1. Introduction
Changing legal regulations have forced industries to care more about the purity of atmospheric air. Implementation of the Directive on industrial emissions (2010/75/EU) [
1], followed by the Directive on medium combustion plants (2015/2193/EU) [
2] and the so-called BAT conclusions for large combustion plants (2017/1442/EU) [
3] and waste incineration (2019/2010/EU) [
4], have shown that the challenges related to emissions reduction from combustion processes (combustion of both fuels and waste for energy production) have again become extremely important. The tightening of standards for nitrogen oxide emissions enforces the use of effective technologies to limit these emissions. For waste incineration, as a result of the adoption of BAT (Best Available Technology) conclusions, standards for the emissions of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (dioxins and furans, respectively) have also been tightened.
Nitrogen oxides (NOx) formed during combustion processes are dangerous atmospheric contaminants, causing lung diseases in people and damage to nature and building structures due to strong acidification of precipitation (acid rain). They also form part of smog, both the acidic London type and the photochemical Los Angeles type. In most industrialized countries, NOx is one of the most harmful anthropogenic air pollutants. Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans, also formed in thermal processes including combustion, are endocrine disrupters—chemical compounds that interfere with the functioning of the human endocrine system. Although not carcinogenic as was previously thought during the 1990s, the harmful effects of dioxins and furans are so important that their emissions are widely studied and limited in accordance with the provisions of the Stockholm Convention on Persistent Organic Pollutants (POPs).
Both pollutants, i.e., nitrogen oxides (NO
x, the sum of nitrogen oxide NO, nitrogen dioxide NO
2, and nitrous oxide N
2O) and polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), have much in common. They are pollutants formed during combustion processes, and their concentrations in exhaust gas depend only in part on the content of the respective element in the combusted fuel: nitrogen in the case of NO
x and chlorine in the case of PCDD/Fs [
5]. Another similarity is the high efficiency of their decomposition (reduction to ammonia for NO
x) or oxidation (for PCDD/Fs) on an oxide catalyst, including vanadium, tungsten, chromium, or molybdenum, using selective catalytic reduction technologies known as SCR [
6,
7]. Both NO
x and dioxins have three known mechanisms of formation; however, these mechanisms are significantly different [
5,
7].
A technology commonly used to reduce emissions of nitrogen oxides from combustion is selective non-catalytic reduction (SNCR). It has shown high effectiveness with much lower investment costs compared to SCR. This technology was first developed in the United States in 1975 by Lyon [
8] and further developed by Arand [
9,
10], Perry [
11], Brogan [
12], and Dean [
13]. Generally, it is based on the high-temperature reaction of ammonia with nitric oxide to produce free nitrogen, which is then emitted into the atmosphere.
Commercially available technologies essentially differ only in the source of ammonia used: gaseous ammonia or its aqueous solution (ammonia water) for
Thermal DeNOx technology [
8], aqueous urea solution for
NOxOUT [
9], or cyanuric acid for
RAPRENOx [
11]. In other variations of SNCR technology, methylamine, ethylamine, ethylenediamine, and diethylenetriamine are also used as reducers and the sources of ammonia. [
14,
15]. However, the most commonly used reducers are ammonia water, NH
4OH, or aqueous urea solution, (NH
2)
2CO:
The average efficiency of SNCR technology is approximately 40–70%, depending on the installation structure, the way the reducer is introduced, temperature, and its distribution in the reaction zone (i.e., in the boiler). Detailed descriptions of the process and the results of kinetic studies during non-catalytic reduction of NO
x of the reaction have been described in detail in previous publications [
16,
17].
Compounds used in the SNCR process are chosen because they decompose with the release of ammonia at high temperature, which is the basic reactant for the process of reducing nitrogen oxides. However, nitrogen-containing compounds, as well as sulfur and alkali metal oxides and hydroxides [
18,
19,
20,
21,
22,
23,
24], can reduce the amount of dioxins emitted from thermal process; acting as inhibitors in the de novo synthesis is the most important of the dioxin formation pathways during thermal processes [
25]. Among the chemical compounds containing nitrogen in their molecules that have inhibitory properties towards the synthesis of PCDD/Fs, urea, ammonia, melamine, ammonium hydroxide, ammonium hydrogen phosphate, EDTA (Ethylenediaminetetraacetic acid), amines, and other compounds containing an amino group or groups in their structure have inhibitory activity. The reduction effect of PCDD/Fs emissions recorded for them reaches almost 90% [
26,
27,
28,
29,
30,
31,
32].
