog. Mag. 2016, 112, 53–59. [Google Scholar]
- American Institute of Chemical Engineers (AIChE). What is CCU/S? Available online: http://www.aiche.org/CCU/Snetwork/what-CCU/S (accessed on 22 February 2019).
- European Commission. Novel Carbon Capture and Utilisation Technologies; Supported by SAPEA Evidence Review Report No 2; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Meylan, F.D.; Moreau, V.; Erkan, S. CO2 utilization in the perspective of industrial ecology, an overview. J. CO2 Util. 2015, 12, 101–108. [Google Scholar] [CrossRef]
- Rydberg, N.; Langlet, D. CCS in the Baltic Sea Region—Bastor 2: Work Package 4—Legal & Fiscal Aspects; Elforsk report 14:48; Elforsk AB: Stockholm, Sweden, 2014. [Google Scholar]
- Snellings, R.; Nielsen, P.; Baciocchi, R.; Costa, G.; Quaghebeur, M. Carbonate-bonded construction materials from alkaline residues. Rilem Tech. Lett. 2017, 2, 53–58. [Google Scholar] [CrossRef]
- Baumann, H.; Tillman, A.-M. The Hitch Hiker’s Guide to LCA; Studentlitteratur AB: Lund, Sweden, 2004. [Google Scholar]
- Gulliksson, H.; Holmgren, U. Hållbar Utveckling: Livskvalitet, Beteende, Teknik (Sustainable Development: Quality of Life, Behaviour and Techniques), 2nd ed.; Studentlitteratur AB: Lund, Sweden, 2015. [Google Scholar]
- Carlson, R.; Pålsson, A.-C. Livscykelanalys Ringar på Vattnet, 2nd ed.; SIS förlag (SIS Publishers): Stockholm, Sweden, 2011. [Google Scholar]
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manag. 2009, 9, 1–21. [Google Scholar] [CrossRef]
- Sveriges lantbruksuniversitet (SLU). Vad är en Livscykelanalys? What is a Life-Cycle Analysis? Available online: http://www.slu.se/institutioner/energi-teknik/forskning/lca/vadar/ (accessed on 18 May 2019).
- International Energy Agency Greenhouse Gas R&D Program (IEAGHG). Environmental Evaluation of CCS Using Life Cycle Assessment (LCA); Rapport 2010/TR2; IEAGHG, Orchard Business Centre: Cheltenham, UK, 2010. [Google Scholar]
- Singh, B. Environmental Evaluation of Carbon Capture and Storage Technology and Large-Scale Deployment Scenarios. Ph.D. Thesis, Norges Teknisk-Naturvitenskapelige Universitet, Trondheim, Norway, 2011. [Google Scholar]
- Swiss Centre for Life Cycle Inventories. Ecoinvent Database v.3. 2014. Available online: http://www.ecoinvent.org/ (accessed on 23 April 2020).
- Björklund, A. Survey of Approaches to Improve Reliability in LCA. Int. J. Life Cycle Assess. 2002, 7, 64. [Google Scholar] [CrossRef]
- Brander, M.; Tipper, R.; Hutchison, C.; Davis, G. Consequential and Attributional Approaches to LCA: A Guide to Policy Makers with Specific Reference to Greenhouse Gas LCA of Biofuels; Technical Paper TP-090403-A.; Ecometrica Press: Edinburgh, Scotland, 2009. [Google Scholar]
| Industrial Process | Type of Use | TRL | Conversion Factor |
|---|---|---|---|
| Lignin production | CO2 used in black liquor pH regulation | 7–8 | 0.22 ton CO2 per t of lignin produced |
| Methanol production | Electrochemical reduction of CO2 | 7 | 1.7 t CO2 per t of methanol produced |
| Polyurethane production | CO2 used as raw material to produce plastics and fibers | 7 | 0.1–0.3 t CO2 per t of polyols |
| Polypropylene carbonate (PPC) production | CO2 used as raw material to produce plastics and fibers | 7 | 0.43 t CO2 per t of PPC produced |
| Concrete curing (Concrete blocks) | CO2 used for precast concrete curing | 7–8 | 0.03 t CO2 per t of block produced 0.12 t CO2 per t of precast concrete |
| Mineral carbonation | CO2 reacted with calcium or magnesium containing minerals | 7–8 | 0.25 t CO2 per t of steel slag |
| Bauxite residue carbonation | CO2 is used to neutralize bauxite residues | 9 | 0.053 t CO2 per t of red mud |
| Horticulture production | CO2 supplementation on plant growth | 9 | 0.5–0.6 kg CO2/h/100m2 160 t CO2 per ha (for tomatoes in Sweden) |
| Urea production | Urea production from ammonia and CO2 | 9 | 0.74 t CO2 per ton of urea |
| Parameter | Value | Notation in Equations |
|---|---|---|
| Cargo ship capacity (t) | 3500 | S |
| Cost per trip (SEK/trip) | 700 | SEK/trip |
| Annually captured CO2 (t/year) | 650,000 | MCO2 |
| Transport stretch (km) | 1504 | ds |
| GHG emissions for the sea transport (kg CO2-equivalents/tkm) | 0.0267 | Ktkm,s |
| Slag | Quantity (tons) |
|---|---|
| Argon-Oxygen Decarburization | 108,000 |