According to previous reports, sulfur dioxide (SO
2) formed during combustion or high-temperature decomposition of sulfur-containing compounds by reaction with the chlorine present in the flue gas significantly reduces its concentration. The reaction was described by Griffin [
33] and Lindbauer [
34]:
If we assume that de novo synthesis is at one of its stages a kind of Friedel–Crafts reaction [
35] (consecutive chlorination of the aromatic ring), then the reduction of the chlorine concentration in the de novo reaction zone (outside the combustion zone, at 250–400 °C) will reduce the amount of dioxins and furans formed, particularly highly chlorinated compounds. The pathway of the Deacon reaction, known for over 100 years [
36], shows that it is not possible to remove all of the chlorine in a molecular form, capable of chlorination according to the Friedel–Crafts mechanism:
The inhibitory action of nitrogen compounds has usually been explained as blocking and deactivating the surface of a copper catalyst for the de novo synthesis using nitrogen to form imines and nitrides.
The ammonia necessary for these reactions obviously comes from the decomposition of compounds used as inhibitors, e.g., ammonium sulfate, persulfate, or thiosulfate:
and thiourea:
However, assuming that the ammonia present in the de novo reaction zone released from ammonia water resulting from the decomposition of urea or other compounds containing nitrogen in the molecule can directly react with chlorine:
we can demonstrate another explanation for the inhibition phenomenon. This thesis is confirmed by the inhibitory effects of alkaline compounds, e.g., calcium oxide or hydroxide, which also effectively act as inhibitors of the de novo synthesis. These compounds can bind chlorine present in exhaust gases, thus reducing the amount participating in the de novo synthesis:
Earlier studies [
37] have confirmed the clear dependence of the amount of PCDD/Fs formed on the amount of inhibitor used, in particular the amount of ammonia and sulfur dioxide released as a result of the decomposition of the inhibitor. This opens the way to use of cheap inorganic compounds containing sulfur and nitrogen to release ammonia as a result of decomposition of the inhibitors of de novo synthesis, significantly reducing the amount of dioxins and furans formed in thermal processes. When these compounds release ammonia as a result of thermal decomposition, it seems possible to apply them in the SNCR process and thus use them to simultaneously reduce emissions of NO
x and PCDD/Fs.
The purpose of this work was to investigate the possibility of using chemical compounds containing sulfur and nitrogen as reagents in the SNCR method, while ensuring the reduction of nitrogen oxide and polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran emissions in flue gases from waste incineration processes.
2. Materials and Methods
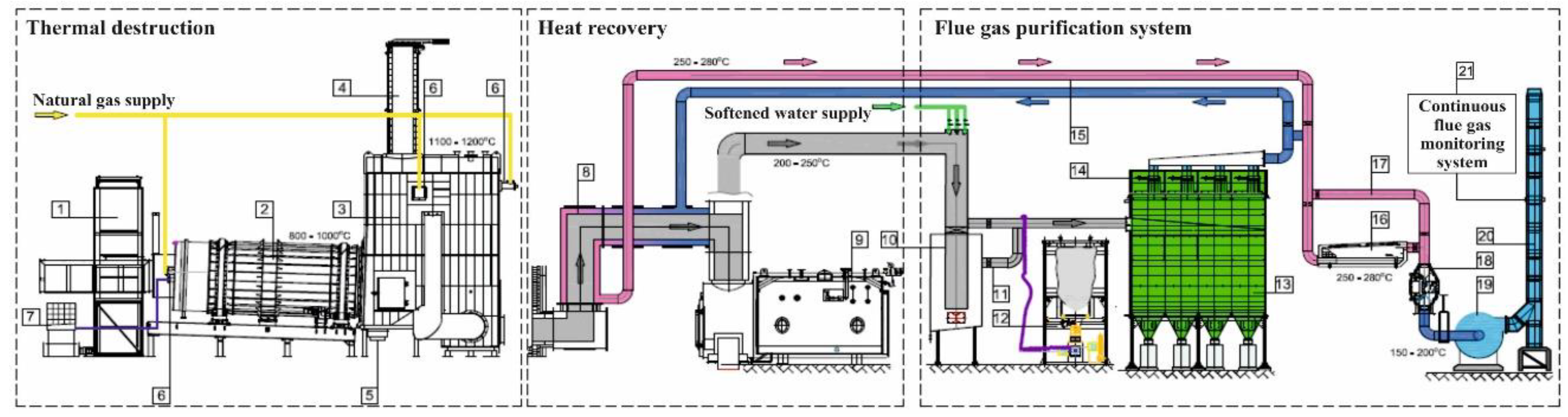
Tests were carried out at the Medical Waste Disposal Plant (MWDP) of the Bydgoszcz Oncology Center. The installation for thermal waste treatment located in MWDP is a medical waste incineration plant equipped with a rotary kiln with an average hourly capacity of approximately 400 kg/h. The schematic of the incineration plant is shown in
Figure 1.
The installation consists of a rotary kiln (combustion chamber) in the form of a cylindrical drum, inclined at an angle of 2–6°. The kiln moves on rollers placed on a special frame and is driven by a chain transmission with the number of revolutions controlled between 1 and 12 revolutions/hour and operates at temperatures from 700 to 1100 °C. Slag and ashes formed during the process have a low organic content, not exceeding 3%. The stationary front plate of the furnace is fitted with a sluice for the loading system, a stub pipe for feeding liquid waste, a gas burner, an additional air pipe connector, and an inspection sightglass. The kiln is equipped with an automatically switched-on gas burner used to heat during start-up (initiation of the combustion process) and to maintain the required temperature in the kiln during the operation of the installation, depending on the adopted technological regime and the type of neutralized waste. The air needed for combustion in the rotary kiln is sucked through the pipe in the front of the rotary kiln. A vacuum of approximately 20–30 Pa is created in the kiln by the main exhaust fan, which prevents gases from escaping from the chamber into the room.
During the combustion process with a controlled air stream, the thermal decomposition of waste into solid and gas products occurs. Solid products from the waste incineration process in the form of ash and slag are collected at the end of the rotary kiln.
Gas products from the rotary kiln pass into a thermoreactor (afterburning chamber) with a heat-resistant lining. In the afterburning chamber, thermal decomposition of organic substances and their oxidation to the final products of combustion takes place at a high temperature of at least 1100 °C. The dimensions of the afterburning chamber ensure that the flue gases remain in the chamber for more than 2 s. The temperature in the afterburning chamber is regulated automatically by two gas burners with variable capacity. The air to the afterburning chamber is supplied by nozzles located on all of its walls in the initial part of the chamber.
Another device on the flue gas path is the heat recovery system. Its main element is a 3.0 MW fire tube recovery boiler. Hot gases leaving the afterburning chamber pass through the heating channel and enter the boiler, where they are rapidly initially cooled to a temperature of approximately 200 °C. The cooled gases are directed to the exhaust aftertreatment system. However, the heat removed from the gas stream is used to generate saturated steam.
In the next stage of the technological process, pre-cooled gases, after passing through the heat recovery system, are sent to the waste gas treatment system, which consists of:
Injection systems for aqueous urea solution for SNRC of nitrogen oxides (between thermoreactor and boiler—8 in
Figure 1);
Quench (spray cooler and exhaust gas humidifier);
Sorbent silo with a dispenser;
Fabric filter;
Heat exchanger;
Catalytic converter (at the stage of research out of service).
A stream of cooled gas at approximately 200 °C passes through a humidification system called the quench. Cold water is sprayed to additionally cool the gas stream by 5–10 °C and moisturize it to accelerate the reaction of binding acidic impurities by the dry method with the use of calcium reagents. The reactant, a mixture of activated carbon dust and calcium hydroxide, is dosed into the flue gas channel. This process involves chemical neutralization of acidic compounds, i.e., SO2, HCl, and HF, as well as adsorption of organic micropollutants and mercury on organic carbon. The next stage of flue gas purification is a four-section fabric filter in a system of vertically arranged bags. Because of a high content of heavy metals, dioxins and furans in the waste separated from flue gases are neutralized at the hazardous waste deposition site. After passing through the entire purification system, the purified gases are released to the atmosphere at a temperature not lower than 140 °C by an exhaust fan through a chimney with a diameter of 0.5 m and a height of 20 m. The installation is equipped with a system for continuous monitoring of pollutant emissions. The continuous measurements include flue gas flow rate, humidity, oxygen content, temperature, and pressure. In addition, quantitative analyses ensure continuous measurement of the following pollutants: SO2, NOx, HCl, HF, CO, CO2, O2, and volatile organic compounds as total organic carbon (TOC).