| Linz Donawitz steel slag | 18,000 |
| Arc furnace slag (highly alloyed) | 80,000 |
| Arc furnace slag (low alloyed) | 10,000 |
| Ladle slag | 51,000 |
| Total | 267,000 |
| Parameter | Value | Notation in Equations |
|---|---|---|
| Truck capacity (t/truck) | 40 | Tcap |
| Cost per trip (SEK/trip) | 9 | SEK/trip |
| Transport stretch (km) | 154 | dr |
| Slag available (Mt) | 0.267 | Ms |
| GHG emissions for the truck transport (kg CO2-equivalents/tkm) | 0.0584 | Ktkm,r |
| Mass of building blocks | Mb |
| Trips | Cost/Trip | Total Cost | GWP100 | CO2-Sink |
|---|---|---|---|---|
| per year | mSEK | mSEK/year | kt CO2-eq/year | kt-CO2/year |
| 186 | 2.45 | 455.7 | 26.1 | 623 |
| Annual CO2 emissions stand at 700 kilotons | ||
| Increase in distance | Increase in GWP100 | Percentage increase in GWP100 |
| Kilometers | kt CO2-eq/year | % |
| 25 | 0.467 | 1.7 |
| 50 | 0.934 | 3.3 |
| 100 | 1.869 | 6.7 |
| Annual CO2 emissions stand at 650 kilotons | ||
| Increase in distance | Increase in GWP100 | Percentage increase in GWP100 |
| Kilometers | kt CO2-eq/year | % |
| 25 | 0.433 | 1.7 |
| 50 | 0.867 | 3.3 |
| 100 | 1.735 | 6.7 |
| Annual CO2 emissions stand at 600 kilotons | ||
| Increase in distance | Increase in GWP100 | Percentage increase in GWP100 |
| Kilometers | kt CO2-eq/year | % |
| 25 | 0.400 | 1.7 |
| 50 | 0.801 | 3.3 |
| 100 | 1.602 | 6.7 |
| Annual CO2 emissions stand at 550 kilotons | ||
| Increase in distance | Increase in GWP100 | Percentage increase in GWP100 |
| Kilometers | kt CO2-eq/year | % |
| 25 | 0.367 | 1.7 |
| 50 | 0.734 | 3.3 |
| 100 | 1.461 | 6.7 |
| CO2 to building block production | tons per year | 114,429 |
| Number of truck trips | per year | 2861 |
| Building block production | tons | 381,429 |
| Total cost for the CCU/S part | mSEK/year | 3.96 |
| CO2 which has to be handled by CCS | tons per year | 535,571 |
| Number of cargo ship trips | per year | 153 |
| Total cost for the CCS part | mSEK | 374.85 |
| GWP100 for the CCU/S part | kt CO2-eq/y | 1.03 |
| GWP100 for the CCS part | kt CO2-eq/y | 21.5 |
| Total GWP100 | kt CO2-eq/y | 22.53 |
| CO2-sink | kt CO2-eq/y | 627.4 |
| CO2 emissions captured from stack—650 kton | |||||||
| Slag mass available (kton) | CO2 in concrete block (kton) | Mass of concrete blocks (kton) | Cost for the CCU/S (mSEK/year) | GWP100 (kt CO2-eq) per year CCUS | GWP100 (kt CO2-eq) per year CCUS+CCS | Total cost for CCU/S + CCS(mSEK/year) | |
| 267 | 114.4 | 381.4 | 3.96 | 1.03 | 22.5 | 378.8 | |
| 400 | 171.4 | 471.4 | 5.94 | 1.54 | 20.8 | 341.6 | |
| 500 | 214.2 | 714.3 | 7.42 | 1.92 | 19.5 | 313.7 | |
| CO2 emissions captured from stack—550 kton | |||||||
| Slag mass available (kton) | CO2 in concrete block (kton) | Mass of concrete blocks (kton) | Cost for the CCU/S (mSEK/year) | GWP100 (kt CO2-eq) per year CCUS | GWP100 (kt CO2-eq) per year CCUS+CCS | Total cost for CCU/S + CCS(mSEK/year) | |
| 267 | 114.4 | 381.4 | 3.96 | 1.03 | 18.5 | 307.8 | |
| 400 | 171.4 | 471.4 | 5.94 | 1.54 | 16.7 | 270.5 | |
| 500 | 214.2 | 714.3 | 7.42 | 1.92 | 15.4 | 242.6 | |
| CO2 emissions captured from stack—700 kton | |||||||
| Slag mass available (kton) | CO2 in concrete block (kton) | Mass of concrete blocks (kton) | Cost for the CCU/S (mSEK/year) | GWP100 (kt CO2-eq) per year CCU/S | GWP100 (kt CO2-eq) per year CCU/S+CCS | Total cost for CCU/S + CCS(mSEK/year) | |
| 267 | 114.4 | 381.4 | 3.96 | 1.03 | 24.5 | 413.1 | |
| 400 | 171.4 | 471.4 | 5.94 | 1.54 | 22.8 | 375.9 | |
| 500 | 214.2 | 714.3 | 7.42 | 1.92 | 21.5 | 347.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhelkis, L.; Govindarajan, V. Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden. Sustainability 2020, 12, 5922. https://doi.org/10.3390/su12155922
Mikhelkis L, Govindarajan V. Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden. Sustainability. 2020; 12(15):5922. https://doi.org/10.3390/su12155922
Chicago/Turabian StyleMikhelkis, Lena, and Venkatesh Govindarajan. 2020. "Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden" Sustainability 12, no. 15: 5922. https://doi.org/10.3390/su12155922
APA StyleMikhelkis, L., & Govindarajan, V. (2020). Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden. Sustainability, 12(15), 5922. https://doi.org/10.3390/su12155922