An aqueous solution of 32.5% urea (trade name AdBlue) and an aqueous solution of ammonium sulfate with a concentration of approximately 20% were used as the reactant during the process of simultaneous removal of NOx and PCDD/Fs from flue gases. In addition, tests were carried out using aqueous solutions of ammonium persulfate ((NH4)2S2O8), ammonium thiosulfate ((NH4)2S2O3), and thiourea ((NH2)2CS) at a concentration of approximately 20%.
Two basic series of tests were carried out on the impact of the addition of aqueous urea solution and aqueous ammonium sulfate solution dosed at different rates to the installation in the zone between the afterburning chamber and the recovery boiler at approximately 950 °C on nitrogen oxide emission. Averaged results of the measurements of NOx concentrations were taken from the continuous monitoring system of the installation. In addition, one series of tests was carried out on the impact of both tested reagents (urea and ammonium sulfate solutions) on the amounts of polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran emissions from the installation. An additional three measurements were made to check the efficiency of other reagents, including ammonium persulfate, ammonium thiosulfate, and thiourea. Determination of PCDD/F content in flue gases was made in accordance with the EN–1948 standard. The waste gas samples were taken from the flue gas stream before the flue gas cleaning installation, directly after the recovery boiler. The duration of the measurement of NOx was 2 h (averaged results from 2 h of operation of the installation under specific conditions), while the time for collecting samples for the determination of dioxins was 4 h preceded by a 3 h period of work in the new experimental conditions to ensure sufficient time for the de novo synthesis to occur under the new conditions supplying the reducer. Measurements of dioxin and furan emissions were made by EMIPRO Ltd. from Krakow, while dioxin determinations were made by the Laboratory of Trace Analysis of the Faculty of Chemistry at the Cracow University of Technology.
The thermal waste treatment installation worked stably with a capacity of approximately 360–380 kg/h, which resulted in an average flue gas stream of approximately 5870 m3N/h (under normal conditions, i.e., 273 K and 101.3 kPa). The average NOx concentration in flue gas under normal conditions was approximately 163.3 mg/m3N. The average composition of flue gas from the incineration plant (based on data from the continuous emission monitoring system) during the tests was as follows: SO2—15.9 mg/m3N, HCl—6.3 mg/m3N, HF—0.02 mg/m3N, CO—25.6 mg/m3N, TOC—0.2 mg/m3N, CO2—7.7%, H2O—10.1%, and O2—13.2%. The variability of composition and the flue gas stream did not exceed 10%.
3. Results
The use of urea and ammonium sulfates as nitrogen oxide reducers in the SNCR process and simultaneously as inhibitors during the de novo synthesis contributes to the reduction of NO
x as well as dioxins and furans. The results of experiments are presented in the tables below.
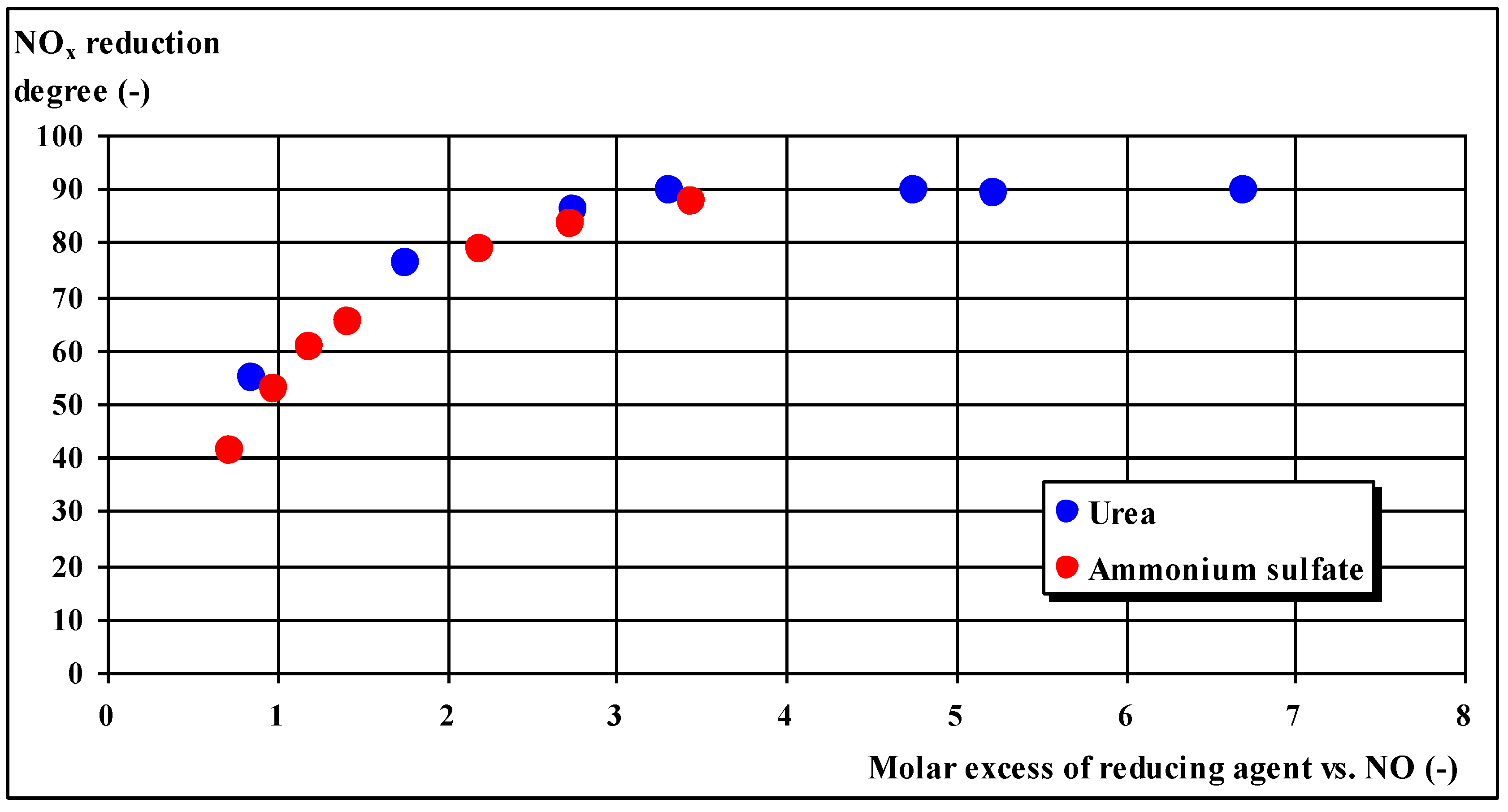
Table 1 shows the impact of excess urea in the reduction of NO
x, and
Table 2 shows the effects of excess ammonium sulfate on the reduction of NO
x. The obtained results are additionally illustrated in
Figure 2.
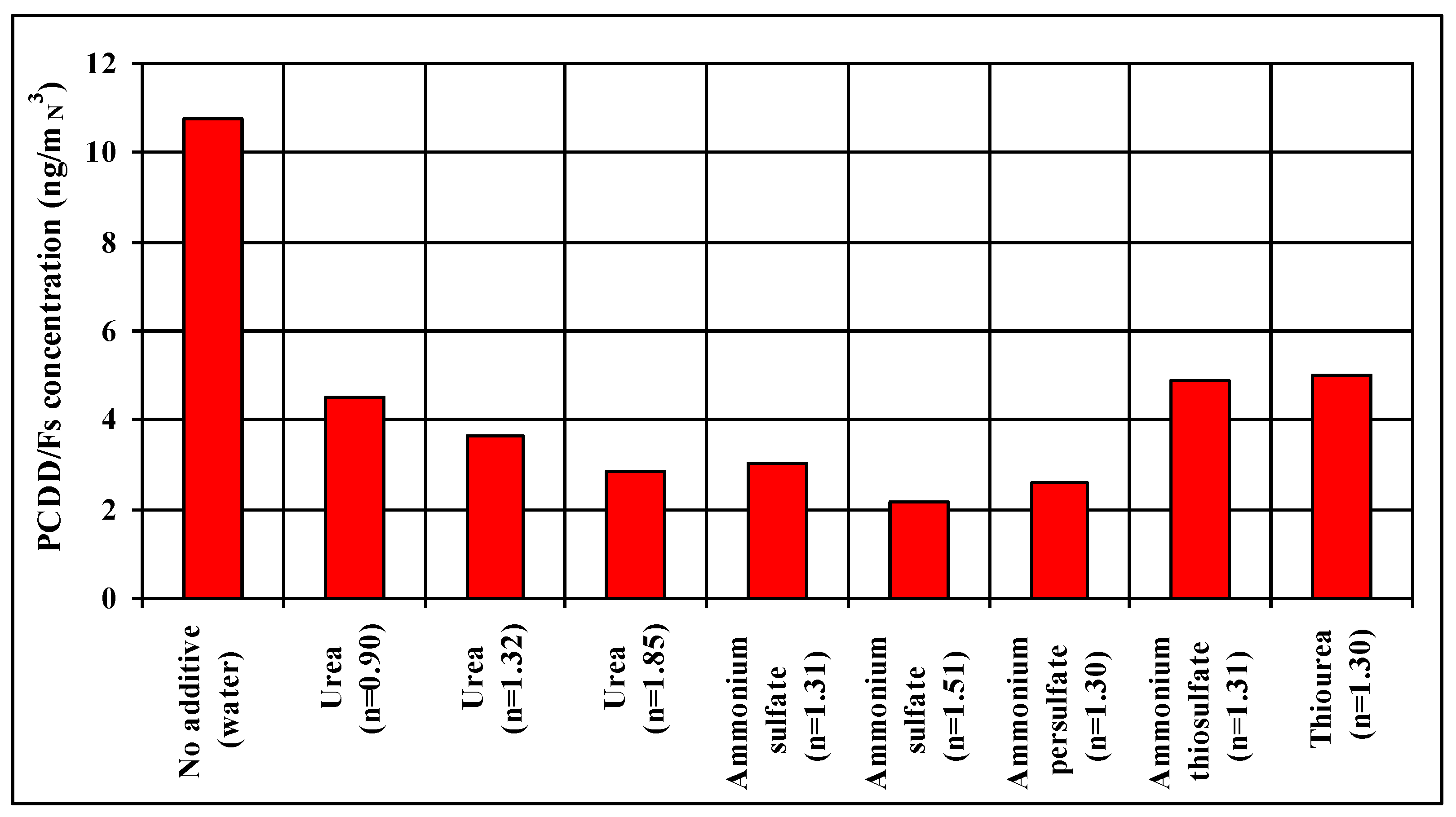
The results of the simultaneous removal of nitrogen oxides and dioxins and furans from flue gases, including the results of measurements of dioxin emissions, are summarized in
Table 3 and illustrated in
Figure 3.
It is difficult to compare the results obtained with literature data. The effectiveness of the reduction of nitrogen oxides with ammonia, ammonia water, aqueous urea solution, or cyanuric acid is widely known. However, there are no literature reports regarding the use of such compounds as ammonium sulfate, ammonium persulfate, ammonium thiosulfate, or thiourea in the process of reducing nitrogen oxides. On the other hand, the use of the abovementioned compounds as de novo synthesis inhibitors are known; however, according to known literature descriptions, they were usually introduced into the waste gas stream immediately before the de novo synthesis temperature zone. The introduction of de novo synthesis inhibitors as reducing agents in the SNCR process is a novelty not yet described in the literature.
4. Conclusions
To investigate the possibility of simultaneous reduction of NOx and PCDD/Fs emissions from thermal processes (including combustion), tests were carried out in a thermal hazardous waste treatment plant (incineration of medical waste) equipped with an SNCR system and continuous emissions monitoring, which provided the opportunity to assess the effect of the change of reactant on the SNCR process efficiency. As part of the project, the effects of the addition of NOx emission reducers and the de novo PCDD/Fs synthesis inhibitors, including urea and chemical compounds containing both nitrogen and sulfur, i.e., ammonium sulfate, ammonium persulfate, ammonium thiosulfate, and thiourea, were examined.
The ammonia formed as a result of the decomposition of the compounds used confirmed its NOx reduction properties during the SNCR process. Simultaneously, the presence of both ammonia and the second degradation product, SO2, proved to be effective factors inhibiting PCDD/Fs synthesis according to the de novo mechanism.
As a result of these tests, the following maximum degrees of simultaneous emission reduction were obtained (for the molar coefficient of excess reducer in relation to nitrogen oxides equal to approximately 1.3):
NOx: 63–67%,
PCDD/Fs: 52–80%.
The best results (simultaneously maximum NOx and PCDD/Fs emission reduction) were obtained from a water solution of ammonia sulfate and persulfate.
These results suggest that it is possible to simultaneously reduce NOx emissions and dioxins from the combustion process using reducers/inhibitors, such as urea, ammonium sulfate, ammonium persulfate, ammonium thiosulfate, and thiourea. The efficiency of the tested reagents in the reduction of NOx emissions is similar, while the degree of reduction of PCDD/Fs emissions of up to 75% is very interesting from a technological point of view. Preliminary tests confirm the effectiveness of the technology for simultaneous reduction of NOx and PCDD/Fs emissions from thermal processes.